Abstract
Background
Adrenocortical carcinoma (ACC) is a rare tumor with a poor prognosis. Often, the physicians who first treat patients with ACC have no prior experience with the disease. The aim of our study was to evaluate the quality of medical care for patients with ACC in Germany.
Methods
Data from the German ACC registry were analyzed with regard to the patients’ preoperative diagnostic evaluation, histopathological reporting, and clinical follow-up. The findings were compared with the recommendations of the European Network for the Study of Adrenal Tumors (ENSAT).
Results
Data were analyzed from 387 patients who had been given an initial diagnosis of ACC in the years 1998 to 2009. 21% of them underwent no hormonal evaluation before surgery, and 59% underwent an inadequate hormonal evaluation. This exposed the patients to unnecessary perioperative risks and impaired their follow-up. 48% did not undergo CT scanning of the chest, even though the lungs are the most frequent site of metastases of ACC. For 13% of the patients, the diagnosis of ACC was later revised by a reference pathologist. For 11% of the patients, the histopathology report contained no information about resection status, even though this is an important determinant of further treatment and prognosis. Optimal management requires re-staging at three-month intervals, yet some patients underwent re-staging only after a longer delay, or not at all.
Conclusion
We have identified significant deficits in the care of patients with ACC in Germany. We suspect that the situation is similar for other rare diseases. The prerequisite to better care is close and early cooperation of the treating physicians with specialized centers.
As with other rare diseases, patients suffering from adrenocortical carcinoma (ACC) are often confronted with the problem that the physician initially providing treatment has no prior experience with the disease and is therefore uncertain of the diagnostic and therapeutic measures required. Complicating matters further, patients with ACC suffer not only from the malignant mass itself but often also from the consequences of an excess of hormones, which is not always recognized in the early stages.
ACC (C74.0) can develop at any age, and more than half the patients are less than 45 years of age (1). The incidence is approximately one disease per million population (2, e1). Women are more frequently afflicted than men with a female : male ratio of 1.5 : 1 (1, 3, e2). In approximately 60% of cases the clinical repercussions of autonomous hormone production lead to a diagnosis—generally Cushing’s syndrome or rapidly worsening androgenization in women (4, 5). ACC may also be endocrinologically inactive, however, and may only be noticed due to local abdominal symptoms caused by the mass or they may be incidentally discovered during an imaging procedure. The prognosis is essentially dependent on complete resection of the tumor and thus on the initial tumor stage. If radical surgery is not possible, the median survival is only 12 months (6). The adrenotoxic substance mitotane and various chemotherapy protocols may only control tumor growth in the advanced stages for short periods (7, 8).
Due to the rarity of the disease, information about ACC is based predominantly on retrospective analyses with low case numbers or on personal experiences from individual centers. Because adrenal masses are very common, however (prevalence approximately 2% to 4%) (9), recommendations for preoperative diagnostics have been in place for a number of years (10, 11, e3) and have been confirmed by a National Institutes of Health (NIH) Consensus Conference (12, e4).
In the last ten years, the efforts of a number of working groups and a close international network has significantly improved the available data. In 2003, the German Adrenocortical Carcinoma Registry (Nebennierenkarzinomregister, NKR) was established, and German, French, Italian, and British centers have merged to form the European Network for the Study of Adrenal Tumors (www.ensat.org) (e5). The aim of the present article was to evaluate the medical care of patients with ACC using data from the German ACC Registry (NKR).
Methods
Data acquisition and patient collective
After approval by the Ethics Committee of the University Hospital of Würzburg, the acquisition of data for the German ACC Registry (NKR) began in 2003. Initially, retrospective data were collected. With growing awareness of the registry (due also in part to the homepage www.nebennierenkarzinom.de), greater numbers of cases of newly diagnosed ACC were reported by treating physicians, but also increasingly by patients themselves. The telephone number of the NKR has evolved into a sort of hotline for patients, relatives, and physicians seeking advice.
Currently, an estimated two-thirds of German ACC patients are registered in the NKR registry. However, in some cases contact is only made after recurrence of the carcinoma. To date, 42 patients initially diagnosed in 2008 and 35 patients initially diagnosed in 2009 have been registered, whereas on average more than 60 cases per year have been registered from the years 2005–2007.
