Abstract
Clinical reports, primarily with Parkinson’s patients, note an association between the prescribed use of pramipexole (and other direct-acting dopamine agonist medications) and impulse control disorders, particularly pathological gambling. Two experiments examined the effects of acute pramipexole on rats’ impulsive choices where impulsivity was defined as selecting a smaller-sooner over a larger-later food reward. In Experiment 1, pramipexole (0.1 to 0.3 mg/kg) significantly increased impulsive choices in a condition in which few impulsive choices were made during a stable baseline. In a control condition, in which impulsive choices predominated during baseline, pramipexole did not significantly change the same rats’ choices. Experiment 2 explored a wider range of doses (0.01 to 0.3 mg/kg) using a choice procedure in which delays to the larger-later reinforcer delivery increased across trial blocks within each session. At the doses used in Experiment 1, pramipexole shifted choice toward indifference regardless of the operative delay. At lower doses of pramipexole (0.01 & 0.03 mg/kg), a trend toward more impulsive choice was observed at the 0.03 mg/kg dose. The difference in outcomes across experiments may be due to the more complex discriminations required in Experiment 2; i.e., multiple discriminations between changing delays within each session.
Keywords: Pramipexole, D2/D3 agonist, Impulsivity, Choice, Gambling
The Diagnostic and Statistical Manual of Mental Disorders (4th ed., text revision DSM-IV-TR; American Psychiatric Association, 2000) classifies pathological gambling (PG) as one of several impulse control disorders. Several recent clinical reports suggest that individuals diagnosed with Parkinson’s disease (PD) who are treated with dopamine (DA) agonist medications may demonstrate increased impulsivity and gambling activities which in some cases reaches pathological levels (for a review see Potenza et al., 2007). Other cases of DA agonist-related problem behavior have involved compulsive shopping and hypersexuality (Dodd et al., 2005; Driver-Dunckley et al., 2007; Giovannoni et al., 2000; Klos et al., 2005; McKeon et al., 2007; Munhoz et al., 2009; Voon et al., 2006a; Weintraub et al., 2006); two activities also classified by the DSM-IV-TR as impulse control disorders. A causal role of these DA agonist medications in the development of impulsive behaviors is suggested by the absence (or socially acceptable frequency) of impulse control disorders prior to drug therapy and the subsequent resolution of the problematic behavior once drug use is discontinued (e.g., Mamikonyan et al., 2008).
Dodd et al. (2005) reviewed the literature on problem gambling in PD patients and reported that 84% of the reported cases of PG occurred while patients were taking one of three DA agonists: pramipexole (59%), pergolide, or ropinirole (25% of the cases combined). Similarly, in a prospective prevalence study, Voon et al. (2006b) reported that 7.2% of their PD patients prescribed one of these three DA agonists (either as monotherapy or combined with levodopa) currently or in the past met DSM-IV diagnostic criteria for PG after they began taking the DA agonist medication. None of the patients taking levodopa alone were diagnosed with PG (see Garcia et al., 2007; Grosset et al., 2007; Imamura et al., 2008; and Weintraub et al., 2006 for similar findings). When Voon et al. (2006a) included hypersexuality and compulsive shopping as instances of impulse control disorders, 13.7% of PD patients taking one of these three DA agonists engaged in abnormal impulsive behavior after taking the agonist alone or combined with levodopa. Szarfman et al. (2006) examined cumulative reports of gambling incidents in the May 2005 US Food and Drug Administration’s Adverse Event Reporting System. They found that among the 4,400 drugs in the system, these same three DA agonists accounted for 76.1% of the reports of gambling incidents; with pramipexole accounting for 58% of all gambling-related reports in the FDA records.
Our experiments focused on pramipexole, which is a DA D2/D3 receptor agonist with high selective affinity for the D3-receptor subtype (Bennett & Piercey, 1999). DA D3 receptors are expressed predominantly in the mesocorticolimbic dopamine pathway (Grace, 2002; Sokoloff et al., 1990, 2006). Limbic structures in this region are more active during impulsive decision making (e.g., McClure et al., 2004, 2007) and play an important role in drug cues reinstating drug self-administration (e.g., See, 2005). Conversely, expression of DA D3 receptors in the prefrontal cortex is low (Levesque et al., 1992). The latter brain region is thought to play an important role in executive function (Miller & Cohen, 2001), forgoing impulsive choice (McClure et al., 2004, 2007), and tends to be hypoactive in drug-dependent populations (e.g., Hester & Garavan, 2004).
To date, only two experiments have examined the effects of pramipexole on gambling-like activities or impulsivity. Riba et al. (2008) reported that 0.5 mg pramipexole had no effect on healthy male volunteers’ decisions to wager small or large amounts of money (5 or 25 Euro cents), but it did increase the probability of making a second large wager when the preceding one was followed by an unexpected big win (50 Euro cents). The other experiment examined the effects of pramipexole on impulsive choice using a delay-discounting procedure in which humans chose between hypothetical monetary rewards delivered immediately or following a delay (Hamidovic et al., 2008). Acute pramipexole (0.25 & 0.5 mg) did not significantly increase impulsive choices. However, there was a nonsignificant trend toward more impulsive choices at the higher dose, and the sample size (n = 8) was smaller than is typical of human studies using similar delay-discounting procedures (e.g., Richards et al., 1999).
In sum, the clinical literature suggests a linkage between DA D2/D3 agonists and impulse control disorders, such as pathological gambling, but these reports lack appropriate control conditions or systematic replication. The experimental research with pramipexole and impulsive/risky choices is small and has yet to reveal systematic effects. Therefore, the present research experimentally investigated the effect of acute pramipexole on impulsive decision making in rats. Impulsivity was defined as selecting a small amount of food delivered immediately while forgoing a larger amount of food available after a brief delay. This definition of impulsivity captures many of the features of impulse control disorders outlined by the DSM-IV-TR (e.g., a choice which demonstrates an inability to resist immediate temptation and results in a long-term loss; in this case, a loss of within-session “income”). The social validity of this definition of impulsivity is suggested by studies demonstrating substantial differences in this form of impulsivity between drug-addicted and control participants (e.g., Madden et al., 1997; Vuchinich & Simpson, 1998) and between pathological gamblers and controls (e.g., Alessi & Petry, 2003; Dixon et al., 2003, 2006; Petry, 2001). Finally, studying the effects of pramipexole on impulsive choice in rats allows for an examination of the effects of the drug against a known behavioral baseline.
Experiment 1
Method
Subjects
Nine male Wistar rats (Harlan Sprague-Dawley, Indianapolis, IN) served as subjects. Seven rats had prior experience choosing between small-immediate and large-delayed food reinforcers and were approximately 6 months old at the start of this experiment. The remaining two rats (G1B1 & G1B2) were experimentally naïve and were 3 months old at the start of the experiment. Rats were weighed daily and maintained at approximately 85% of free-feeding growth curve weights through supplemental, post-session feeding provided 120 min after each session. Between sessions, rats were housed individually in plastic cages within a temperature-controlled colony room providing a 12:12 hr light/dark cycle (lights on at 6:00 am). Water was available continuously in the home cage. All procedures were approved by the Kansas University Animal Care and Use Committee and conformed to the guidelines established by the NIH Guide for the Use of Laboratory Animals.
