Abstract
The Cu(I)-catalyzed azide-alkyne cycloaddition (CuAAC) is the standard method for bioorthogonal conjugation. However, current Cu(I) catalyst formulations are toxic, hindering their use in living systems. Here we report that BTTES, a tris(triazolylmethyl)amine-based ligand for Cu(I), promotes the cycloaddition reaction rapidly in living systems without apparent toxicity. This catalyst allows, for the first time, noninvasive imaging of fucosylated glycans during zebrafish early embryogenesis. We microinjected embryos with alkyne-bearing GDP-fucose at the one-cell stage and detected the metabolically incorporated unnatural sugars using the biocompatible click chemistry. Labeled glycans could be imaged in the enveloping layer of zebrafish embryos between blastula and early larval stages. This new method paves the way for rapid, noninvasive imaging of biomolecules in living organisms.
Introduction
The recent development of bioorthogonal click chemistry has led to an explosion of interest in the selective covalent labeling of biomolecules in cells and living organisms.1, 2 In these labeling reactions one of the two bioorthogonal functional groups is first incorporated into target biomolecules via genetic3 or metabolic approaches.4, 5 A biophysical probe, functionalized in a complementary fashion, is introduced in the second step, allowing detection or isolation of the target of interest. To minimize perturbations to the physiological state of the cells or organisms probed, an ideal ligation reaction must proceed in water at neutral pH and at temperatures between 25 to 37 °C without any cytotoxic effects. Further, the reactive partners participating in this transformation must be inert to the native functional groups present in the biological system.6, 7
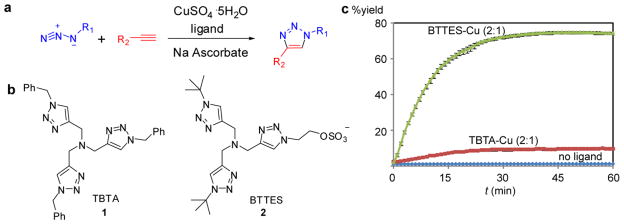
Few chemical reactions satisfy both the bioorthogonal and click requirements. Discovered by Sharpless-Fokin and Meldal in 2002, the Cu(I)-catalyzed azide-alkyne cycloaddition (CuAAC) is the quintessential bioorthogonal click reaction for chemical biologists (Fig. 1a).8, 9 This transformation is accelerated by approximately seven orders of magnitude compared to the uncatalyzed version.10, 11 As a ligand-assisted process, the reaction is further accelerated by Cu(I)-stabilizing ligands (i.e. tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine, TBTA, 1, Fig. 1b, 1c and tris(3-hydroxypropyltriazolylmethyl)amine, THPTA, 3).12, 13 The fast reaction kinetics and exquisite selectivity of CuAAC has gained it widespread utilization in chemical biology and materials science.14, 15
Figure 1.
Copper(I)-catalyzed azide-alkyne cycloaddtion (CuAAC) is accelerated by Cu(I)-stabilizing ligands. (a) CuAAC of azides and terminal alkynes to form 1,4-disubstituted 1,2,3-triazoles. (b) Structures of CuAAC-accelerating ligands. (c) Conversion–time profiles of CuAAC in the presence/absence of accelerating ligands. Reaction conditions: propargyl alcohol (50 μM), 3-azido-7-hydroxy-coumarin (100 μM), CuSO4 (75 μM), 0.1 M potassium phosphate buffer (pH 7.0)/DMSO 95:5, sodium ascorbate (2.5 μM), room temperature. Error bars represent the standard deviation of three replicate experiments.
To date, however, the use of CuAAC in living systems has been hampered by the toxicity associated with the catalyst formulation (CuSO4 or CuBr + sodium ascorbate + TBTA).7 TBTA, the ligand utilized in the optimized CuAAC conditions to stabilize the Cu(I) oxidation state, has very poor water solubility, which mandates the use of high Cu loading (0.2–1 mM) to achieve reasonable reaction rates. Free Cu(I) ions that escape from the coordination sphere of TBTA promote the generation of reactive oxygen and nitrogen species and induce detrimental consequences to cellular metabolism.16 For example Escherichia coli that incorporated azidohomoalanine into their outer membrane protein OmpC survived the initial treatment with 100 μM CuBr for 16 h, but were no longer able to divide.17 Similarly, greater than 90% of mammalian cells underwent apoptosis and cell lysis within 20 min when treated with 1 mM Cu(I) under optimized CuAAC conditions.7 Zebrafish embryos exhibited a similar sensitivity to Cu(I). When embryos were treated with 1 mM CuSO4, 1.5 mM sodium ascorbate, and 0.1 mM TBTA ligand, all the embryos were dead within 15 min.7 As presently formulated, labeling of biomolecules via CuAAC is not feasible in living systems.
To improve upon the biocompatibility of the azide-alkyne cycloaddition, Bertozzi and coworkers developed a copper-free [3+2] cycloaddition by employing ring strains as an alternative means for alkyne activation.18, 19 Among the cycloalkynes examined, a difluorinated cyclooctyne, DIFO,20 and a biarylazacyclooctynone, BARAC,21 showed rapid kinetics in biomolecular labeling experiments. DIFO-fluorophore conjugates are particularly sensitive for imaging azide-tagged glycans within complex biological systems, including live cells,20 C. elegans22 and zebrafish embryos,23, 24 with very low background fluorescence. However, recent in vivo studies revealed that DIFO-based probes bind to mouse serum albumin non-specifically, presumably via covalent-bond formation between the cyclooctyne and cysteine residues.25 In addition, the construction of these cyclooctyne-based probes usually involves multistep linear syntheses, which can be a challenge.20, 26 A major goal in this field is to identify a new copper catalyst formulation that can promote rapid azide-alkyne cycloaddition in living systems without cytotoxicity.
