Abstract
Background
Chronic rhinosinusitis (CRS) is among the most common causes of olfactory loss. The loss of the sense of smell is thought to result from structural and functional changes occurring in the olfactory epithelium caused by inflammation. However, the cellular mechanisms underlying CRS-associated olfactory loss remain incompletely understood.
Methods
Transgenic mice expressing TNF-alpha specifically within the olfactory epithelium were used as a model for CRS-associated olfactory loss. TNF-alpha expression was induced over different time intervals, and olfactory epithelial tissue was assessed for the expression of neuronal markers by laser scanning confocal microscopy and Western blot.
Results
TNF-alpha expression results in an inflammatory infiltrate in the olfactory epithelium, thinning of the olfactory neuron layer, and a progressive loss of olfactory function. Reduced expression of markers for neurons and mature olfactory neurons (neural cell adhesion molecule [NCAM] and olfactory marker protein [OMP], respectively) was observed in the neuroepithelium and in the subepithelial axon bundles. Expression of growth-associated protein (GAP) 43, a marker for immature neurons, was also reduced. These alterations were reversed when TNF-alpha expression was discontinued.
Conclusion
TNF-alpha expression in a transgenic model of CRS-associated olfactory loss results in progressive loss of olfactory neurons. Decreased GAP-43 expression suggests that TNF-alpha–associated inflammation inhibits differentiation of progenitor cells into immature olfactory neurons. Therefore, reduced regeneration of olfactory neurons may be an important mechanism underlying olfactory loss in CRS, in addition to neuronal loss or apoptosis. This mouse model represents a potential tool in the development of novel therapeutic strategies for the prevention of olfactory neuron loss in CRS.
Keywords: GAP-43, inflammation, markers, NCAM, olfaction, OMP, rhinosinusitis, TNF, transgenic
Defects in the sense of smell are common clinical problems that can be remarkably frustrating for those affected. Although multiple etiologies for clinical olfactory loss exist, one of the most common is chronic rhinosinusitis (CRS).1 However, the pathophysiological mechanisms that produce olfactory dysfunction in patients with CRS are not clearly understood. Mucosal edema and narrowing of the nasal passages may certainly contribute by preventing odorants from effectively reaching sensory olfactory neurons. However, many CRS patients with olfactory loss have seemingly patent olfactory clefts, suggesting an alternative cause for hyposmia or anosmia in this patient group.
Histologically, the olfactory epithelium of patients with CRS is characterized by an inflammatory cell infiltrate that largely mirrors characteristics seen in the respiratory sinonasal mucosa.2 These invading macrophages and lymphocytes have the ability to produce substantial quantities of inflammatory cytokines and chemokines, including RANTES, eotaxin, TNF-α, IL-1β, IL-6, and IL-13. Certain of these mediators, viz., TNF-α, are universally associated with chronic sinonasal disease, regardless of histological subtype or etiology.3,4 Like other inflammatory mediators, TNF-α is produced chiefly by infiltrating lymphocytes and macrophages, and, to a lesser degree, by resident dendritic cells, macrophages, epithelial cells, and fibroblasts. TNF-α, in turn, is capable of inducing the expression of additional inflammatory mediators from a variety of local sources.
Few studies have addressed the role of CRS-associated inflammation in functional olfactory loss. This is largely because of the inaccessibility of human olfactory tissue and the inherent difficulty of maintaining olfactory neurons in standard cell culture conditions. Furthermore, no animal models exist for the study of this disease entity. Our group recently reported the development of a transgenic mouse model that replicates CRS-associated inflammation via a temporally controlled, tissue-specific expression of individual inflammatory mediators within the olfactory epithelium.5 This model replicates the inflammatory disease state seen in CRS and provides a template for the study of individual pathways and mechanisms that contribute to CRS-associated olfactory dysfunction.
In this study, we examined the effect of TNF-α expression on the structure and characteristics of the mouse olfactory epithelium. As previously reported, local production of TNF-α results in inflammation and loss of olfactory neurons.5 In this report, we specifically examine the effect of TNF-α on the expression of neuronal marker proteins, including growth-associated protein 43 (GAP) 43, neural cell adhesion molecule (NCAM), and olfactory marker protein (OMP). We feel that this mouse model represents a potential tool in the development of novel therapeutic strategies for the prevention of olfactory neuron loss in CRS.