Apparatus
Nine identical operant chambers (Med Associates, St. Albans, VT) were used. Each chamber measured 24.1 cm wide, 30.5 cm long, and 21 cm high. One wall of the chamber was an intelligence panel equipped with a nonretractable center lever (11 cm above the floor) and two retractable side levers (horizontally aligned 11 cm apart and 6.5 cm above the floor). Above each lever was a white, 28-volt cue light (2.5 cm in diameter and 6 cm above each lever). A feeder (Coulbourn Instruments, Allentown, PA) equipped with a pellet detection unit (Pinkston et al., 2008) delivered 45-mg grain-based food pellets (Bio-serv, Frenchtown, NJ; no. F0165) into a receptacle in the center of the intelligence panel (3 cm wide and 4 cm long) equipped with a 28-volt light (1 cm above the floor and 10 cm below the center lever). Chambers were enclosed within a light- and sound-attenuation cubicle (Med Associates, St. Albans, VT) equipped with a ventilation fan and a white noise speaker. A Med Associates® interface system controlled the sessions and collected data.
Procedures
For the two naïve rats, lever pressing was autoshaped (Brown & Jenkins, 1968). Prior to beginning the experiment proper, all rats completed several sessions in which responses alternated between pressing the left and right side levers to earn food reinforcers. This ensured that the rats had a recent history of equivalent responding and reinforcement on both of the side levers.
For the remainder of the experiment, sessions were composed of 42 trials, organized into seven blocks of six trials. Each block consisted of two forced-choice trials (randomized with one on the left lever and the other on the right) followed by four free-choice trials. The light above the center lever signaled the start of every trial. On forced-choice trials, a response on the center lever extinguished the cue light and caused one side lever to be inserted into the chamber with the light above that lever simultaneously lit. A single response on the side lever retracted the lever and began the pellet delivery sequence. During this sequence, either the light above the lever was extinguished and one food pellet was delivered immediately (0.01-s delay; the smaller-sooner reinforcer [SS]), or the light flashed in 0.25-s intervals during a delay after which three pellets were delivered (the larger-later reinforcer [LL]) and the cue light was extinguished. A flash of light in the food receptacle accompanied the delivery of each pellet.
Pellet deliveries were followed by an inter-trial interval (ITI) during which no stimuli were presented. The duration of the ITI was adjusted to ensure that the start of each trial was separated by exactly 100 s regardless of response latency and delay associated with the lever selected. Within each trial, a 30-s limited hold was in effect: If either center- or side-lever response latency exceeded 30 s following the illumination of the relevant cue light, the trial was terminated, the ITI was initiated, and the trial was scored as an omission.
Each rat completed two conditions, with five rats completing these in one sequence and the other four rats in the other sequence. In one condition, a baseline of self-control (i.e., 0–20% choice of the SS reinforcer) was established to determine if pramipexole would increase impulsive choices. The other condition established a baseline of impulsive choices (80–100% SS choices) which served as a control: From a baseline of stable impulsive choices would pramipexole increase choice of the LL reinforcer? If it did, then any effect of pramipexole on choice in the self-control baseline would be attributable to a drug-related non-specific disruption of choice. The assignment of the SS and LL reinforcers to the left and right levers was counterbalanced across rats (see Table 1). It should be noted that prior to this condition, the experienced rats made choices between SS and LL rewards with delay to the LL adjusted in an attempt to produce a baseline of indifference. Stable indifference proved to be difficult to achieve and could not be reliably recovered following drug sessions. Therefore the two baseline procedures described below were pursued.
Table 1.
Sequence of pramipexole doses, larger-later (LL) lever assignment, and order in which baseline conditions were experienced for each subject in Experiment 1. For each baseline condition, sessions to stability and stable LL delay are shown.
| Subject | Dose Sequence |
LL Lever |
1st Baseline Condition |
Sessions to Stability |
Delay to LL |
2nd Baseline Condition |
Sessions to Stability |
Delay to LL |
|---|---|---|---|---|---|---|---|---|
| B1Br3 | 0.1, 0.18, 0.3 | Right | Self-control | 38 | 8 s | Impulsive | 98 | 31 s |
| P1Br1 | 0.3, 0.18, 0.1 | Right | Self-control | 121 | 13 s | Impulsive | 121 | 32 s |
| B1P1 | 0.3, 0.18, 0.1 | Right | Self-control | 114 | 8 s | Impulsive | 81 | 28 s |
| B1Br2 | 0.1, 0.18, 0.3 | Left | Self-control | 38 | 3 s | Impulsive | 68 | 20 s |
| B1Bl2 | 0.1, 0.18, 0.3 | Left | Self-control | 26 | 6 s | Impulsive | 49 | 22 s |
| P1Br2 | 0.1, 0.18, 0.3 | Right | Impulsive | 31 | 14 s | Self-control | 35 | 3 s |
| B1Bl3 | 0.3, 0.18, 0.1 | Left | Impulsive | 169 | 18 s | Self-control | 48 | 3 s |
| G1B1 | 0.3, 0.18, 0.1 | Left | Impulsive | 74 | 24 s | Self-control | 127 | 11 s |
| G1B2 | 0.3, 0.18, 0.1 | Right | Impulsive | 113 | 27 s | Self-control | 166 | 3 s |
Self-control baseline
Sessions were conducted as described above with the delay to the LL reinforcer set at 6 s. After each session, if the percentage of free-choices emitted on the SS lever was greater than 20% for three consecutive sessions, then the delay to the LL reinforcer was decreased by 2 s. After choice of the SS reinforcer initially fell in the 0–20% range, all subsequent delay adjustments were in 1 s increments. In an attempt to maintain some choice of the SS reinforcer within each session, the delay to the LL reinforcer was increased by 1 s if the rat chose the LL alternative on all free-choice trials for three consecutive sessions. This between-session adjusting-delay procedure was continued until (a) SS choices were at or below 20% for ten consecutive sessions, (b) at least one of the stable sessions contained nonexclusive choice, and (c) no omissions occurred in the stable sessions. Once a stable baseline had been achieved, pre-session injections were initiated (see below) and no further adjustments to the delay could be made. The delays at which each rat stabilized in each condition are shown in Table 1. Rats that first completed the self-control baseline condition also completed the impulsive baseline condition after all pre-session pramipexole injections had been administered.
Impulsive baseline
With two exceptions this condition was identical to the self-control baseline condition. First, the delay to the LL reinforcer was increased by 1 s if the percentage of free-choices emitted on the SS lever was less than 80% for two consecutive days. Second, this delay was decreased by 1 s if a subject exclusively chose the SS alternative in all free-choice trials for three consecutive days. The stability criteria outlined above were used here except that the range of SS choices was required to be at or above 80% (i.e., predominantly impulsive choices). Rats assigned to complete this condition first also completed the self-control baseline condition after all pre-session pramipexole injections had been administered.
Pharmacological procedures
Pramipexole was provided by Drs. Shaomeng Wang and Jianyong Chen (University of Michigan, Ann Arbor, MI). Pramipexole was dissolved in physiological saline. Three doses of pramipexole (0.1, 0.18, 0.3 mg/kg) or saline vehicle were administered subcutaneously 10 min prior to the session at a volume of 1.0 ml/kg. This range of doses has been shown to be behaviorally active (e.g., Collins et al., 2005), while higher doses have been found to greatly decrease locomotor activity during the post-injection period in which our sessions were conducted (Lagos et al., 1998).
Drug tests were separated by no-injection days in which sessions were completed as usual. No-injection days continued until choice was in the baseline range for at least four consecutive days (median: 4.5; IQR: 4–5 days). The saline vehicle was given first and tests with pramipexole followed in either an increasing or decreasing sequence (see Table 1). Once each dose was tested, the series was repeated twice (in the same order) in both baseline conditions.
Data analysis
Separate two-way (dose × series) repeated-measures ANOVAs were conducted to determine if pramipexole significantly affected omissions, response latencies, and impulsive choice (SPSS 16.0, SPSS Inc., Chicago, IL). Where data were not normally distributed and remained significantly non-normal following standard transformations, Greenhouse-Geisser corrected degrees of freedom were used. Bonferroni t-tests were used for post hoc comparisons.