Results and Discussion
In nature copper is a bioessential element and the second most abundant transition metal in the human organism.27 With Cu(II)/Cu(I) redox potential between 0.0 to 0.8 V, copper-containing enzymes are prevalent, participating particularly in reactions involving dioxygen transport and utilization,27–30 as well as in the degradation of unwanted side products of O2 metabolism such as O2·− radicals.31 The activities of these enzymes are elegantly orchestrated by the ligands surrounding the copper ions in the active sites. Applying lessons from nature, we sought to design a new ligand for Cu(I) that could extend the utilization of CuAAC to living systems. When coordinating with Cu(I), the ligand would engage in forming an active copper catalyst to promote the azide-alkyne cycloaddition at micromolar Cu(I) concentrations, while sequestering the copper-associated cytotoxicity.
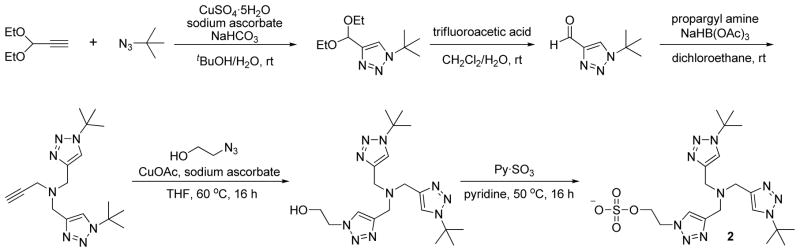
To develop a non-toxic Cu(I) catalyst that is suitable for applications in living systems, we screened a library of 14 TBTA analogues (Figure S1, Supporting Information), most of which showed improved water solubility except 4, 8, 11 and 15. From all monomeric TBTA analogs that are water soluble, we discovered that the higher number of bulky tert-butyl groups a ligand bears, the faster the corresponding cycloaddition reaction becomes (Figure S2d, Supporting Information). This screen led to the discovery of a bis(tert-butyltriazoly) ligand, BTTES (2, Scheme 1), which contains the ideal balance between reactivity and solubility. This ligand was the most effective in promoting the CuAAC among all water-soluble ligands screened (Figure S2d, Supporting Information), and dramatically accelerated the rate of the azide-alkyne cycloaddition by coordination with the in situ generated Cu(I) (Fig. 1c); It also bears a sulfate functionality designed to minimize the cell membrane permeability of the coordinated copper. BTTES was synthesized via CuAAC by reacting 2-azidoethanol with N,N-bis(triazolmethyl)propargylamine capped with bulky tert-butyl groups (Scheme 1). In a fluorogenic assay (Figure S2a, Supporting Information), 75 μM of CuSO4 premixed with BTTES at various ratios (between 2:1 to 6:1, [ligand]:[copper]) yielded > 50% cycloaddition product within 20 min (Figure S2b, Supporting Information). By contrast, the TBTA-Cu(I)-catalyzed reaction was significantly slower, leveling off with < 20% yield, and the reaction rate decreased gradually when more than one equivalent of the ligand was used (Figure S2c, Supporting Information).
Scheme 1.
The synthesis of BTTES.
Biocompatibility evaluation of the BTTES-Cu(I) complex
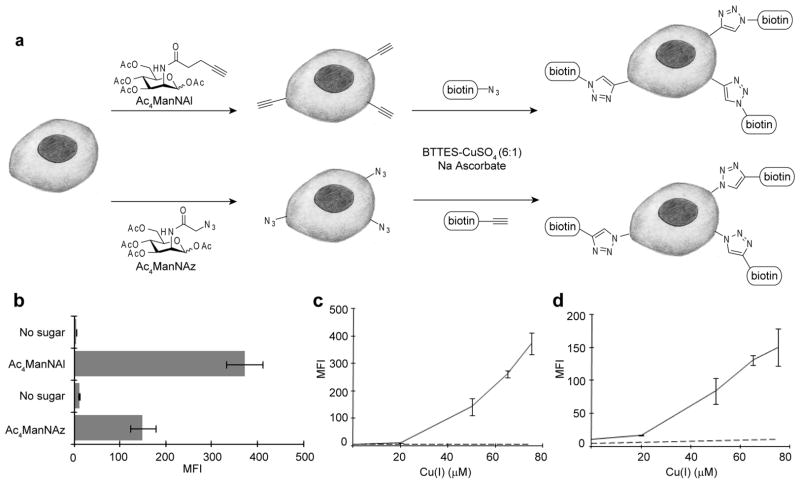
As the first step toward extending the use of CuAAC to living systems, we sought to evaluate the cytotoxicity of the new ligand-copper complex by 7-aminoactinomycin D (7-AAD) staining and flow cytometry analysis. Jurkat cells, a human T lymphocyte cell line, were incubated with 50 μM peracetylated N-(4-pentynoyl)mannosamine (Ac4ManNAl) or peracetylated N-azidoacetylmannosamine (Ac4ManNAz) to introduce the corresponding sialic acid (SiaNAl or SiaNAz) into their cell-surface glycoconjugates.32 Three days later, the cells were reacted with biotin-azide (or biotin-alkyne, 100 μM), sodium ascorbate (2.5 mM) and CuSO4 (25–75 μM) premixed with BTTES or in the absence of BTTES. The reactions were quenched after 5 min with bathocuproine sulphonate (BCS), a biocompatible copper chelator.33 Significant cell death was observed when the cells were treated with the in situ generated Cu(I) alone, and cell death increased along with increasing copper concentrations (Figure S3, Supporting Information). Interestingly, we observed that cells bearing alkynes on their surface underwent apoptosis much faster than their azide-bearing counterparts, presumably due to the formation of the reactive Cu(I)-acetylide on their surface. In stark contrast, when cells were treated with the BTTES-Cu(I) complexes, cell death decreased dramatically. In the presence of BTTES-Cu(I) 6:1 complex, cell death was completely suppressed to the same level as untreated cells. Similar results were obtained using Pro−5 Chinese Hamster Ovary (CHO) cells (Figure S4, Supporting Information).
