Abstract
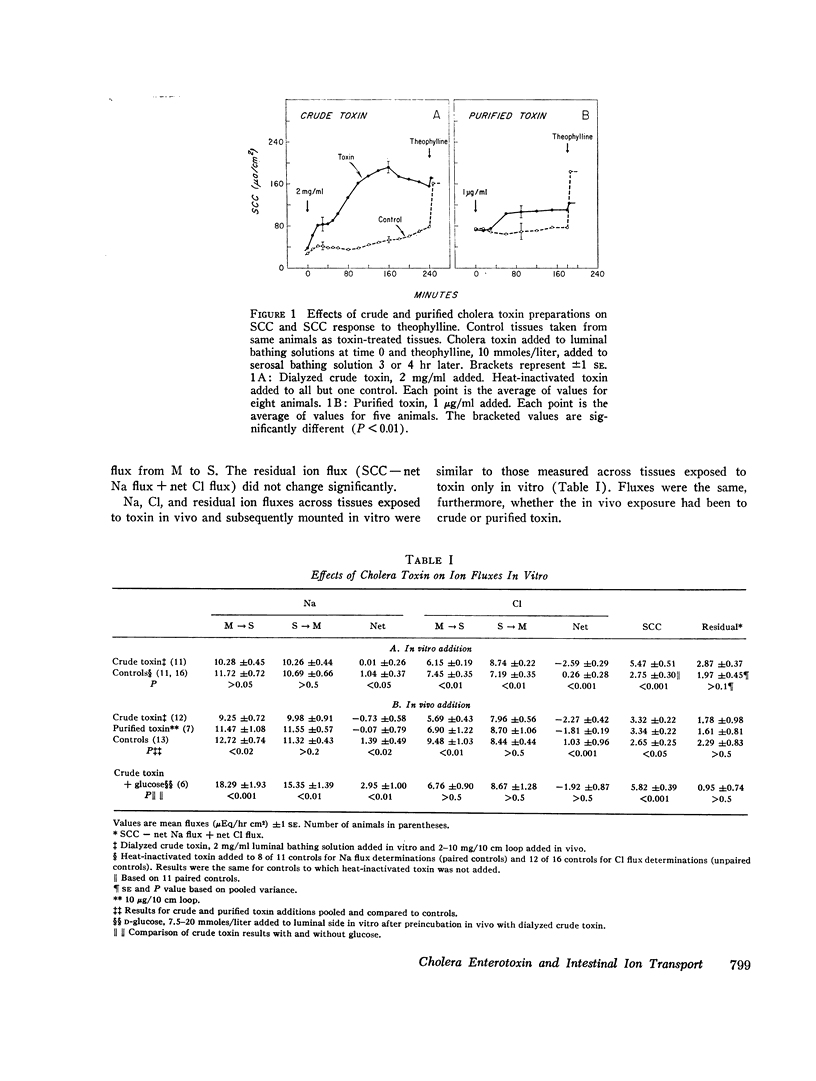
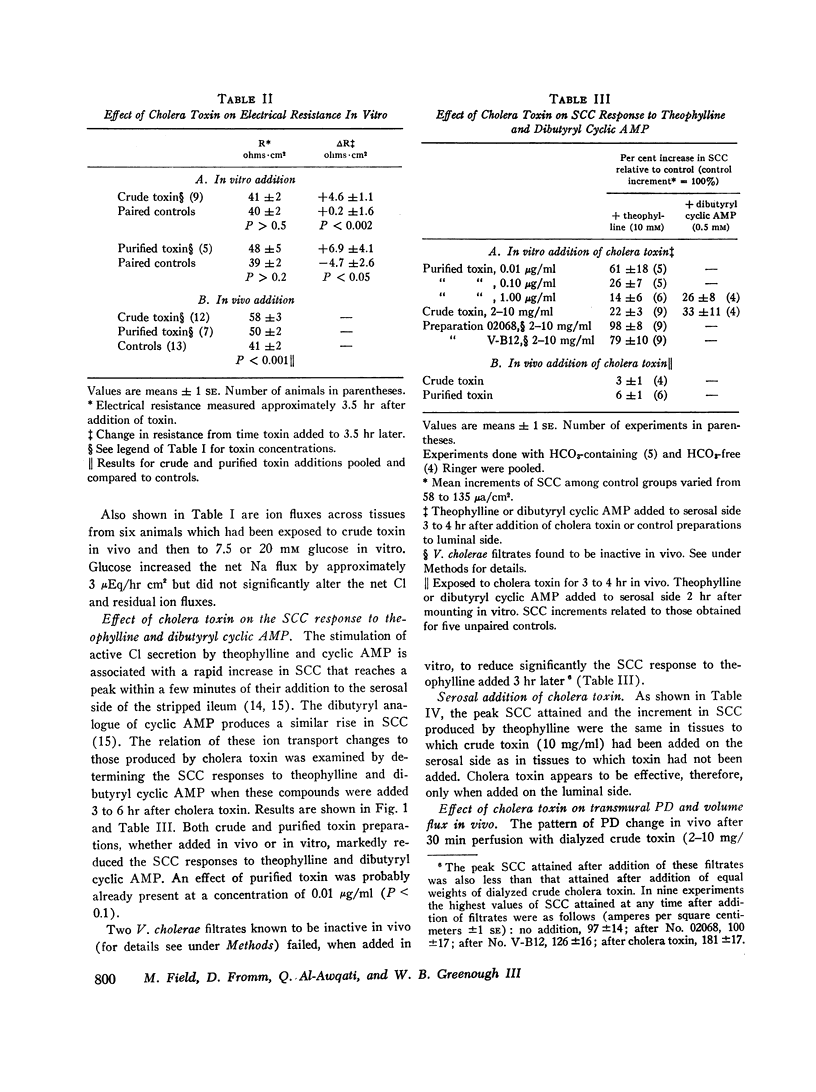
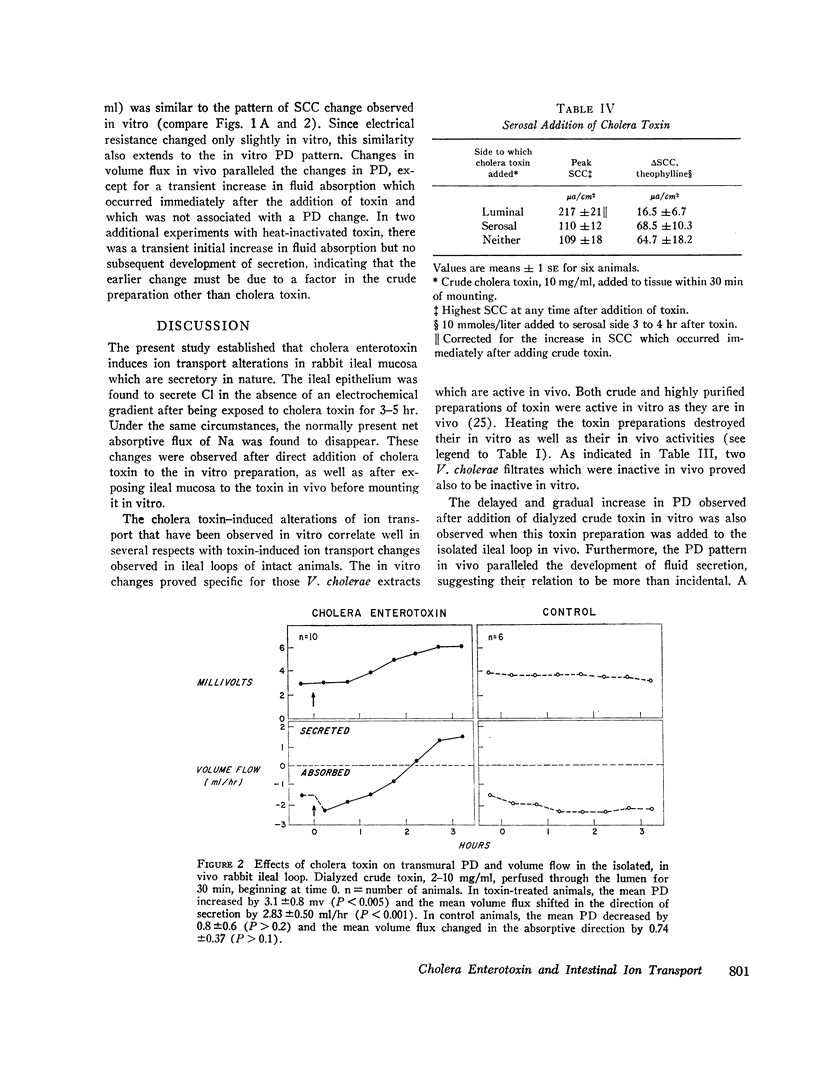
The effects of cholera enterotoxin on intestinal ion transport were examined in vitro. Addition of dialyzed filtrate of Vibrio cholerae (crude toxin) to the luminal side of isolated rabbit ileal mucosa caused a delayed and gradually progressive increase in transmural electric potential difference (PD) and shortcircuit current (SCC). A similar pattern was observed upon addition of a highly purified preparation of cholera toxin, although the changes in PD and SCC were smaller. Na and Cl fluxes across the short-circuited mucosa were determined with radioisotopes 3-4 hr after addition of crude toxin or at a comparable time in control tissues. The toxin caused a net secretory flux of Cl and reduced to zero the net absorptive flux of Na. Similar flux changes were observed when either crude or purified toxin was added in vivo and tissues were mounted in vitro 3-4 hr later. Additon of D-glucose to the luminal side of toxin-treated mucosa produced a large net absorptive flux of Na without altering the net Cl and residual ion fluxes.
Adenosine 3′,5′-cyclic phosphate (cyclic AMP) and theophylline had previously been shown to cause a rapid increase in SCC and ion flux changes similar to those induced by cholera toxin. Pretreatment of ileal mucosa with either crude or purified cholera toxin greatly reduced the SCC response to theophylline and dibutyryl cyclic AMP, which, together with the flux data, suggest that both cyclic AMP and cholera toxin stimulate active secretion by a common pathway. Inhibition of the SCC response to theophylline was observed after luminal but not after serosal addition of toxin. In vitro effects of cholera toxin correlated closely with in vivo effects: heating toxin destroyed both; two V. cholerae filtrates which were inactive