Results
In the self-control baseline condition (≥ 80% choice of the LL reinforcer), an average of 79.2 (SE = 16.5) sessions were required to reach an average stable adjusted-delay of 6.4 s (SE = 1.2 see Table 1). In the impulsive baseline condition, an average of 89.3 (SE = 13.4) sessions were required to achieve a stable adjusted-delay of 24.0 s (SE = 1.9). In one condition, two rats’ (B1P1 & G1B2) stable baseline choices could not be recovered during no-injection days between the second and third series of doses. These rats did not complete the third dose series and missing data from this series were interpolated by SPSS using the between-subjects average. These interpolations did not affect the outcome of any analysis.
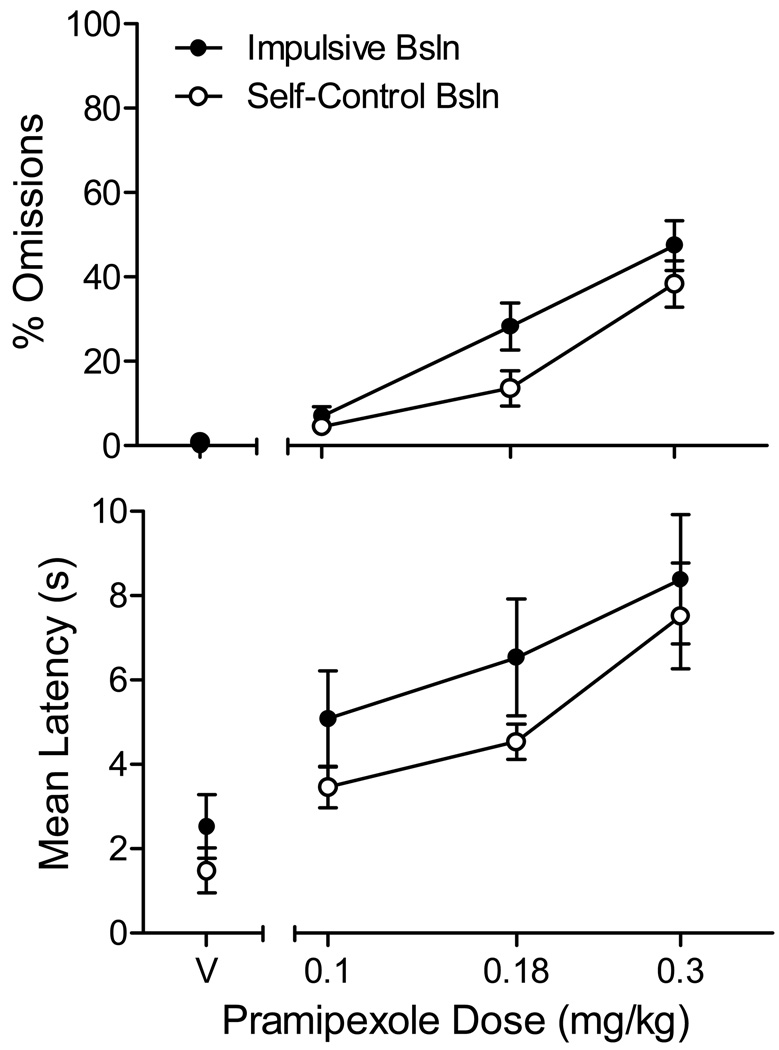
The top panel of Figure 1 shows the average percent of trials on which the rat failed to respond within 30 s (an omission) in the vehicle (V) and pramipexole sessions. When compared with vehicle, pramipexole dose-dependently increased the number of trials scored as omissions in both the impulsive (F(3, 48) = 30.8, p < .001, ηp2= 0.79; linear contrast: p < .001) and self-control (F(1.5, 17.4) = 15.3, p = .001, ηp2= 0.66; linear contrast: p = .001) baselines. The difference in omission frequencies across the two baseline conditions approached, but did not achieve conventional levels of significance (main effect of baseline, p = .06). No interactions were significant. A separate two-way ANOVA revealed no significant main effect of series (i.e., the first, second, or third pass through the sequence of saline and active injections, p = .17) and the dose × series interaction was not significant (p = .45).
Figure 1.
Average (SEM) percent of trials omitted per session (top panel) and mean center lever response latency (bottom panel) as a function of saline and pramipexole doses in Experiment 1.
As shown in the bottom panel of Figure 1, pramipexole similarly affected center-lever response latencies on trials in which a response was made. The main effect of dose was significant in both the impulsive (F(3, 48) = 17.3, p < .001, ηp2= 0.68; linear contrast: p = .001) and self-control baselines (F(3, 48) = 16.9, p < .001, ηp2= 0.68; linear contrast: p < .001); however, no significant difference in latencies was observed across baselines (p = .17). A separate two-way ANOVA revealed no significant main effect of series (p = .93) and the dose × series interaction was not significant (p = .71).
In five sessions in which injections were administered (2.2% of all injection sessions), four rats failed to complete any free- or forced-choice trials (one rat did this twice at the highest dose). This occurred twice in the self-control baseline and three times in the impulsive baseline and was restricted to the two highest doses (0.18 and 0.3 mg/kg). In these cases, the missing data were interpolated from between-subject averages; these interpolations did not affect the outcome of any analysis.
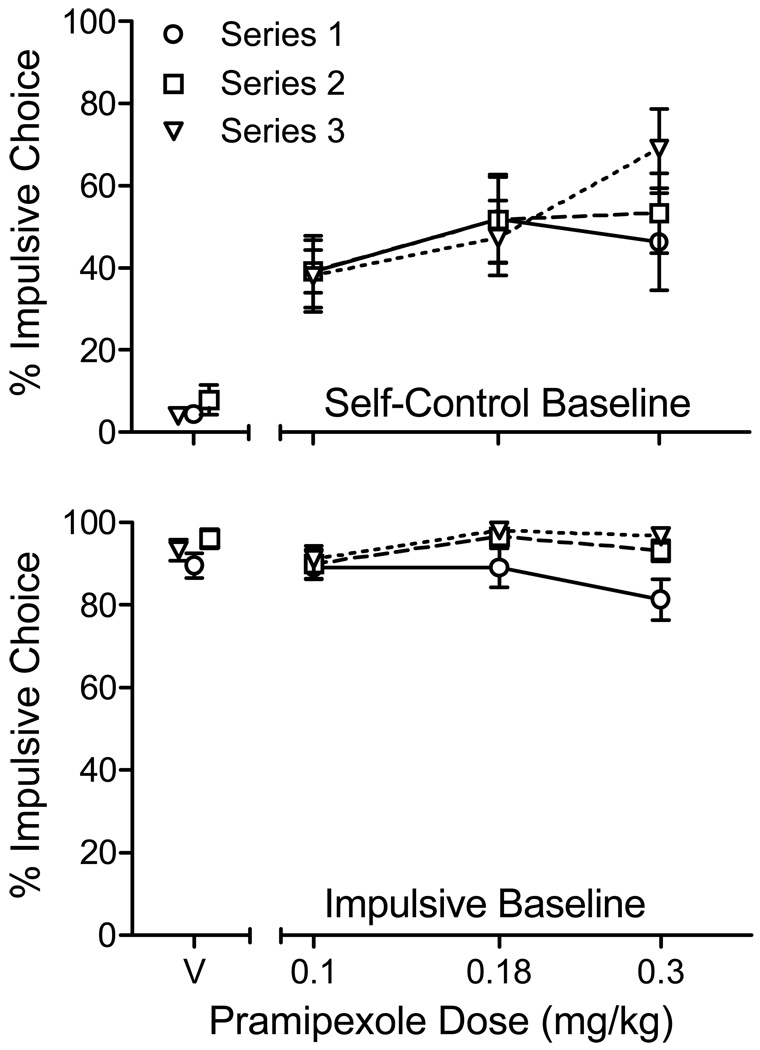
The top panel of Figure 2 shows the average percentage of impulsive choices made in the self-control baseline condition. Separate data paths illustrate the effects of pramipexole in the three dosing series. In this condition, impulsive choices were infrequent on vehicle (V) days. By contrast, pramipexole increased impulsive choices above saline levels (F(3,48) = 21.3, p < .001; ηp2= 0.73; linear contrast: p < .001). The main effect of dosing series was not significant (p < .48). However the dose × series interaction approached significance (p = .06), reflecting the higher prevalence of impulsive choices on the final dosing series at the highest dose.
