Abstract
Biological agents, including TNF inhibitors, have revolutionized the treatment of RA in recent years. Certolizumab pegol (CZP) is a novel pegylated anti-TNF approved for the treatment of adult patients with moderately to severely active RA. This article provides an overview of three published clinical trials of CZP in RA in patients with active disease who have shown an inadequate response to DMARDs, including MTX: RA prevention of structural damage (RAPID) 1 and 2, which evaluated the efficacy and safety of CZP added to MTX when dosed every 2 weeks, and efficacy and safety of CZP – 4 weekly dosage in rheumatoid arthritis (FAST4WARD), which evaluated CZP monotherapy when dosed every 4 weeks. In the trials, CZP plus MTX or as monotherapy significantly improved the signs and symptoms of RA and RA disease activity, and CZP plus MTX significantly inhibited the progression of radiographic joint damage as early as Week 16 of the treatment. In addition, CZP treatment significantly improved patient-reported outcome measures, providing significant reductions in pain and fatigue and improvements in physical function as early as Week 1 of treatment; improvements in health-related quality of life were evident at the first assessment at Week 12. CZP treatment improved productivity at work, significantly reducing the number of days of missed work as well as the number of days with reduced productivity, and also increased productivity within the home and improved participation in family, social and leisure activities. CZP was generally well tolerated when used either as monotherapy or added to MTX; most adverse events were mild or moderate. Taken together, the results of these trials suggest that CZP is an effective new option for the treatment of RA.
Keywords: Certolizumab pegol, Methotrexate, Monotherapy, Rheumatoid arthritis, TNF-α inhibitor
Introduction
The introduction of biological TNF-α inhibitors was a significant advance in the management of RA [1]. However, while many patients respond well to these agents, others may never respond, and some patients may stop responding over time or may need to switch or discontinue therapy because of tolerability issues. There is thus a continuing need for new RA treatments.
Certolizumab pegol (CZP) is a novel pegylated anti-TNF, consisting of a Fab′ attached to a 40-kDa PEG moiety. Attachment of PEG to the Fab′ increases the plasma half-life of CZP to ∼2 weeks, allowing dosing every 2 or 4 weeks, and may contribute to the preferential distribution of the drug to inflamed tissues that has been observed in animal models [2]. CZP lacks an Fc region, so it does not induce complement- or antibody-dependent cell-mediated cytotoxicity, which has been observed in vitro with adalimumab, etanercept and infliximab [3]. CZP is approved in the USA, Canada and Europe for the treatment of adult patients with moderately to severely active RA, and in the USA and Switzerland for the treatment of patients with Crohn’s disease.
The efficacy and safety of CZP in adult patients with active RA were established in three Phase III clinical trials, in which CZP was administered with MTX or as monotherapy [4–6]. These studies demonstrated that CZP reduces the clinical signs and symptoms of active RA and inhibits the progression of structural joint damage. The trials also assessed a number of patient-reported outcomes (PROs), including health-related quality of life (HRQoL), fatigue, pain, physical function and household/work productivity. PROs assess the impact of RA on everyday life from the patient’s perspective and are being increasingly recognized as important measures for inclusion in RA clinical trials. Together with physician-reported outcomes, PROs help to provide a more comprehensive evaluation of the efficacy of RA therapy. This article thus provides an overview of the efficacy and safety data for CZP from these three pivotal trials, with a particular focus on the PRO and productivity results.
Article search
The PubMed database was searched (all years) to identify articles reporting data from Phase III clinical trials of CZP in RA, using the search terms ‘certolizumab pegol’ or its trade name as marketed by UCB in the title. Additional articles were identified from abstracts published on major rheumatology congress web sites including the EULAR (2006–09) and ACR (2006–09).
Clinical efficacy of CZP
CZP plus MTX
The RA prevention of structural damage (RAPID) 1 and 2 trials were Phase III, multi-centre, randomized, double-blind placebo-controlled trials, which evaluated the efficacy and safety of CZP plus MTX in adults (n = 982 and 619, respectively) with active RA despite treatment with MTX [4, 5]. RAPID 1 was a 52-week trial of a lyophilized formulation of CZP, while RAPID 2 was a 24-week trial of a liquid formulation. Patients aged ≥18 years with adult-onset RA (≥6 months but <15 years) were randomized 2 : 2 : 1 to one of two regimens of s.c CZP (400 mg at Weeks 0, 2 and 4, followed by 200 or 400 mg) plus MTX every 2 weeks, or placebo plus MTX. Notably, withdrawal for ACR20 non-response was mandatory for these trials; patients who failed to demonstrate ACR20 improvement at both Weeks 12 and 14 were to be withdrawn and allowed to enter an open-label extension study of CZP 400 mg plus MTX every 2 weeks. Patients who completed the studies were also allowed to enter open-label treatment. The primary efficacy endpoint for both trials was the proportion of patients achieving a 20% improvement in the ACR response criteria at Week 24 [7]. Mean change from baseline in modified total Sharp score (mTSS) at Week 52 was a co-primary endpoint in RAPID 1. Secondary endpoints in both studies included ACR50 and ACR70 responder rates, mean change from baseline in 28-joint DAS assessment (ESR) [DAS-28 (ESR)] and PROs.
The majority of patients had high disease severity at baseline (Table 1). In both trials, the onset of symptom relief with CZP was rapid. ACR20 response rates were significantly higher with CZP plus MTX than placebo plus MTX at Week 1 (22.9 and 14.3% with CZP 200 mg plus MTX vs 5.6 and 3.3% with placebo plus MTX in the RAPID 1 and 2 trials, respectively) [4, 5]. ACR20 response rates peaked at Week 12 in both studies (63.8 and 62.7% for CZP 200 mg vs 18.3 and 12.7% for placebo in RAPID 1 and 2, respectively; both P < 0.001). At Week 24, ACR20 response rates were 58.8 and 57.3% for patients receiving CZP 200 mg plus MTX, respectively, vs 13.6 and 8.7%. Significantly higher ACR50 and ACR70 response rates for CZP vs placebo groups were seen from Weeks 2 and 4 in RAPID 1, and Weeks 6 and 20 in RAPID 2, respectively. Responses were sustained to the end of the trials (Week 52 in RAPID 1 and Week 24 in RAPID 2; Table 2), and were similar in the CZP 400 mg plus MTX groups. CZP treatment also yielded significant improvements in all ACR core component scores, including reductions in swollen and tender joint scores and improvements in both patient’s and physician’s global assessments of disease activity, by Week 1 that were sustained throughout both studies [4, 5].