BTTES-Cu(I) catalyzed azide-alkyne cycloaddition allows rapid detection of sialylated glycans on live cell surface
Next, we investigated the utility of the BTTES-Cu(I) catalyst for live cell labeling (the catalyst formulation: BTTES-CuSO4 ([ligand]:[Cu] = 6:1) complex and 2.5 mM sodium ascorbate). LNCaP cells, a human prostate adenocarcinoma cell line, bearing alkynyl (SiaNAl) or azido sialic acid (SiaNAz) residues within their cell-surface glycans were reacted with biotin-azide or biotin-alkyne (100 μM) catalyzed by various concentrations of the copper catalyst for 1–2.5 min at room temperature and then stained with Alexa Fluor 488-streptavidin and analyzed by flow cytometry. As shown in Fig. 2c and 2d, cells displaying alkyne or azide showed a dose-dependent increase in fluorescence upon treatment with the catalyst. Cells lacking alkynyl or azido residues only showed background labeling (Fig. 2b, Figure S5, Supporting Information). Similar results were observed for HEK 293T, a human embryonic kidney cell line, Jurkat and Pro−5 CHO cells (Figure S6–S8, Supporting Information). In all cell lines profiled, significantly higher fluorescence was detected in cells displaying alkyne on the cell surface than the corresponding azide-displaying counterparts, with robust labeling achieved within one min. This observation is consistent with our previous discovery that Ac4ManNAl metabolism is more efficient than Ac4ManNAz in these mammalian cell lines.25
Figure 2.
Detection of glycoconjugates on the surface of live cells via biocompatible CuAAC. (a) Schematic representation of metabolic labeling and detection of cell-surface sialic acids using Ac4ManNAl and Ac4ManNAz and BTTES-Cu(I)-catalyzed click chemistry. (b, c, d) Flow cytometry data of cell surface labeling experiments described in (a) using LNCaP cells. (b) Cells were treated with biotin-azide or biotin-alkyne (100 μM) in the presence of the BTTES-Cu(I) catalyst ([Cu] = 75μM) for 1 or 2.5 min respectively before probing with streptavidin-Alexa Fluor 488 conjugates. In all cases, cells cultured in the absence of sugar displayed mean fluorescence intensity (MFI, arbitrary units) values < 15. (c) Cells were labeled with biotin-azide (100 μM) for 1 min in the presence of 25–75 μM Cu(I). (d) Cells were labeled with biotin-alkyne (100 μM) for 2.5 min in the presence of 25–75 μM Cu(I). Error bars represent the standard deviation of three replicate experiments. Solid line, + Ac4ManNAl (c) or Ac4ManNAz (d); dashed line, no sugar.
To measure the kinetics of the BTTES-promoted CuAAC on the cell surface, we reacted SiaNAz-expressing Pro−5 CHO cells with biotin-alkyne and the BTTES-Cu(I) catalyst ([Cu] = 75 μM) for 2–17 min, followed by staining with Alexa Fluor 488-streptavidin. As quantified by flow cytometry analysis, the cell-associated Alexa Fluor 488 fluorescence reached a plateau around 14 min, indicating the completion of the ligation reaction between biotin-alkyne and the cell surface reagent-accessible SiaNAz (Fig. 3). Importantly, during the 17-min course of the reaction, no cell apoptosis was observed (Figure S9, Supporting Information).
Figure 3.
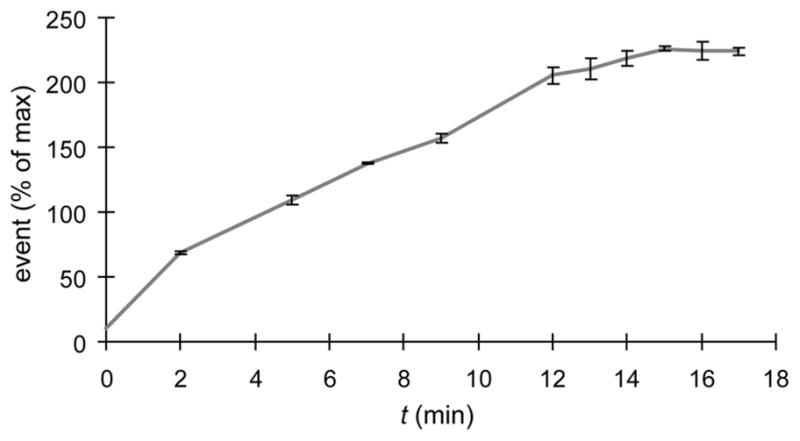
Analysis of CuAAC kinetics on the surface of CHO cells by flow cytometry. CHO cells were incubated for 3 days in untreated medium or medium supplemented with 50 μM Ac4ManNAz, followed by labeling with 100 μM biotin-alkyne in the presence of BTTES-Cu(I) catalyst ([Cu] = 75 μM). The reaction was quenched at various time points with BCS and probed with Alexa Fluor 488-streptavidin conjugates. Error bars represent the standard deviation of three replicate experiments.
