Abstract
Plasticity is a hallmark of neural systems, including the neural system controlling breathing (Mitchell and Johnson, 2003). Despite its biological and potential clinical significance, our understanding of mechanisms giving rise to any form of respiratory plasticity remains incomplete. Here we discuss recent advances in our understanding of cellular mechanisms giving rise to phrenic long-term facilitation (pLTF), a long-lasting increase in phrenic motor output induced by acute intermittent hypoxia (AIH). Recently, we have come to realize that multiple, distinct mechanisms are capable of giving rise to long-lasting phrenic motor facilitation (PMF); we use PMF as a general term that includes AIH-induced pLTF. It is important to begin an appreciation and understanding of these diverse pathways. Hence, we introduce a nomenclature based on upstream steps in the signaling cascade leading to PMF. Two pathways are featured here: the “Q” and the “S” pathways, named because they are induced by metabotropic receptors coupled to Gq and Gs proteins, respectively. These pathways appear to interact in complex and interesting ways, thus providing a range of potential responses in the face of changing physiological conditions or the onset of disease.
1. Introduction
Plasticity is a fundamental property of the neural system controlling breathing (Mitchell and Johnson, 2003). In this context, plasticity is defined as a change in future system behavior based on experience (Mitchell and Johnson, 2003). Our current understanding of mechanisms giving rise to any form of respiratory plasticity remains incomplete despite recent progress.
Here we update our understanding of mechanisms giving rise to long-lasting facilitation in respiratory motor output of the phrenic nerve (phrenic motor facilitation, PMF). The most extensively studied form of PMF is phrenic long-term facilitation (pLTF) following acute intermittent hypoxia (AIH) (for review see: Mahamed and Mitchell, 2007a). However, recent evidence has revealed that multiple, distinct cellular mechanisms give rise to PMF. A major challenge will be to understand the biological significance and possible therapeutic implications of this complexity.
2. Multiple Pathways to PMF
At least five distinct mechanisms of PMF have been identified. The first underlies AIH-induced pLTF (see figure 1a). Following brief hypoxic episodes, pLTF is observed through a mechanism that requires activation of spinal serotonin type 2 receptors (5-HT2; Kinkead and Mitchell, 1999; Fuller et al., 2001), a metabotropic receptor coupled to Gq proteins. This same mechanism is simulated by episodic presentation of either 5-HT2A or 5-HT2B receptor agonists in the cervical spinal cord (MacFarlane and Mitchell, 2007; MacFarlane and Mitchell, 2008a), demonstrating that 5-HT2 receptor activation is necessary and sufficient for pLTF. Since Gq-coupled Alpha-1 adrenergic receptors also appear to be necessary (Neverova et al., 2007) and sufficient (figure 1b; MacFarlane and Mitchell, unpublished) for PMF, we suspect a common mechanism. We refer to this PMF pathway as the “Q pathway” since multiple Gq protein-coupled metabotropic receptors (Bockaert et al., 2006) initiate the response. Metabotropic receptors coupled to Gs proteins in the cervical spinal cord also elicit PMF, specifically adenosine 2A (Golder et al., 2008) and 5-HT7 receptors (figure 1c; Hoffman and Mitchell, 2008). We refer to this PMF pathway as the “S pathway” since multiple Gs protein-coupled receptors initiate the response. Three distinct pathways to PMF are induced by: 1) spinal vascular endothelial growth factor (VEGF; Dale-Nagle and Mitchell, 2008a), 2) spinal erythropoietin (Dale-Nagle and Mitchell, 2008b) and 3) phrenic inactivity due to hypocapnia or vagal feedback (Mahamed and Mitchell, 2007b; Zhang et al., 2004). Here, we focus on the “Q” and the “S” pathways as models to understand interactions between pathways to PMF.
Figure 1.