Figure 2.
Average (SEM) percent choice of the impulsive (SS) alternative in impulsive and self-control baseline conditions of Experiment 1.
In the impulsive baseline condition (bottom panel of Figure 2), impulsive choices predominated in vehicle sessions. No significant main effect of pramipexole was detected (p = .34); however, there was a significant effect of dosing series (F(2,48) = 9.5, p < .01; ηp2= 0.54) reflecting a reduction in impulsive choice at the 0.3 mg/kg dose in the first, relative to the second and third, series of pramipexole doses. Where this reduction was observed (three sessions), it occurred in sessions in which more than 60% of the trials were omitted (i.e., the trial was terminated because the rat failed to respond within 30 s).
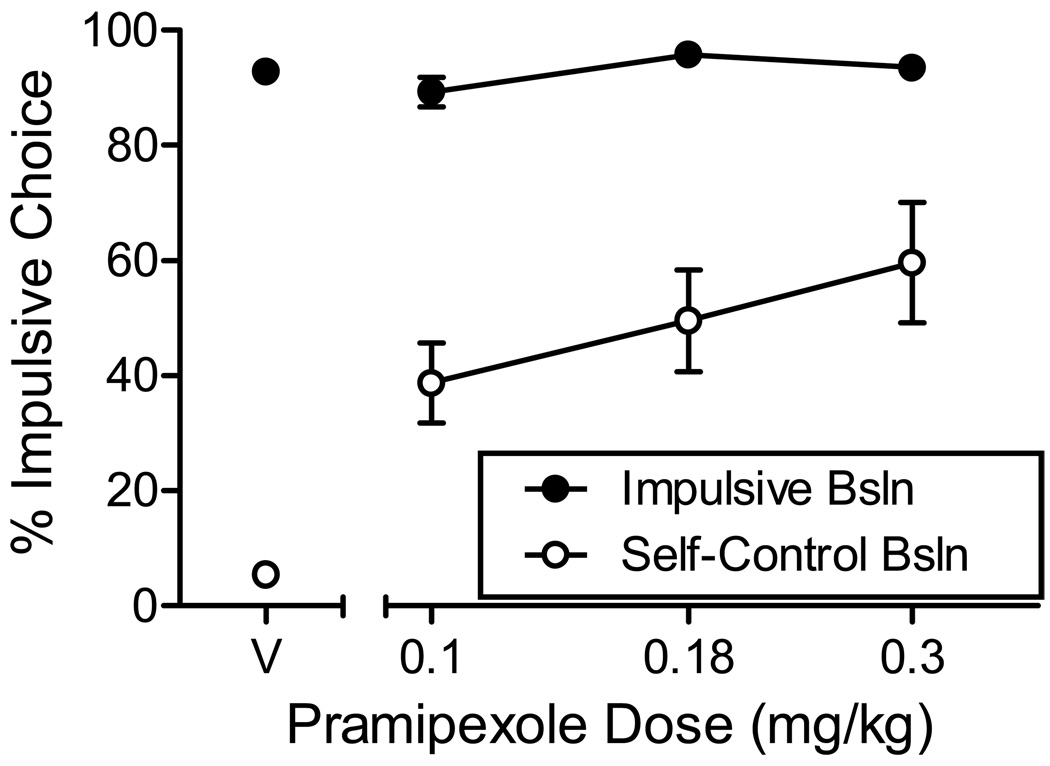
To reduce the probability that conclusions about the effects of pramipexole on impulsive choice would be influenced by sessions in which few choice responses occurred, a second series of analyses were conducted in which data were excluded if the rat did not complete at least 50% of the free-choice trials. This exclusion criterion resulted in the exclusion of 7.4% of the data in the self-control baseline condition and 10.8% in the impulsive baseline condition. To allow statistical analyses with these missing data, a single average was calculated at each dose for each rat across dosing series. These data are shown in Figure 3.
Figure 3.
Average (SEM) percent choice of the impulsive (SS) alternative in impulsive and self-control baseline conditions of Experiment 1. Data were excluded from analysis if the rats completed fewer than 50% of free-choice trials in a session.
As before, a significant effect of pramipexole was observed in the self-control baseline condition (F(3,24) = 16.1, p < .001; ηp2= 0.67), but not in the impulsive baseline (p = .12). Post-hoc comparisons of self-control baseline choices revealed significant differences between saline and all pramipexole doses (p < .03 in all cases); however, no significant differences were detected between doses. In the impulsive baseline, post-hoc comparisons revealed no differences between doses or between any single dose and saline.
Discussion
At doses ranging from 0.1 to 0.3 mg/kg, pramipexole significantly increased impulsive choices in male Wistar rats. From a stable self-control baseline, the average minimum increase in impulsive choice was 31% (range: 4 to 72%) and the average maximum increase was 50% (range: 13 to 92%). These increases would appear not to be due to a disruption in choice produced by active doses of pramipexole. If this were the case, choice should have been disrupted similarly from the baseline of stable impulsive choice. This, however, was not the case. From a stable impulsive baseline, the average pramipexole-related change in impulsive choice ranged from −4 to 7%.
Further evidence against this choice-disruption hypothesis comes from two putative measures of response disruption – center-lever response latencies (i.e., trial-initiation responses) and omissions (i.e., trials in which latencies exceeded 30 s). For both measures, pramipexole produced similar dose-related disruptions during both the impulsive and self-control baseline conditions. If longer latencies and/or more omissions had been observed in the self-control baseline, one might reasonably argue that choice too was more disrupted by pramipexole in this condition. However, no difference in latencies was observed across conditions, and there was a trend toward more omissions in the impulsive baseline condition (p = .06); the opposite of what one would expect if increased impulsive choices in the self-control condition were due to drug-related disruptions in responding.
While these findings provide preliminary evidence that pramipexole increased impulsive choice in male Wistar rats, it is important to note that, with a few exceptions, the drug did not produce strong preferences for the SS over the LL reward. Three rats chose the SS reward >60% of the time at the 0.18 and 0.3 mg/kg doses, but the rest did not. Thus, pramipexole increased impulsive choice, but it did so only to the point of indifference between a SS and a LL reinforcer. Given this tendency toward indifference, the possibility must be considered that choice in the self-control baseline may have been more easily disrupted by any perturbation than was choice in the impulsive baseline condition. Indirect evidence supporting this hypothesis is provided by Experiment 4 of Nevin (1974). In this study, pigeons’ pecked a single key on a multiple schedule of reinforcement. In one component of the multiple schedule, pecks were reinforced immediately (0.4-s signaled delay), while in the other component reinforcers were delayed (9.6-s signaled delay). When free food was delivered during a blackout period (a standard disruptor in studies of this type) the percentage decrease below baseline response rate was larger in the component in which the pigeon had previously obtained delayed reinforcers. If this outcome, obtained in a single-response context, can be translated to the choices made in the two baselines of the present experiment, then one might expect strong preference for a delayed reinforcer (self-control baseline condition) to be more susceptible to disruption than a comparable (but opposite) preference for an immediate reinforcer (impulsive baseline condition). If so, then the shift toward more impulsive choices observed in the self-control baseline of Figure 3 may be more related to non-specific choice-disrupting effects of pramipexole than to any impulsivity-increasing effects of this drug. Arguing against this hypothesis are findings suggesting that drugs such as cocaine do not serve as behavioral disruptors in tests of behavioral momentum (Cohen, 1986; Harper, 1999; Pinkston et al., 2009).