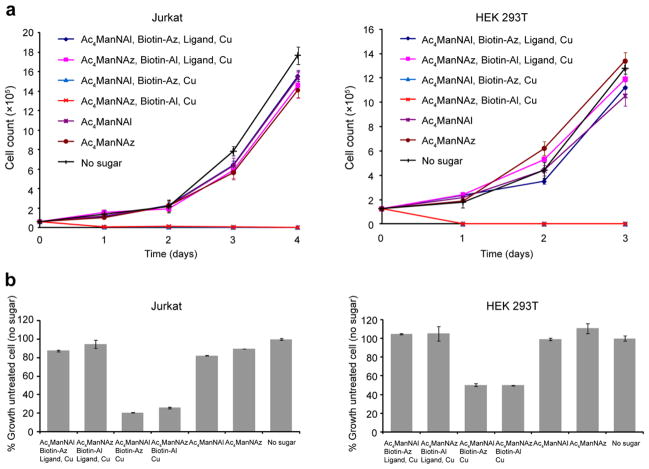
To evaluate if BTTES-Cu(I)-catalyzed click chemistry causes any long term perturbation to the treated cells, we labeled Jurkat and HEK 293T cells bearing SiaNAz or SiaNAl residues with biotin-alkyne or biotin-azide in the presence of the catalyst ([Cu] = 75 μM) for 3 min. The reactions were then quenched with BCS. The catalyst-treated cells were cultured for three to four days. In control experiments, we cultured untreated cells and cells treated with in situ generated Cu(I) in the absence of ligand. Viable cells, based on Trypan Blue assay, were counted each day. Cells treated with the BTTES-Cu(I) catalyst proliferated at similar rates as untreated cells (Fig. 4a). By contrast, all cells labeled in the absence of BTTES underwent lysis within 24 h of the labeling experiments. Similar results were obtained using an alamarBlue cell viability assay (Fig. 4b). Taken together, the discovery of BTTES creates a nontoxic and highly efficient Cu(I) catalyst for azide-alkyne cycloaddition, setting the stage to test its use for in vivo imaging of azide- and alkyne-tagged biomolecules.
Figure 4.
BTTES-Cu(I)-catalyzed click chemistry has no long term perturbation to Jurkat and HEK cells. (a) Cell growth curve after click chemistry treatment. (b) Cell viability measured using an alamarBlue assay (Invitrogen). Error bars represent the standard derivation for three replicates.
Microinjection of GDP-FucAl allows imaging of fucosylated glycans during the blastula and tissue segmentation periods of zebrafish embryogenesis
The development of a multicellular organism as it grows from a single zygote to a complex system of tissues is accompanied by complex changes of glycosylation on the cell surface. To test if the biocompatible copper catalyst could be extended to image the dynamic changes of glycans in vivo, we took advantage of the rapid embryonic development and optical clarity of the zebrafish embryo as a vertebrate model system.
The first class of glycans we chose to explore in developing zebrafish was fucosylated glycans. Specific terminal glycan fucosylation confers unique properties to cell surface glycoconjugates and is often regulated in cellular differentiation and embryogenesis.34 There is evidence that Lewis X (Lex), an α1,3 fucosylated trisaccharide, serves as a biomarker for murine pluripotent stem cells, in which it plays an important role in adhesion, guiding the migration of cells in the preimplantation embryo.34, 35 Studies have also shown that during development of the zebrafish nervous system, neuroepithelial cells require fucosylated glycans to guide the migration of vagus motor neuron progenitors in the developing hindbrain.36 Despite the physiological significance of these fucosylated glycans, there is currently no method for imaging them in live cells or organisms.37, 38
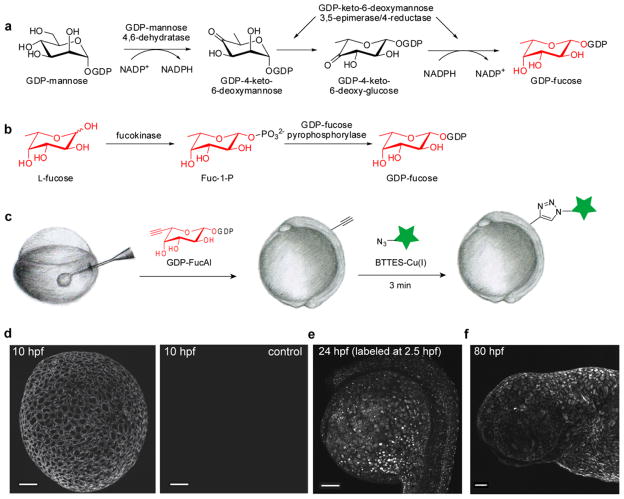
In nature, fucosylated glycans are synthesized by fucosyltranferases, enzymes that transfer the activated fucose from GDP-fucose (GDP-Fuc) to the acceptor substrates. GDP-Fuc is formed either through a de novo biosynthetic pathway or a salvage pathway.39 In the de novo pathway, GDP-mannose is converted into GDP-Fuc via GDP-mannose 4,6-dehydratase (GMD) and GDP-keto-6-deoxymannose 3,5-epimerase/4-reductase (also named FX) (Fig. 5a). By contrast, the salvage pathway begins with the uptake of free fucose, which is converted into fucose-1-phosphate by fucokinase, and thence to GDP-fucose by GDP-fucose pyrophosphorylase (Fig. 5b). In vertebrates, the de novo biosynthetic pathway usually accounts for 90% of the total GDP-Fuc production, whereas the remaining 10% of GDP-Fuc is contributed by the salvage pathway. Discovered by Wong and Bertozzi, the salvage pathway of cultured mammalian cells can be hijacked by unnatural fucose analogs. Their studies demonstrated that 6-azidofucose is cytotoxic, preventing its use in live cell imaging via the cyclooctyne-based copper-free click chemistry.40 However, FucAl, a 5-alkyne-modified fucose analogue, is well tolerated by several mammalian cell lines.37, 38
Figure 5.