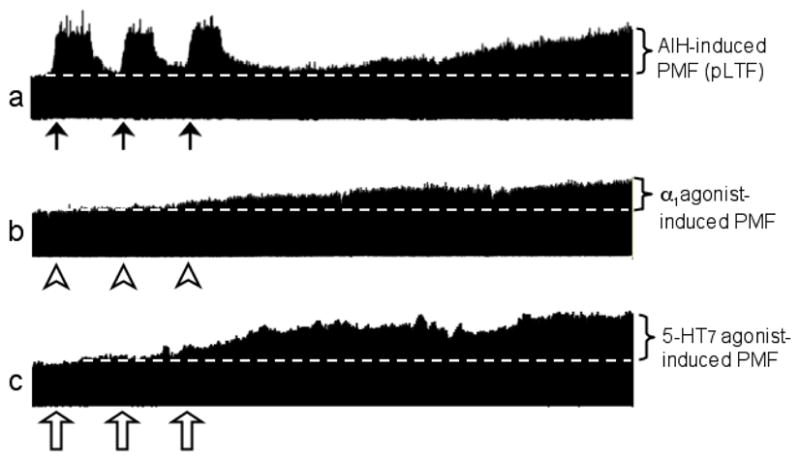
Representative traces of phrenic motor facilitation (PMF) induced by: a. acute intermittent hypoxia (i.e. pLTF, the Q pathway; tracing from Mitchell, 2007); b. episodic intrathecal α1 adrenergic agonist administration (phenylephrine; i.e. Q pathway, MacFar-lane and Mitchell, unpublished); and c. episodic intrathecal 5-HT7 receptor agonist administration (AS19; i.e. S pathway, Hoffman and Mitchell, 2008). Arrows indicate hypoxic episodes or agonist injections. Progressive increase in integrated phrenic burst amplitude above baseline (dotted white line) is PMF (brackets on right).
3. The “Q” and “S” Pathways to PMF
The Q Pathway
Phrenic LTF (pLTF) was originally described as a persistent increase in phrenic activity following repeated carotid sinus nerve stimulation (Millhorn et al 1980a,b), but is also induced by acute intermittent hypoxia (AIH; Hayashi et al., 2003; Bach and Mitchell 1996). AIH-induced pLTF is shown in Figure 1a and our working cellular model is shown in Figure 2 (left side). pLTF requires spinal serotonin receptor activation for induction, but not maintenance (Fuller et. al., 2001; Baker-Herman and Mitchell, 2002). Episodic serotonin and 5-HT2 receptor agonists are sufficient to elicit PMF without AIH (MacFarlane and Mitchell, 2007; MacFarlane and Mitchell, 2008). Thus, AIH-induced pLTF arises predominantly from the Q pathway since 5-HT2 receptors are coupled to Gq proteins (Bockaert et al., 2006). pLTF maintenance requires new protein synthesis (Baker-Herman and Mitchell, 2002), particularly new synthesis of brain derived neurotrophic factor (Baker-Herman et al., 2004). Activation of the high affinity BDNF receptor, TrkB, is both necessary and sufficient for pLTF (Baker-Herman et al., 2004). Extracellular regulated kinase MAP kinases (ERK) are a relevant downstream signaling molecule since: 1) BDNF increases ERK phosphorylation in motor neurons (Kishino and Nakamaya, 2003); 2) AIH increases ERK phosphorylation in ventral cervical segments associated with the phrenic motor nucleus (Wilkerson and Mitchell, 2009); and 3) spinal MEK (the kinase that phosphorylates ERK) inhibition abolishes pLTF (Hoffman and Mitchell, unpublished). Although downstream signaling events from ERK are less clear, glutamate receptor phosphorylation and/or membrane insertion may increase glutamatergic transmission within phrenic motor neurons, thereby establishing pLTF (Fuller et al., 2000; Bocchiaro and Feldman, 2004; Mahamed and Mitchell, 2007a; McGuire et al., 2008).
Figure 2.