Table 1.
Baseline characteristics of patients in the RAPID 1, 2 (ITT populations) and FAST4WARD (modified ITT population) trials [4–6]
| Baseline characteristics | RAPID 1 (n = 982) | RAPID 2 (n = 619) | FAST4WARD (n = 220) |
|---|---|---|---|
| Age, mean (s.d.), years | 52.0 (11.6) | 51.9 (11.5) | 53.8 (12.2) |
| Sex, female, % | 83.2 | 81.6 | 83.6 |
| Duration of RA, mean (s.d.), years | 6.1 (4.3) | 6.2 (4.2) | 9.5 (8.9) |
| No. of previous DMARDs, mean (s.d.) | 2.3 (1.3) | 2.2 (1.3) | 2.0 (1.2) |
| MTX dose, mean, mg/week | 13.6 | 12.5 | NA |
| RF positive, ≥14 IU/ml, % | 81.8 | 76.9 | 100 |
| Tender joint count, mean (s.d.) | 30.7 (12.9) | 30.2 (14.0) | 29.0 (13.1) |
| Swollen joint count, mean (s.d.) | 21.5 (9.8) | 21.0 (9.8) | 20.5 (9.7) |
| Patient’s assessment of arthritis pain (0–100 mm VAS), mean (s.d.) | 63.1 (18.9) | 60.9 (20.2) | 56.5 (21.4) |
| HAQ-DI, mean (s.d.) | 1.7 (0.6) | 1.6 (0.6) | 1.5 (0.6) |
| DAS-28 (ESR), mean (s.d.) | 6.9 (0.8) | 6.8 (0.8) | 6.3 (1.0) |
| CRP, geometric mean (CV), mg/l | 14.7 (144.2) | 13.6 (180.9) | 11.5 (233.1) |
| FAS (0–10), mean (s.d.) | 6.5 (2.0) | 6.5 (1.9) | 6.3 (2.2) |
| SF-36 PCS, mean (s.d.) | 30.8 (6.5) | 30.9 (6.2) | 27.9 (7.8) |
| SF-36 MCS, mean (s.d.) | 39.4 (11.2) | 39.3 (11.0) | 44.7 (11.5) |
The ITT populations for RAPID 1 and 2 consisted of all patients who were randomized into the studies; the modified ITT population for FAST4WARD consisted of all randomized patients who had taken one or more dose of study medication. Adapted from Mease [21] with permission of Future Medicine Ltd. CV: coefficient of variation; ITT: intention-to-treat; NA: not applicable.
Table 2.
ACR response rates (%) at study end in RAPID 1 (Week 52), RAPID 2 (Week 24) and FAST4WARD (Week 24) [4–6]
| Study | ACR20 | ACR50 | ACR70 |
|---|---|---|---|
| RAPID 1a,b | |||
| Placebo + MTX Q2 W (n = 199) | 13.6 | 7.6 | 3.5 |
| CZP 200 mg + MTX Q2 W (n = 393) | 53.1, P < 0.001 | 38.0, P < 0.001 | 21.2, P < 0.001 |
| CZP 400 mg + MTX Q2 W (n = 390) | 54.9, P < 0.001 | 39.9, P < 0.001 | 23.2, P < 0.001 |
| RAPID 2a,b | |||
| Placebo + MTX Q2 W (n = 127) | 8.7 | 3.1 | 0.8 |
| CZP 200 mg + MTX Q2 W (n = 246) | 57.3, P < 0.001 | 32.5, P < 0.001 | 15.9, P ≤ 0.01 |
| CZP 400 mg + MTX Q2 W (n = 246) | 57.6, P < 0.001 | 33.1, P < 0.001 | 10.6, P ≤ 0.01 |
| FAST4WARDc,d | |||
| Placebo Q4 W (n = 109) | 9.3 | 3.7 | 0 |
| CZP 400 mg Q4 W (n = 111) | 45.5, P < 0.001 | 22.7, P < 0.001 | 5.5, P ≤ 0.05 |
aITT population: analyses performed using non-responder imputation. bDosing every 2 weeks (Q2 W). cModified ITT population: analyses performed using non-responder imputation. dDosing every 4 weeks (Q4 W). P-values vs placebo plus MTX or placebo alone. ACR20/ACR50/ACR70 responses for the CZP and placebo groups were compared using logistic regression with treatment and geographical region as factors in the RAPID trials or a Cochran–Mantel–Haenszel test stratified by country in the FAST4WARD trial. Details of the statistical analyses are provided in the primary publications for the trials [4–6]. ITT: intention-to-treat.
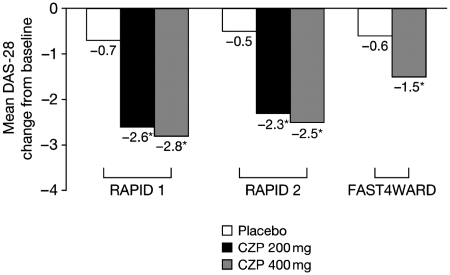
Treatment with CZP plus MTX was associated with significantly greater improvements in disease activity from Week 1, as evidenced by DAS-28 (ESR) scores, throughout both trials (P < 0.001 at all time points) [4, 5]. At Week 1, mean change from baseline in DAS-28 was −0.8 with CZP 200 mg and −0.3 with placebo in RAPID 1, and −0.8 with CZP 200 mg and −0.2 with placebo in RAPID 2. Improvements were sustained to the end of both studies (52 or 24 weeks, respectively; Fig. 1), and were similar with the CZP 400 mg dose. In RAPID 2, DAS-28 remission was observed in 9.4% of patients treated with CZP 200 mg plus MTX compared with only 0.8% of patients in the placebo group [5].
Fig. 1.