In vivo imaging of fucosylated glycans during early zebrafish embryogenesis via BTTES-Cu(I)-catalyzed click chemistry. (a) GDP-Fuc de novo biosynthetic pathway. (b) GDP-Fuc salvage pathway. (c) Microinjection combined with the BTTES-Cu(I)-catalyzed click chemistry enables the labeling of fucosylated glycans in zebrafish embryos. Zebrafish embryos were microinjected with a single dose of GDP-FucAl and allowed to develop to 10 hpf (d), 2.5 hpf (e) and 80 hpf (f). The embryos were then reacted with Alexa Fluor 488-azide catalyzed by BTTES-Cu(I) and imaged using confocal microscopy. The maximum intensity z-projection fluorescence image for e was acquired at 24 hpf. Scale bar: 100 μm.
To image fucosylated glycans in zebrafish embryos, we conceived a strategy of bypassing the salvage pathway and using the downstream GDP-FucAl directly as a metabolic substrate. We synthesized GDP-FucAl chemoenzymatically41 and microinjected one-cell stage embryos with 20 pmol of GDP-FucAl (Fig. 5c). We dechorionated the embryos and reacted them at 10 hours post fertilization (hpf) with Alexa Fluor 488-azide (100 μM) in the presence of the BTTES-Cu(I) catalyst ([Cu] = 50 μM). Immediately following a 3-min reaction, we were able to observe robust labeling of the treated embryos, and only background fluorescence was detected for control embryos microinjected with GDP-Fuc (Fig. 5d, Figure S10, Supporting Information). We also reacted embryos at the blastula period (2.5 hpf) and achieved strong labeling of fucosides after a 3-min click reaction; this labeling remained visible at 24 hpf (Fig. 5e).
To examine the developmental period during which a single dose of GDP-FucAl could yield a detectable alkyne-dependent fluorescent signal, we extended our labeling time course to 80 hpf, at which point the zebrafish has completed most of its morphogenesis and reached the early larval stage. Specifically, we examined 80 hpf larvae, which are distinguishable by progressive development of the pectoral fins, the median fin fold, and the pigmentation pattern produced by the melanophores (Figure S11, Supporting Information). After labeling with Alexa Fluor 488-azide in the presence of the BTTES-Cu(I) catalyst, we could observe robust labeling for the zebrafish larva at 80 hpf (Fig. 5f), indicating that the exogenously supplied GDP-FucAl persisted and remained accessible for the labeling reaction. As revealed by examining individual z-planes obtained using confocal fluorescence microscopy, only the enveloping layer of the blastodisc was labeled by the Alexa fluorophore due to the low tissue penetration depth of the click chemistry reagents (Movie S1, Supporting Information).
Recently, Finn and coworkers reported that THPTA (3), a water-soluble member of the tris(triazolylmethyl)amine family, is a superior ligand to TBTA for cowpea mosaic virus bioconjugation.13 To evaluate if this ligand could be used for in vivo labeling and to compare its labeling efficiency to that of BTTES, we conducted labeling experiments using zebrafish embryos metabolically labeled with GDP-FucAl (n = 24). At regular intervals starting from the tissue segmentation period, we reacted the alkyne-bearing embryos with Alexa Fluor 488-azide in the presence of the THPTA-Cu(I) catalyst under the same conditions as specified for the BTTES-Cu(I) catalyst. In contrast to the BTTES-Cu(I)-mediated reaction, we could barely detect any fluorescence signal above the background with the THPTA-Cu(I) catalyst (Figure S13, Supporting Information). The exact mechanism of the different catalytic activities between these structurally similar ligands is a complex and unresolved issue42 that is currently under thorough investigation in our labs.
Conclusion
Imaging the glycome within a cellular environment is now possible using new tools from the emerging field of bioorthogonal click chemistry.43 With the discovery of the tris(triazolylmethyl)amine-based ligand BTTES, we have identified a practical and biocompatible click reaction that allows the imaging of glycan biosynthesis during zebrafish early development. Robust labeling, which requires one hour of reaction time when using the cyclooctyne-based copper-free click chemistry, was achieved within minutes.24 We observed little toxicity or developmental abnormalities resulting from treatments with the Cu(I) catalyst through four days post click reaction. Among the 120 embryos microinjected with various nucleotide sugar analogues and treated with the click reagents, less than twelve embryos exhibited minor developmental defects, i.e. impaired posterior body development characterized by a shorter anterior-posterior axis (Figure S11, Supporting Information). Comparable phenotypes were observed in 10% of embryos that were injected but were not treated with the click reagents (n ~ 1000), suggesting that the defects may be due to microinjection rather than the click reaction (Figure S12, Supporting Information).
