Abstract
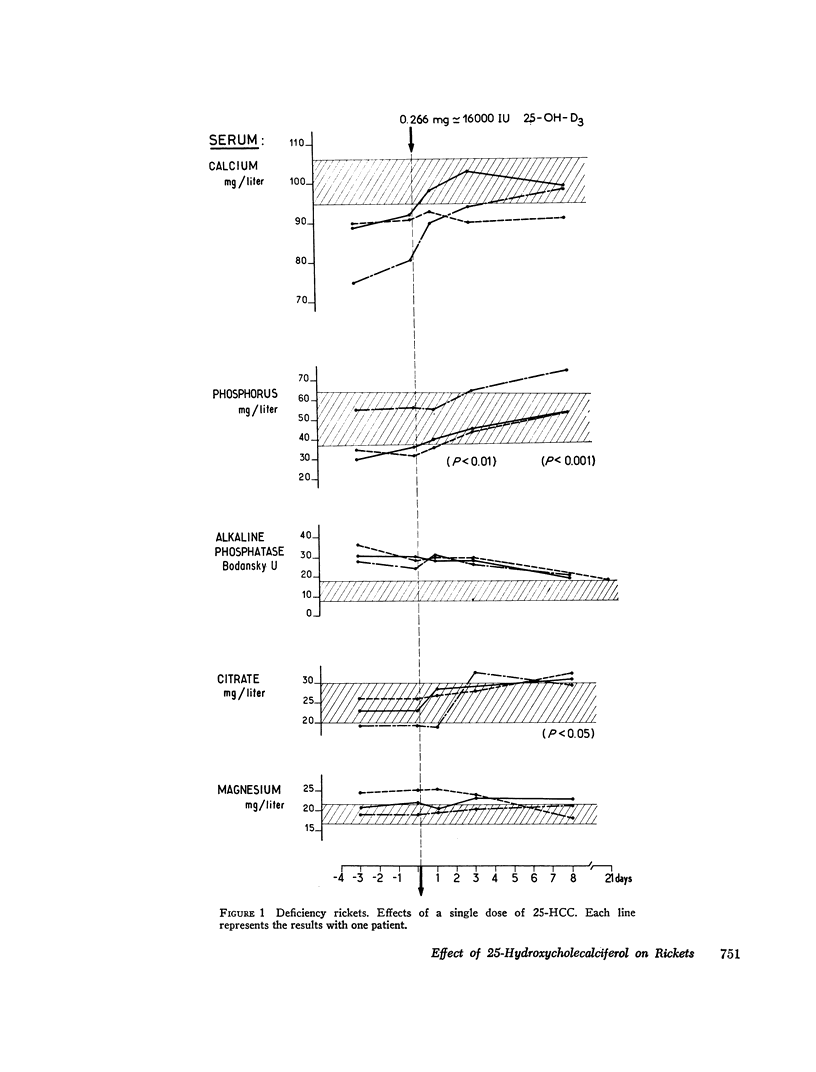
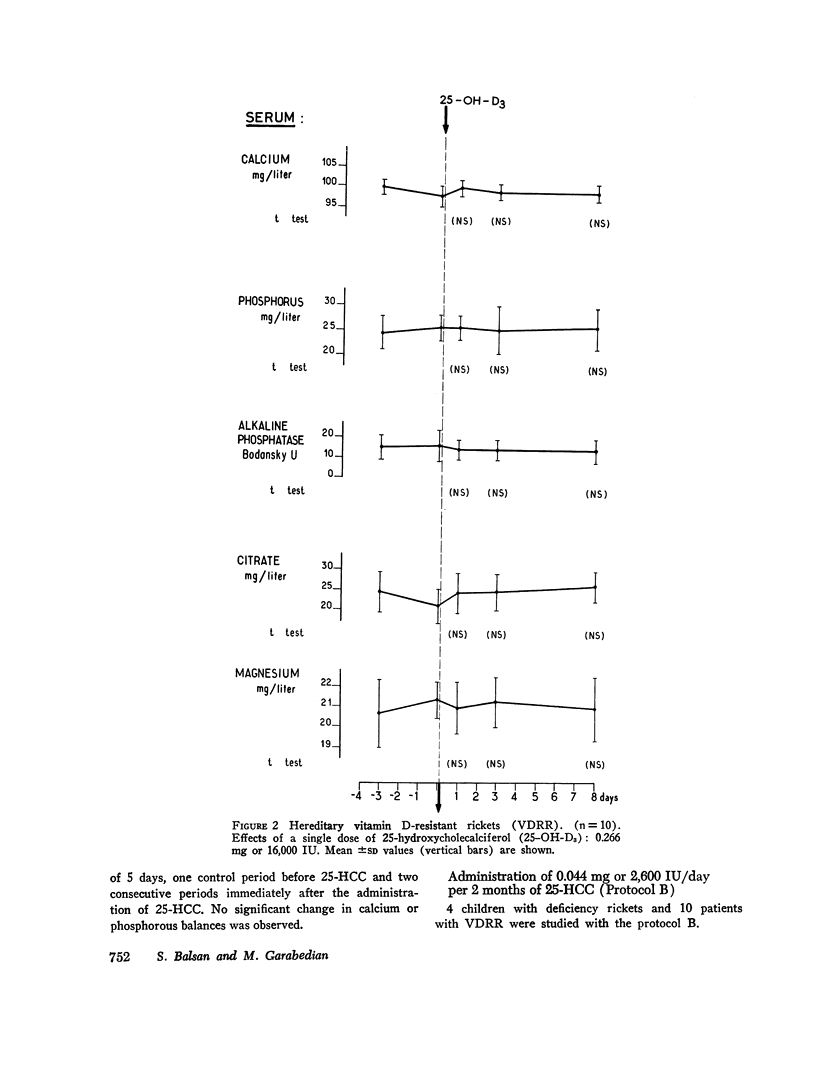
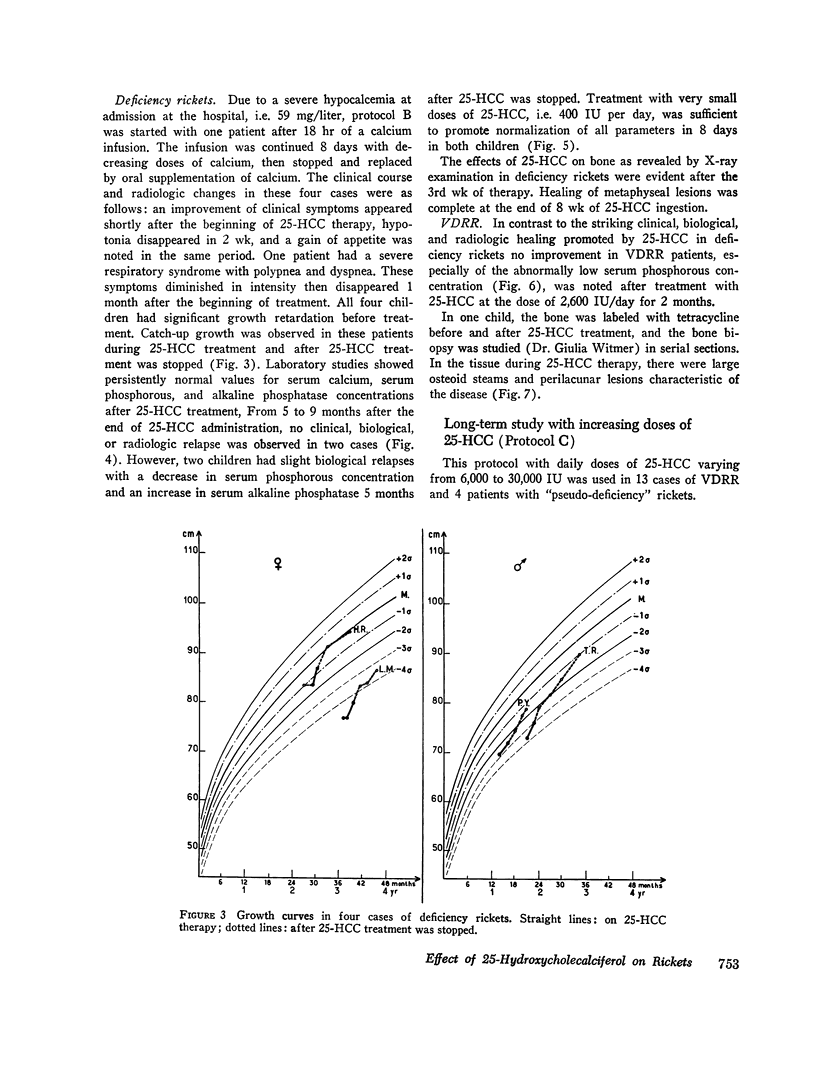
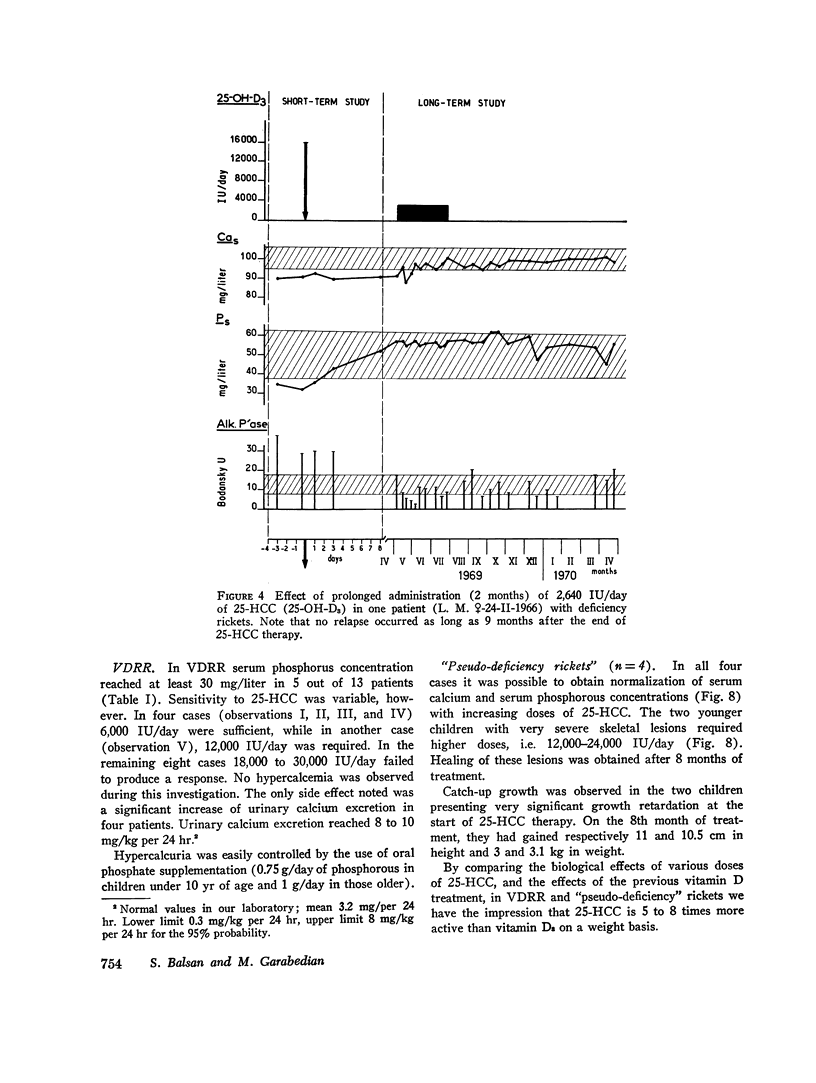
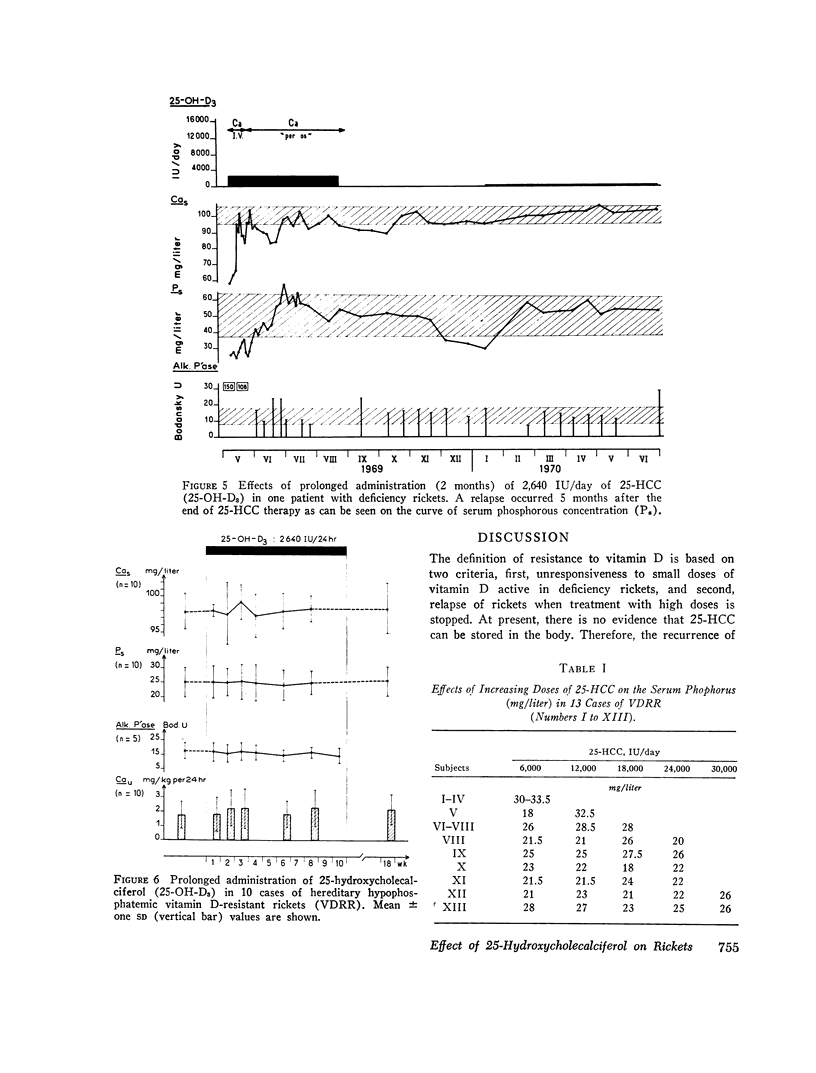
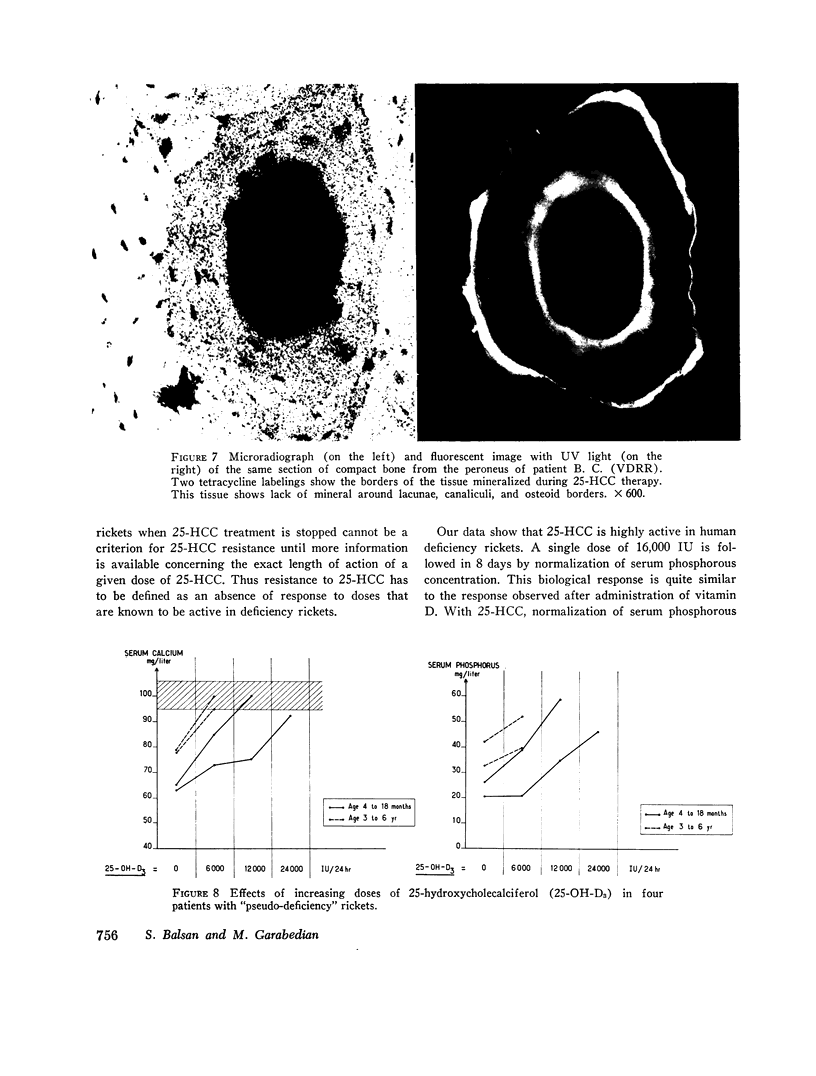
The effects of 25-hydroxycholecalciferol were studied in 4 children with deficiency rickets and 22 children with D-resistant rickets, including patients with hereditary hypophosphatemic D-resistant rickets, “pseudo-deficiency” rickets, and rickets secondary to cystinosis or to tyrosinosis. Three protocols were used. (a) 8 days after a single oral dose of 16,000 IU of 25-hydroxycholecalciferol, normalization of all biological parameters was observed in all cases of deficiency rickets. A complete lack of response was observed in the different types of resistant rickets. (b) Under prolonged administration of 2,640 IU/day for 2 months, clinical-biological symptoms and X-ray lesions disappeared, and a catch-up growth pattern was observed in deficiency rickets; no relapse of rickets occurred up to 5 months after therapy was stopped. The same dose had no significant effect in 10 patients with hereditary hypophosphatemic D-resistant rickets. A bone biopsy performed in one case showed the persistence of characteristic lesions. (c) With increasing doses