METHODS
Inducible Olfactory Inflammation (IOI) Mouse
The IOI mouse line was created, as previously described, by introduction of the Teton genetic system into the mouse genome under the control of the olfactory-specific cyp2g1 promoter.5 Briefly, the reverse tetracycline transactivator gene was knocked into the cyp2g1 coding region in mouse embryonic stem cells. The tet-response element (TRE)–TNF-α construct containing the murine TNF-α gene under control of the tetracycline-responsive element was generated by cloning the murine TNF-α gene6 into the pTRE vector (Clontech, Mountain View, CA). The TRE–TNF-α vector was injected into fertilized mouse eggs to generate transgenic mice. Transgenic lines were maintained by mating to BL/6 mice. The cyp2g1-rtTA mice were bred to wild-type BL/6 mice to establish germline transmission. True-breeding strains of TRE–TNF-α and cyp2g1-rtTA were housed under specific pathogen-free conditions. The two lines were crossed to create the IOI mouse, with the presence of both constructs determined by polymerase chain reaction.
Histological Analyses
For histology, mouse nasal cavities were embedded in paraffin or were processed for frozen sections. After death by CO2 inhalation, mice were decapitated and the heads were fixed and decalcified by immersion in TBD2 solution (Shandon, Pittsburgh, PA) for 24 hours. The heads were then embedded in paraffin, and 12-μm sections were obtained and collected on glass slides for hematoxylin and eosin staining. For frozen section analysis, mice were anesthetized by i.p. injection of 100 mg/kg of xylaket (Sigma, St. Louis, MO), before intracardiac perfusion with 4% paraformaldehyde. The olfactory tissue was then dissected, postfixed in 4% paraformaldehyde, and transferred to a solution of 30% sucrose and 250 mM of EDTA for 48 hours. The decalcified heads were then infiltrated with OCT tissue-tek compound (Miles, Elkhart, IN) and frozen on dry ice into a plastic mold. Sections of mouse olfactory tissue in OCT were cut on a cryostat (12 μM), placed on Super-frost plus slides (Fisher Scientific, Pittsburg, PA), and dried 60 minutes before use. Images shown are representative of at least three separate experiments for a minimum of three individual mice.
Immunostaining
Cryostat sections were blocked for 1 hour in PBS containing 5% normal secondary serum and then incubated overnight at 4°C in 5% normal serum containing primary antibody to either NCAM (Chemicon, Temecula, CA), OMP (Wako, Richmond, VA), or GAP-43 (Serotec, Raleigh, NC). Primary antibodies were detected using fluorescent-tagged secondary antibodies, conjugated to Al-exa488 or Alexa594 (Invitrogen, Carlsbad, CA). Each sample was counterstained by addition of DAPI mounting medium (Vector Labs, Burlingame, CA) before coverslipping. Slides were viewed using a LSM510 confocal microscope (Carl Zeiss Micro-imaging, Thornwood, NJ) per the manufacturers’ instructions. Images shown are representative of at least three separate experiments for a minimum of three individual mice.
Immunoblotting
IOI mice were maintained under standard conditions with drinking water supplemented with 0.5 mg/mL of doxycycline. Three mice per time point were killed by CO2 inhalation and decapitated. The heads were then bisected in a sagittal plane and the septal and turbinate mucosa was removed by microdissection. Tissue was snap frozen in liquid nitrogen before the addition of ice-cold lysis buffer (50 mM of Tris-HCl, pH 8.0, 150 mM of NaCl, and 1% Triton X-100). Each sample was sonicated for 15 seconds and then centrifuged for 10 minutes at 14,000 ×g. Clarified extracts were assessed for protein concentration using the modified bicinchoninic acid protein assay (Pierce, Rockford, IL). Equal amounts of each triplicate sample were then pooled, combined with 4× SDS loading buffer and separated by SDS-PAGE. Separated proteins were transferred to polyvinylidene fluoride and incubated with primary antibody to either GAP-43, NCAM, or Actin, followed by incubation with an alkaline-phosphatase–conjugated secondary antibody. Proteins were detected using chemiluminescence by exposure to Kodak X-AR film. Densitometry was performed using the ImageJ (NIH, Bethesda, MD) image analysis software.