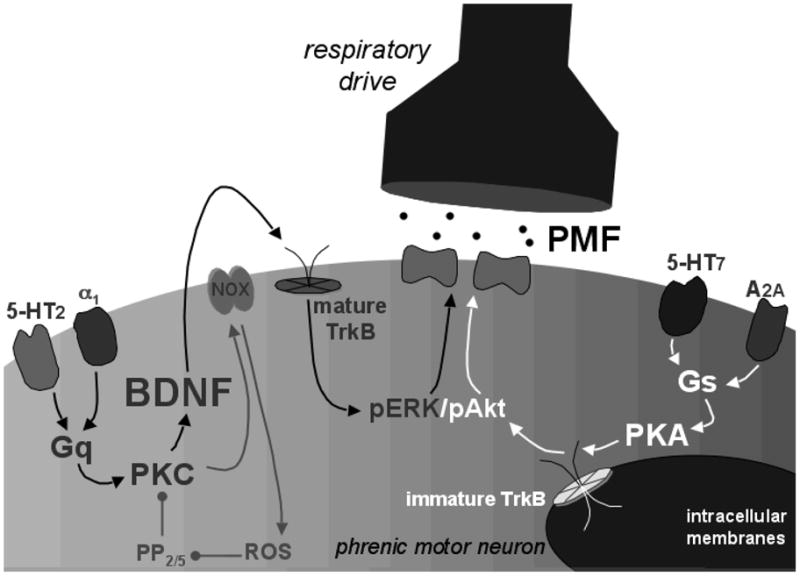
Current working model of convergent pathways to PMF. The “Q” pathway (left, black arrows) is elicited by intermittent activation of Gq-coupled metabotropic receptors (e.g. 5-HT2 or α1). Subsequent activation of protein kinase C (PKC) initiates new BDNF synthesis and increases NADPH oxidase (NOX) activity. BDNF activates TrkB and then ERK MAP kinases (pERK). Protein phosphatases (PP2/5) normally constrain pLTF, but are regulated via NADPH oxidase (NOX) dependent ROS formation. The “S” pathway (right; white arrows) is elicited by Gs-coupled metabotropic receptors (eg. 5-HT7 and A2A) coupled to protein kinase A (PKA). PKA may induce new synthesis of an immature TrkB isoform, which auto-phosphorylates and signals from inside the cell via Akt activation (pAkt). We postulate that both pERK and pAkt phosphorylate glutamate receptors, thereby giving rise to greater synaptic strength and PMF. We cannot rule out changes in motor neuron excitability as a cause of PMF, for example via membrane insertion of ion channels.
pLTF expression is constrained by serine/threonine protein phosphatases (likely PP2A and PP5) during continuous, but not intermittent, hypoxia (Wilkerson et al., 2008). These phosphatases are, in turn, constrained by increased ROS formation via NADPH oxidase activity since: 1) NADPH oxidase activity is necessary for AIH-induced pLTF (MacFarlane et al., 2008; MacFarlane et al., 2009); 2) phosphatase inhibition does not affect AIH-induced pLTF (Wilkerson et al., 2008); and 3) spinal phosphatase inhibition restores AIH-induced pLTF in rats pretreated with ROS scavengers (MacFarlane et al., 2008). NADPH oxidase, ROS and PP2A may constitute a “regulatory cassette” that modulates pLTF expression and confers pattern sensitivity (Wilkerson et al., 2007; MacFarlane et al., 2008).
The S Pathway
Activation of Gs protein-coupled metabotropic receptors activates adenylate cyclase, cyclic AMP and protein kinase A (PKA). Spinal activation of Gs protein-coupled A2A (Golder et al., 2008) and 5-HT7 receptors (Hoffman and Mitchell, 2008) is sufficient to elicit PMF through a distinct cellular mechanism from the Q pathway (i.e. the S pathway); 5-HT7 receptor-induced PMF is exemplified in figure 1c and our working model is illustrated on the right side of in figure 2.