in vivo proved also to be inactive in vitro; PD and volume flow measurements in isolated, in vivo ileal loops of rabbit revealed that the PD pattern after addition of toxin is similar to that seen in vitro and also correlates closely with changes in fluid movement. The results suggest that stimulation by cholera toxin of a cyclic AMP-dependent active secretory process of the intestinal epithelial cells is a major cause of fluid loss in cholera.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banwell J. G., Pierce N. F., Mitra R., Caranasos G. J., Keimowitz R. I., Mondal A., Manji P. M. Preliminary results of a study of small intestinal water and solute movement in acute and convalescent human cholera. Indian J Med Res. 1968 May;56(5):633–639. [PubMed] [Google Scholar]
- Carpenter C. C., Greenough W. B., 3rd, Sack R. B. The relationship of superior mesenteric artery blood flow to gut electrolyte loss in experimental cholera. J Infect Dis. 1969 Feb;119(2):182–193. doi: 10.1093/infdis/119.2.182. [DOI] [PubMed] [Google Scholar]
- Carpenter C. C., Sack R. B., Feeley J. C., Steenberg R. W. Site and characteristics of electrolyte loss and effect of intraluminal glucose in experimental canine cholera. J Clin Invest. 1968 May;47(5):1210–1220. doi: 10.1172/JCI105810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson T. W. The transport of salt and water across isolated rat ileum. Evidence for at least two distinct pathways. J Gen Physiol. 1967 Jan;50(3):695–727. doi: 10.1085/jgp.50.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott H. L., Carpenter C. C., Sack R. B., Yardley J. H. Small bowel morphology in experimental canine cholera. A light and electron microscopic study. Lab Invest. 1970 Feb;22(2):112–120. [PubMed] [Google Scholar]
- Field M., Fromm D., McColl I. Ion transport in rabbit ileal mucosa. I. Na and Cl fluxes and short-circuit current. Am J Physiol. 1971 May;220(5):1388–1396. doi: 10.1152/ajplegacy.1971.220.5.1388. [DOI] [PubMed] [Google Scholar]