Based on physician’s letters, surgery reports, and pathology and laboratory results, information about symptoms, diagnostic procedures, and treatment was entered into structured data collection forms by trained medical personnel. These data were then entered into a special pseudonymized database. About every three months follow-up surveys were conducted, based on evaluation of the submitted physician’s letters or on telephone queries with the treating physicians. All patients gave their written consent to data acquisition and evaluation after appropriate explanations.
At the time the database for this study was closed (December 2009), 591 patients had been included in the NKR registry. Patients for whom no follow-up had been collected (n = 25), patients with a diagnosis prior to 1998 (n = 129), and children less than 16 years of age (n = 34) were excluded from the study. Patients who were initially diagnosed abroad were also not included in the analysis (n = 16). The initial diagnoses for the 387 study patients were done in more than a hundred different clinics or practices.
Histopathological diagnosis and tumor stage
For most of the 387 patients, results from the local pathologist regarding the primary tumor were available (n = 348). Confirmation of the diagnosis by the reference pathologist Professor Saeger (Marienhospital Hamburg) was sought for as many patients as possible and was achieved for 213 study patients (55%).
Tumor stage—Tumor stages were determined using ENSAT classification (13):
Stage I: tumor ≤ 5 cm
Stage II: tumor > 5 cm
Stage III: tumor of any size with infiltration of surrounding tissue, regional lymph node involvement, or venous tumor thrombosis in the inferior vena cava or renal vein
Stage IV: distant metastases.
Resection status—The resection status was divided into:
R0: microscopic tumor-free margin
R1: microscopically verifiable residual tumor
R2: macroscopically verifiable residual tumor, including the presence of metastases
Rx: no statement about resection status.
Assessment of the clinical care
The assessment of the quality of the particular measures was based on treatment guidelines outlined at an international consensus conference in Ann Arbor (USA) in 2003 and the recommendations from ENSAT on the care of patients with ACC (7, 14, 15, e6) (www.ensat.org/acc.htm)—which do not fundamentally differ from earlier recommendations (10, 11) (Table 1).
Table 1. Current recommendations for preoperative diagnostics with suspected adrenocortical carcinoma (ACC)*.
| Preoperative hormonal work-up with suspected ACC | |
| Glucocorticoids (minimum 3 of 4 tests) |
|
| Mineralocorticoids |
|
| Sexual steroids and steroid precursors |
|
| Exclusion of a pheochromocytoma |
|
| Preoperative imaging with suspected ACC | |
| |
*Recommendations from ENS@T (European Network for the Study of Adrenal Tumors), (www.ensat.org/acc.htm); DHEAS, dehydroepiandrosterone sulfate
Preoperative hormonal work-up—The recommended tests for endocrine assessment of adrenocortical carcinomas are listed in Table 1. In the current study clarification of the hormonal situation in the patients studied was defined as complete when the following tests had been carried out:
At least one of the tests indicated in Table 1 to exclude hypercortisolism
Testing of sexual hormones/steroid precursors
A test to exclude pheochromocytoma
The hormonal work-up was otherwise classified as incomplete.
Imaging: Computed tomography (CT), or alternatively magnetic resonance imaging (MRI), of the abdomen is recommended as well as CT of the thorax, because it has been known for many years that the lungs are the most common site for metastasis (16). Bone scintigraphy should be carried out if there is clinical suspicion of bone metastases. Although initial data verify the value of positron emission tomography using F-18 fluorodeoxyglucose (FDG-PET) to discriminate adrenocortical carcinoma (17), this diagnostic tool is still not valid as a standard; it may be useful in individual cases, however.
Follow-up care: Because of the high risk of recurrence, systematic and long-term follow-up is recommended. In the first two years after the initial surgery, this includes three-monthly follow-up examinations using CT or MRI of the abdomen and CT of the thorax as well as detection of endocrine tumor markers with hormonally active tumors.