RESULTS
TNF-α Expression Results in a Time-dependent Reduction in Olfactory Epithelium Thickness and a Loss of Olfactory Neurons
The mouse olfactory epithelium consists of multiple layers of olfactory sensory neurons with a multipotent basal layer of stem cells and a superficial layer of supporting sustentacular cells. We have previously reported that expression of TNF-α in the IOI mouse produces significant morphological changes in the olfactory epithelium.5 In this system, expression of TNF-α is augmented >20-fold over baseline levels, as assessed by ELISA and immunohistochemistry.5 As shown in Fig. 1, expression of TNF-α for 2 weeks results in few noticeable histological changes. There remain several layers of olfactory neurons and a subepithelium with well-demarcated axon bundles. After 5 weeks of TNF-α expression, the olfactory epithelium is significantly thinned, with less than a few layers of olfactory neurons. Likewise, the axon bundles have become disorganized and there appears to be a massive infiltrate of inflammatory cells within the subepithelium, particularly just under the basement membrane. Of note, despite being the source for TNF-α production in this model, the superficial layer of sustentacular cells remains completely intact at all time points. These results suggest that TNF-α expression produces a thinning of the olfactory epithelium and a loss of olfactory neurons. Furthermore, there is a substantial infiltration of inflammatory cells within the submucosa, which we previously reported to be largely macrophages.
Figure 1.
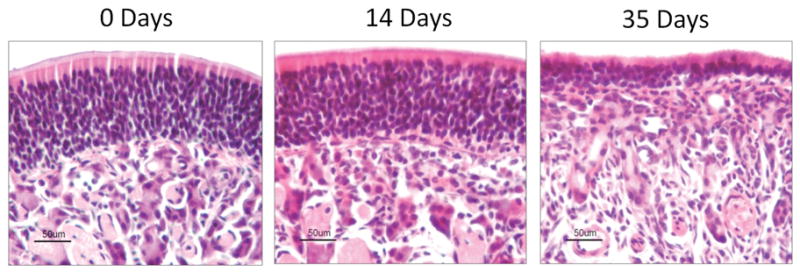
TNF-α produces histological changes in the mouse olfactory epithelium. Inducible olfactory inflammation (IOI) mice were administered 0.5 mg/mL of doxycycline in their drinking water between 0 and 5 weeks. The mice were then killed and their nasal cavities were embedded in paraffin and processed as described in the Methods section. (left) Control mouse olfactory epithelium exhibits multiple layers of well-organized olfactory neurons with an overlying layer of sustentacular cells. The subepithelium contains several discrete axon bundles. (middle) After 2 weeks, the IOI mouse olfactory epithelium appears largely unchanged. The thickness of the olfactory neuron layer is unchanged and the sustentacular cells are undisturbed. There is a moderate increase in inflammatory cells in the subepithelium. (right) After 5 weeks, the olfactory epithelium is significantly thinner with only a few remaining layers of olfactory neurons. The overlying layer of sustentacular cells remains intact. There is a considerable infiltration of inflammatory cells in the subepithelium and the axon bundles have largely degenerated.
TNF-α Produces a Loss of NCAM Expression in the Olfactory Epithelium and Axon Bundles
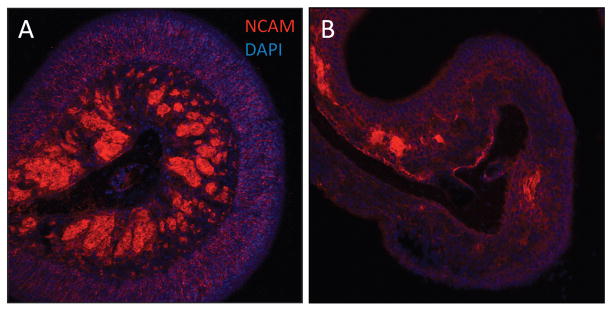
The olfactory neuroepithelium consists of three major cell types: basal cells, olfactory neurons, and sustentacular cells. Olfactory neurons behave like many other neuronally derived cells in the central nervous system. They are bipolar neurons that extend axons through the basement membrane as they travel toward the olfactory bulb. Likewise, they express many of the same neuronal proteins as neurons in the brain and spinal cord. Using Immunofluorescence, we examined the expression and localization of the NCAM within mouse olfactory tissue. This protein is expressed in most neuronally derived cells and is found in both mature and immature olfactory sensory neurons. As shown in Fig. 2 A, under normal conditions, NCAM is highly expressed in axon bundles within the subepithelium, as well as within the cytoplasm of individual olfactory neurons of the epithelium. In contrast, after expression of TNF-α for 5 weeks, the organized architecture of the olfactory epithelium is lost (Fig. 2 B). The axon bundles are difficult to identify and the expression of NCAM is drastically reduced in both the bundles and the epithelium. This suggests that TNF-α expression results in a loss of neuronal cells in olfactory tissue of the IOI mouse.