of 25-hydroxycholecalciferol varying from 6,000 to 30,000 IU/day and a follow-up of 6 months up to 2 yr duration, clinical-biological-radiologic recovery and catch-up growht was obtained in all cases of “pseudo-deficiency” rickets. In hypophosphatemic hereditary D-resistant rickets, 5 out of 13 patients' serum concentration of phosphorus reached at least 30 mg/liter, but a catch-up growth pattern was not observed. These results indicate that (a) 25-hydroxycholecalciferol is highly active in deficiency rickets; (b) a defect in the conversion of vitamin D3 to its active 25-hydroxy metabolite is probably not the metabolic defect in any of the different types of vitamin D-resistant rickets studied.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antener I., Vuataz L., Bruschi A., Kaeser M. Determination of citric acid in urine and in serum. Z Klin Chem Klin Biochem. 1966 Nov;4(6):296–298. doi: 10.1515/cclm.1966.4.6.296. [DOI] [PubMed] [Google Scholar]
- Avioli L. V., Birge S., Lee S. W., Slatopolsky E. The metabolic fate of vitamin D3-3H in chronic renal failure. J Clin Invest. 1968 Oct;47(10):2239–2252. doi: 10.1172/JCI105909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avioli L. V., Williams T. F., Lund J., DeLuca H. F. Metabolism of vitamin D3-3H in vitamin D-resistant rickets and familial hypophosphatemia. J Clin Invest. 