The BTTES-Cu(I) catalyst, combined with yolk-cell microinjection, allows for the first time rapid imaging of fucosylated glycans in the enveloping layer of zebrafish embryos as early as 2.5 hours post fertilization. Since transplantation can be easily performed from labeled donor embryos to unlabeled hosts beginning with blastula stages (4 hpf) through the onset of gastrulation (5.3 hpf), exploiting this manipulation may allow us to follow the allocation of these glycans in specific lineages of the embryo during development.
Importantly, this work adds the alkynyl functionality to the bioorthogonal reagent repertoire of in vivo imaging. As the synthesis of BTTES can be easily scaled up to multigrams and many azide- and alkyne-functionalized reagents are commercially available, only genetic or metabolic manipulations are required to generate the tagged biomolecules in vivo for this new procedure of click modification. Thus, the chemical tools reported here can be directly applied for studying other sectors of the glycome and be generalized for dynamic in vivo imaging or profiling of other biomolecules, i.e. proteins, lipids and cofactors, many of which are already targets of the canonical click chemistry in vitro.14
Experimental section
Labeling of alkynyl or azido sialic acids on mammalian cell surface with biotinylated probes via CuAAC and analyzing by flow cytometry
Jurkat, CHO, HEK and LNCaP cells were incubated for 3 days in untreated medium or medium containing 50 μM Ac4ManNAz or Ac4ManNAl. The cells then were distributed into a 96-well round bottom tissue culture plate (0.4–0.5 million cells/well), pelleted (300 × g, 3 min), and washed 2 × with 200 μL of labeling buffer (PBS, pH 7.4, containing 1% FCS). Cells were then resuspended in 92 μL labeling buffer (stored at 4 °C), followed by addition of 100 μM biotin-alkyne (for azide-bearing cells) or biotin-azide (for alkyne-bearing cells), 2.5 mM sodium ascorbate and BTTES-CuSO4 complex (CuSO4 concentrations from 20 μM to 75 μM, BTTES-CuSO4 ratio from 5:1 to 7:1) in labeling buffer at room temperature. The reactions were quenched by adding 2 μL of copper chelator BCS (50 mM) at various time points. Then the cells were pelleted, washed 3 × with labeling buffer (4 °C), and resuspended in the same buffer containing 1 μg/mL streptavidin-Alexa Fluor 488 conjugate (Invitrogen). After a 30-min incubation on ice (in the dark), the cells were washed 3 × with 200 μL of cold labeling buffer, and then resuspended in 400 μL FACS buffer (Hank’s Balanced Salt Solution, pH = 7.4, 1% BCS, 2 μg/mL 7-AAD, 0.2% NaN3) for flow cytometry analysis.
AlamarBlue cell viability and cell growth assay
Jurkat and HEK 293T cell were cultured in medium or medium containing 50 μM Ac4ManNAz or Ac4ManNAl. After 72 hours all cells were harvested, with HEK cells harvested by trypsinization (Trypsin EDTA, 1 ×). Cells were washed twice with 10 mL PBS (1% FBS) and resuspended in the same buffer to a final concentration of 1 M viable cells per 100 μL. Cells cultured with Ac4ManNAz were reacted with or without biotin-alkyne and cells cultured with Ac4ManNAl were reacted with or without biotin-azide in a 100 μL reaction containing 75 μM CuSO4, 450 μM BTTES, and 2.5 mM freshly prepared sodium ascorbate at room temperature. As a positive apoptosis control to determine the copper toxicity in the absence of the ligand, cells (cultured in the absence of the unnatural sugars) were reacted with 75 μM CuSO4 and 2.5 mM sodium ascorbate. After 3 min all reactions were quenched with BCS (1 mM). Untreated cells cultured in medium or medium containing Ac4ManNAz or Ac4ManNAl were included as negative controls. Following the reactions cells were washed twice with 0.5 mL PBS (1% FBS) and once with 0.5 mL complete media. Cells were resuspended in 1 mL complete media and total cell numbers were determined. In most cases, ~75% cells were recovered. All cells were then normalized to the lowest concentration. Cell viability was determined by AlamarBlue colorimetric assay (Invitrogen). Briefly, treated or untreated Jurkat were seeded at 80,000 cells/100 μL complete media and HEK 293T cells were seeded at 10,000 cells/100 μL complete media. AlamarBlue stock solution was diluted to 10 % and incubated with the Jurkat cells and HEK 293T cells for 6 and 20 h respectively, at which time the percentage of the reduced form of resorufin (alamarBlue) reached ~50% (the percentage of the reduced form of resorufin is proportional to cell viability). The absorbance of resorufin was measured at 540 (Red) and 600 (Ox) nm. To determine cell growth following the reaction, Jurkat and HEK 293T cell were seeded at 60,000 cells/mL and 125,000 cells/mL, respectively. The viable cells were counted every 24 hours for 3–4 days using Trypan blue dye exclusion method.
