Abstract
Background
The plasma B-type natriuretic peptide (BNP) level is a useful prognostic marker in heart failure and valvular heart disease. In patients with isolated severe tricuspid regurgitation (TR), little is known about the determinants of plasma BNP levels and the correlation with future outcome. The purpose of this study was to identify the determinants of plasma BNP levels in patients with isolated severe TR and the value of the BNP level in predicting postoperative outcomes after corrective surgery.
Methods
We prospectively enrolled 39 patients with isolated, severe TR undergoing corrective surgery. A plasma BNP assay and cardiac magnetic resonance (CMR) imaging were performed before surgery. The combined end-point was the occurrence of cardiac death or readmission due to heart failure.
Results
Linear regression analysis showed that the left ventricular ejection fraction and right ventricular end systolic volume were the most important determinants of the BNP levels (p = 0.002, R2 = 0.315). Based on the receiver operating characteristics (ROC) curve, we were able to derive an optimal cutoff value (200 pg/mL) to predict postoperative cardiac death with a sensitivity of 80% and a specificity of 85%. The one-year survival rate was 96% in patients with a BNP < 200 pg/mL and 53% in patients with a BNP ≥ 200 pg/dL (p = 0.001).
Conclusion
An elevation in the BNP level is determined by the functional status of the right and left ventricles in patients with isolated, severe TR. An elevated BNP predicts adverse events after corrective surgery. Therefore, the BNP level should be included in the clinical evaluation and risk stratification of patients with isolated TR.
Keywords: B-type natriuretic peptide, Tricuspid regurgitation, Surgery, Cardiac magnetic resonance
Introduction
Tricuspid regurgitation (TR) is a common finding in patients who have previously undergone left-sided valve surgery.1),2) Determining the optimal time for corrective surgery remains a difficult clinical problem in patients with severe TR. Subjective symptoms in patients with severe TR are often non-specific, progress very slowly, and only become evident after irreversible right ventricular (RV) dysfunction occurs. Echocardiographic or cardiac magnetic resonance (CMR) measurement of the complex geometry of the RV have been investigated to predict the impact of TR on long-term prognosis independent of the left ventricular (LV) ejection fraction or pulmonary artery pressure3),4) because high surgical mortality and morbidity have been reported in these patients;5-7) however, it is extremely difficult to obtain accurate and reproducible information regarding RV haemodynamics in a quantitative manner using echocardiography, the technique most frequently used to assess cardiac haemodynamics.8) Although CMR imaging has emerged as the reference standard imaging modality for quantitative assessment of RV volumes, systolic function, and valve function, CMR imaging is not readily available because of the high cost, and CMR imaging is a time-consuming process. Therefore, it is of paramount importance to provide a surrogate marker to predict clinical outcomes after surgery and to determine surgical timing for patients with severe TR. B-type natriuretic peptide (BNP) is primarily secreted from the cardiac ventricles in response to ventricular volume and pressure overload;9),10) the stimulus for BNP release is an increase in ventricular wall stress.11) The plasma BNP level is elevated in patients with heart failure12) and hypertrophy.13) In patients with valvular heart disease, such as aortic stenosis and mitral regurgitation, increased levels of BNP correlate with poor functional capacity, the severity of valve disease, and the clinical outcome;14),15) however, there, have been no studies dealing with this important issue in patients with TR. In the present study, we prospectively enrolled patients with isolated severe TR to evaluate the role of BNP as a predictor of clinical outcomes after surgery.
Methods
Study subjects
We prospectively enrolled consecutive patients with isolated severe TR who underwent corrective surgery between January 2006 and March 2009. All patients satisfied the following 3 criteria for severe TR: (1) a TR jet area > 30% of the right atrial area; (2) inadequate cusp coaptation; and (3) systolic flow reversal in the hepatic vein. Patients with significant left-sided valve disease who underwent concomitant left-sided valve surgery were excluded from the study. Ultimately, 39 patients were enrolled. The mean patient age was 55.9 years of age; 33 patients (84.6%) were women. In all patients, CMR imaging was performed before surgery and clinical follow-up was performed after surgery. The occurrence of clinical events was determined by reviewing the hospital records and telephone interview if needed. The study protocol was approved by the Institutional Review Board of Seoul National University Hospital.