1967 Dec;46(12):1907–1915. doi: 10.1172/JCI105680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsan S. 25-hydroxycholecalciferol: effects in idiopathic vitamin D-resistant rickets. Calcif Tissue Res. 1970;(Suppl):45–46. doi: 10.1007/BF02152346. [DOI] [PubMed] [Google Scholar]
- Balsan S., Guivarch J., Sachs C., Garabedian M. 25-hydroxycholécalciférol. Effet d'une dose unique chez l'enfant normal. Rev Eur Etud Clin Biol. 1970 May;15(5):515–521. [PubMed] [Google Scholar]
- Blunt J. W., DeLuca H. F., Schnoes H. K. 25-hydroxycholecalciferol. A biologically active metabolite of vitamin D3. Biochemistry. 1968 Oct;7(10):3317–3322. doi: 10.1021/bi00850a001. [DOI] [PubMed] [Google Scholar]
- Blunt J. W., DeLuca H. F. The synthesis of 25-hydroxycholecalciferol. A biologically active metabolite of vitamin D3. Biochemistry. 1969 Feb;8(2):671–675. doi: 10.1021/bi00830a031. [DOI] [PubMed] [Google Scholar]
- Blunt J. W., Tanaka Y., DeLuca H. F. The biological activity of 25-hydroxycholecalciferol, a metabolite of vitamin D3. Proc Natl Acad Sci U S A. 1968 Oct;61(2):717–718. doi: 10.1073/pnas.61.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. C., Weber J. C., DeLuca H. F. On the subcellular location of vitamin D metabolites in intestine. J Biol Chem. 1970 Aug 10;245(15):3776–3780. [PubMed] [Google Scholar]