Repeated spinal A2A receptor activation elicits PMF through a mechanism of TrkB “trans-activation” that is independent of new BDNF synthesis or BDNF/TrkB binding (Golder et al., 2008). A2A-induced PMF requires new synthesis of an immature TrkB isoform which auto-dimerizes, auto-phosphorylates and signals from within phrenic motor neurons (Golder et al., 2008). Once activated, intracellular TrkB elicits PMF via PI3 kinase activation, increasing the phosphorylation of protein kinase B or Akt, but not ERK (Golder et al., 2008). Episodic spinal 5-HT7 receptor activation also elicits PMF, confirming that multiple Gs protein-coupled metabotropic receptors induce PMF (Figure 1c; Hoffman and Mitchell, 2008). 5-HT7 receptor-induced PMF requires new TrkB (not BDNF) synthesis and Akt activation, confirming that this form of PMF occurs via the S pathway (Hoffman and Mitchell, unpublished). Thus, although the “S” and “Q” pathways converge on TrkB signaling, both upstream and downstream signaling events are distinct.
4. Interactions between the “Q” and “S” Pathways to PMF
Because both serotonin and ATP/adenosine are released in the vicinity of phrenic motor neurons during hypoxia, we tested whether A2A or 5-HT7 receptors contribute to PMF following AIH (i.e. pLTF). However, when selective antagonists for A2A (Hoffman and Mitchell, 2007) or 5-HT7 receptors (Hoffman and Mitchell, unpublished) are applied to the cervical spinal cord, AIH-induced pLTF is greatly enhanced, and not diminished as predicted. These surprising findings demonstrate that the S and Q pathways are both initiated during AIH, but interact in complex ways. We propose that these pathways exhibit “cross-talk inhibition,” a characteristic of some G-protein signaling cascades (Rhyzov et al., 2006; Meszaros et al., 2000; Roy et al., 2006). Current research in our laboratory is focused on understanding mechanisms and implications of such mutual inhibition.
6. Conclusions and significance
We have recently come to appreciate the role of plasticity in respiratory motor control. A frequently studied model of plasticity in our laboratory is PMF, a long-lasting spinally-mediated increase in phrenic motor output that can be triggered by multiple, distinct mechanisms. Based on an emerging understanding of PMF induced by Gq and Gs coupled metabotropic receptors, it has become clear that mechanisms leading to PMF interact in interesting and complex ways. A major goal of our laboratory is to understand this seemingly bewildering array of potential responses, and to harness this plasticity in the treatment of devastating ventilatory control disorders for which there are few effective therapies and no known cures. For example, by harnessing mechanisms of PMF, we may be able to reverse deficits in breathing capacity caused by cervical spinal injury or motor neuron disease (Mitchell, 2007).
Acknowledgments
Supported by NIH HL080209 and NS05777.
Contributor Information
Erica A. Dale-Nagle, Email: edale@wisc.edu.
Gordon S. Mitchell, Email: mitchell@svm.vetmed.wisc.edu.