Metabolic labeling of zebrafish embryos by microinjection with GDP-FucAl and detection by the BTTES-Cu(I) catalyzed click chemistry
Zebrafish embryos at the one-cell or two-cell stage were microinjected in the yolk with 1 nL of a 20 mM solution of GDP-FucAl (or GDP-fucose) and either rhodamine-dextran (5% w/v) or phenol red (0.1% w/v) in 0.2 M KCl as a tracer. The embryos were allowed to develop to 5 hpf, at which point they were manually dechorionated. The dechorionated embryos were cultured in E3 embryo medium at 28 °C for an additional 3 h before transferring to 1% agarose-coated 96-well plates containing 92 μL embryo medium using a fire-polished wide-bore Pasteur pipette. Alexa Fluor-488 azide (100 μM from a 1.75 mM stock in H2O) was added to each well, followed by BTTES-CuSO4 6:1 complex ([Cu] = 50 μM from 2.5 mM stock). The solutions were gently shaken, and freshly prepared sodium ascorbate (2.5 mM from 100 mM stock in embryo medium) was added to initiate the click reactions. After 3 min, the reaction was quenched with BCS (1 mM from 50 mM stock in water) and diluted immediately with 100 μL embryo medium. The treated embryos were washed 2 × with 15 mL embryo medium and were anesthetized with 0.2% (w/v) Tricaine in embryo medium. After mounting on a slide or 50 mm glass bottom dishes (Matek) in 1.2% ultra-low melt agarose in E3 medium, the embryos were ready for imaging by confocal microscopy.
Supplementary Material
Acknowledgments
This work was partially supported by the National Institutes of Health (GM080585, P.W.), the Mizutani Foundation for Glycoscience (P.W.) and startup funds from Albert Einstein College of Medicine. C.B. is supported by the NIH training grant GMT3207491. Y.L. and part of the ligand synthesis were supported by the Office of Science, Office of Basic Energy Sciences, of the U. S. Department of Energy under contract No. DE-AC02-05 CH11231. We thank Profs. K. Barry Sharpless and Valery Fokin for providing six azide and alkyne precursors for initial ligand screening, Prof. Pamela Stanley for providing Pro−5 CHO cells, Spartak Kalinin for zebrafish care and Liana M. Klivansky for help on chemical synthesis. P.W. thanks Prof. Carolyn Bertozzi for insightful discussion of copper-free click chemistry, Prof. Valery Fokin for discussion of the mechanism of CuAAC, Prof. Erik Snapp for assistance in fluorescence image acquiring and processing, and Profs. Carolyn Bertozzi, Vern Schramm and Dr. Penny Drake for critical comments on the manuscript.
Footnotes
Supporting Information Available: Synthetic procedures, spectral data for BTTES, protocol for kinetic measurement, experimental procedures for flow cytometry, fluorescent microscopy, zebrafish husbandry and strains. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Kolb HC, Finn MG, Sharpless KB. Angew Chem Int Ed. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 2.Baskin JM, Bertozzi CR. Qsar Comb Sci. 2007;26:1211–1219. [Google Scholar]
- 3.Wang L, Schultz PG. Angew Chem Int Ed. 2004;44:34–66. doi: 10.1002/anie.200460627. [DOI] [PubMed] [Google Scholar]
- 4.Laughlin ST, Agard NJ, Baskin JM, Carrico IS, Chang PV, Ganguli AS, Hangauer MJ, Lo A, Prescher JA, Bertozzi CR. Methods Enzymol. 2006;415:230–250. doi: 10.1016/S0076-6879(06)15015-6. [DOI] [PubMed] [Google Scholar]
- 5.Dieterich DC, Lee JJ, Link AJ, Graumann J, Tirrell DA, Schuman EM. Nat Protc. 2007;2:532–540. doi: 10.1038/nprot.2007.52. [DOI] [PubMed] [Google Scholar]
- 6.Prescher JA, Bertozzi CR. Nat Chem Biol. 2005;1:13–21. doi: 10.1038/nchembio0605-13. [DOI] [PubMed] [Google Scholar]
- 7.Sletten EM, Bertozzi CR. Angew Chem Int Ed. 2009;48:6974–6998. doi: 10.1002/anie.200900942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew Chem Int Ed. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 9.Tornoe CW, Christensen C, Meldal M. J Org Chem. 2002;67:3057–3064. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 10.Wu P, Fokin VV. Aldrich Acta. 2007;40:7–17. [Google Scholar]
- 11.Meldal M, Tornoe CW. Chem Rev. 2008;108:2952–3015. doi: 10.1021/cr0783479. [DOI] [PubMed] [Google Scholar]
- 12.Chan TR, Hilgraf R, Sharpless KB, Fokin VV. Org Lett. 2004;6:2853–2855. doi: 10.1021/ol0493094. [DOI] [PubMed] [Google Scholar]
- 13.Hong V, Presolski SI, Ma C, Finn MG. Angew Chem Int Ed. 2009;48:9879–9883. doi: 10.1002/anie.200905087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolb HC, Sharpless KB. Drug Discov Today. 2003;8:1128–1137. doi: 10.1016/s1359-6446(03)02933-7. [DOI] [PubMed] [Google Scholar]