BNP assay
All of the venous blood samples were collected in EDTA tubes for measurement of BNP levels. Assays for BNP were performed with a triage B-type Natriuretic Peptide test (Biosite Diagnostics, Inc., San Diego, CA, USA) which is a fluorescence immunoassay for the quantitative determination of BNP in whole blood and plasma specimens. The triage B-type Natriuretic Peptide test has a turnaround time of 15 minutes and a measurement range of 5 - 5,000 pg/mL; this method correlates the fluorescence measurement to the BNP concentration using an internal calibration curve. The pre-operative BNP levels were obtained within 1 week prior to surgery.
CMR imaging
CMR imaging examinations were performed on a 1.5 Tesla system (Sonata Magnetom, Siemens, Erlangen, Germany). All images were acquired by a phased-array body surface coil during breath holds, and were electrocardiogram-triggered. After localizer imaging, cine true-fast imaging with steady-state precession imaging (TrueFISP; repetition time/echo time, 45 ms/1.3 ms; flip angle 80°; matrix 256×169; field of view 330×330 mm; slice thickness, 8 mm) was performed in several long-axis planes (2-, 3-, and 4-chamber views) of the heart. Parallel short-axis sections from the valve plane to the apex were acquired for volumetric analysis. Velocity-encoding cine CMR imaging with free breathing and prospective electrocardiography (ECG) gating (repetition time/echo time, 45 ms/3.2 ms; flip angle 30°; matrix 256×238; field of view 330×330 mm; slice thickness, 5 mm) was performed in a plane orthogonal to the right and left pulmonary arteries to allow net forward volume of right ventricle (RV) to be calculated. RV and LV end-diastolic (EDV) and end-systolic volumes (ESV), stroke volume, cardiac output, ejection fraction (EF), and flow profiles of the right and left pulmonary arteries were measured by one independent observer on a personal computer using dedicated software (QMASS MR; Medis, Leiden, The Netherlands). Ventricular volumes, stroke volume, and cardiac output were corrected for body surface area. The amount of TR was calculated by subtracting the net pulmonary blood volume of the right and left pulmonary arteries from the RV stroke volume. The TR fraction was calculated by dividing the TR amount by the RV stroke volume (expressed as a percentage).
Statistical analysis
Data are expressed as the mean ± SEM or median (range) for continuous variables and as numbers (percentages) for categorical variables. For comparison of continuous and categorical variables between patients with clinical events and those without clinical events, the Mann-Whitney U test and Fisher exact test were used, respectively. Clinical end points or events were defined as cardiac death and readmission due to heart failure. Using receiver operating characteristic (ROC) curves, we examined the sensitivities and specificities of various cut-off points that reliably predicted cardiac deaths. Linear regression analysis with the use of forward selection based on the likelihood ratio test was used to determine independent variables for the level of BNP. A Kaplan-Meier curve was constructed to demonstrate the survival difference in relation to the cut-off level of BNP according to the presence or absence of cardiac deaths with the use of the log-rank test. SPSS (version 17.0) was used for statistical analyses. p values < 0.05 were considered statistically significant.