Results
Features of the study collective
The characteristics of the 387 study patients are listed in Table 2. The study included 242 women and 145 men aged between 16 and 86 years (median 52 years). Cushing’s syndrome, local symptoms due to the mass (for example, feeling of pressure in the abdomen or back or flank pain) or virilization most commonly lead to a diagnosis of adrenocortical carcinoma. Feminization (gynecomastia, testicular atrophy) due to estrogen-producing tumors was rather rare. The high proportion of tumors that were incidentally discovered during imaging for other reasons (“incidentaloma”, 16%) was remarkable.
Table 2. Characteristics of the study collective.
| Total | 387 |
|
242 |
|
145 |
| Age | |
|
52 |
|
16–86 |
| Stage (ENSAT classification*1) | |
|
19 (5%) |
|
150 (39%) |
|
96 (25%) |
|
119 (30%) |
|
3 (1%) |
| Symptoms that led to diagnosis | |
| (multiple entries in part) | |
|
115 (29%) |
|
91 (24%) |
|
59 (24% of the women) |
|
8 (6% of the men) |
|
39 (10%) |
|
26 (7%) |
|
61 (16%) |
|
3 (<1%) |
|
14 (4%) |
|
26 (7%) |
*1(13); *2imaging done for other reasons with previously unsuspected adrenocortical carcinoma
At the initial diagnosis most patients had stage II (39%) disease, followed by an already metastasized disease (stage IV) in 30% of cases. In 89% of patients the primary tumor had been operated on.
Preoperative hormonal work-up
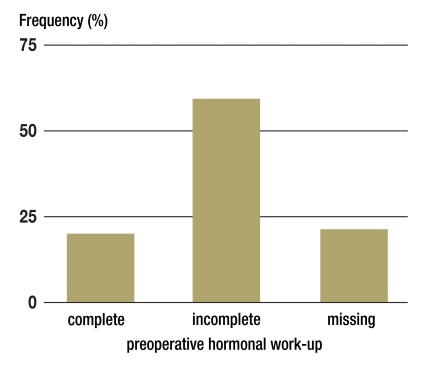
Precise information about the type and extent of the hormonal work-ups carried out were available for 352 of the 387 study patients. Despite the generous definition used compared to the ENSAT recommendations, the hormonal work-up could be assessed as complete in only 20% of patients (Figure 1). In most patients (59%) testing was incomplete, meaning that in women, for example, only androgens were determined but no testing for Cushing’s syndrome was carried out. In 31 patients only pheochromocytoma was excluded preoperatively. In every fifth patient no hormonal work-up was carried out prior to surgery (n = 73, 21%).
Figure 1.
Preoperative hormonal work-up in patients with adrenocortical carcinoma in Germany (1998–2009, n = 352, 16–86 years)
Definitions:
Complete preoperative hormonal work-up:
at least 1 test to diagnose hypercortisolism
+ at least 1 sexual hormone/steroid precursor test
+ exclusion of pheochromocytoma;
Incomplete preoperative hormonal work-up:
all other combinations;
Missing preoperative hormonal work-up:
no endocrine testing
Imaging
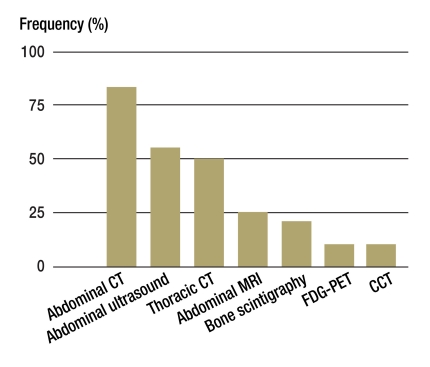
Information about imaging at the initial diagnosis was available for 370 study patients. Figure 2 shows the frequency of the imaging procedures used. A crucial result is that in almost half the patients (48%) no thoracic cross-sectional imaging was carried out. Of the 194 patients for whom thoracic CT was carried out, lung metastases were detected in a good third of cases (n = 65, 34%) at the time of the initial diagnosis.
Figure 2.