Nonetheless, to begin to address this alternative account of the results of Experiment 1, Experiment 2 employed a commonly used procedure for assessing drug effects on impulsive choice. In this procedure, developed by Evenden and Ryan (1996), the delay to the larger food reward is initially set at zero and is progressively increased across trial blocks within each session. According to the choice-disruption hypothesis just described, choice should be least disruptable in the first trial block because all reinforcers are immediate. In subsequent trial blocks, as more delayed rewards are selected, choice should be more disruptable and preference should shift toward indifference. However, if pramipexole increases impulsive choice, then (barring a floor effect) it should decrease choice of the LL reinforcer in all trial blocks except the first.
Experiment 2
Method
Subjects and Apparatus
Twelve experimentally naïve male Wistar rats (Harlan Sprague-Dawley, Indianapolis, IN), approximately 3 months old at the start of the experiment, served as subjects. Rats were housed as in Experiment 1 and were provided supplemental feeding to maintain their weight at 85% of adult free-feeding weight. All procedures were approved by the Kansas University Animal Care and Use Committee.
Procedures
Initial training was accomplished as with the experimentally naïve rats in Experiment 1. Once lever pressing was reliably occurring on all three levers, the experiment began. The procedures used in the experimental sessions were based on those outlined by Evenden and Ryan (1996). Sessions were composed of 40 trials presented in blocks of 10 trials. Each block was composed of four forced- and then six free-choice trials. Two forced-choice trials were completed on each lever in random order. Forced- and free-choice trials operated as in Experiment 1. The lever to which the LL reward was assigned was counterbalanced across subjects (see Table 2).
Table 2.
Larger-later (LL) lever assignment and sessions to reach stability for each subject in Experiment 2
| Subject | LL Lever |
Sessions to Stability |
|---|---|---|
| B1G1 | Right | 41 |
| B1G2 | Left | 50 |
| B1G3 | Right | 43 |
| B1G4 | Left | 41 |
| G1R1 | Right | 40 |
| G1R2 | Left | 40 |
| P1R1 | Right | 43 |
| P1R3 | Right | 44 |
| P1R4 | Left | 69 |
The delay to the larger food reward was increased within-session between trial blocks. In block 1, the delay to the larger reward was 0 s, so the rat simply chose between more (3 pellets) and less (1 pellet) food. Within the second, third, and fourth trial blocks the delay to the larger reward was set at 10, 20, and then 30 s (in that order). In addition to the forced-choice trials, two procedures were used throughout the experiment to enhance the salience of the between-block changes in delays. First, trial blocks were separated by a 180-s blackout. Second, on a randomly determined one out of seven days, no delay was imposed between choice and delivery of the larger reward in any trial block. This no-delay procedure, originally used by Evenden and Ryan (1996), may increase the probability that subjects attend to the delays arranged in each trial block, rather than to the passage of time or signaled transitions between trial blocks.
Stability of choice within each trial block was assessed after a minimum of 20 baseline sessions. Within a block, choice was considered stable when the mean percent LL choice over the last 6 sessions differed from the previous 6-session mean by no more than 10% with no monotonic trend. Baseline sessions continued until these stability criteria were met in all four trial blocks and no omissions occurred in the last 12 sessions. Of the 12 rats that began the experiment, 3 demonstrated either hyper- or hypo-sensitivity to the within-session delay increases during no-injection sessions (floor and ceiling effects, respectively). These rats were excluded from the study because their baseline preference for the LL reward could only increase (hypersensitivity to delay) or only decrease (hyposensitivity).
Pharmacological procedures
Pramipexole (0.01, 0.03, 0.1, 0.18, 0.3 mg/kg) or saline vehicle was administered subcutaneously 10 min prior to the session at a volume of 1.0 ml/kg. The lower doses were added after it was observed that doses of 0.1 and above disrupted preference for the large over the small reinforcer during the first trial block (0-s delay to both rewards). Two rats had already completed the first series of 0.1–0.3 mg/kg doses before this decision was made. Therefore, for these rats, the smaller doses were included only in the second and third dose series. The lower doses have been found to be behaviorally active (e.g., Collins et al., 2005; Lagos et al., 1998).
Within each dosing series, saline vehicle was given first and tests with pramipexole followed in a descending order of doses. The descending order was selected because we have occasionally observed in previous work with dopamine agonists that the effects of smaller doses change after the administration of larger doses, and so beginning the sequence with the larger dose minimizes variability. Once each dose was tested, the series of doses was repeated twice (in the same order). Drug tests were separated by four no-injection sessions. Pramipexole was not administered during the occasional sessions in which the delay remained at zero throughout.
Data analysis
A preliminary two-way (dose × series) repeated-measures ANOVA was used to evaluate if the effects of pramipexole varied as a function of repeated series of doses. To include delay within this analysis, area under the percent LL choice curve was calculated for each subject at each dose. Because no main effect of series or dose × series interaction was detected, the choice data were collapsed across series in the remaining analyses. A two-way (dose × delay) repeated-measures ANOVA was conducted to determine if pramipexole significantly affected percent impulsive choice. Bonferroni-corrected t-tests were used for post hoc comparisons.
Results & Discussion
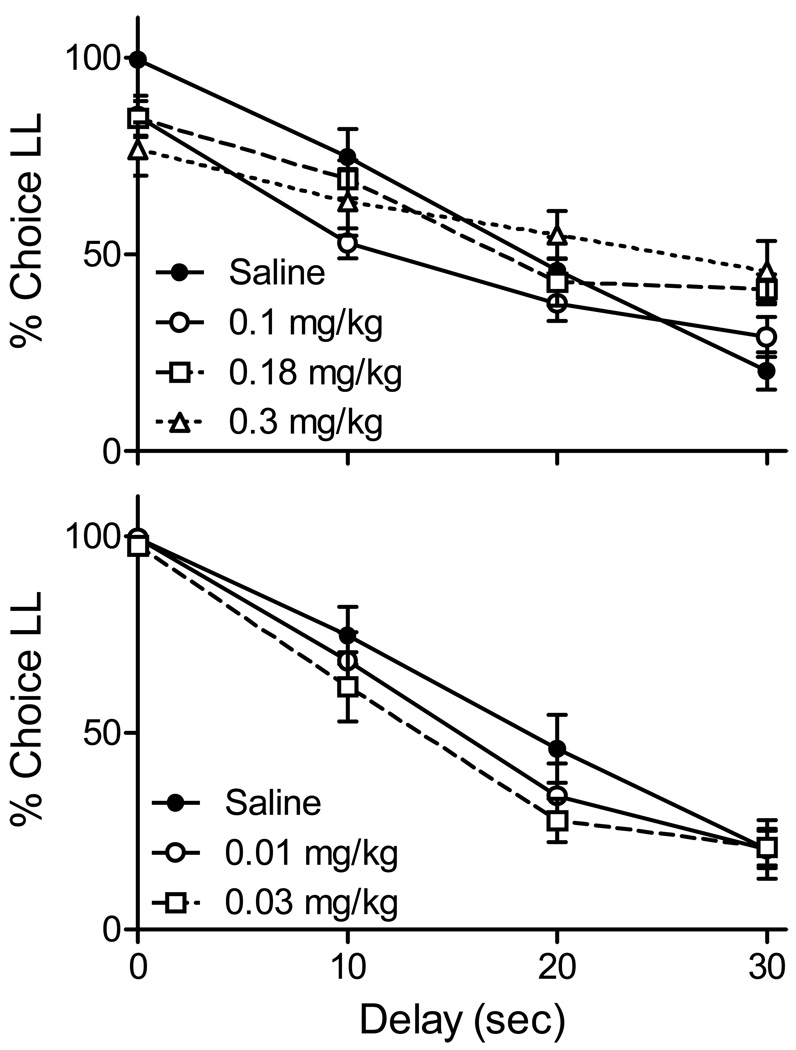
Figure 4 shows that as delays to the LL reward were increased within the session, percent choice of this reward significantly declined during saline sessions (F(3,32) = 31.5, p < .001). There was no significant difference between saline and no-injection sessions (F(1,24) = 0.19, p > .05) and the latter data are omitted from the graphs. Also not shown are choices made during sessions in which the delay remained at 0 s through all trial blocks. Across rats, the larger reward was selected 98.9% of the time in the first trial block, and declined in the three subsequent trial blocks to 94.4, 92.1, and 93.3%. A one-way ANOVA revealed that this decline was significant (F(3,24) = 6.25, p < .01) and Bonferroni-corrected post hoc comparisons revealed that choice of the larger reward was significantly higher in trial block 1 than in block 4 (p ≤ .02) but not blocks 2 or 3 (p’s > .08).