Results
Baseline characteristics
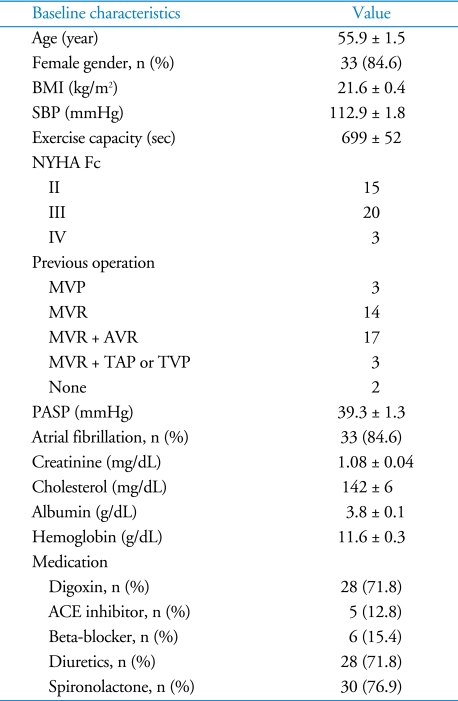
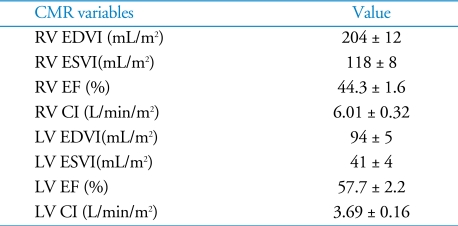
Thirty-nine patients were included in the analysis; their baseline characteristics of the patients are shown in Table 1. Thirty-seven patients (95%) had previous left-sided valve surgery. Three of the 37 patients underwent tricuspid valve surgery. Two patients (5%) had no previous cardiac surgery. The pulmonary artery systolic pressure, as estimated by echocardiography, was not elevated, suggesting LV dysfunction did not affect the development of isolated TR in this group of patients. The median BNP level was 64 pg/mL and the interquartile range (25 - 75%) was 40 - 181 pg/mL. The CMR variables are described in Table 2. End diastolic and end systolic RV volume were significantly increased. In contrast to the RV volume, the LV volume was mildly increased and the mean LV EF was normal.
Table 1.
Baseline characteristics
Values: mean ± SEM. BMI: body mass index, SBP: systolic blood pressure, exercise capacity: exercise time on treadmill by Bruce protocol, NYHA Fc: New York Heart Association Functional class, MVP: mitral valvuloplasty, MVR: mitral valve replacement, AVR: aortic valve replacement, TAP: tricuspid annuoplasty, TVP: tricuspid valvuloplasty, PASP: estimated pulmonary artery systolic pressure by echocardiography
Table 2.
CMR variables
Values: mean ± SEM. CMR: cardiac magnetic resonance, RV: right ventricle,LV: left ventricle, EDVI: end diastolic volume index, ESVI: end systolic volume index, EF: ejection fraction, CI: cardiac index
RV ESV index and LV EF determine the plasma BNP level in patients with isolated, severe TR
To identify important CMR determinants associated with the plasma BNP level in patients with isolated, severe TR, we performed linear regression analysis with the forward stepwise selection method (criteria to include, probability of F < 0.050; criteria to exclude, probability of F > 0.100). The dependent variable was the value of logarithmic transformation of the plasma BNP level because distribution of BNP level was not normal. Logarithmic transformation resulted in a normal distribution of the dependent variable which was evaluated by Kolmogorov-Smirnov test. The independent variables were CMR parameters. The LV EF and RV ESV were the 2 most important determinants of the plasma BNP level [p = 0.002, R2 = 0.315, logBNP = 0.003×[RV end systolic volume index (ESVI)] - 0.014×(LV EF) + 2.346]. Regression diagnostics by residual plots revealed no relevant violations. On ROC curve analysis, we found that a LV EF of 46% (sensitivity, 75%; and specificity, 85%) and RV ESVI 140 mL/m2 (sensitivity, 86% and specificity, 85%) predicted a BNP ≥ 200 pg/mL most effectively.
Plasma BNP level predicts cardiac death after open heart surgery for isolated, severe TR
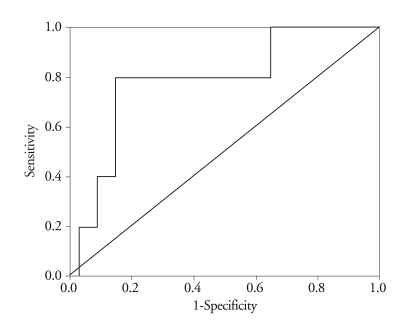
The area of the ROC curve relating BNP levels to postoperative mortality was 0.788 (95% confidence interval: 0.573 - 1.003; p = 0.04)(Fig. 1). Based on the ROC curve, we were able to derive an optimal cut-off value (200 pg/mL) to predict post-operative cardiac death.
Fig. 1.
ROC curve for determining cut-off value to predict outcome after surgery. ROC: receiver operating characteristic.