Frequency of imaging procedures used at the time of the initial diagnosis in patients with adrenocortical carcinoma in Germany (1998–2009, n = 370, 16–86 years)
Documentation of the resection status
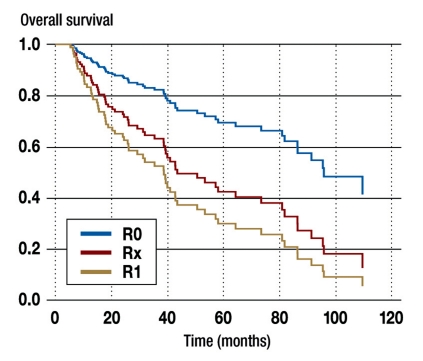
The documentation of the resection results (R status) was assessed using surgery reports and histological results. Only patients with stage I–III disease and with no intraoperative damage to the tumor capsule were included in the analysis (n = 192). Sufficient documents were available for 180 of the 192 patients. In 80% of the cases R0 resection was carried out, although this could only be indirectly inferred from the pathology report in many cases. In 7% of the patients microscopically verifiable tumor residue remained (R1 resection) and in 2% macroscopically verifiable tumor residue remained (R2 resection). In 11% the information in the pathology report was insufficient to enable statements to be made about the success of resection (Rx). In a survival analysis using Cox regression it was shown that patients with Rx resection—exactly as for R1 resection—have a significantly worse (tumor stage-adjusted) overall survival than patients with R0 resection (Figure 3).
Figure 3.
Tumor stage–adjusted survival (Cox regression analysis) by resection status in patients with adrenocortical carcinoma in Germany (1998–2009, n = 180);
patients in stage IV and patients with damage to the tumor capsule were excluded.
Patients with R2 resection were not analyzed because of the low case numbers (n = 6).
R0 = microscopically complete resection (n = 149)
R1 = microscopically verifiable tumor residue (n = 16); hazard ratio for death compared to R0 group 3.3; (95% confidence interval: 1.8–6.0; p<0,01)
Rx = no statement about resection status made (n = 19); hazard ratio for death compared to R0 group 2.3; (95% confidence interval: 1.07–5.2; p = 0,03)
Misdiagnoses
A surprisingly high number of misdiagnoses became apparent during the evaluation of the pathology results. Between 2006 and 2009 a second assessment of the tumor material was requested by the reference pathologist in 161 of the patients registered in the NKR registry. In 21 patients (13%) the diagnosis of ACC had to be revised as a result. In a number of cases adrenal cortex metastases of other malignancies, malignant pheochromocytoma, kidney cell carcinoma, or sarcoma were involved.
Follow-up care
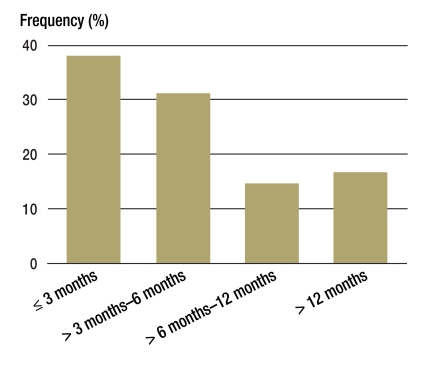
There was information available about the first restaging for 350 of the 387 study patients: of these patients, initial imaging check-ups were carried out within three months for less than 40% (Figure 4). For about one third the first follow-up examination took place 7 months after surgery or even later and for 16.5% only after more than one year.
Figure 4.
Time to first postoperative follow-up (imaging) in patients with adrenocortical carcinoma in Germany (1998–2009, n = 350, 17–86 years)
Discussion
The present evaluation of data from the German Adrenocortical Carcinoma Registry reveals a wide range of deficits in the medical care of patients with ACC in Germany. For most patients the preoperative hormonal work-up, staging, pathology diagnostics or follow-up care were inadequate, despite recommendations for preoperative diagnostics having been available for a number of years (7, 10– 12, e3).