Figure 2.
Expression of neural cell adhesion molecule (NCAM) is reduced in the inducible olfactory inflammation (IOI) mouse. IOI mice were administered 0.5 mg/mL of doxycycline in their drinking water between 0 and 5 weeks. The mice were then killed and nasal cavity frozen sections were evaluated using confocal microscopy as detailed in the Methods section. (A) Control mice express NCAM diffusely within the cytoplasm of olfactory sensory neurons as well as within the axon bundles. (B) After 5 weeks, NCAM expression in the olfactory epithelium is lost, concomitant with a loss of most olfactory neurons. Likewise, NCAM expression in the axon bundles is nearly undetectable.
TNF-α Expression Results in a Loss of Mature and Immature Olfactory Neurons in the IOI Mouse
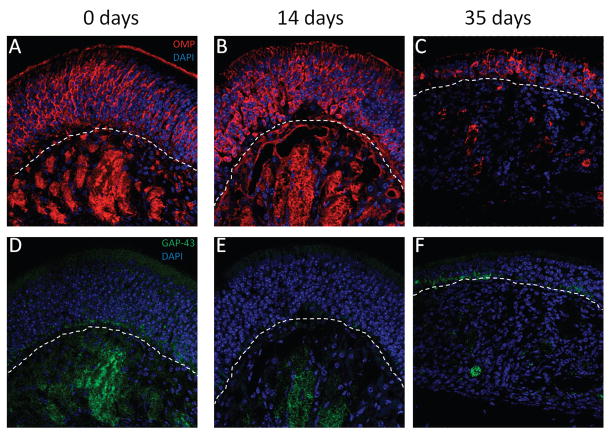
We next wanted to verify that the neurons lost in the IOI mouse are, in fact, olfactory neurons. Mature olfactory neurons can be identified by the presence of OMP, a 19-kDa cytosolic protein of unknown function, expressed specifically in this cell type. Likewise, immature olfactory neurons were identified by the expression of GAP-43, a 43-kDa cytosolic protein expressed only in developing neurons. As shown in Fig. 3 A, OMP displays a similar expression pattern as NCAM, being heavily localized to the axon bundles and cytoplasm of olfactory neurons. In contrast, GAP-43 is expressed only in cells within the basal region of the olfactory epithelium, as well as in the axon bundles (Fig. 3 D). This expression pattern has been reported previously, as immature olfactory neurons are continuously being generated from basal cells adjacent to the basement membrane. OMP expression is not significantly reduced after 2 weeks of TNF-α expression; however, the precise organization of OMP expression is clearly lost (Fig. 3 B). GAP-43 expression is considerably reduced in the axon bundles while no expression could be detected within the olfactory epithelium (Fig. 3 D). After 5 weeks of TNF-α expression, the olfactory epithelium is clearly thinner. Of note, only a few OMP+ neurons remain (Fig. 3 C). Likewise, OMP expression in the axon bundles is completely lost. GAP-43 expression is essentially undetectable at this point (Fig. 3 F). These results suggest a loss of both mature and developing immature olfactory neurons with TNF-α expression.
Figure 3.
Growth-associated protein (GAP) 43 and olfactory marker protein (OMP) expression is reduced in the inducible olfactory inflammation (IOI) mouse. (A and D) The expression pattern for OMP in control mice is similar to that observed for neural cell adhesion molecule (NCAM), with expression in olfactory neurons and axon bundles. GAP-43 is expressed only in the basal layer of the olfactory epithelium and in the axon bundles, consistent with expression only in developing immature neurons. (B and E) After 2 weeks, OMP and GAP-43 expression is moderately reduced and there is some disorganization to the olfactory epithelium. (C and F) After 5 weeks, OMP and GAP-43 expression is almost completely lost with only a few OMP+ and/or GAP-43+ neurons remaining in the epithelium. The axon bundles are difficult to identify.