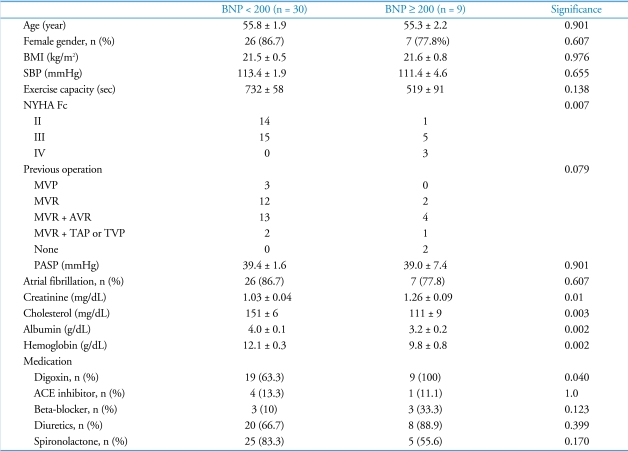
The baseline characteristics of patients according to BNP level are compared in Table 3. No significant differences were observed between both groups with respect to age, gender, body mass index, exercise capacity, and the presence of atrial fibrillation. Patients with a BNP level ≥ 200 pg/mL had a significantly higher New York Heart Association functional class and a lower level of serum creatinine, cholesterol, albumin, and hemoglobin. Among baseline characteristics, the serum albumin level was the most important factor in determining the plasma BNP level [R2 = 0.258, p = 0.004, logBNP = 3.219 - 0.324×(albumin)]. The pulmonary artery systolic pressure, as estimated by echocardiography, was not different between the two groups, suggesting that the RV afterload was comparable between the groups.
Table 3.
Baseline characteristics according to BNP levels
Values: mean ± SEM. BNP: B-type natriuretic peptide, BMI: body mass index, SBP: systolic blood pressure, exercise capacity: exercise time on treadmill by Bruce protocol, NYHA Fc: New York Heart Association Functional class, MVP: mitral valvuloplasty, MVR: mitral valve replacement, AVR: aortic valve replacement, TAP: tricuspid annuloplasty, TVP: tricuspid valvuloplasty, PASP: estimated pulmonary artery systolic pressure by echocardiography
The CMR variables of patients according to BNP level are compared in Table 4. RV volume was significantly larger and LV EF was significantly lower in the patients with a BNP level ≥ 200 pg/mL that in the patients with a BNP level < 200 pg/mL.
Table 4.
CMR variables according to BNP levels
Values: mean ± SEM. CMR: cardiac magnetic resonance, BNP: B-type natriuretic peptide, RV: right ventricle, LV: left ventricle, EDVI: end diastolic volume index, ESVI: end systolic volume index, EF: ejection fraction, CI: cardiac index, LV: left ventricle
The high BNP group showed worse survival after corrective surgery for isolated, severe TR
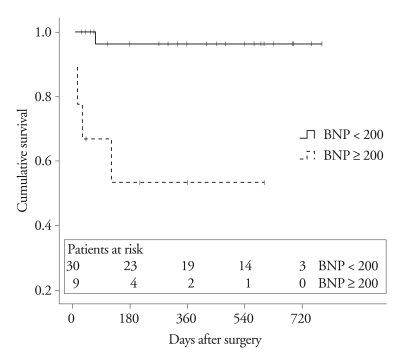
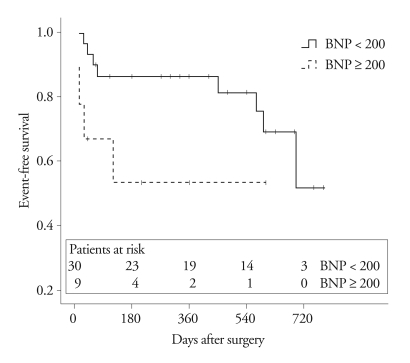
Thirty-eight patients underwent tricuspid valve replacement and one patient underwent tricuspid valve repair and annuloplasty. The median duration of follow-up after surgery was 420 days (range, 11 - 780 days). Five of the 39 patients died after surgery (1 patient in the lower BNP group and 4 patients in the higher BNP group); all of 5 patients died due to congestive heart failure. Kaplan-Meier curves and log-rank analysis revealed a significant difference between the 2 BNP groups (p = 0.001)(Fig. 2). The 1-year survival rate was 96 ± 4% in patients with a BNP < 200 pg/mL, and 53 ± 17% in patients with a BNP ≥ 200 pg/dL. Combined events, including death and readmission due to congestive heart failure, occurred in 12 among 39 patients during the follow-up period. The patients with BNP < 200 pg/mL had fewer events within 1 year following surgery. Kaplan-Meier survival curves and log-rank analysis showed a significant difference between the two groups during follow-up (p = 0.049)(Fig. 3).