Mean change in DAS-28 from baseline to study end in the RAPID 1 (Week 52), RAPID 2 (Week 24) and FAST4WARD (Week 24) trials [4–6]. RAPID 1 and 2 compared CZP plus MTX Q2 W vs placebo plus MTX Q2 W; FAST4WARD compared CZP monotherapy Q4 W vs placebo alone Q4 W. *P ≤ 0.001 vs placebo plus MTX.
Both trials investigated the effects of CZP on the progression of joint damage. In RAPID 1, the mean (s.d.) change in mTSS from baseline to Week 52, which was a co-primary endpoint of the study, was significantly lower in patients receiving CZP 200 mg plus MTX [0.4 (5.7) in the CZP 200 mg group] compared with patients receiving placebo plus MTX [2.8 (7.8); P < 0.001] [4]. The changes were also significantly lower in the CZP plus MTX groups vs the placebo plus MTX group at Week 24 (P < 0.001). At both time points, significantly lower mean changes from baseline in both erosion (Week 24: 0 vs 0.7, Week 52: 0.1 vs 1.5; P < 0.001) and joint space narrowing subscores (Week 24: 0.2 vs 0.7, Week 52: 0.4 vs 1.4; P ≤ 0.01) were observed in patients receiving CZP 200 mg plus MTX. Similarly, in RAPID 2, the mean (s.d.) change in mTSS from baseline at Week 24 was significantly lower in patients receiving CZP 200 mg plus MTX [0.2 (2.7)] compared with patients receiving placebo plus MTX [1.2 (4.1)] (P ≤ 0.01) [5]. Patients in the CZP 200 mg group in RAPID 2 also had significantly lower erosion (mean change from baseline: 0.1 vs 0.7) and joint space narrowing (mean change from baseline: 0.1 vs 0.5) subscores (P ≤ 0.01). Results for patients receiving the 400-mg dose were similar. An analysis of joint damage in patients who withdrew from the trials at Week 16 due to ACR20 non-response at Weeks 12 and 14 (as mandated by the study protocol) found that radiographic progression was inhibited by CZP plus MTX despite the fact that these patients did not meet the threshold for a clinical response [4, 5]. These observations suggest that the rapid effects of CZP may lead to long-term benefits for patients in terms of slowing disease progression.
As mentioned above, patients who either withdrew from the RAPID 1 and 2 trials due to ACR20 non-response or who completed the studies were allowed to enter an open-label extension study of CZP 400 mg plus MTX. Recently reported results from this study indicate that the improvements in RA signs and symptoms and disease activity and the inhibition of joint damage progression were sustained over 2 years [8].
CZP monotherapy
Efficacy and safety of CZP – 4 weekly dosage in rheumatoid arthritis (FAST4WARD) was a Phase III, 24-week, multi-centre, randomized, double-blind placebo-controlled trial evaluating the efficacy and safety of CZP monotherapy in 220 adults with active RA who had failed therapy with at least one prior DMARD [6]. Patients aged 18–75 years with adult-onset RA were randomized to receive a lyophilized formulation of CZP 400 mg or placebo subcutaneously every 4 weeks. Patients completing the trial or withdrawing on or after Week 12 were offered entry into an open-label study of CZP 400 mg every 4 weeks unless they were withdrawn because of non-compliance or possible treatment-related adverse events (AEs). The primary endpoint was ACR20 response at Week 24; radiographic assessments were not performed in this trial. Secondary endpoints included ACR50 and ACR70 responder rates, DAS-28 (ESR) and PROs.
Patients in this trial also had high disease activity at baseline (Table 1). ACR20 response rates were significantly higher with CZP than placebo from Week 1 (36.7 vs 6.6%; P < 0.001) onwards [6]. At Week 12, ACR20 response rates were 47.7 and 8.5% for patients taking CZP 400 mg and placebo, respectively (P < 0.001), and remained significantly higher for CZP until the study end (Table 2). ACR50 and ACR70 responses were significantly higher with the CZP 400 mg group from Weeks 1 and 8 onwards, respectively, and significantly greater improvements in DAS-28 (ESR) were observed with CZP monotherapy from Week 1 onwards (P < 0.001 at all time points) [6]. At Week 1, least square mean changes from baseline in DAS-28 (ESR) were −0.9 and −0.3 in the CZP and placebo groups, respectively. Improvements were sustained to Week 24 (Fig. 1).
Effect of CZP on HRQoL, fatigue, pain and physical function
PROs were also assessed as secondary endpoints in all three trials and included HRQoL, fatigue, arthritis pain and physical function. HRQoL was assessed using the short-form 36 (SF-36)-item health survey questionnaire (which assesses physical functioning, role physical, bodily pain, general health, vitality, social functioning, role emotional and mental health) [9]. Scores for the physical component summary (PCS, mainly comprising the physical functioning, role physical, bodily pain and general health domains) and mental component summary (MCS, mainly comprising the vitality, social functioning, role emotional and mental health domains) were also obtained. Fatigue over the previous week was assessed using the Fatigue Assessment Scale (FAS), a numerical rating scale [10], and arthritis pain was assessed using a 0- to 100-mm visual analogue scale (VAS) [11]. Daily pain assessments were also performed in FAST4WARD during the first week using a modified brief pain inventory (mBPI), which asked patients to rate their worst pain in the past 24 h, average pain in the past 24 h and pain right now. Physical function was assessed using the HAQ-disability index (HAQ-DI) [12, 13].
The proportion of patients achieving minimum clinically important differences (MCIDs) in HRQoL, fatigue, pain and physical function was also determined. MCIDs for the SF-36 domains are defined as ≥5.0-point increases from baseline, and for the PCS and MCS as ≥2.5-point increases from baseline [14]. The MCIDs for the HAQ-DI, pain and FAS are defined as a ≥0.22-point decrease from baseline [15], a ≥10-mm reduction from baseline [11, 15, 16] and a 1-point reduction from baseline [17], respectively.