The results of the assessment of the preoperative hormonal work-up are alarming. For a large proportion of the patients clarification of the hormonal situation was incomplete; in a fifth of patients no preoperative hormonal work-up was carried out at all, although careful endocrine diagnostic evaluation prior to surgery of adrenal tumors has been obligatory for many years (10– 12, e7). This exposed the patients to unnecessary perioperative risks. With Cushing’s syndrome patients experience adrenal insufficiency peri- and postoperatively and appropriate glucocorticoid replacement is vital (18). It must be noted that appreciable hypercortisolism may be present even if no clinical indication of Cushing’s syndrome can be identified (subclinical Cushing’s syndrome). Furthermore, pheochromocytoma must always be excluded preoperatively because surgical resection of pheochromocytoma without premedication may lead to life-threatening complications (19). Cross-sectional imaging alone often does not differentiate between pheochromocytoma and ACC. In addition, hormonal work-up provides information about the type of the tumor. High serum concentrations of androgens, secretion of steroid precursors, or estrogen production in men indicate a carcinoma, for example. The preoperative hormonal work-up also serves to establish the presence of tumor markers.
Incomplete imaging at the time of initial diagnosis may also have serious consequences for patients. Classification into the wrong tumor stage often leads to inadequate therapeutic decisions. Although it has been known for a long time that the lungs are the organ most commonly affected by metastases (1, 16), in almost half the patients no thoracic CT was carried out. More than one third of those patients that underwent a thoracic CT already presented with lung metastases at the time of the initial diagnosis, meaning it can be assumed that lung metastases remain undetected in a large number of the remaining patients and disseminated disease (stage IV) was thus not captured.
To assess the prognosis and determine the future course of treatment, both correct stage classification and a precise description of the resection outcome (R status) are of considerable importance. Patients with R1 or Rx resection status have—even after adjustment for tumor stage—a significantly poorer prognosis than patients with R0 resection status. Consequently, adjuvant radiotherapy of the tumor bed is recommended in addition to mitotane therapy for the first two groups (20, e8). It can be assumed that the Rx group contains patients with R0 and with R1 resection status, meaning that individualized therapy decisions are made more difficult.
The high rate of misdiagnoses underlines the need to create a reference histology, particularly for endocrine-inactive tumors of the adrenal glands, because histological differentiation of ACC from other malignancies, and in some cases also from benign lesions of the adrenal glands, is difficult.
In the case of a recurrence repeat surgery plays an important role, because there are indications that this improves the overall survival of patients (7, 21, 22, e9). For this reason regular follow-up examinations are necessary so that the point for recurrence surgery is not missed. In approximately one-third of patients the first restaging did not take place within the first six months, and for 17% it took place after more than one year. With this approach, some patients lose the chance for optimal treatment of a recurrence.
Assuming curative surgery, follow-up examinations were often omitted in the past for patients with ACC. The risk of recurrence is, however, high. Recurrence rates of up to 80% after radical resection have been reported (23, e10). As well as early detection of recurrences, adjuvant treatment is also increasingly significant in the postoperative phase. Recently, an Italian-German study showed that adjuvant therapy with mitotane significantly reduced the risk of recurrence and was able to significantly prolong survival (median recurrence-free survival with mitotane 42 months compared to 10 and 25 months in the Italian and German control groups respectively, p <0.001 and p = 0.005 respectively) (24). Treatment with mitotane, a derivative of the insecticide DDT, requires considerable experience and careful monitoring of patients (25, e11), meaning that collaboration with a specialist center is essential. The hormone situation should also be evaluated during follow-up so that possible underfunction or renewed hormone excess can be treated promptly.
Limitations of the study
The present study is limited by the in part retrospective nature of data acquisition. In addition, the formal evidence level of the underlying recommendations are to be classified as low (“expert opinion”). Furthermore, missing or incomplete documentation does not always necessarily equate to diagnostic procedures having been omitted—we did attempt to take this into consideration. On the other hand it can be assumed that those cases reported in the registry involve patients who are receiving better care than unreported cases, meaning that the actual extent of the deficits in patient care are possibly even greater than described.
Summary
During the evaluation of data from the German ACC Registry (NKR), significant deficits were identified in the medical care of patients with ACC in Germany. Optimal diagnostic evaluation and therapy for rare diseases are—particularly given the limited time available—a challenge. The deficits described here are thus not unexpected and probably paradigmatic for rare diseases. Early cooperation with a specialized center is a critical tool. Fortunately, the option to treat ACC patients within clinical trials is becoming increasingly possible. The first randomized study of ACC (www.firm-act.org), which is still ongoing, will provide a foundation for evidence-based therapy of the advanced disease for the first time. More information about current trials is available on the NKR homepage (www.nebennierenkarzinom.de). Developments over recent years give cause for hope that the care of these patients will improve in future.