Figure 4.
Average (SEM) percent choice of the LL reward as a function of LL delay across trial blocks in Experiment 2. The top panel displays choices made at the same doses used in Experiment 1. The bottom panel shows choices made at two smaller doses.
The upper panel of Figure 4 shows Experiment 2 choices made at the doses used in Experiment 1. At these doses the percent choice of the larger food reward was significantly decreased in the first trial block in which both rewards were immediately available (Bonferroni corrected p < .05 at all three doses). This outcome is not consistent with the alternative account of the results of Experiment 1. That is, if choices maintained by immediate rewards are less susceptible to disruptors (such as a non-specific effect of a drug) then choice in the first trial block of Experiment 2 should have been as difficult to disrupt, as was choice in the impulsive baseline of Experiment 1.
Similar shifts toward indifference were observed at the 30-s delay (significant at the 0.1 and 0.18 mg/kg doses; p < .05). At the Experiment 1 doses, pramipexole flattened the choice function (toward indifference) at all delays but 20 s; the delay at which indifference was observed in saline sessions. This flattening was dose dependent, as linear functions fit to choices made at each dose revealed significantly more shallow slopes when 0.1 mg/kg was compared with saline and 0.3 mg/kg was compared to 0.1 mg/kg (p < .05 in both cases). Because the doses of pramipexole used in Experiment 1 significantly shifted choice toward indifference in these first and final trial blocks, the remainder of the choice curve could not be interpreted as a drug effect on impulsive choice. Therefore, the data were not further analyzed.
The lower panel of Figure 4 shows the effects of the two lower doses of pramipexole on choice, exclusively used in Experiment 2. These doses did not shift choice toward indifference in either the initial or final trial block (0 and 30 s, respectively); thus, any significant reductions in choosing the LL reward at the 10- and 20-s delays could be interpreted as a drug related increase in impulsivity. Across all delays, there was no significant main effect of dose (p = .13) or delay × dose interaction (p = .19). Post-hoc tests conducted at the 10- and 20-s delays failed to reveal a significant increase in impulsive choice at either of the low doses of pramipexole. However, the differences at 0.03 mg/kg approached significance at the 10- and 20-s delays (one-tailed p’s = .04 and .03, respectively; which fell short of the critical value of the Bonferroni adjusted p = .01).
Although this experiment started with 12 rats, 3 were excluded from dosing because their baseline choices made it impossible to see either an increase or a decrease in impulsivity; hyper- or hypo-sensitivity to delay, respectively. The smaller than anticipated sample size of 9 rats may have been insufficient to detect the effects of the 0.01 and 0.03 mg/kg doses. Thus, future research should employ additional subjects and may benefit from exploring doses between 0.03 and 0.1 mg/kg. These doses may produce a significant increase in impulsive choice while not disrupting choice in the first and final trial blocks.
General Discussion
Two experiments examined the effects of acute pre-session pramipexole injections on rats’ impulsive choice. In Experiment 1, acute pramipexole at doses between 0.1 and 0.3 mg/kg significantly increased impulsive choice. The same doses did not significantly disrupt choice (toward indifference) in a control baseline in which the rats preferred the SS reinforcer. The second experiment employed a procedure in which delays to the LL reinforcer were increased within session. In Experiment 2, acute pramipexole in the 0.1–0.3 mg/kg range produced a non-specific disruption of choice (toward indifference). These choice-disrupting effects were not observed at lower acute doses (0.01 and 0.03 mg/kg), but the highest of these two doses produced only a trend toward more impulsive choice.
Experiment 1 included a control condition in which the effects of pramipexole were examined from a baseline of predominantly impulsive choices (i.e., preference for the SS reinforcer). Because acute pramipexole at doses ranging from 0.1 to 0.3 mg/kg did not disrupt choice in this impulsive baseline, there is reason to believe that the significant increase in impulsive choice observed in the self-control baseline was due to an impulsivity-inducing effect of pramipexole, rather than a non-specific disruption of discriminations underlying choice. The caveat to this interpretation is that choice in the self-control baseline may have been more easily disrupted than in the other baseline because the consequences maintaining choice in the self-control baseline were delayed reinforcers.
Experiment 2 was designed to address this alternative explanation by examining the effects of the same doses of pramipexole when immediate reinforcers followed all choices at the beginning of the session (presumably producing the least choice disruption) and when reinforcers became progressively more delayed through the remainder of the session. Contrary to expectation, acute pramipexole (0.1–0.3 mg/kg) significantly disrupted choice in the first block of choice trials when all reinforcers were delivered immediately.
Why pramipexole disrupted choice in the first block of trials in Experiment 2 but not in the impulsive baseline of Experiment 1 deserves comment. One hypothesis is that stimulus control was more strongly established in Experiment 1 than in Experiment 2. Strong stimulus control in Experiment 1 might be expected because the same outcomes followed presses on the left and right levers throughout each session and throughout each baseline (a period of approximately 150 days of consistent outcomes within each baseline). By contrast, in Experiment 2 the delay to the larger reinforcer increased from 0 to 30 s within-session across trial blocks. This may have decreased the likelihood that the lever and cue light associated with the larger reward would acquire as strong a delay-cuing function as was established in Experiment 1. The finding that behavior under weak stimulus control is more sensitive to the rate-altering effects of drugs than behavior under strong stimulus control (Laties, 1975; Laties & Weiss, 1966; Moerschbaecher, Boren, Schrot, & Fontes, 1979) may help to explain the choice disruptions observed in Experiment 2 at doses employed in Experiment 1. If these speculations are correct, then Experiment 2 may not have adequately tested the alternative account of the results of Experiment 1. Clearly more research is necessary.
Three future research directions are suggested by the present findings and their relation to clinical reports of increased impulse control disorders among PD patients treated with pramipexole. First, because PD patients take daily doses of pramipexole, often for months or more before the development of impulse control disorders, future research should examine the effects of chronic pramipexole on impulsive choice. Second, there may be merit in exploring the effects of pramipexole in Parkinson’s rats, as this is the group of humans who have most often been reported to develop PG when given this drug or other dopamine agonists (Ondo & Lai, 2008); although it should be noted that a number of clinical reports have documented a relation between pramipexole and impulse control disorders in individuals treated for restless leg syndrome (Potenza, 2007). Third, the role of DA, and how it might interact with the serotonergic system to affect impulsive choice is not well understood (Winstanley, 2009). While the present findings tentatively suggest a role of DA D2 and D3 receptors in impulsive choice, this hypothesis was not directly tested by comparing the effects of pramipexole with those of other drugs such as DA agonists that affect different DA receptors or DA receptor-specific antagonists.