- Field M. Ion transport in rabbit ileal mucosa. II. Effects of cyclic 3', 5'-AMP. Am J Physiol. 1971 Oct;221(4):992–997. doi: 10.1152/ajplegacy.1971.221.4.992. [DOI] [PubMed] [Google Scholar]
- Field M., Plotkin G. R., Silen W. Effects of vasopressin, theophylline and cyclic adenosine monophosphate on short-circuit current across isolated rabbit ileal mucosa. Nature. 1968 Feb 3;217(5127):469–471. doi: 10.1038/217469a0. [DOI] [PubMed] [Google Scholar]
- GANGAROSA E. F., BEISEL W. R., BENYAJATI C., SPRINZ H., PIYARATN P. The nature of the gastrointestinal lesion in asiatic cholera and its relation to pathogenesis: a biopsy study. Am J Trop Med Hyg. 1960 Mar;9:125–135. doi: 10.4269/ajtmh.1960.9.125. [DOI] [PubMed] [Google Scholar]
- Gill D. M., Pappenheimer A. M., Jr, Brown R., Kurnick J. T. Studies on the mode of action of diphtheria toxin. VII. Toxin-stimulated hydrolysis of nicotinamide adenine dinucleotide in mammalian cell extracts. J Exp Med. 1969 Jan 1;129(1):1–21. doi: 10.1084/jem.129.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilles-Baillien M., Schoffeniels E. Fluxes of inorganic ions across the isolated intestinal epithelium of the greek tortoise. Arch Int Physiol Biochim. 1967 Dec;75(5):754–762. doi: 10.3109/13813456709084932. [DOI] [PubMed] [Google Scholar]
- Grady G. F., Madoff M. A., Duhamel R. C., Moore E. W., Chalmers T. C. Sodium transport by human ileum in vitro and its response to cholera enterotoxin. Gastroenterology. 1967 Nov;53(5):737–744. [PubMed] [Google Scholar]
- Grady G. F., Madoff M. A., Duhamel R. C., Moore E. W., Chalmers T. C. Sodium transport inhibition by cholera toxin: the role of non-ionic diffusion of ammonia. J Infect Dis. 1968 Jun;118(3):263–270. doi: 10.1093/infdis/118.3.263. [DOI] [PubMed] [Google Scholar]