CZP plus MTX
From the initial assessment at Week 12, significantly more patients treated with CZP plus MTX than placebo plus MTX reported statistically significant and clinically meaningful improvements in all SF-36 domains, PCS and MCS that were sustained through 52 (RAPID 1) or 24 (RAPID 2) weeks (Table 3 shows PCS and MCS scores at study end) (P < 0.001) [5, 18–20]. At Week 52 in RAPID 1, 42 and 39% of patients treated with CZP 200 mg plus MTX achieved improvements greater than or equal to MCID in SF-36 PCS and MCS scores, respectively, compared with only 11 and 10% of patients in the placebo group (P < 0.001), while at Week 24 in RAPID 2, 44 and 40% of CZP 200 mg-treated patients achieved improvements greater than or equal to MCID in SF-36 PCS and MCS scores, respectively, vs only 9 and 8% of placebo patients. Of note were the significant improvements in SF-36 MCS scores following CZP treatment, which have not previously been observed with other TNF inhibitors [19]. Results were similar in patients who received CZP 400 mg plus MTX.
Table 3.
Mean change from baseline to study end in SF-36 PCS, SF-36 MCS, FAS, pain VAS and HAQ-DI scores in RAPID 1, 2 and FAST4WARD (ITT population) [4–6, 18, 21, 22]
| RAPID 1,a,b,c adjusted mean change (s.e.m.) | Placebo + MTX Q2 W (n = 199) | CZP 200 mg + MTX Q2 W (n = 393) | CZP 400 mg + MTX Q2 W (n = 390) |
|---|---|---|---|
| Week 52 | |||
| SF-36 PCS | 1.7 (0.6) | 7.8 (0.4), P < 0.001 | 8.6 (0.4), P < 0.001 |
| SF-36 MCS | 2.1 (0.8) | 6.4 (0.6), P < 0.001 | 6.4 (0.6), P < 0.001 |
| Fatigue (FAS) | −0.8 (0.2) | −2.6 (0.1), P < 0.001 | −2.5 (0.1), P < 0.001 |
| Arthritis pain (VAS) | −8.8 (1.6) | −31.0 (1.2), P < 0.001 | −33.5 (1.2), P < 0.001 |
| Physical function (HAQ-DI) | −0.18 (0.04) | −0.60 (0.03), P < 0.001 | −0.63 (0.03), P < 0.001 |
| RAPID 2,a,b,c adjusted mean change (s.e.m.) | Placebo + MTX Q2 W (n = 127) | CZP 200 mg + MTX Q2 W (n = 246) | CZP 400 mg + MTX Q2 W (n = 246) |
|---|---|---|---|
| Week 24 | |||
| SF-36 PCS | 0.9 (0.7) | 5.2 (0.5), P < 0.001 | 5.5 (0.5), P < 0.001 |
| SF-36 MCS | 1.6 (0.9) | 6.1 (0.7), P < 0.001 | 6.3 (0.7), P < 0.001 |
| Fatigue (FAS) | −0.5 (0.2) | −2.0 (0.1), P < 0.001 | −2.2 (0.1), P < 0.001 |
| Arthritis pain (VAS) | −4.7 (1.9) | −23.7 (1.4), P < 0.001 | −26.1 (1.4), P < 0.001 |
| Physical function (HAQ-DI) | −0.14 (0.04) | −0.50 (0.03), P < 0.001 | −0.50 (0.03), P < 0.001 |
| FAST4WARD,d,e,f least square mean change | Placebo Q4 W (n = 109) | CZP 400 mg Q4 W (n = 111) | |
|---|---|---|---|
| Week 24 | |||
| SF-36 PCS | NA | NA | NA |
| SF-36 MCS | NA | NA | NA |
| Fatigue (FAS)e | −0.3 | NA | −1.7, P < 0.001 |
| Arthritis pain (VAS)b | 1.7 | NA | −20.6, P < 0.001 |
| Physical function (HAQ-DI)b | 0.13 | NA | −0.36, P < 0.001 |
aITT population. bAnalyses performed using last observation carried forward approach. cDosing every 2 weeks. dModified ITT population. eAnalyses based on observed data. fDosing every 4 weeks. P-values vs placebo plus MTX or placebo alone. Analyses were performed using analysis of covariance, with treatment and geographical region as factors and baseline value as covariate. ITT: intention-to-treat; NA: not available.
Statistically significant reductions in fatigue and arthritis pain scores were reported by patients receiving CZP plus MTX at Week 1 and were sustained to either Week 52 (RAPID 1) or Week 24 (RAPID 2) (P < 0.001 for both CZP dose groups; Table 3) [4, 5, 21, 22]. CZP-treated patients also reported significant improvements from baseline in physical function compared with patients receiving placebo plus MTX, as early as Week 1 (P < 0.001 for both CZP dose groups) [4, 5, 20]. At Week 1, mean changes from baseline in HAQ-DI scores were −0.27 and −0.20 in the CZP 200 mg groups in RAPID 1 and 2 vs −0.11 and −0.05 in the placebo groups, respectively (P < 0.001). These rapid benefits continued to improve and were sustained throughout both trials (Table 3). Clinically meaningful improvements in physical function were also reported following CZP treatment, as defined by improvements greater than or equal to MCID, from Week 1 in RAPID 1 (43% for patients receiving CZP 200 mg plus MTX vs 25% of placebo patients; P < 0.001) and Week 2 in RAPID 2 [4, 5, 20, 22]. By study end, 47 and 57% of patients receiving CZP 200 mg plus MTX reported improvements in HAQ-DI greater than or equal to MCID in RAPID 1 (Week 52) and 2 (Week 24), respectively, compared with 13 and 11% of patients receiving placebo plus MTX (P < 0.001) [5, 18].
In patients who successfully completed the RAPID 1 trial and entered the open-label extension study of CZP 400 mg plus MTX every 2 weeks, improvements in HRQoL and physical function and reductions in pain and fatigue were maintained through 100 weeks of treatment at average levels at least three times higher than the thresholds for meaningful improvement [23].
CZP monotherapy
Patients treated with CZP monotherapy reported statistically significant improvements in HRQoL (all eight SF-36 domains as well as PCS and MCS scores) at Week 24 vs placebo (P < 0.001) [6]. Significantly more CZP-treated patients also reported improvements in HRQoL that met or exceeded MCID throughout the study period in all SF-36 domains, PCS and MCS (P ≤ 0.05) with the exception of the role emotional domain at Week 4 (P = 0.613) and Week 12 (P = 0.091) [24]. Clinically meaningful improvements in PCS and MCS were achieved by 46 and 34% of patients receiving CZP at Week 24, respectively, compared with 16 and 7% of patients receiving placebo (P < 0.001) [6].
