Abstract
Background
Peripheral blood stem cells (PBSC) have become the preferred stem cell source for autologous hematopoietic transplantation. A critical aspect of this treatment modality is cryopreservation of the stem cell products, which permits temporal separation of the PBSC mobilization/collection phase from the subsequent high-dose therapy. While controlled rate freezing and liquid nitrogen storage have become “routine” practice in many cell processing facilities, there is clearly room for improvement, as current cryopreservation media formulations still result in significant loss and damage to the stem/progenitor cell populations essential for engraftment, and can also expose the patients to relatively undefined serum components and larger volumes of DMSO that can contribute to the morbidity and mortality of the transplant therapy.
Methods
This study compared cryopreservation of PBSC in a novel intracellular-like, fully defined, serum- and protein-free preservation solution, CryoStor™ (BioLife Solutions, Inc.), with a standard formulation used by the Fred Hutchinson Cancer Research Center (FHCRC). Briefly, human PBSC apheresis specimens were collected and 5 × 107 cells/1 ml sample vial were prepared for cryopreservation in the following solutions: 1) FHCRC standard – Normosol-R, 5% HSA, 10% DMSO, and 2) CryoStor™ CS10 (final diluted conc. of 5% DMSO). A standard controlled-rate freezing program was employed, and frozen vials were stored in the vapor phase of a liquid nitrogen freezer for a minimum of one week. Vials were then thawed and evaluated for TNC, Viability, CD34, and granulocytes by flow cytometry, along with colony-forming activity in methylcellulose.
Results
The PBSC samples frozen in CryoStor™ CS10 yielded significantly improved post-thaw recoveries for total viable CD34+, CFU, and viable granulocytes. Specifically, relative to the FHCRC standard formulation, cryopreservation with CS10 resulted in an average 1.8 fold increased recovery of viable CD34+ cells (P = 0.005), a 1.5 fold increase in CFU-GM numbers (P = 0.030), and a 2.3 fold increase in granulocyte recovery (P = 0.045).
Discussion
This study indicates that use of CryoStor™ for cryopreservation can yield significantly improved recovery and in vitro functionality of the stem/progenitor cells in PBSC products. In addition, it is important to note that these improved recoveries were obtained while also not introducing any extra serum or serum-derived proteins, and reducing the final concentration/volume of DMSO by half. Further in vitro and in vivo studies are clearly necessary, however these findings imply use of CryoStor™ for cryopreservation might ultimately result in improved engraftment for those patients with lower content of CD34+ cells in their PBSC collections, along with reducing the requirement for additional apheresis collections, and decreasing the risk of adverse infusion reactions associated with higher exposure to DMSO.
Keywords: Cryopreservation, CryoStor, CD34+, CFU-GM, PBSC
Introduction
High-dose therapy, with autologous stem cell support, is recognized as the standard of care for a variety of malignant diseases, including multiple myeloma, lymphoma, and some types of solid tumors [1], and peripheral blood stem cells (PBSC) have become the preferred source for hematopoietic reconstitution in this setting [2–6]. Furthermore, published studies have shown that, following stem cell transplantation, the cell subset most closely correlated with the rate of neutrophil and platelet engraftment are the CD34+ cells [4–8]. In addition to CD34+ progenitors, other mononuclear cells (MNC) including NK cells, CD8+ T cells have been implicated in engraftment [9–10]. Currently, acceptable stem/progenitor cell numbers range from 2–5 × 106 CD34+ cells/kg patient body weight, which typically means that multiple apheresis procedures are required to achieve these minimum cell dose goals. Cryopreservation of the PBSC products is also required, in order to retain the functional capacity of the stem/progenitor cells during the lengthy time interval between the apheresis collection and treatment with the high-dose conditioning regimen.
PBSC products are routinely cryopreserved in a saline solution mixture such as Normosol-R or Plasma-Lyte, supplemented with a protein source such as albumin or autologous plasma, and cryoprotectants such as Dimethyl Sulfoxide (DMSO) and/or dextran-40 [1, 9]. The products are subjected to controlled rate freezing and then stored at very cold temperatures (below −150°C) until the units are required; at which time the products are thawed rapidly at 37°C, sometimes washed to remove the cryoprotectant DMSO, and administered to the patient [4–6, 11]. Unfortunately, these traditional cryopreservation media formulations and freezing methods introduce several distinct problems. First and foremost, the overall cryopreservation process itself can damage many cell types present in the apheresis collections, including some of the CD34+ stem/progenitor cells essential for rapid engraftment and hematopoietic reconstitution. Second, the relatively high amounts of DMSO (typically final concentrations of 6–10%) and large volumes used have been associated with serious adverse reactions after infusion of these cryopreserved products, contributing to the overall morbidity and mortality of the transplant regimens [4–6, 12].