TNF-α Regulates Expression of Neuronal Proteins in a Temporally Controlled Manner
We next wanted to examine the time dependence of neuronal marker expression in the IOI mouse. Using Western blot analysis, the expression of GAP-43 and NCAM were detected in olfactory tissue from IOI mice fed doxycycline for up to 5 weeks. As shown in Fig. 4 A, GAP-43 expression is immediately reduced to almost undetectable levels within 2 days. There is a brief return of expression at around 7 days, but GAP-43 otherwise remains undetectable for the duration of TNF-α expression. Similarly, NCAM expression is also decreased rapidly after TNF-α production is induced, with a brief recovery at around 7 days. When displayed graphically, it becomes apparent that NCAM expression is reduced to ~50% of control levels, while GAP-43 expression becomes almost undetectable (Fig. 4 B). We next wanted to determine whether there was any recovery of neuronal marker protein expression when doxycycline was removed from the mouse’s diet, and, consequently, TNF-α expression discontinued. As shown in Fig. 4, A and B, GAP-43 and NCAM expression return to near normal levels almost immediately and remain elevated. Of note, two different major isoforms of NCAM are detected in most neuronal cells, one with a molecular weight of 140 kDa and a second with a weight of 180 kDa. In our experiments, the smaller isoform is detected at greater levels in control mice and mice reared in the presence of doxycycline. However, during the apparent recovery period when TNF-α expression is discontinued, there is a reverse in this expression, with the larger isoform being detected at higher levels. Interestingly, the 180-kDa isoform of NCAM is associated with neuronal proliferation and differentiation, suggesting that this may indicate active recovery and growth of olfactory neurons at these time points.7
Figure 4.
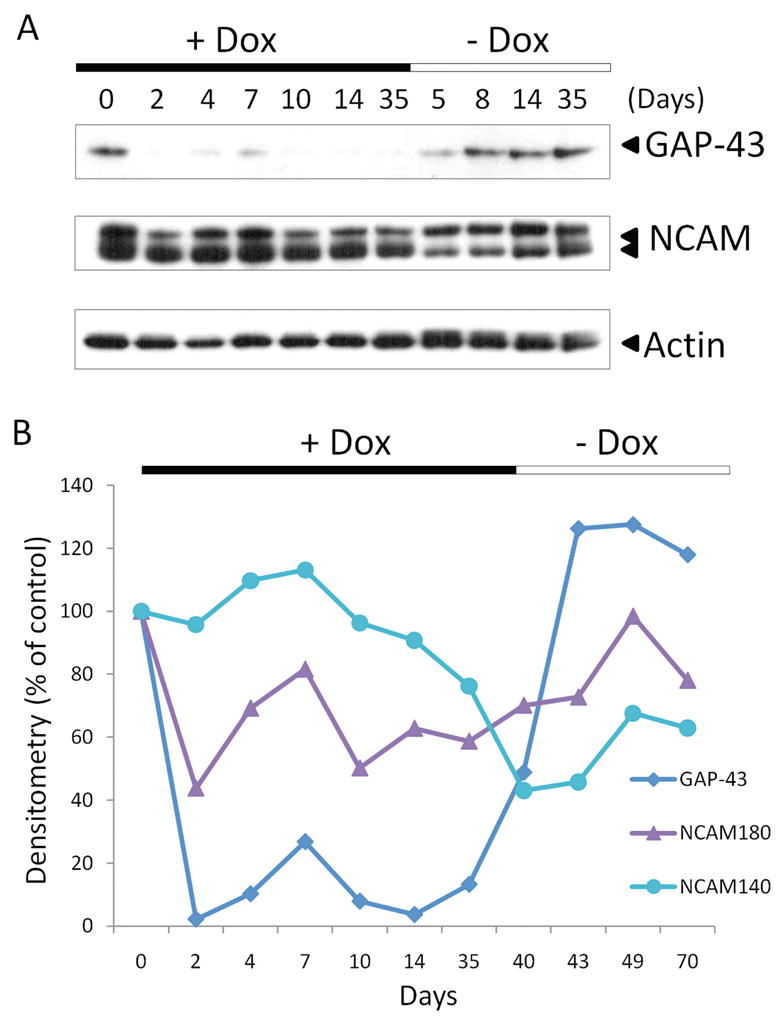
Expression of growth-associated protein (GAP) 43 and neural cell adhesion molecule (NCAM) is reduced with TNF-α expression, but recovers quickly when TNF-α expression is discontinued. Inducible olfactory inflammation (IOI) mice administered 0.5 mg/mL of doxycycline in their drinking water for 0–5 weeks were killed and olfactory tissue was isolated and examined by Western blot for expression of neuronal marker proteins. (A) NCAM and GAP-43 expression are reduced in a time-dependent manner after induction of TNF-α. When TNF-α expression is discontinued, GAP-43 and NCAM expression returns to near normal levels. (B) Western blots were examined by densitometry and expressed graphically. As shown, GAP-43 and NCAM expression are reduced time dependently during TNF-α expression, with a brief increase in expression at around 7 days. In the absence of TNF-α, expression levels return to near normal levels.