References
- Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol. 1996;104:251–260. doi: 10.1016/0034-5687(96)00017-5. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci. 2002;22:6239–46. doi: 10.1523/JNEUROSCI.22-14-06239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci. 2004;7:48–55. doi: 10.1038/nn1166. [DOI] [PubMed] [Google Scholar]
- Bocchiaro CM, Feldman JL. Synaptic activity-dependent persistent plasticity in endogenously active mammalian motoneurons. Proc Natl Acad Sci USA. 2004;101:4292–5. doi: 10.1073/pnas.0305712101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockaert J, Claeysen S, Bécamel C, Dumuis A, Marin P. Neuronal 5HT metabotropic receptors: fine-tuning of their structure, signaling and roles in synaptic modulation. Cell Tissue Tes. 2006;326:553–72. doi: 10.1007/s00441-006-0286-1. [DOI] [PubMed] [Google Scholar]
- Dale-Nagle EA, Mitchell GS. Intrathecal administration of vascular endothelial growth factor (VEGF) elicits phrenic motor facilitation. FASEB J. 2008a;22:1232.6. [Google Scholar]
- Dale-Nagle EA, Mitchell GS. Cervical spinal erythropoietin elicits phrenic motor facilitation in rats. Soc Neurosci Abstr 2008b [Google Scholar]
- Fuller DD, Bach KB, Baker TL, Kinkead R, Mitchell GS. Long term facilitation of phrenic motor output. Respir Physiol. 2000;121:135–146. doi: 10.1016/s0034-5687(00)00124-9. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Zabka A, Baker TL, Mitchell GS. Physiological and Genomic Consequences of Intermittent Hypoxia: Selected Contribution: Phrenic long-term facilitation requires 5-HT receptor activation during but not following episodic hypoxia. J Appl Physiol. 2001;90:2001–2006. doi: 10.1152/jappl.2001.90.5.2001. [DOI] [PubMed] [Google Scholar]
- Golder FJ, Ranganathan L, Satriotomo I, Hoffman M, Lovett-Barr MR, Watters JJ, Baker-Herman TL, Mitchell GS. Spinal adenosine A2a receptor activation elicits long-lasting phrenic motor facilitation. J Neurosci. 2008;28:2033–42. doi: 10.1523/JNEUROSCI.3570-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi F, Hinrichsen CF, McCrimmon DR. Short-term plasticity of descending synaptic input to phrenic motoneurons in rats. J Physiol. 2003;94(4):1421–30. doi: 10.1152/japplphysiol.00599.2002. [DOI] [PubMed] [Google Scholar]
- Hoffman MS, Mahamed S, Golder FJ, Mitchell GS. Adenosine A2A Receptors Constrain Phrenic Long Term Facilitation Following Acute Intermittent Hypoxia. FASEB J. 2007:918.12. doi: 10.1113/jphysiol.2009.180075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman MS, Mitchell GS. Episodic Spinal 5-HT7 Receptor Activation Induces Phrenic Motor Facilitation. FASEB J. 2008:1232.8. doi: 10.1113/jphysiol.2010.201657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinkead R, Mitchell GS. Time-dependent hypoxic ventilatory responses in rats: effects of ketanserin and 5 carboxamidotryptamine. Am J Physiol. 1999;277(2 Pt2):R658–66. doi: 10.1152/ajpregu.1999.277.3.R658. [DOI] [PubMed] [Google Scholar]
- Kishino A, Nakamaya C. Enhancement of BDNF and activated ERK immunoreactivity in spinal motor neurons after peripheral administration of BDNF. Brain Res. 2003;964:56–66. doi: 10.1016/s0006-8993(02)04066-0. [DOI] [PubMed] [Google Scholar]
- MacFarlane PM, Mitchell GS. Serotonin-induced phrenic long-term facilitation requires reactive oxygen species signaling via the NADPH oxidase complex. Soc Neurosci Abst 2007 [Google Scholar]
- MacFarlane PM, Mitchell GS. Respiratory long-term facilitation following intermittent hypoxia requires reactive oxygen species formation. Neuroscience. 2008;152:189–97. doi: 10.1016/j.neuroscience.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane PM, Wilkerson JE, Lovett-Barr MR, Mitchell GS. Reactive oxygen species and respiratory plasticity following intermittent hypoxia. Respir Physiol Neurobiol. 2008;164:263–71. doi: 10.1016/j.resp.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane PM, Satriotomo I, Windelborn JA, Mitchell GS. NADPH oxidase activity is necessary for acute intermittent hypoxia-induced phrenic long-term facilitation. J Physiol. 2009;587(Pt9):1931–42. doi: 10.1113/jphysiol.2008.165597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahamed S, Mitchell GS. Is there a link between intermittent hypoxia-induced respiratory plasticity and obstructive sleep apnoea? Exp Physiol. 2007a;92:27–37. doi: 10.1113/expphysiol.2006.033720. [DOI] [PubMed] [Google Scholar]