- 15.Lutz JF. Angew Chem Int Ed. 2007;46:1018–1025. doi: 10.1002/anie.200604050. [DOI] [PubMed] [Google Scholar]
- 16.Gaetke LM, Chow CK. Toxicology. 2003;189:147–163. doi: 10.1016/s0300-483x(03)00159-8. [DOI] [PubMed] [Google Scholar]
- 17.Link AJ, Vink MK, Tirrell DA. J Am Chem Soc. 2004;126:10598–10602. doi: 10.1021/ja047629c. [DOI] [PubMed] [Google Scholar]
- 18.Agard NJ, Prescher JA, Bertozzi CR. J Am Chem Soc. 2004;126:15046–15047. doi: 10.1021/ja044996f. [DOI] [PubMed] [Google Scholar]
- 19.Jewett JC, Bertozzi CR. Chem Soc Rev. 2010;39:1272–1279. doi: 10.1039/b901970g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baskin JM, Prescher JA, Laughlin ST, Agard NJ, Chang PV, Miller IA, Lo A, Codelli JA, Bertozzi CR. Proc Natl Acad Sci U S A. 2007;104:16793–16797. doi: 10.1073/pnas.0707090104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jewett JC, Sletten EM, Bertozzi CR. J Am Chem Soc. 2010;132:3688–3690. doi: 10.1021/ja100014q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laughlin ST, Bertozzi CR. ACS Chemical Biology. 2009;4:1068–1072. doi: 10.1021/cb900254y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laughlin ST, Baskin JM, Amacher SL, Bertozzi CR. Science. 2008;320:664–667. doi: 10.1126/science.1155106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baskin JM, Dehnert KW, Laughlin ST, Amacher SL, Bertozzi CR. Proc Natl Acad Sci U S A. 2010;107:10360–10365. doi: 10.1073/pnas.0912081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang PV, Prescher JA, Sletten EM, Baskin JM, Miller IA, Agard NJ, Lo A, Bertozzi CR. Proc Natl Acad Sci U S A. 2010;107:1821–1826. doi: 10.1073/pnas.0911116107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poloukhtine AA, Mbua NE, Wolfert MA, Boons GJ, Popik VV. J Am Chem Soc. 2009;131:15769–15776. doi: 10.1021/ja9054096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaim W, Rall J. Angew Chem Int Ed. 1996;35:43–60. [Google Scholar]
- 28.Solomon EI, Tuczek F, Root DE, Brown CA. Chem Rev. 1994;94:827–856. [Google Scholar]
- 29.Magnus KA, Tonthat H, Carpenter JE. Chem Rev. 1994;94:727–735. [Google Scholar]
- 30.Solomon EI, Chen P, Metz M, Lee SK, Palmer AE. Angew Chem Int Ed. 2001;40:4570–4590. doi: 10.1002/1521-3773(20011217)40:24<4570::aid-anie4570>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 31.Pierre J-L, Chautemps P, Refaif S, Beguin C, El Marzouki A, Serratrice G, Saint-Aman E, Rey P. J Am Chem Soc. 1995;117:1965–1973. [Google Scholar]
- 32.Chang PV, Chen X, Smyrniotis C, Xenakis A, Hu T, Bertozzi CR, Wu P. Angew Chem Int Ed. 2009;48:4030–4033. doi: 10.1002/anie.200806319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohindru A, Fisher JM, Rabinovitz M. Nature. 1983;303:64–65. doi: 10.1038/303064a0. [DOI] [PubMed] [Google Scholar]
- 34.Becker DJ, Lowe JB. Glycobiology. 2003;13:41R–53R. doi: 10.1093/glycob/cwg054. [DOI] [PubMed] [Google Scholar]
- 35.Saito S, Liu B, Yokoyama K. Hum Cell. 2004;17:107–115. doi: 10.1111/j.1749-0774.2004.tb00026.x. [DOI] [PubMed] [Google Scholar]
- 36.Ohata S, Kinoshita S, Aoki R, Tanaka H, Wada H, Tsuruoka-Kinoshita S, Tsuboi T, Watabe S, Okamoto H. Development. 2009;136:1653–1663. doi: 10.1242/dev.033290. [DOI] [PubMed] [Google Scholar]
- 37.Hsu TL, Hanson SR, Kishikawa K, Wang SK, Sawa M, Wong CH. Proc Natl Acad Sci U S A. 2007;104:2614–2619. doi: 10.1073/pnas.0611307104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sawa M, Hsu TL, Itoh T, Sugiyama M, Hanson SR, Vogt PK, Wong CH. Proc Natl Acad Sci U S A. 2006;103:12371–12376. doi: 10.1073/pnas.0605418103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma B, Simala-Grant JL, Taylor DE. Glycobiology. 2006;16:158R–184R. doi: 10.1093/glycob/cwl040. [DOI] [PubMed] [Google Scholar]
- 40.Rabuka D, Hubbard SC, Laughlin ST, Argade SP, Bertozzi CR. J Am Chem Soc. 2006;128:12078–12079. doi: 10.1021/ja064619y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang W, Hu T, Frantom PA, Zheng T, Gerwe B, Del Amo DS, Garret S, Seidel RD, 3rd, Wu P. Proc Natl Acad Sci U S A. 2009;106:16096–16101. doi: 10.1073/pnas.0908248106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Presolski SI, Hong V, Cho SH, Finn MG. J Am Chem Soc. 2010 doi: 10.1021/ja105743g. ASAP article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laughlin ST, Bertozzi CR. Proc Natl Acad Sci U S A. 2009;106:12–17. doi: 10.1073/pnas.0811481106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.