- Cousins R. J., DeLuca H. F., Chen T., Suda T., Tanaka Y. Metabolism and subcellular location of 25-hydroxycholecalciferol in intestinal mucosa. Biochemistry. 1970 Mar 17;9(6):1453–1459. doi: 10.1021/bi00808a021. [DOI] [PubMed] [Google Scholar]
- DeLuca H. F., Lund J., Rosenbloom A., Lobeck C. C. Metabolism of tritiated vitamin D3 in familial vitamin D-resistant rickets with hypophosphatemia. J Pediatr. 1967 May;70(5):828–832. doi: 10.1016/s0022-3476(67)80342-1. [DOI] [PubMed] [Google Scholar]
- DeLuca H. F. Recent advances in the metabolism and function of vitamin D. Fed Proc. 1969 Sep-Oct;28(5):1678–1689. [PubMed] [Google Scholar]
- DeLuca H. F., Suda T., Schnoes H. K., Tanaka Y., Holick M. F. 25,26-dihydroxycholecalciferol, a metabolite of vitamin D3 with intestinal calcium transport activity. Biochemistry. 1970 Nov 24;9(24):4776–4780. doi: 10.1021/bi00826a022. [DOI] [PubMed] [Google Scholar]
- Earp H. S., Ney R. L., Gitelman H. J., Richman R., DeLuca H. F. Effects of 25-hydroxycholecalciferol in patients with familial hypophosphatemia and vitamin-D-resistant rickets. N Engl J Med. 1970 Sep 17;283(12):627–630. doi: 10.1056/NEJM197009172831204. [DOI] [PubMed] [Google Scholar]