To conclude, the present findings tentatively support the hypothesis that pramipexole increases impulsivity when defined as preference for a smaller-sooner over a larger-later reinforcer. The link between pramipexole and impulse control disorders (such as human pathological gambling) must be interpreted with caution because the term impulsivity has several meanings (see Evenden, 1999), only one of which was measured in the present experiments. Although human pathological gamblers favor smaller-sooner over larger-later rewards (see Petry & Madden, 2009 for a review), the role that this form of impulsivity may play on the decision to gamble remains theoretical (e.g., Madden et al., 2007; Rachlin, 1990).
Acknowledgments
This research was supported by NIH grant DA023564. Pramipexole was provided under NIH grant DA 020669 (James H. Woods, University of Michigan, Ann Arbor). Jonathan Pinkston is now at the University of Texas Health Science Center.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/pha.
References
- Alessi SM, Petry NM. Pathological gambling severity is associated with impulsivity in a delay discounting procedure. Behavioural Processes. 2003;64:345–354. doi: 10.1016/s0376-6357(03)00150-5. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed., text rev. Washington, DC.: Author; 2000. [Google Scholar]
- Bennett JP, Piercey MF. Pramipexole -- a new dopamine agonist for the treatment of Parkinson’s disease. Journal of Neurological Science. 1999;163:23, 31. doi: 10.1016/s0022-510x(98)00307-4. [DOI] [PubMed] [Google Scholar]
- Brown PL, Jenkins HM. Autoshaping of the pigeon’s key-peck. Journal of the Experimental Analysis of Behavior. 1968;11:1–8. doi: 10.1901/jeab.1968.11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SL. A pharmacological examination of the resistance-to-change hypothesis of response strength. Journal of the Experimental Analysis of Behavior. 1986;46:363–379. doi: 10.1901/jeab.1986.46-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Witkin JM, Newman AH, Svensson KA, Grundt P, Jianjing C, Woods JH. Dopamine agonist-induced yawning in rats: A dopamine D3 receptor-mediated behavior. Journal of Pharmacology and Experimental Therapeutics. 2005;314:310–319. doi: 10.1124/jpet.105.085472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon MR, Marley J, Jacobs EA. Delay discounting by pathological gamblers. Journal of Applied Behavior Analysis. 2003;36:449–458. doi: 10.1901/jaba.2003.36-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon MR, Jacobs EA, Sanders S. Contextual control of delay discounting by pathological gamblers. Journal of Applied Behavior Analysis. 2006;39:413–422. doi: 10.1901/jaba.2006.173-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd ML, Klos KJ, Bower JH, Geda YE, Josephs KA, Ahlskog JE. Pathological gambling caused by drugs used to treat Parkinson disease. Archives of Neurology. 2005;62:1377–1381. doi: 10.1001/archneur.62.9.noc50009. [DOI] [PubMed] [Google Scholar]
- Driver-Dunckley ED, Noble BN, Hentz JG, Evidente VG, Caviness JN, Parish J, Krahn L, Adler CH. Gambling and increased sexual desire with dopaminergic medications in restless legs syndrome. Clinical Neuropharmacology. 2007;30:249–255. doi: 10.1097/wnf.0b013e31804c780e. [DOI] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology (Berl) 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Evenden JL, Ryan CN. The pharmacology of impulsive behaviour in rats: The effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology (Berl) 1996;128:161–170. doi: 10.1007/s002130050121. [DOI] [PubMed] [Google Scholar]
- Garcia RF, Ordacgi L, Mendlowicz MV, de Freitas GR, Rosso AL, Nazar BP, Fontenelle LF. Treatment of juvenile Parkinson disease and the recurrent emergence of pathologic gambling. Cognitive and Behavioral Neurology. 2007;20:11–14. doi: 10.1097/WNN.0b013e31802b6c34. [DOI] [PubMed] [Google Scholar]
- Gerlach M, Double K, Arzberger T, Leblhuber F, Tatschner T, Riederer P. Dopamine receptor agonists in current clinical use: Comparative dopamine receptor binding profiles defined in the human striatum. Journal of Neural Transmission. 2003;110:1119–1127. doi: 10.1007/s00702-003-0027-5. [DOI] [PubMed] [Google Scholar]
- Giladi N, Weitzman N, Schreiber S, Shabtai H, Peretz C. New onset heightened interest or drive for gambling, shopping, eating or sexual activity in patients with Parkinson’s disease: The role of dopamine agonist treatment and age at motor symptoms onset. Journal of Psychopharmacology. 2007;21:501–506. doi: 10.1177/0269881106073109. [DOI] [PubMed] [Google Scholar]
- Giovannoni G, O'Sullivan JD, Turner K, Manson AJ, Lees AJ. Hedonistic homeostatic dysregulation in patients with Parkinson’s disease on dopamine replacement therapies. Journal of Neurology Neurosurgery and Psychiatry. 2000;68:423–428. doi: 10.1136/jnnp.68.4.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA. Dopamine. In: Davis KL, Charney D, Coyle JT, Nemeroff C, editors. Neuropsychopharmacology: The fifth generation of progress. New York: Lippincott Williams & Wilkins; 2002. pp. 119–132. [Google Scholar]
- Grosset KA, Macphee G, Pal G, Stewart D, Watt A, Davie J, Grosset DG. Problematic gambling on dopamine agonists: Not such a rarity. Movement Disorders. 2006;21:2206–2208. doi: 10.1002/mds.21110. [DOI] [PubMed] [Google Scholar]
- Hamidovic A, Kang UJ, de Wit H. Effects of low to moderate acute doses of pramipexole on impulsivity and cognition in healthy volunteers. Journal of Clinical Psychopharmacology. 2008;28:45–51. doi: 10.1097/jcp.0b013e3181602fab. [DOI] [PubMed] [Google Scholar]
- Harper DN. Behavioral resistance to haloperidol and clozapine. Behavioural Processes. 1999;46:1–13. doi: 10.1016/S0376-6357(98)00056-4. [DOI] [PubMed] [Google Scholar]
- Hester R, Garavan H. Executive dysfunction in cocaine addiction: Evidence for discordant frontal, cingulate, and cerebellar activity. Journal of Neuroscience. 2004;24:11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura A, Geda YE, Slowinski J, Wszolek ZK, Brown LA, Uitti RJ. Medications used to treat Parkinson’s disease and the risk of gambling. European Journal of Neurology. 2008;15:350–354. doi: 10.1111/j.1468-1331.2008.02081.x. [DOI] [PubMed] [Google Scholar]
- Klos KJ, Bower JH, Josephs KA, Matsumoto JY, Ahlskog JE. Pathological hypersexuality predominantly linked to adjuvant dopamine agonist therapy in Parkinson’s disease and multiple system atrophy. Parkinsonism and Related Disorders. 2005;11:381–386. doi: 10.1016/j.parkreldis.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Lader M. Antiparkinsonian medication and pathological gambling. CNS Drugs. 2008;22:407–416. doi: 10.2165/00023210-200822050-00004. [DOI] [PubMed] [Google Scholar]
- Lagos P, Scorza C, Monti JM, Jantos H, Reyes-Parada M, Silveira R, Ponzoni A. Effects of the D3 preferring dopamine agonist pramipexole on sleep and waking, locomotor activity and striatal dopamine release in rats. European Neuro-psychopharmacology. 1998;8:113–120. doi: 10.1016/s0924-977x(97)00054-0. [DOI] [PubMed] [Google Scholar]
- Laties VG. The role of discriminative stimuli in modulating drug action. Federation Proceedings. 1975;34:1880–1888. [PubMed] [Google Scholar]