Cryopreservation exposes cells to an abnormal condition that results in significant metabolic changes in response to the low temperature excursions [13]. In addition to potential damage caused by ice crystal formation during the freezing process, an interval of hypothermic insult is also experienced. The benefits of low temperature-induced reduction in cellular metabolism is offset by other cellular changes such as reduced membrane fluidity, a reduction in ionic gradient control by ATP-driven pumps, generation of enzymatic intermediates, and an increase in free radicals [13–15]. All of these secondary changes are stresses that can lead to the induction of apoptotic and necrotic responses as a result of the low temperature exposure [14–15]. By formulating solutions that better balance the cell environment at these lower temperatures, innovative products have been developed that yield improved cold temperature preservation. In contrast to extracellular-like solutions (such as culture media or normal saline) that have historically been the basis for hypothermic storage and cryopreservation solutions, the CryoStor™ platform is an intracellular-like formulation that better maintains the ionic balance of cells specifically at hypothermic and freezing temperatures. It has the added benefit of also being both serum- and protein- free [14–16]. Use of CryoStor™ solutions has been reported to consistently improve yield, enhance cell survival, and allow for more rapid recovery by reducing cryopreservation-induced cell stress and damage [11, 14–17].
The aims of this study were to establish whether an intracellular-like preservation solution, CryoStor™, could be used to cryopreserve PBSC specimens, and to determine whether cryopreservation with CryoStor™ could improve the in vitro yield and functionality of the stem/progenitor cells when compared to the FHCRC standard, clinically applicable freezing media. The data indicate that freezing in CryoStor™ results in significantly improved recovery of total viable CD34+ stem/progenitor cells, colony-forming cells, and granulocytes following cryopreservation and thaw.
Materials and Methods
PBSC Sample Collection
Under IRB approved research protocols, de-identified specimens from PBSC apheresis collections were obtained from both autologous patient and normal allogeneic donors. Aliquots of 50 × 106 cells were removed from each sample (standard FHCRC cell volume for cryopreservation in cryovials), centrifuged, and resuspended using Normosol-R (Hospira, Inc., Lake Forest, IL, USA) to a final volume adjusted to 0.5ml.
Cryopreservation
For the FHCRC controls, samples were mixed with an equal volume of 2X freeze media consisting of Normosol-R (Baxter Inc., Northfield, IL, USA), 10% Human Serum Albumin (Talecris Biotheraputics, Inc., Research Triangle Park, NC, USA), and 20% DMSO (Edwards Lifesciences Cryoserv, Ben Venue Laboratories, Inc., Bedford, Oh, USA). Cryostor™ CS5 and CS10 (BioLife Solutions, Inc., Bothell, WA) sample vials were prepared in a similar manner, added directly to the PBSC/Normosol-R solution as a 1:1 dilution. The vials were frozen using a Cryomed 1010 controlled rate freezer. Briefly, samples were cooled at 1°C/minute until heat of fusion, then 1C/minute until −40°C, and then 10°C/minute until −90°C. Once frozen the vials were placed in cardboard boxes and stored in racks in the vapor phase of a liquid nitrogen (LN2) freezer. A total of 10 samples were investigated for each of the solutions as an initial feasibility study. One sample was removed from the data analysis due to sample contamination.
Thawing
Samples were thawed according to standard FHCRC procedures. Briefly, after a minimum time of one week storage, vials were removed from the freezer and immediately placed in a 37°C water bath until ice crystals were just disappearing. The contents of each sample vial were transferred to a 50 ml centrifuge tube and serially diluted 1:2 over a 10–15 minute timespan with 0.2μM filtered PBS with 1% FBS to a final volume of 32ml. These were then centrifuged at 1500 RCF for 10 minutes at room temperature. Excess supernatant was removed and a final volume was determined.