The IOI Mice Recover from TNF-α–Induced Olfactory Neuron Loss Rapidly after Removal of TNF-α from the Local Environment
As shown in the previous section, NCAM and GAP-43 expression rapidly returns when TNF-α is removed from the local environment. We verified this finding using immunofluorescence and histology. As shown in Fig. 5, after a 1-week recovery period, the olfactory epithelium of the IOI mouse is beginning to return to its baseline appearance. There are additional olfactory neurons and a thickened epithelium. There is also a return of NCAM expression in the axon bundles, as well as a return of NCAM+ mature olfactory neurons. There is also an increase in GAP-43+ olfactory neurons in the olfactory epithelium, suggesting that there is a proliferation of immature, developing neurons during the recovery period. After 5 weeks of recovery, the IOI mouse olfactory epithelium has returned to normal in appearance (Fig. 5 D).
Figure 5.
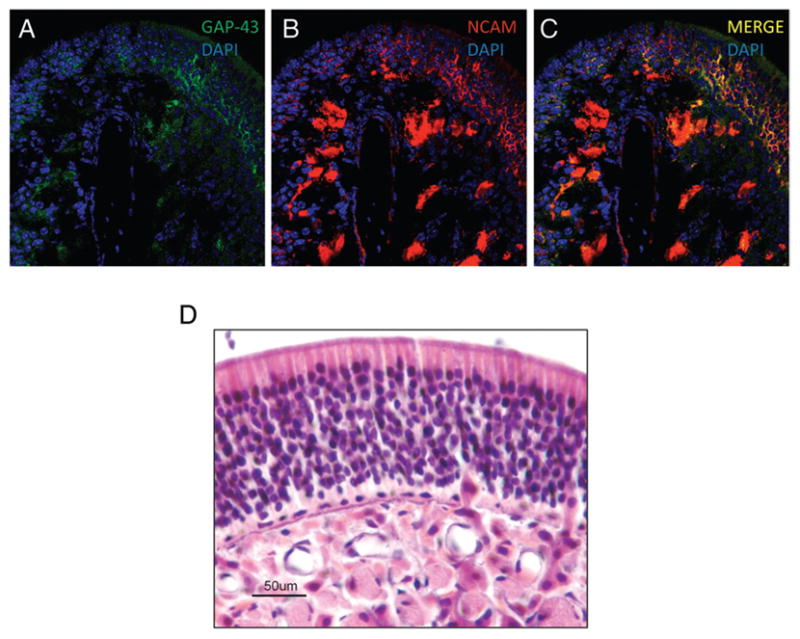
Neural cell adhesion molecule (NCAM) and growth-associated protein (GAP) 43 expression recovers within 1 week and mice appear histologically normal after 5 weeks. (A–C) After a 1-week recovery, new NCAM+ and GAP-43+ olfactory neurons are apparent in the epithelium. Significant expression has also returned in the axon bundles. (D) After a 5-week recovery, the olfactory tissue appears essentially normal, with multiple layers of olfactory neurons and well-circumscribed axon bundles.