- Hirschhorn N., Kinzie J. L., Sachar D. B., Northrup R. S., Taylor J. O., Ahmad S. Z., Phillips R. A. Decrease in net stool output in cholera during intestinal perfusion with glucose-containing solutions. N Engl J Med. 1968 Jul 25;279(4):176–181. doi: 10.1056/NEJM196807252790402. [DOI] [PubMed] [Google Scholar]
- Hubel K. A. Effect of luminal chloride concentration on bicarbonate secretion in rat ileum. Am J Physiol. 1969 Jul;217(1):40–45. doi: 10.1152/ajplegacy.1969.217.1.40. [DOI] [PubMed] [Google Scholar]
- Kimberg D. V., Field M., Johnson J., Henderson A., Gershon E. Stimulation of intestinal mucosal adenyl cyclase by cholera enterotoxin and prostaglandins. J Clin Invest. 1971 Jun;50(6):1218–1230. doi: 10.1172/JCI106599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitch G. J., Burrows W. Experimental cholera in the rabbit ligated intestine: ion and water accumulation in the duodenum, ileum and colon. J Infect Dis. 1968 Oct;118(4):349–359. doi: 10.1093/infdis/118.4.349. [DOI] [PubMed] [Google Scholar]
- Moore W. L., Jr, Bieberdorf F. A., Morawski S. G., Finkelstein R. A., Fordtran J. S. Ion transport during cholera-induced ileal secretion in the dog. J Clin Invest. 1971 Feb;50(2):312–318. doi: 10.1172/JCI106496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz M., Iber F. L., Moore E. W. Rabbit cholera: relation of transmural potentials to water and electrolyte fluxes. Am J Physiol. 1971 Jul;221(1):19–24. doi: 10.1152/ajplegacy.1971.221.1.19. [DOI] [PubMed] [Google Scholar]
- Munck B. G. Interactions between lysine, Na+ and Cl- transport in rat jejunum. Biochim Biophys Acta. 1970 Jun 2;203(3):424–433. doi: 10.1016/0005-2736(70)90182-3. [DOI] [PubMed] [Google Scholar]