Significant and clinically meaningful reductions in fatigue were reported by patients receiving CZP monotherapy vs placebo at Week 1 (P ≤ 0.01) and were sustained to Week 24 (P < 0.001; Table 3), when clinically meaningful reductions in fatigue were reported by 46% of CZP patients vs 17% of placebo patients (P < 0.001) [6]. Arthritis pain, which was assessed daily (mBPI scale) during the first week of treatment in the FAST4WARD study, was significantly reduced in patients receiving CZP vs placebo by Day 2 (P ≤ 0.05) [6]. At Week 1, the first pain assessment using pain VAS, mean changes from baseline were −16.7 vs −5.2 for the CZP and placebo groups (P < 0.001), respectively, and pain relief continued to improve to Week 24 (P < 0.001; Table 3). At study end (Week 24), significantly more patients receiving CZP monotherapy reported clinically meaningful reductions in arthritis pain (47 vs 17%, respectively; P < 0.001) [6].
Patients treated with CZP 400 mg monotherapy reported statistically significant improvements in physical function compared with patients receiving placebo from Week 1 (−0.23 vs 0.04, respectively) through Week 24 (−0.36 vs 0.13; P < 0.001 for both time points; Table 3) [6]. By study end (Week 24), 49% of patients receiving CZP reported clinically meaningful improvements in physical function vs 12% of those receiving placebo (P < 0.001) [6].
Effects on home and work productivity
The validated RA-specific Work Productivity Survey (WPS-RA) questionnaire was used to assess the impact of RA on productivity in the workplace and in the home and on participation in family, social and leisure activities [25]. The survey assesses employment status, productivity at work for employed patients, productivity at home and daily activities.
CZP plus MTX
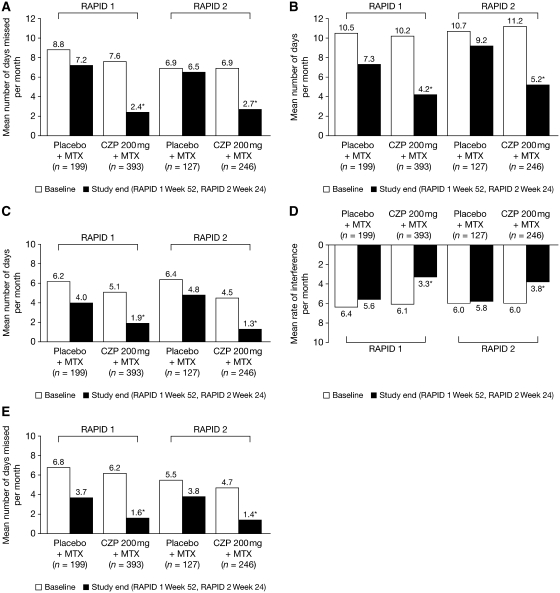
Patients receiving CZP plus MTX reported less loss of productivity at home compared with placebo patients; this improvement began as early as Week 4 (first assessment), when CZP 200 mg plus MTX patients reported (on average) significantly fewer household work days missed per month vs placebo plus MTX patients (6.9 vs 7.6, respectively), as well as significantly fewer household work days per month with productivity reduced by at least half (8.1 vs 9.8, respectively), fewer days per month with outside hired help (3.5 vs 4.1, respectively) and a lower rate of RA interference with household work productivity (5.0 vs 5.9, respectively, on a 0–10 scale, with 0 = no interference and 10 = complete interference; P ≤ 0.05; RAPID 1 study) [26]. These improvements were sustained to the end of both RAPID 1 (52 weeks) and RAPID 2 (24 weeks), as is evidenced in Fig. 2, which shows home productivity at baseline and study end. Over 1 year, treatment with CZP 200 mg plus MTX resulted in an annual average of 52.1 fewer full household work days missed and 36.6 fewer days with reduced productivity due to RA compared with placebo plus MTX [26].
Fig. 2.
Effect of CZP on productivity at home and on social, family and leisure activities in the RAPID 1 (Week 52) and RAPID 2 (Week 24) trials [26]. Results are shown at baseline and study end. (A) Household work days missed due to arthritis per month. (B) Days with household work productivity reduced by ≥50% due to arthritis per month. (C) Days with outside help hired due to arthritis per month. (D) RA interference with household work productivity per month (0–10 scale, 0 = no interference, 10 = complete interference). (E) Days of lost family, social and leisure activities per month. The analysis population in RAPID 1 and 2 trials was the intention-to-treat population. *P ≤ 0.05 vs placebo plus MTX. Analyses were performed using a non-parametric bootstrap t-test and the last observation carried forward approach. Adapted from Kavanaugh et al. [26] with permission of John Wiley & Sons Inc.
Patients receiving CZP plus MTX also reported significant reductions in the number of lost days of family, social and leisure activities due to RA compared with patients receiving placebo plus MTX by Week 4 (first assessment) [26]. For example, patients receiving CZP 200 mg plus MTX in RAPID 1 reported an average of 4.3 days of lost participation at Week 4 compared with 5.2 days for placebo plus MTX patients (P ≤ 0.05). Improvements were again sustained to study end (Fig. 2). Over 1 year in RAPID 1, these reductions translated into an average cumulative gain of 26.8 days of family, social and leisure activities compared with placebo plus MTX [26]. Results for all household productivity and family activity measures were similar for the CZP 400 mg plus MTX group in RAPID 1 (data not shown) and both CZP plus MTX groups in RAPID 2 (data not shown for CZP 400 mg plus MTX) [26].