Fig. 2.
Kaplan-Meier survival curve for death after surgery according to BNP level. BNP: B-type natriuretic peptide.
Fig. 3.
Kaplan-Meier survival curve for death and re-admission due to heart failure after surgery according to BNP level. BNP: B-type natriuretic peptide.
Discussion
This is the first study to determine the BNP levels in patients with severe, isolated TR in relation to CMR parameters, and to evaluate the role of BNP as a surrogate marker to predict future outcomes after surgery. We found that the following: (1) the BNP was determined by the LV EF and RV ESVI in patients with severe, isolated TR; (2) a BNP ≥ 200 pg/mL was the best cut-off value to predicted poor outcome after corrective surgery; and (3) patients with a BNP ≥ 200 pg/mL had higher mortality and morbidity after surgery.
The occurrence of functional TR after left-sided surgery is not an infrequently event and is well-known to be closely linked to exercise intolerance and to portend a poor prognosis.8),16),17) In an earlier study performed at our institution, corrective TR surgery was associated with a high operative mortality and morbidity.4) Therefore, the decision on whether or not to proceed to TR surgery is difficult, which made us search hemodynamic parameters of echocardiography and CMR imaging predicting prognosis in patients with severe TR.3),4) In addition, we would like to have a simple and easily available surrogate marker to predict the prognosis of patients with severe, isolated TR. Patients with severe, isolated TR need repeated evaluation because the isolated functional TR normally occurs long after left-sided surgery. BNP activation is a well-known phenomenon in patients with heart failure, but the role of BNP in patients with isolated function TR is not known.
The BNP level was determined by the LV EF and RV ESVI in the present study. In a previous study, BNP was reported to be independent of LV and RV systolic function in 105 patients with RV or LV dysfunction by first-pass radionuclide ventriculography and multiple ECG-gated equilibrium radionuclide ventriculography, respectively.18) Based on multivariate analysis, the authors showed that only RVEF and LVEF remained significant. In the present study, analyzing patients with severe, isolated TR, we also arrived at the same conclusion that the BNP level is dependent on LV and RV systolic function. However, we found that not the RV EF, but the RV ESVI, was a significant parameter in determining the BNP level using CMR imaging. Severe TR induces a chronic volume overload of the RV, which leads to progressive RV dilation, dysfunction, and finally RV failure. The pathophysiologic sequence suggests that RV ESVI may be a more sensitive factor to be associated with BNP elevation in patients with severe TR. Our previous report showing that timely-performed surgery for patients with severe functional TR can preserve RV function (RV EF) also supports this idea.3)
LV EF was the most important factor in determining plasma BNP in patients with severe TR in the present study. Pulmonary arterial pressure may be an important factor which determines the hemodynamic significance of severe TR. A low LV EF frequently elevates the pulmonary arterial pressure, which may activate BNP. However, there was no difference in terms of pulmonary artery pressure estimated by echocardiography between the low BNP group and the high BNP group. Therefore, the LV EF may have affected the BNP level independent of RV function in the present study.
The complex geometry of the RV is an obstacle to performing research, because it is extremely difficult using echocardiography to obtain accurate and reproducible information regarding RV hemodynamics in a quantitative manner, the technique most frequently used to assess cardiac haemodynamics.8) CMR imaging has emerged as a reference standard imaging modality for quantitative assessment of RV volumes, systolic function, and valve function.19),20) With this technology, we here attempted to determine the effects of RV and LV hemodynamics on the BNP level in patients with severe TR in the absence of left-sided valve dysfunction.