Key Messages.
In Germany there are considerable deficits in terms of hormonal work-up, imaging, histological diagnosis, and follow-up for patients with adrenocortical carcinoma (ACC).
Incomplete or missing hormonal work-up prior to surgery on an adrenocortical carcinoma exposes patients to unnecessary risks such as the danger of peri- or postoperative adrenal insufficiency.
Treatment decisions are made more difficult because of incomplete staging examinations and a lack of information about the resection status.
To improve the medical care of patients with ACC, early contact with a specialized center is recommended.
A number of structural changes such as the establishment of national registries and the founding of the European Network for the Study of Adrenal Tumors (ENSAT) as well as initiation of several controlled prospective trials of ACC have all led in recent years to an improved body of data and will in the near future enable evidence-based treatment standards to be established for the first time (current information: www.nebennierenkarzinom.de).
Acknowledgments
Translated from the original German by language & letters.
Footnotes
Conflict of interest statement
HRA Pharma provided financial support to enable the researchers from Würzburg to conduct in vitro tests of mitotane in adrenal gland cells.
PD Dr. Fassnacht and Professor Allolio are Investigators in a clinical trial sponsored by HRA Pharma (France) on the pharmacokinetics of mitotane.
PD Dr. Fassnacht is also Principle Investigator and Professor Allolio is Investigator in an investigator-intiated clinical trial of sunitinib supported by Pfizer.
Dr. Willenberg has received financial support from HRA Pharma for the study of mitotane action on the function of bovine adrenal cells in vitro.
Dr. Johanssen, Dr. Hahner, Prof. Saeger, PD Dr. Quinkler, Prof. Beuschlein, Prof. Dralle, Ms Haaf, Dr. Jurowich, Dr. Dr. Kroiss, Prof. Langer, Prof. Oelkers, Dr. Spahn, and Dr. Mäder declare that no conflict of interest exists according to the guidelines of the International Committee of Medical Journal Editors.
References
- 1.Fassnacht M, Allolio B. Clinical management of adrenocortical carcinoma. Best Pract Res Clin Endocrinol Metab. 2009;23:273–289. doi: 10.1016/j.beem.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Kebebew E, Reiff E, Duh QY, Clark OH, McMillan A. Extent of Disease at Presentation and Outcome for Adrenocortical Carcinoma: Have We Made Progress? World J Surg. 2006;30:872–878. doi: 10.1007/s00268-005-0329-x. [DOI] [PubMed] [Google Scholar]
- 3.Bilimoria KY, Shen WT, Elaraj D, et al. Adrenocortical carcinoma in the United States: treatment utilization and prognostic factors. Cancer. 2008;113:3130–3136. doi: 10.1002/cncr.23886. [DOI] [PubMed] [Google Scholar]
- 4.Abiven G, Coste J, Groussin L, et al. Clinical and biological features in the prognosis of adrenocortical cancer: poor outcome of cortisol-secreting tumors in a series of 202 consecutive patients. J Clin Endocrinol Metab. 2006;91:2650–2655. doi: 10.1210/jc.2005-2730. [DOI] [PubMed] [Google Scholar]
- 5.Johanssen S, Fassnacht M, Brix D, et al. Das Nebennierenkarzinom - Diagnostik und Therapie. [Adrenocortical carcinoma: Diagnostic work-up and treatment] Urologe A. 2008;47:172–181. doi: 10.1007/s00120-007-1578-0. [DOI] [PubMed] [Google Scholar]