- Fraser D. R., Kodicek E. Unique biosynthesis by kidney of a biological active vitamin D metabolite. Nature. 1970 Nov 21;228(5273):764–766. doi: 10.1038/228764a0. [DOI] [PubMed] [Google Scholar]
- Haussler M. R., Boyce D. W., Littledike E. T., Rasmussen H. A rapidly acting metabolite of vitamin D3. Proc Natl Acad Sci U S A. 1971 Jan;68(1):177–181. doi: 10.1073/pnas.68.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick M. F., Schnoes H. K., DeLuca H. F. Identification of 1,25-dihydroxycholecalciferol, a form of vitamin D3 metabolically active in the intestine. Proc Natl Acad Sci U S A. 1971 Apr;68(4):803–804. doi: 10.1073/pnas.68.4.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsting M., DeLuca H. F. In vitro production of 25-hydroxycholecalciferol. Biochem Biophys Res Commun. 1969 Jul 23;36(2):251–256. doi: 10.1016/0006-291x(69)90322-2. [DOI] [PubMed] [Google Scholar]
- Kodicek E., Lawson D. E., Wilson P. W. Biological activity of a polar metabolite of vitamin D. Nature. 1970 Nov 21;228(5273):763–764. doi: 10.1038/228763a0. [DOI] [PubMed] [Google Scholar]
- Lund J., DeLuca H. F. Biologically active metabolite of vitamin D3 from bone, liver, and blood serum. J Lipid Res. 1966 Nov;7(6):739–744. [PubMed] [Google Scholar]