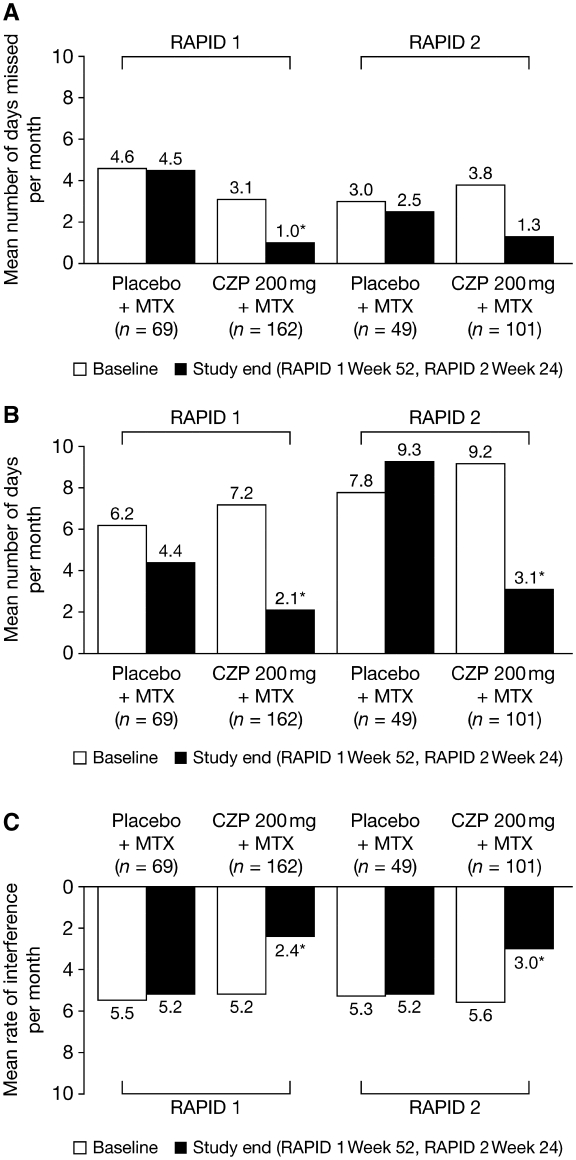
In addition to improving home productivity, treatment with CZP plus MTX rapidly improved productivity at work [26]. At Week 4 in RAPID 1, patients receiving CZP 200 mg plus MTX reported an average of 1.5 work days missed per month and 4.3 work days per month with productivity reduced by at least half (presenteeism), compared with 2.5 and 6.5 days, respectively, in the placebo plus MTX group (P ≤ 0.05). The monthly rate of RA interference with work productivity was also significantly lower in CZP-treated patients (3.5 vs 4.2, on a 0–10 scale, with 0 = no interference and 10 = complete interference). These improvements were again sustained to study end (Fig. 3). Over 1 year in RAPID 1, this resulted in an average cumulative gain of 41.9 full work days and 29.4 fewer days with reduced productivity due to RA compared with treatment with placebo plus MTX [26]. Similar trends were reported by patients receiving CZP 400 mg plus MTX in the RAPID 1 trial (data not shown), and by patients in both CZP plus MTX groups in RAPID 2 (data not shown for CZP 400 mg plus MTX), although reductions in absenteeism did not attain statistical significance in RAPID 2.
Fig. 3.
Effect of CZP on productivity in the workplace in the RAPID 1 (Week 52) and RAPID 2 (Week 24) trials [26]. Results are shown at baseline and study end. (A) Work days missed (absenteeism) due to arthritis per month. (B) Days with work productivity reduced by ≥50% (presenteeism) due to arthritis per month. (C) RA interference with work productivity per month (0–10 scale, 0 = no interference, 10 = complete interference). The analysis population in RAPID 1 and 2 trials was the intention-to-treat population (employed patients only). *P ≤ 0.05 vs placebo plus MTX. Analyses were performed using a non-parametric bootstrap t-test and the last observation carried forward approach. Adapted from Kavanaugh et al. [26] with permission of John Wiley & Sons Inc.
A post hoc analysis of these trials found that these improvements in productivity were closely reflected by similar changes in pain, physical function and fatigue. Patients who achieved improvements greater than or equal to MCID in fatigue, pain and physical function reported greater improvements in productivity at work and home and increased participation in family, social and leisure activities [27].
CZP monotherapy
CZP monotherapy also provided rapid and sustained improvements in home and work productivity as early as Week 4 compared with placebo. At Week 4, CZP-treated patients reported 5.2 days of household work lost compared with 8.4 days in the placebo group, 5.8 days of household work with productivity reduced by ≥50% vs 9.4 days for placebo, and 1.5 lost days of family, social and leisure activities compared with 2.9 days with placebo [20]. CZP monotherapy decreased the number of work days with reduced productivity to an average of 2.8 compared with 4.6 days for the placebo group at Week 4, and employed patients reported a lower mean rate of interference by RA compared with placebo-treated patients (2.9 vs 4.3) [20]. At Week 24, CZP 400 mg monotherapy was associated with cumulative gains of 25.5 additional full household work days; 27.6 additional productive household days; 14.0 days of family, social and leisure activities; 4.1 full paid work days; and 21.0 more productive work days compared with patients who received placebo [28].
Safety and tolerability
CZP plus MTX
Mandatory withdrawal of ACR20 non-responders at Week 16 and the 2 : 1 randomization ratio in RAPID 1 and 2 resulted in longer mean exposure to study drug in the CZP plus MTX groups than the placebo plus MTX groups [4, 5]. The pooled frequencies of treatment-emergent AEs (TEAEs) in these two trials were therefore expressed as the incidence rates per 100 patient-years (pt-yrs; Table 4).
Table 4.
| Pooled data for RAPID 1 and 2 Rate per 100 pt-yrs |
FAST4WARD Patients, n% |
||||
|---|---|---|---|---|---|
| Adverse events | Placebo + MTX (n = 324) | CZP 200 mg + MTX (n = 640) | CZP 400 mg + MTX (n = 635) | Placebo (n = 109) | CZP 400 mg (n = 111) |
| Exposure, n, pt-yrs | 132.0 | 406.7 | 419.5 | – | – |
| Any TEAE | 264.4 | 239.1 | 221.1 | 63 (57.8) | 84 (75.7) |
| Intensity | |||||
| Mild | 155.5 | 162.3 | 156.4 | 43 (39.4) | 62 (55.9) |
| Moderate | 96.7 | 79.0 | 75.6 | 40 (36.7) | 52 (46.8) |
| Severe | 14.2 | 12.5 | 12.9 | 11 (10.1) | 8 (7.2) |
| TEAE related to study drug | 66.9 | 78.1 | 74.4 | 24 (22.0) | 27 (24.3) |
| TEAE leading to withdrawal | 3.8 | 7.2 | 7.0 | 2 (1.8) | 5 (4.5) |
| Any TE infection | 73.2 | 80.9 | 76.7 | 16 (14.7) | 33 (29.7) |
| Serious TEAEs | 11.9 | 16.3 | 16.6 | 3 (2.8) | 8 (7.2) |
| TEAE leading to death | 0.8 | 0.7 | 1.2 | 0 | 0 |
| Serious infections | 1.5 | 6.0 | 7.1 | 0 | 2 (1.8) |
| Tuberculosis | 0 | 1.2 | 1.2 | 0 | 0 |
| Malignancy | 1.5 | 2.0 | 1.2 | 0 | 2 (1.8) |
| Cardiac disorders | 5.3 | 4.7 | 4.8 | 2 (1.8) | 0 |
aAll patients who received at least one dose of study medication. Reproduced from Mease [21] with permission of Future Medicine Ltd.