- 6.Icard P, Goudet P, Charpenay C, et al. Adrenocortical carcinomas: surgical trends and results of a 253-patient series from the French Association of Endocrine Surgeons study group. World J Surg. 2001;25:891–897. doi: 10.1007/s00268-001-0047-y. [DOI] [PubMed] [Google Scholar]
- 7.Schteingart DE, Doherty GM, Gauger PG, et al. Management of patients with adrenal cancer: recommendations of an international consensus conference. Endocr Relat Cancer. 2005;12:667–680. doi: 10.1677/erc.1.01029. [DOI] [PubMed] [Google Scholar]
- 8.Assie G, Antoni G, Tissier F, et al. Prognostic parameters of metastatic adrenocortical carcinoma. J Clin Endocrinol Metab. 2007;92:148–154. doi: 10.1210/jc.2006-0706. [DOI] [PubMed] [Google Scholar]
- 9.Mansmann G, Lau J, Balk E, Rothberg M, Miyachi Y, Bornstein SR. The clinically inapparent adrenal mass: update in diagnosis and management. Endocr Rev. 2004;25:309–340. doi: 10.1210/er.2002-0031. [DOI] [PubMed] [Google Scholar]
- 10.Kloos RT, Gross MD, Francis IR, Korobkin M, Shapiro B. Incidentally discovered adrenal masses. Endocr Rev. 1995;16:460–484. doi: 10.1210/edrv-16-4-460. [DOI] [PubMed] [Google Scholar]
- 11.Allolio B, Reincke M. Das Nebenniereninzidentalom: Die Kunst der Beschränkung in Diagnostik und Therapie. Dtsch Arztebl. 1995;92(11):A 764–A 770. [Google Scholar]
- 12.Grumbach MM, Biller BM, Braunstein GD, et al. Management of the clinically inapparent adrenal mass („incidentaloma“) Ann Intern Med. 2003;138:424–429. doi: 10.7326/0003-4819-138-5-200303040-00013. [DOI] [PubMed] [Google Scholar]
- 13.Fassnacht M, Johanssen S, Quinkler M, et al. Limited prognostic value of the 2004 International Union Against Cancer staging classification for adrenocortical carcinoma: proposal for a Revised TNM Classification. Cancer. 2009;115:243–250. doi: 10.1002/cncr.24030. [DOI] [PubMed] [Google Scholar]
- 14.Fassnacht M, Hahner S, Banfelder N, Weismann D, Allolio B. Diagnostik und Therapie des Nebennierenrinden-Karzinoms. Dtsch Arztebl. 2005;102(23):A 1670–A 1675. [Google Scholar]
- 15.Libe R, Fratticci A, Bertherat J. Adrenocortical cancer: pathophysiology and clinical management. Endocr Relat Cancer. 2007;14:13–28. doi: 10.1677/erc.1.01130. [DOI] [PubMed] [Google Scholar]
- 16.Hutter AM Jr, Kayhoe DE. Adrenal cortical carcinoma. Clinical features of 138 patients. Am J Med. 1966;41:572–580. doi: 10.1016/0002-9343(66)90219-1. [DOI] [PubMed] [Google Scholar]
- 17.Groussin L, Bonardel G, Silvera S, et al. 18F-Fluorodeoxyglucose positron emission tomography for the diagnosis of adrenocortical tumors: a prospective study in 77 operated patients. J Clin Endocrinol Metab. 2009;94:1713–1722. doi: 10.1210/jc.2008-2302. [DOI] [PubMed] [Google Scholar]
- 18.Bornstein SR. Predisposing factors for adrenal insufficiency. N Engl J Med. 2009;360:2328–2339. doi: 10.1056/NEJMra0804635. [DOI] [PubMed] [Google Scholar]
- 19.Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet. 2005;366:665–675. doi: 10.1016/S0140-6736(05)67139-5. [DOI] [PubMed] [Google Scholar]
- 20.Polat B, Fassnacht M, Pfreundner L, et al. Radiotherapy in adrenocortical carcinoma. Cancer. 2009;115:2816–2823. doi: 10.1002/cncr.24331. [DOI] [PubMed] [Google Scholar]
- 21.Bellantone R, Ferrante A, Boscherini M, et al. Role of reoperation in recurrence of adrenal cortical carcinoma: results from 188 cases collected in the Italian National Registry for Adrenal Cortical Carcinoma. Surgery. 1997;122:1212–1218. doi: 10.1016/s0039-6060(97)90229-4. [DOI] [PubMed] [Google Scholar]