Study limitations
First, in most of our study subjects, the cause of TR was functional and occurred late after left-sided valve surgery, and thus our findings may not be directly applicable to TR patients with other organic valve diseases. Second, because we did not have a comparative group, we cannot conclude that surgery is better than medical therapy for patients with elevated BNP levels. This is an inherent limitation of clinical studies involving valvular heart disease because of the relatively low prevalence of the disease and the duration of time required for the hemodynamic disturbance to affect chamber remodeling and function. Finally, the cut-off value of the BNP level by the ROC curve could not be validated in a subsequent patient group because of the low prevalence of severe TR. Despite these limitations, we believe that the values suggested in the present study can aid in clinical decision-making and can guide future research regarding this issue.
In conclusion, the present study demonstrated that in patients with isolated, severe TR, an elevation in BNP level is present and biologically active and reflects the hemodynamic interaction of RV and LV. Furthermore, elevated levels of BNP are independent predictors of mortality and morbidity after corrective surgery. Thus, the BNP level emerged as a biomarker of the severity of TR consequences and of poor clinical outcome in patients with isolated TR. Measurement of the BNP should be considered in patients with isolated TR to support the clinical decision-making process. These findings should be further evaluated in larger clinical trials.
Acknowledgements
This study was supported by a grant from Korea Institute of Medicine and the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare, and Family Affairs, Republic of Korea (A090458).
References
- 1.Czer LS, Maurer G, Bolger A, DeRobertis M, Kleinman J, Gray RJ, Chaux A, Matloff JM. Tricuspid valve repair. Operative and follow-up evaluation by Doppler color flow mapping. J Thorac Cardiovasc Surg. 1989;98:101–110. discussion 110-1. [PubMed] [Google Scholar]
- 2.Kim HK, Kim YJ, Kim KI, Jo SH, Kim KB, Ahn H, Sohn DW, Oh BH, Lee MM, Park YB, Choi YS. Impact of the maze operation combined with left-sided valve surgery on the change in tricuspid regurgitation over time. Circulation. 2005;112:I14–I19. doi: 10.1161/CIRCULATIONAHA.104.524496. [DOI] [PubMed] [Google Scholar]
- 3.Kim HK, Kim YJ, Park EA, Bae JS, Lee W, Kim KH, Kim KB, Sohn DW, Ahn H, Park JH, Park YB. Assessment of haemodynamic effects of surgical correction for severe functional tricuspid regurgitation: cardiac magnetic resonance imaging study. Eur Heart J. 2010;31:1520–1528. doi: 10.1093/eurheartj/ehq063. [DOI] [PubMed] [Google Scholar]
- 4.Kim YJ, Kwon DA, Kim HK, Park JS, Hahn S, Kim KH, Kim KB, Sohn DW, Ahn H, Oh BH, Park YB. Determinants of surgical outcome in patients with isolated tricuspid regurgitation. Circulation. 2009;120:1672–1678. doi: 10.1161/CIRCULATIONAHA.109.849448. [DOI] [PubMed] [Google Scholar]
- 5.Kuwaki K, Morishita K, Tsukamoto M, Abe T. Tricuspid valve surgery for functional tricuspid valve regurgitation associated with left-sided valvular disease. Eur J Cardiothorac Surg. 2001;20:577–582. doi: 10.1016/s1010-7940(01)00786-2. [DOI] [PubMed] [Google Scholar]
- 6.Kwon DA, Park JS, Chang HJ, Kim YJ, Sohn DW, Kim KB, Ahn H, Oh BH, Park YB, Choi YS. Prediction of outcome in patients undergoing surgery for severe tricuspid regurgitation following mitral valve surgery and role of tricuspid annular systolic velocity. Am J Cardiol. 2006;98:659–661. doi: 10.1016/j.amjcard.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 7.Staab ME, Nishimura RA, Dearani JA. Isolated tricuspid valve surgery for severe tricuspid regurgitation following prior left heart valve surgery: analysis of outcome in 34 patients. J Heart Valve Dis. 1999;8:567–574. [PubMed] [Google Scholar]