- Martin D. L., Melancon M. J., Jr, DeLuca H. F. Vitamin D stimulated, calcium-dependent adenosine triphosphatase from brush borders of rat small intestine. Biochem Biophys Res Commun. 1969 Jun 27;35(6):819–823. doi: 10.1016/0006-291x(69)90697-4. [DOI] [PubMed] [Google Scholar]
- Olson E. B., DeLuca H. F. 25-hydroxycholecalciferol: direct effect on calcium transport. Science. 1969 Jul 25;165(3891):405–407. doi: 10.1126/science.165.3891.405. [DOI] [PubMed] [Google Scholar]
- PRADER A., ILLIG R., HEIERLI E. [An unusual form of primary vitamin D-resistant rickets with hypocalcemia and autosomal-dominant hereditary transmission: hereditary pseudo-deficiency rickets]. Helv Paediatr Acta. 1961 Dec;16:452–468. [PubMed] [Google Scholar]
- Ponchon G., DeLuca H. F. The role of the liver in the metabolism of vitamin D. J Clin Invest. 1969 Jul;48(7):1273–1279. doi: 10.1172/JCI106093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelig M. S. Vitamin D and cardiovascular, renal, and brain damage in infancy and childhood. Ann N Y Acad Sci. 1969 Sep 26;147(15):539–582. doi: 10.1111/j.1749-6632.1967.tb41272.x. [DOI] [PubMed] [Google Scholar]
- Smith J. E., Goodman D. S. The turnover and transport of vitamin D and of a polar metabolite with the properties of 25-hydroxycholecalciferol in human plasma. J Clin Invest. 