- Mahamed S, Mitchell GS. Facilitation of phrenic motor output following sustained hypocapnia in rats. FASEB J. 2007b;21:918.16. [Google Scholar]
- McGuire M, Liu C, Cao Y, Ling L. Formation and maintenance of ventilatory long-term facilitation require NMDA but not non-NMDA receptors in awake rats. J Appl Physiol. 2008;105(3):942–50. doi: 10.1152/japplphysiol.01274.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meszaros JG, Gonzalez AM, Endo-Mochizuki Y, Villegas S, Villarreal F, Brunton LL. Identification of G protein-coupled signaling pathways in cardiac fibroblasts: cross talk between G(q) and G(s) Amer J Physiol Cell Physiol. 2000;278:C154–162. doi: 10.1152/ajpcell.2000.278.1.C154. [DOI] [PubMed] [Google Scholar]
- Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by a new central neural mechanism. Respir Physiol. 1980a;41:87–103. doi: 10.1016/0034-5687(80)90025-0. [DOI] [PubMed] [Google Scholar]
- Millhorn DE, Eldridge FL, Waldrop TG. Prolonged stimulation of respiration by endogenous central serotonin. Respir Physiol. 1980b;42:171–88. doi: 10.1016/0034-5687(80)90113-9. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J Appl Physiol. 2003;94:358–74. doi: 10.1152/japplphysiol.00523.2002. [DOI] [PubMed] [Google Scholar]
- Mitchell GS. Respiratory plasticity following intermittent hypoxia: a guide for novel therapeutic approaches to ventilatory control disorders. In: Gaultier C, editor. Genetic Basis for Respiratory Control Disorders. New York: Springer Publishing Company; 2007. [Google Scholar]
- Neverova NV, Saywell SA, Nashold LF, Mitchell GS, Feldman JL. Episodic stimulation of alpha1-adrenoreceptors induces protein kinase C-dependent persistent changes in motoneuronal excitability. J Neurosci. 2007;27:4435–42. doi: 10.1523/JNEUROSCI.2803-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AA, Nunn C, Ming H, Zou MX, Penninger J, Kirshenbaum LA, Dixon SJ, Chidiac P. Upregulation of endogenous RGS2 mediates cross desensitization between Gs and Gq signaling in osteoblasts. J Biol Chem. 2006;281:32684–93. doi: 10.1074/jbc.M604416200. [DOI] [PubMed] [Google Scholar]
- Rhyzov S, Goldstein AE, Biaggioni I, Feokistov I. Cross-talk between G(s) and G(q)- coupled pathways in regulation of interleukin-4 by A(2B) adenosine receptors in human mast cells. Mol Pharmacol. 2006;70:727–35. doi: 10.1124/mol.106.022780. [DOI] [PubMed] [Google Scholar]
- Wilkerson JE, MacFarlane PM, Hoffman MS, Mitchell GS. Respiratory plasticity following intermittent hypoxia: roles of protein phosphatases and reactive oxygen species. Biochem Soc Trans. 2007;35(Pt5):1269–72. doi: 10.1042/BST0351269. [DOI] [PubMed] [Google Scholar]
- Wilkerson JE, Satriotomo I, Baker-Herman TL, Watters JJ, Mitchell GS. Okadaic acid-sensitive protein phosphatases constrain phrenic long-term facilitation after sustained hypoxia. J Neurosci. 2008;28:2949–58. doi: 10.1523/JNEUROSCI.5539-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkerson JE, Mitchell GS. Daily intermittent hypoxia augments spinal BDNF levels, ERK phosphorylation and respiratory long-term facilitation. Exp Neurol. 2009;217(1):116–23. doi: 10.1016/j.expneurol.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, McGuire M, White DP, Ling L. Serotonin receptor subtypes involved in vagus nerve stimulation-induced phrenic long-term facilitation in rats. Neurosci Lett. 2004;363(2):108–11. doi: 10.1016/j.neulet.2004.03.067. [DOI] [PubMed] [Google Scholar]