Testing
Immediately after thawing, washing and resuspension, the samples were analyzed for total nucleated cell count (TNC), viability, % CD34+, burst forming units (BFU), and colony-forming activity (CFU). Cell counts were obtained on a Coulter ZM cell counter. CD34+ analysis and viability were determined using flow cytometry as previously described (4). Briefly, specimens were stained with CD14 FITC and CD34 PE (BD, Biosciences, CA, USA) for 25 minutes on ice, washed once and incubated for 5 minutes with 15ul 7AAD (Sigma-Aldrich), and flow cytometric analysis was performed using a BD FACSCalibur (BD Biosciences, CA, USA). CD14 was used in this case as an exclusionary stain for CD34+ cell determination. Granulocyte percentages were obtained from flow cytometry using the forward versus side scatter histograms. The histogram differentiates cell size and shape allowing various cell populations, including granulocytes, to be determined.
Colony-forming assays (CFA) were performed by plating Methocult GF H4434 (StemCell Technologies, Vancouver, B.C., Canada) containing cell concentrations of 1×104 cells/ml and 3×104 cells/ml into 3ml 35×10 culture dishes (Nunc Rochester, NY, USA). Two concentrations were used to ensure that there were sufficient, but not overly dense, colonies to count following 14 days incubation. As a baseline control, each sample was plated for CFA prior to cryopreservation. Post-thaw CFA results were expressed as a percentage of baseline; comparisons of these data were then made between experimental treatment groups. Plates were incubated in humidified incubator at 37°C with 5% CO2 for 14 days, after which time colonies > 50 cells were tallied and recorded. A total of 4 plates at each concentration were set up and the average number of colonies was determined.
Statistical Analysis
This study was designed to address whether improvements in post-thaw outcomes could be realized using an alternative cryopreservation solution. Current practice when assessing post-thaw outcome is to compare pre-freeze results (as baseline) to post-thaw results; in this scenario, the ideal is that pre-freeze will be as close to baseline as possible, thus illustrating minimal loss attributed to the freeze-thaw. To address whether there were significant differences between cryopreservation groups, post-thaw data were expressed as a percent of pre-freeze, and a two-tailed, paired samples t-test was applied assuming an alpha-level of 0.050. Data were initially analyzed using Microsoft Excel (Microsoft, WA) and later verified using SPSS v17.0 (SPSS, Inc, IL).
Results
Initial studies were performed to compare CS5 and CS10 with the FHCRC standard method as a means to determine which CryoStor solution would be more effective at cryopreserving PBSC’s. Samples were collected as described in the methods and cryopreserved samples were compared on the basis of TNC, BFU, CD34+ cells, and CFU. A total of 9 samples were collected and evaluated for this comparison.
Based on the initial investigation, more favorable results were obtained with the CS10 when compared to the CS5 in each of the tested parameters post-thaw (Figure 1). On average, TNC recovery was 1.2 fold greater, while BFU, CD34, and CFU recoveries were 1.35 fold greater using the CS10 as compared with the CS5 (Figure 1). Given the initial results, CS10 was used for the remainder of the study and compared to the FHCRC standard solution for the cryopreservation of PBSC’s.
Figure 1.

Cryopreservation of PBSC’s: A comparison of CryoStor CS5 to CS10. Following cryopreservation of PBSC’s, TNC recovery, BFU, viable CD34+, and CFU assays were performed to determine cryopreservation efficacy. The average fold difference of overall improvement afforded by the CS10 was determined by comparison to CS5 recovery.
CryoStor significantly improves viable CD34+ cell recovery
The use of CS10 to cryopreserve PBSC samples resulted in improved post-thaw recovery of viable CD34+ cells in all 9 samples when compared with the FHCRC standard (Figure 2). On average, there was a 1.68 fold increase over the FHCRC standard with a significant p value = 0.005 (Figure 5; Table 1).
Figure 2.

Post-thaw recovery of viable CD34+ progenitor cells following cryopreservation with CryoStor or FHCRC standard. Viable CD34+ cell counts were performed as described and paired samples from each patient were plotted as a fold comparison to the FHCRC standard. Individual paired samples are shown.
Figure 5.