- Nalin D. R., Cash R. A., Rahman M., Yunus M. Effect of glycine and glucose on sodium and water adsorption in patients with cholera. Gut. 1970 Sep;11(9):768–772. doi: 10.1136/gut.11.9.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris H. T., Curran P. F., Schultz S. G. Modification of intestinal secretion in experimental cholera. J Infect Dis. 1969 Feb;119(2):117–125. doi: 10.1093/infdis/119.2.117. [DOI] [PubMed] [Google Scholar]
- Pierce N. F., Banwell J. G., Rupak D. M., Mitra R. C., Caranasos G. J., Keimowitz R. I., Mondal A., Manji P. M. Effect of intragastric glucose-electrolyte infusion upon water and electrolyte balance in Asiatic cholera. Gastroenterology. 1968 Sep;55(3):333–343. [PubMed] [Google Scholar]
- Powell D. W., Malawer S. J., Plotkin G. R. Secretion of electrolytes and water by the guinea pig small intestine in vivo. Am J Physiol. 1968 Nov;215(5):1226–1233. doi: 10.1152/ajplegacy.1968.215.5.1226. [DOI] [PubMed] [Google Scholar]
- Schafer D. E., Lust W. D., Sircar B., Goldberg N. D. Elevated concentration of adenosine 3':5'-cyclic monophosphate in intestinal mucosa after treatment with cholera toxin. Proc Natl Acad Sci U S A. 1970 Oct;67(2):851–856. doi: 10.1073/pnas.67.2.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp G. W., Hynie S. Stimulation of intestinal adenyl cyclase by cholera toxin. Nature. 1971 Jan 22;229(5282):266–269. doi: 10.1038/229266a0. [DOI] [PubMed] [Google Scholar]
- Swallow J. H., Code C. F. Intestinal transmucosal fluxes of bicarbonate. Am J Physiol. 1967 Mar;212(3):717–723. doi: 10.1152/ajplegacy.1967.212.3.717. [DOI] [PubMed] [Google Scholar]
- Taylor A. E., Wright E. M., Schultz S. G., Curran P. F. Effect of sugars on ion fluxes in intest-ine. Am J Physiol. 1968 Apr;214(4):836–842. doi: 10.1152/ajplegacy.1968.214.4.836. [DOI] [PubMed] [Google Scholar]
- Turnberg L. A., Bieberdorf F. A., Morawski S. G., Fordtran J. S. Interrelationships of chloride, bicarbonate, sodium, and hydrogen transport in the human ileum. J Clin Invest. 1970 Mar;49(3):557–567. doi: 10.1172/JCI106266. [DOI] [PMC free article] [PubMed] [Google Scholar]