- Laties VG, Weiss B. Influence of drugs on behavior controlled by internal and external stimuli. Journal of Pharmacology and Experimental Therapeutics. 1966;152:388–396. [PubMed] [Google Scholar]
- Madden GJ, Petry NM, Badger GJ, Bickel WK. Impulsive and self-control choices in opioid-dependent patients and non-drug-using control participants: drug and monetary rewards. Experimental and Clinical Psychopharmacology. 1997;5:256–262. doi: 10.1037//1064-1297.5.3.256. [DOI] [PubMed] [Google Scholar]
- Madden GJ, Ewan EE, Lagorio CH. Toward an animal model of gambling: Delay discounting and the allure of unpredictable outcomes. Journal of Gambling Studies. 2007;23:63–83. doi: 10.1007/s10899-006-9041-5. [DOI] [PubMed] [Google Scholar]
- Mamikonyan E, Siderowf AD, Duda JE, Potenza MN, Horn S, Stern MB, Weintraub D. Long-term follow-up of impulse control disorders in Parkinson’s disease. Movement Disorders. 2008;23:75–80. doi: 10.1002/mds.21770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- McKeon A, Josephs KA, Klos KJ, Hecksel K, Bower JH, Bostwick J, Ahlskog JE. Unusual compulsive behaviors primarily related to dopamine agonist therapy in Parkinson’s disease and multiple system atrophy. Parkinsonism and Related Disorders. 2007;13:516–519. doi: 10.1016/j.parkreldis.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Miller E, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Moerschbaecher JM, Boren JJ, Schrot J, Fontes JC. Effects of cocaine and d-amphetamine on repeated acquisition and performance of conditional discriminations. Journal of the Experimental Analysis of Behavior. 1979;31:127–140. doi: 10.1901/jeab.1979.31-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munhoz RP, Fabiani G, Becker N, Teive HA. Increased frequency and range of sexual behavior in a patient with Parkinson’s disease after use of pramipexole: A case report. Journal of Sexual Medicine. 2009;6:1177–1180. doi: 10.1111/j.1743-6109.2008.00861.x. [DOI] [PubMed] [Google Scholar]
- Nevin JA. Response strength in multiple schedules. Journal of the Experimental Analysis of Behavior. 1974;21:389–408. doi: 10.1901/jeab.1974.21-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondo WG, Lai D. Predictors of impulsivity and reward seeking behavior with dopamine agonists. Parkinsonism and Related Disorders. 2008;14:28–32. doi: 10.1016/j.parkreldis.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Petry NM. Pathological gamblers, with and without substance use disorders, discount delayed rewards at high rates. Journal of Abnormal Psychology. 2001;110:482–487. doi: 10.1037//0021-843x.110.3.482. [DOI] [PubMed] [Google Scholar]
- Petry NM, Madden GJ. Discounting and pathological gambling. In: Madden GJ, Bickel WK, editors. Impulsivity: The behavioral and neurological science of discounting. Washington, DC: American Psychological Association; 2009. pp. 273–294. [Google Scholar]
- Pinkston JW, Ginsburg BC, Lamb RJ. Examination of reinforcement magnitude on the pharmacological disruption of fixed-ratio performance. Experimental and Clinical Psychopharmacology. 2009;17:237–246. doi: 10.1037/a0016609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkston JW, Ratzlaff KL, Madden GJ, Fowler SC. An inexpensive infrared detector to verify the delivery of food pellets. Journal of the Experimental Analysis of Behavior. 2008;90:249–255. doi: 10.1901/jeab.2008.90-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MN, Voon V, Weintraub D. Drug insight: Impulsive control disorders and dopamine therapies in Parkinson’s disease. Nature Clinical Practice Neurology. 2007;3:664–672. doi: 10.1038/ncpneuro0680. [DOI] [PubMed] [Google Scholar]
- Rachlin H. Why do people gamble and keep gambling despite heavy losses? Psychological Science. 1990;1:294–297. [Google Scholar]
- Reuter J, Raedler T, Rose M, Hand I, Gläscher J, Büchel C. Pathological gambling is linked to reduced activation of the mesolimbic reward system. Nature Neuroscience. 2005;8:147–148. doi: 10.1038/nn1378. [DOI] [PubMed] [Google Scholar]
- Riba J, Krämer UM, Heldmann M, Richter S, Münte TF. Dopamine agonist increases risk taking but blunts reward-related brain activity. PLoS ONE. 2008;3:e2479. doi: 10.1371/journal.pone.0002479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JB, Zhang L, Mitchell SH, de Wit H. Delay or probability discounting in a model of impulsive behavior: effect of alcohol. Journal of the Experimental Analysis of Behavior. 1999;71:121–143. doi: 10.1901/jeab.1999.71-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE. Neural substrates of cocaine-cue associations that trigger relapse. European Journal of Pharmacology. 2005;526:140–146. doi: 10.1016/j.ejphar.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347:146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Diaz J, Le Foll B, Guillin O, Leriche L, Bezard E, Gross C. The dopamine D3 receptor: A therapeutic target for the treatment of neuropsychiatric disorders. CNS Neurological Disorder Drug Targets. 2006;5:25–43. doi: 10.2174/187152706784111551. [DOI] [PubMed] [Google Scholar]
- Szarfman A, Doraiswamy PM, Tonning JM, Levine JG. Association between pathological gambling and Parkinsonian therapy as detected in the Food and Drug Administation Adverse Event database. Archives of Neurology. 2006;63:299–300. doi: 10.1001/archneur.63.2.299-b. [DOI] [PubMed] [Google Scholar]
- Tippmann-Peikert M, Park JG, Boeve BF, Shepard JW, Silber MH. Pathologic gambling in patients with restless legs syndrome treated with dopaminergic agonists. Neurology. 68:301–303. doi: 10.1212/01.wnl.0000252368.25106.b6. [DOI] [PubMed] [Google Scholar]
- Tulloch IF. Pharmacologic profile of ropinirole: A nonergoline dopamine agonist. Neurology. 1997;49:S58–S62. doi: 10.1212/wnl.49.1_suppl_1.s58. [DOI] [PubMed] [Google Scholar]
- Voon V, Hassan K, Zurowski M, de Souza M, Thomsen T, Fox S, Lang AE, Miyasaki J. Prevalence of repetitive and reward-seeking behaviors in Parkinson disease. Neurology. 2006a;67:1254–1257. doi: 10.1212/01.wnl.0000238503.20816.13. [DOI] [PubMed] [Google Scholar]
- Voon V, Hassan K, Zurowski M, Duff-Canning S, de Souza M, Fox S, Lang AE, Miyasaki J. Prospective prevalence of pathologic gambling and medication association in Parkinson disease. Neurology. 2006b;66:1750–1752. doi: 10.1212/01.wnl.0000218206.20920.4d. [DOI] [PubMed] [Google Scholar]
- Vuchinich RE, Simpson CA. Hyperbolic temporal discounting in social drinkers and problem drinkers. Experimental and Clinical Psychopharmacology. 1998;6:292–305. doi: 10.1037//1064-1297.6.3.292. [DOI] [PubMed] [Google Scholar]
- Weintraub D, Siderowf AD, Potenza MN, Goveas J, Morales KH, Duda JE, Moberg PJ, Stern MB. Association of dopamine agonist use with impulse control disorders in Parkinson disease. Archives of Neurology. 2006;63:969–973. doi: 10.1001/archneur.63.7.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley C. The neural and neurochemical basis of delay discounting. In: Madden GJ, Bickel WK, editors. Impulsivity: The behavioral and neurological science of discounting. Washington, DC: American Psychological Association; 2009. p. 95.p. 122. [Google Scholar]