1971 Oct;50(10):2159–2167. doi: 10.1172/JCI106710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohs S. J., DeLuca H. F. Subcellular location of vitamin D and its metabolites in intestinal mucosa after a 10-IU dose. Biochemistry. 1967 Nov;6(11):3338–3349. doi: 10.1021/bi00863a002. [DOI] [PubMed] [Google Scholar]
- Suda T., DeLuca H. F., Schnoes H. K., Blunt J. W. The isolation and identification of 25-hydroxyergocalciferol. Biochemistry. 1969 Sep;8(9):3515–3520. doi: 10.1021/bi00837a005. [DOI] [PubMed] [Google Scholar]
- Suda T., DeLuca H. F., Schnoes H. K., Ponchon G., Tanaka Y., Holick M. F. 21,25-dihydroxycholecalciferol. A metabolite of vitamin D3 preferentially active on bone. Biochemistry. 1970 Jul 7;9(14):2917–2922. doi: 10.1021/bi00816a025. [DOI] [PubMed] [Google Scholar]
- Suda T., DeLuca H. F., Tanaka Y. Biological activity of 25-hydroxyergocalciferol in rats. J Nutr. 1970 Sep;100(9):1049–1052. doi: 10.1093/jn/100.9.1049. [DOI] [PubMed] [Google Scholar]
- Trummel C. L., Raisz L. G., Blunt J. W., Deluca H. F. 25-Hydroxycholecalciferol: stimulation of bone resorption in tissue culture. Science. 1969 Mar 28;163(3874):1450–1451. doi: 10.1126/science.163.3874.1450. [DOI] [PubMed] [Google Scholar]
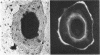
- Witmer G., Balsan S. Biopsie osseuse dans quatre cas de rachitisme vitamino-résistant idiopathique. Pathol Biol. 1968 Apr;16(7):421–429. [PubMed] [Google Scholar]