DISCUSSION
In previous work, our laboratory introduced a transgenic mouse model of inflammation-associated functional olfactory loss. In this model, any inflammatory cytokine can be expressed specifically within olfactory tissue in a time-dependent manner. The phenotype of the TNF-α–expression mouse is notable for a significantly thinned olfactory epithelium with a massive inflammatory cell infiltrate within the subepithelium. Furthermore, these mice completely lose their sense of smell. Using this model, we have evaluated the expression of different neuronal marker proteins within olfactory tissue over different periods of TNF-α expression. We showed that markers for both immature (GAP-43) and mature (NCAM and OMP) olfactory neurons are reduced in a time-dependent manner, concomitant with an apparent loss of olfactory neurons. Of particular interest, these mice rapidly recover when TNF-α expression is discontinued, with return of neuronal protein expression and proliferation of new olfactory neurons.
TNF-α is, perhaps, the most well-studied member of the diverse family of inflammatory cytokines. Receptors for TNF-α are expressed in most tissues, particularly in cells of the immune system. Most TNF-α production, in turn, originates from macrophages, with the balance coming from a variety of other cell sources including endothelial cells, mast cells, fibroblasts, and neuronal cells. TNF-α is a major proinflammatory mediator and has been associated with both cell proliferation and apoptosis, depending on the cell type and environment. In the central nervous system, TNF-α is expressed mostly from activated microglia and astrocytes, and most studies suggest that it produces a proapoptotic effect on surrounding cells. For example, in cultured embryonic sensory neurons, inhibitors of TNF-α signaling rescue nerve growth factor–deprived neurons from cell death.8 Likewise, neurons from TNF-α knockout mice exhibit improved survival compared with wild-type neurons.9 A recent study also found that microglia-derived TNF-α is an early signal for delayed cell death in spinal cord motoneurons.10 However, a smaller number of dichotomous studies report that TNF-α can also be neuroprotective, and under certain circumstances may actually spare neurons from local inflammation and injury. For example, mice lacking the TNF-α receptor display poorer outcomes after ischemic brain injury.11 Of note, however, this observation holds true mostly in the acute phase after brain injury, and TNF-α likely again plays a proapoptotic role under chronic models of brain injury.
The literature exploring TNF-α signaling in olfactory neurons is much more limited. Farbman et al. found that mRNA for both TNF-α and the TNF-α receptor (TNFR1) is expressed within the olfactory epithelium.12 Likewise, addition of TNF-α to organotypic cultures of fetal rat olfactory epithelium results in the formation of apoptotic bodies within 6 hours.13 In vitro, this proapoptotic effect appears to be mediated, at least in part, by intracellular caspases. In the present study, we have used a transgenic mouse model that expresses TNF-α specifically within olfactory tissues. These mice lose their sense of smell within 4–6 weeks and show signs of chronic inflammation, including infiltration of macrophages into the olfactory submucosal tissue. Our results show that loss of the sense of smell is concomitant with a decrease in olfactory epithelial thickness. Likewise, expression of proteins that distinguish mature and immature olfactory neurons are largely lost at this point. Of interest, two phases of neuronal marker protein expression were observed in this model. Expression of GAP-43 and NCAM decreased within 48 hours after induction of TNF-α production. However, between 4 and 7 days, there is a brief increase in expression of both proteins, consistent with a proliferative phase of TNF-α signaling. Expression subsequently continues to decrease over subsequent weeks, consistent with the established role of TNF-α in cell death. Thus, in olfactory tissue, as has previously been found in models of brain injury, TNF-α may signal through an acute proliferative phase, as well as a chronic proapoptotic phase.
Perhaps the most interesting finding in the present study is the ability of the IOI mouse to completely recover from chronic TNF-α–mediated inflammation and the loss of most olfactory sensory neurons. This recovery begins within 1 week after TNF-α expression is discontinued and is essentially complete within 6 weeks. The ability of olfactory neurons to regenerate is well established, and these neurons are continuously being replaced by dividing basal cells throughout an organism’s life cycle. However, little is known about the mechanism of olfactory neuron cell death and subsequent regeneration. Our results suggest that TNF-α may play a major role in olfactory neuron degeneration and regeneration in conditions consistent with chronic sinonasal inflammation. Furthermore, the regenerative ability of olfactory neurons in this model suggests that TNF-α and its signaling components may be appropriate therapeutic targets in the treatment of CRS-associated clinical olfactory dysfunction.