- 8.Kim HK, Kim YJ, Park JS, Kim KH, Kim KB, Ahn H, Sohn DW, Oh BH, Park YB, Choi YS. Determinants of the severity of functional tricuspid regurgitation. Am J Cardiol. 2006;98:236–242. doi: 10.1016/j.amjcard.2006.01.082. [DOI] [PubMed] [Google Scholar]
- 9.Mukoyama M, Nakao K, Hosoda K, Suga S, Saito Y, Ogawa Y, Shirakami G, Jougasaki M, Obata K, Yasue H, et al. Brain natriuretic peptide as a novel cardiac hormone in humans. Evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide. J Clin Invest. 1991;87:1402–1412. doi: 10.1172/JCI115146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakagawa M, Tanaka I, Suga S, Ogawa Y, Tamura N, Goto M, Sugawara A, Yoshimasa T, Itoh H, Mukoyama M, et al. Preparation of a monoclonal antibody against mouse brain natriuretic peptide (BNP) and tissue distribution of BNP in mice. Clin Exp Pharmacol Physiol Suppl. 1995;22:S186–S187. doi: 10.1111/j.1440-1681.1995.tb02874.x. [DOI] [PubMed] [Google Scholar]
- 11.Ikeda K, Asahara T, Achiwa K, Hoshino H. Synthesis of N-acetylglucosaminyl- and N-acetylgalactosaminylceramides as cerebroside analogs and their anti-human immunodeficiency virus type 1 activities. Chem Pharm Bull (Tokyo) 1997;45:402–405. doi: 10.1248/cpb.45.402. [DOI] [PubMed] [Google Scholar]
- 12.Rademaker MT, Charles CJ, Espiner EA, Nicholls MG, Richards AM, Kosoglou T. Combined neutral endopeptidase and angiotensin-converting enzyme inhibition in heart failure: role of natriuretic peptides and angiotensin II. J Cardiovasc Pharmacol. 1998;31:116–125. doi: 10.1097/00005344-199801000-00017. [DOI] [PubMed] [Google Scholar]
- 13.Isnard R. NT-BNP/BNP for screening left ventricular hypertrophy in hypertension: what else? Arch Cardiovasc Dis. 2008;101:295–297. doi: 10.1016/j.acvd.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Detaint D, Messika-Zeitoun D, Avierinos JF, Scott C, Chen H, Burnett JC, Jr, Enriquez-Sarano M. B-type natriuretic peptide in organic mitral regurgitation: determinants and impact on outcome. Circulation. 2005;111:2391–2397. doi: 10.1161/01.CIR.0000164269.80908.9D. [DOI] [PubMed] [Google Scholar]
- 15.Pizarro R, Bazzino OO, Oberti PF, Falconi M, Achilli F, Arias A, Krauss JG, Cagide AM. Prospective validation of the prognostic usefulness of brain natriuretic peptide in asymptomatic patients with chronic severe mitral regurgitation. J Am Coll Cardiol. 2009;54:1099–1106. doi: 10.1016/j.jacc.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 16.Groves PH, Lewis NP, Ikram S, Maire R, Hall RJ. Reduced exercise capacity in patients with tricuspid regurgitation after successful mitral valve replacement for rheumatic mitral valve disease. Br Heart J. 1991;66:295–301. doi: 10.1136/hrt.66.4.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nath J, Foster E, Heidenreich PA. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol. 2004;43:405–409. doi: 10.1016/j.jacc.2003.09.036. [DOI] [PubMed] [Google Scholar]
- 18.Vogelsang TW, Jensen RJ, Monrad AL, Russ K, Olesen UH, Hesse B, Kjaer A. Independent effects of both right and left ventricular function on plasma brain natriuretic peptide. Eur J Heart Fail. 2007;9:892–896. doi: 10.1016/j.ejheart.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 19.Grothues F, Moon JC, Bellenger NG, Smith GS, Klein HU, Pennell DJ. Interstudy reproducibility of right ventricular volumes, function, and mass with cardiovascular magnetic resonance. Am Heart J. 2004;147:218–223. doi: 10.1016/j.ahj.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Koskenvuo JW, Järvinen V, Pärkkä JP, Kiviniemi TO, Hartiala JJ. Cardiac magnetic resonance imaging in valvular heart disease. Clin Physiol Funct Imaging. 2009;29:229–240. doi: 10.1111/j.1475-097X.2009.00865.x. [DOI] [PubMed] [Google Scholar]