Most AEs were mild to moderate, and rates of withdrawal due to AEs were low across the groups. TEAEs led to death in seven patients in RAPID 1 and two patients in RAPID 2, but all were considered unlikely to be related or unrelated to administration of the study drug. TEAEs leading to death included myocardial infarction, hepatic neoplasm, cardiac arrest, cerebral stroke, atrial fibrillation and fatigue, and femur fracture and shock. Rates of infection were comparable across the groups. Urinary tract infections and upper respiratory tract infections (including nasopharyngitis) were the most frequently reported infectious AEs [4, 5]. Serious infections occurred more frequently in the CZP plus MTX groups than in the placebo plus MTX groups. The most frequently reported serious infections in the CZP plus MTX groups were tuberculosis, pneumonia and erysipelas. The 10 cases of tuberculosis all occurred in countries with high incidence rates of the disease (Bulgaria, Estonia, Latvia, Poland, Russia and the Ukraine) [4, 5].
Malignant neoplasms occurred at similar rates across the treatment groups. Malignancy affected 12 patients in RAPID 1, including one in the placebo group (thyroid neoplasm; 1.1 per 100 pt-yrs), seven in the CZP 200 mg group (three basal cell carcinomas, including one that metastasized to the brain, one adrenal adenoma, one hepatic neoplasm, one oesophageal carcinoma and one uterine cancer; 2.3 per 100 pt-yrs) and four in the CZP 400 mg group (two tongue neoplasms, one extranodal marginal-zone B-cell lymphoma and one papilloma; 1.3 per 100 pt-yrs) [4]. In RAPID 2, one malignancy was reported in each treatment group (placebo, bladder cancer; CZP 200 mg, testicular cancer; and CZP 400 mg, colon cancer) [5].
The incidence of injection site pain was low for either CZP dose group in both trials (RAPID 1: eight and seven patients in the CZP 200 mg and 400 mg plus MTX groups, respectively; RAPID 2: one patient in the CZP 400 mg plus MTX group; <3 cases per 100 pt-yrs) [4, 5].
CZP monotherapy
In FAST4WARD, AEs occurred in 57.8 and 75.7% of patients in the placebo and CZP 400 mg groups, respectively (Table 4) [6]. The majority of AEs were mild to moderate; the most common AEs in the CZP group were headache, nasopharyngitis, upper respiratory tract infections, diarrhoea and sinusitis. AEs leading to withdrawal were reported for two (1.8%) patients in the placebo group (nausea and pneumonitis) and five (4.5%) patients in the CZP 400 mg group (bacterial arthritis, Salmonella arthritis, increased blood creatinine/increased blood urea, ischaemic stroke and menorrhagia). There were no deaths.
The rate of serious infections was low, occurring in two (1.8%) patients in the CZP 400 mg group and no patients in the placebo group, and there were no reports of tuberculosis. Two cases (1.8%) of benign tumours (uterine fibroids and benign parathyroid tumour) were reported in the CZP 400 mg group, with none in the placebo group. No patients in the CZP 400 mg group reported injection site pain.
Conclusions
Available data from three pivotal clinical trials demonstrate that CZP, either added to MTX or as monotherapy, improves the signs and symptoms of RA as early as Week 1 of treatment. CZP also inhibited the progression of structural joint damage as early as 16 weeks. Patients reported significant improvements in HRQoL and physical function, and significant relief of arthritis pain and fatigue, following treatment with CZP. Patients also experienced significant improvements in productivity within and outside the home, as well as increased participation in family, social and leisure activities with CZP. In the trials, the clinical and functional benefits of CZP were rapid, in many instances occurring by Week 1 of treatment, and were sustained up to study end (1 year when administered with MTX or 6 months when CZP was administered as monotherapy). CZP added to MTX or as monotherapy was well tolerated; the safety profile of CZP is similar to that of other TNF inhibitors and CZP has a low incidence of injection site pain.
Since CZP has only recently been approved, the efficacy and safety data noted in this review are limited to data from controlled clinical trials and do not include clinical registry data. Nevertheless these data suggest that CZP offers an important option to manage moderate to severe RA. Clinical trials are underway to further investigate the benefits of CZP in more clinically representative patient populations, such as those who have received DMARDs other than MTX.

Acknowledgements
The author wishes to thank Linda Wychowski and Karen Munro of PAREXEL for writing and editorial assistance in developing this article, which was funded by UCB, Inc.
Funding: This article was funded by UCB, Inc., which sponsored the clinical trials in which the data were collected. Funding to pay the Open Access publication charges for this article was provided by UCB.
Disclosure statement: P.J.M. has received research grant support, speaker honoraria and consulting fees from UCB.