The IOI mouse is the first transgenic model of inducible cytokine-mediated sinonasal inflammation. It represents a valuable tool for the study of CRS and its role in olfactory loss and dysfunction. Using this model, we have been able to advance our understanding of the effects of inflammatory cytokines on olfactory neuron structure and function. Future studies will likely be able to clarify the signaling mechanisms and associated cellular mediators that contribute to CRS-associated olfactory dysfunction. We hypothesize that local expression of inflammatory cytokines in human olfactory tissue contributes to clinical olfactory loss via their effects on olfactory neurons and progenitor cells. Therefore, the IOI mouse will likely prove to be an invaluable tool for the study of its human disease counterpart.
Footnotes
Presented at the spring meeting of the American Rhinologic Society, Orlando, Florida, May 2, 2008
References
- 1.Loury M, Kennedy DW. Chronic sinusitis and nasal polyposis. In: Getchell TVBL, Doty RL, Snow J, editors. Smell and Taste in Health and Disease. New York: Raven Press; 1991. pp. 711–730. [Google Scholar]
- 2.Kern RC, Conley DB, Haines GK, III, Robinson AM. Pathology of the olfactory mucosa: Implications for the treatment of olfactory dysfunction. Laryngoscope. 2004;114:279–285. doi: 10.1097/00005537-200402000-00018. [DOI] [PubMed] [Google Scholar]
- 3.Kuehnemund M, Ismail C, Brieger J, et al. Untreated chronic rhino-sinusitis: A comparison of symptoms and mediator profiles. Laryngoscope. 2004;114:561–565. doi: 10.1097/00005537-200403000-00032. [DOI] [PubMed] [Google Scholar]
- 4.Lennard CM, Mann EA, Sun LL, et al. Interleukin-1 beta, interleukin-5, interleukin-6, interleukin-8, and tumor necrosis factor-alpha in chronic sinusitis: Response to systemic corticosteroids. Am J Rhinol. 2000;14:367–373. doi: 10.2500/105065800779954329. [DOI] [PubMed] [Google Scholar]
- 5.Lane AP, Turner JH, May L, et al. A genetic model of chronic rhinosinusitis-associated olfactory inflammation reveals reversible functional impairment and dramatic neuroepithelial reorganization. J Neurosci. 2010;30:2324–2329. doi: 10.1523/JNEUROSCI.4507-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001;11:372–377. doi: 10.1016/s0962-8924(01)02064-5. [DOI] [PubMed] [Google Scholar]
- 7.Angata K, Huckaby V, Ranscht B, et al. Polysialic acid-directed migration and differentiation of neural precursors are essential for mouse brain development. Mol Cell Biol. 2007;27:6659–6668. doi: 10.1128/MCB.00205-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barker V, Middleton G, Davey F, et al. TNFalpha contributes to the death of NGF-dependent neurons during development. Nat Neurosci. 2001;4:1194–1198. doi: 10.1038/nn755. [DOI] [PubMed] [Google Scholar]
- 9.Scherbel U, Raghupathi R, Nakamura M, et al. Differential acute and chronic response of tumor necrosis alpha-deficient mice to experimental brain injury. Proc Natl Acad Sci USA. 1999;96:8721–8726. doi: 10.1073/pnas.96.15.8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gary DS, Bruce-Keller AJ, Kindy MS, et al. Ischemic and excitotoxic brain injury is enhanced in mice lacking the p55 tumor necrosis factor receptor. J Cereb Blood Flow Metab. 1998;18:1283–1287. doi: 10.1097/00004647-199812000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Bruce AJ, Boling W, Kindy MS, et al. Altered neuronal and microglial responses to excitotoxic and ischemic brain injury in mice lacking TNF receptors. Nat Med. 1996;2:788–794. doi: 10.1038/nm0796-788. [DOI] [PubMed] [Google Scholar]
- 12.Farbman AI, Buchholz JA, Suzuki Y, et al. A molecular basis of cell death in olfactory epithelium. J Comp Neurol. 1999;414:306–314. doi: 10.1002/(sici)1096-9861(19991122)414:3<306::aid-cne2>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki Y, Farbman AI. Tumor necrosis factor-α-induced apoptosis in olfactory epithelium in vitro: Possible roles of caspase 1 (ICE), Caspase 2 (ICH-1), and caspase 3 (CPP32) Exp Neurol. 2000;165:35–45. doi: 10.1006/exnr.2000.7465. [DOI] [PubMed] [Google Scholar]