- 22.Schulick RD, Brennan MF. Long-term survival after complete resection and repeat resection in patients with adrenocortical carcinoma. Ann Surg Oncol. 1999;6:719–726. doi: 10.1007/s10434-999-0719-7. [DOI] [PubMed] [Google Scholar]
- 23.Stojadinovic A, Ghossein RA, Hoos A, et al. Adrenocortical carcinoma: clinical, morphologic, and molecular characterization. J Clin Oncol. 2002;20:941–950. doi: 10.1200/JCO.2002.20.4.941. [DOI] [PubMed] [Google Scholar]
- 24.Terzolo M, Angeli A, Fassnacht M, et al. Adjuvant mitotane treatment in patients with adrenocortical carcinoma. N Engl J Med. 2007;356:372–380. doi: 10.1056/NEJMoa063360. [DOI] [PubMed] [Google Scholar]
- 25.Daffara F, De Francia S, Reimondo G, et al. Prospective evaluation of mitotane toxicity in adrenocortical cancer patients treated adjuvantly. Endocr Relat Cancer. 2008;15:1043–1053. doi: 10.1677/ERC-08-0103. [DOI] [PubMed] [Google Scholar]
- e1.Golden SH, Robinson KA, Saldanha I, Anton B, Ladenson PW. Clinical review: Prevalence and incidence of endocrine and metabolic disorders in the United States: a comprehensive review. J Clin Endocrinol Metab. 2009;94:1853–1878. doi: 10.1210/jc.2008-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e2.Wooten MD, King DK. Adrenal cortical carcinoma. Epidemiology and treatment with mitotane and a review of the literature. Cancer. 1993;72:3145–3155. doi: 10.1002/1097-0142(19931201)72:11<3145::aid-cncr2820721105>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- e3.Osella G, Terzolo M, Borretta G, et al. Endocrine evaluation of incidentally discovered adrenal masses (incidentalomas) [see comments] J Clin Endocrinol Metab. 1994;79:1532–1539. doi: 10.1210/jcem.79.6.7989452. [DOI] [PubMed] [Google Scholar]
- e4.NIH-State-of-science-art-statement Management of the Clinically Inapparent Adrenal Mass (Incidentaloma) February 4-6, 2002. http://consensus.nih.gov/ta/021/021_statement.htm. 2002. [PubMed]
- e5.Koschker AC, Fassnacht M, Hahner S, Weismann D, Allolio B. Adrenocortical carcinoma—improving patient care by establishing new structures. Exp Clin Endocrinol Diabetes. 2006;114:45–51. doi: 10.1055/s-2006-923808. [DOI] [PubMed] [Google Scholar]
- e6.Allolio B, Fassnacht M. Clinical review: Adrenocortical carcinoma: clinical update. J Clin Endocrinol Metab. 2006;91:2027–2037. doi: 10.1210/jc.2005-2639. [DOI] [PubMed] [Google Scholar]
- e7.Fassnacht M, Kenn W, Allolio B. Adrenal tumors: how to establish malignancy? J Endocrinol Invest. 2004;27:387–399. doi: 10.1007/BF03351068. [DOI] [PubMed] [Google Scholar]
- e8.Fassnacht M, Hahner S, Polat B, et al. Efficacy of adjuvant radiotherapy of the tumor bed on local recurrence of adrenocortical carcinoma. J Clin Endocrinol Metab. 2006;91:4501–4504. doi: 10.1210/jc.2006-1007. [DOI] [PubMed] [Google Scholar]
- e9.Jensen JC, Pass HI, Sindelar WF, Norton JA. Recurrent or metastatic disease in select patients with adrenocortical carcinoma. Aggressive resection vs chemotherapy. Arch Surg. 1991;126:457–461. doi: 10.1001/archsurg.1991.01410280059008. [DOI] [PubMed] [Google Scholar]
- e10.Pommier RF, Brennan MF. An eleven-year experience with adrenocortical carcinoma. Surgery. 1992;112:970–971. 963 70; discussion. [PubMed] [Google Scholar]
- e11.Hahner S, Fassnacht M. Mitotane for adrenocortical carcinoma treatment. Curr Opin Investig Drugs. 2005;6:386–394. [PubMed] [Google Scholar]