References
- 1.Gartlehner G, Hansen RA, Jonas BL, Thieda P, Lohr KN. The comparative efficacy and safety of biologics for the treatment of rheumatoid arthritis: a systematic review and metaanalysis. J Rheumatol. 2006;33:2398–408. [PubMed] [Google Scholar]
- 2.Palframan R, Airey M, Moore A, Vugler A, Nesbitt A. Use of biofluorescence imaging to compare the distribution of certolizumab pegol, adalimumab, and infliximab in the inflamed paws of mice with collagen-induced arthritis. J Immunol Methods. 2009;348:36–41. doi: 10.1016/j.jim.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 3.Nesbitt A, Fossati G, Bergin M, et al. Mechanism of action of certolizumab pegol (CDP870): in vitro comparison with other anti-tumor necrosis factor alpha agents. Inflamm Bowel Dis. 2007;13:1323–32. doi: 10.1002/ibd.20225. [DOI] [PubMed] [Google Scholar]
- 4.Keystone E, van der Heijde D, Mason D, et al. Certolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis: findings of a fifty-two-week, phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum. 2008;58:3319–29. doi: 10.1002/art.23964. [DOI] [PubMed] [Google Scholar]
- 5.Smolen JS, Landewe R, Mease P, et al. Efficacy and safety of certolizumab pegol plus methotrexate in active rheumatoid arthritis: the RAPID 2 study. A randomised controlled trial. Ann Rheum Dis. 2009;68:797–804. doi: 10.1136/ard.2008.101659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fleischmann R, Vencovsky J, van Vollenhoven R, et al. Efficacy and safety of certolizumab pegol monotherapy every 4 weeks in patients with rheumatoid arthritis failing previous disease-modifying antirheumatic therapy: the FAST4WARD study. Ann Rheum Dis. 2009;68:805–11. doi: 10.1136/ard.2008.099291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Felson DT, Anderson JJ, Boers M, et al. American College of Rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum. 1995;38:727–35. doi: 10.1002/art.1780380602. [DOI] [PubMed] [Google Scholar]
- 8.Keystone E, Fleischmann R, Smolen J, et al. The efficacy of certolizumab pegol added to methotrexate is sustained over 2 years in the treatment of rheumatoid arthritis. Arthritis Rheum. 2009;60(Suppl.):S622. [Google Scholar]
- 9.Ware JE, Kosinski M, Keller SK. SF-36 Physical and Mental Health Summary Scales: A User's Manual. Boston: New England Medical Center, The Health Institute; 1994. [Google Scholar]
- 10.Tack BB. Self-reported fatigue in rheumatoid arthritis. A pilot study. Arthritis Care Res. 1990;3:154–7. [PubMed] [Google Scholar]
- 11.Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9:105–21. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum. 1980;23:137–45. doi: 10.1002/art.1780230202. [DOI] [PubMed] [Google Scholar]
- 13.Bruce B, Fries JF. The Stanford health assessment questionnaire: dimensions and practical applications. Health Qual Life Outcomes. 2003;1:20. doi: 10.1186/1477-7525-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strand V, Singh JA. Improved health-related quality of life with effective disease-modifying antirheumatic drugs: evidence from randomized controlled trials. Am J Manag Care. 2007;13(Suppl. 9):S237–51. [PubMed] [Google Scholar]
- 15.Wells GA, Tugwell P, Kraag GR, Baker PR, Groh J, Redelmeier DA. Minimum important difference between patients with rheumatoid arthritis: the patient’s perspective. J Rheumatol. 1993;20:557–60. [PubMed] [Google Scholar]
- 16.Farrar JT, Young JP, Jr, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149–58. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 17.Wells G, Li T, Maxwell L, MacLean R, Tugwell P. Determining the minimal clinically important differences in activity, fatigue, and sleep quality in patients with rheumatoid arthritis. J Rheumatol. 2007;34:280–9. [PubMed] [Google Scholar]
- 18.Strand V, Keininger DL, Tahiri-Fitzgerald E. Certolizumab pegol results in clinically meaningful improvements in physical function and health-related quality of life in patients with active rheumatoid arthritis despite treatment with methotrexate. Arthritis Rheum. 2007;56(Suppl.):393. [Google Scholar]
- 19.CIMZIA. [summary of product characteristics] Bruxelles, Belgium: UCB Pharma, SA; 2009. [Google Scholar]
- 20.Strand V, Brown M, Purcaru O. Certolizumab pegol monotherapy improves productivity in patients with active rheumatoid arthritis: results from a phase III randomized controlled trial. Ann Rheum Dis. 2007;66(Suppl. II):274. [Google Scholar]
- 21.Mease P. Certolizumab pegol for rheumatoid arthritis: effective in combination with methotrexate or as monotherapy. Int J Clin Rheumatol. 2009;4:253–66. [Google Scholar]
- 22.Schiff M, Keininger DL. Certolizumab pegol added onto methotrexate improves physical function and reduces pain in patients with rheumatoid arthritis who have an incomplete response to methotrexate: data from RAPID 2. Ann Rheum Dis. 2007;66(Suppl. II):187. [Google Scholar]
- 23.Strand V, Fleischmann R, Kvien TK, et al. Certolizumab pegol (CZP) added to methotrexate (MTX) provides lasting improvements in patient-reported outcomes (PROs) over 2 years. Arthritis Rheum. 2009;60(Suppl.):S636. [Google Scholar]
- 24.Strand V, Keininger DL, Tahari-Fitzgerald E, Fleischmann R. Certolizumab pegol monotherapy 400 mg every 4 weeks improves health-related quality of life and relieves fatigue in patients with rheumatoid arthritis who have previously failed DMARD therapy. Ann Rheum Dis. 2007;66(Suppl. III):188. [Google Scholar]
- 25.Osterhaus JT, Purcaru O, Richard L. Discriminant validity, responsiveness and reliability of the rheumatoid arthritis-specific Work Productivity Survey (WPS-RA) Arthritis Res Ther. 2009;11:R73. doi: 10.1186/ar2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kavanaugh A, Smolen J, Emery P, et al. Certolizumab pegol with methotrexate improves home and workplace productivity and social activities in patients with active rheumatoid arthritis. Arthritis Care Res. 2009;61:1592–600. doi: 10.1002/art.24828. [DOI] [PubMed] [Google Scholar]
- 27.Hazes J, Purcaru O, Coteur G, Mease P. Increased productivity at work and in household duties associated with reduced fatigue and improved physical function in RA patients. Ann Rheum Dis. 2008;67(Suppl. II):79. [Google Scholar]
- 28.Westhovens R, Purcaru O. Certolizumab pegol in combination with methotrexate or as monotherapy shows cumulative gains over time in work and home productivity in patients with active rheumatoid arthritis; In: The 13th Belgian Congress on Rheumatology, September 23–25, 2009, Mechelen, Belgium. [Google Scholar]