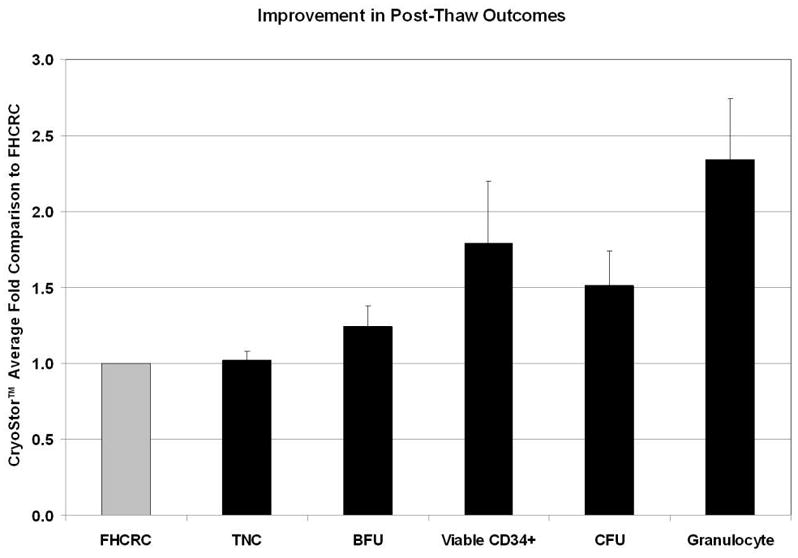
Cryopreservation of PBSC’s: A comparison of CryoStor to FHCRC standard method. Following cryopreservation of PBSC’s, TNC recovery, BFU, viable CD34+, CFU, and granulocyte recovery assays were performed to determine cryopreservation efficacy. The average fold difference of overall improvement afforded by the CS10 was determined by comparison to the FHCRC standard recovery.
Table 1.
Post-Thaw Statistical Analysis: Average fold improvement of CryoStor™ compared to FHCRC standard (Paired samples T-Test)
| Total Nucleated Cell | 1.02 ± 0.06 | P = 0.954 |
| Total CD34+ | 1.47 ± 0.21 | P = 0.005 |
| Viable CD34+ | 1.68 ± 0.32 | P = 0.005 |
| CFU | 1.51 ± 0.23 | P = 0.030 |
| BFU-e | 1.24 ± 0.14 | P = 0.116 |
| Granulocyte | 2.34 ± 0.55 | P = 0.045 |
CryoStor significantly improves post-thaw CFU recovery
For 7 of the 9 samples, a greater number of CFU colonies were observed post-thaw for the samples cryopreserved in CS10 (Figure 3). CS10 yielded an average 1.5 fold increase in CFU compared with the FHCRC standard method and was statistically significant with a p value = 0.030 (Figure 5; Table 1).
Figure 3.
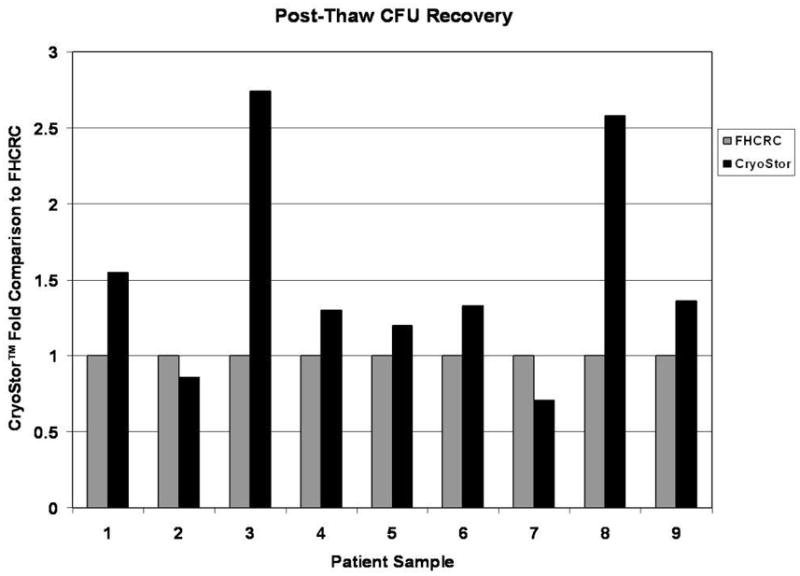
Post-thaw colony forming units following cryopreservation with CryoStor or FHCRC standard. CFU counts were performed as described and paired samples from each patient were plotted as a fold comparison to the FHCRC standard. Individual paired samples are shown.
CryoStor significantly improves post-thaw recovery of granulocytes
Following cryopreservation of PBSC’s the use of CS10 also significantly improved the overall recovery of granulocytes in 8 of the 9 samples (Figure 4). Granulocyte recovery was an average of 2.3 fold higher with CS10 compared with the FHCRC standard and this was statistically significant with a p value = 0.045 (Figure 5; Table 1).
Figure 4.
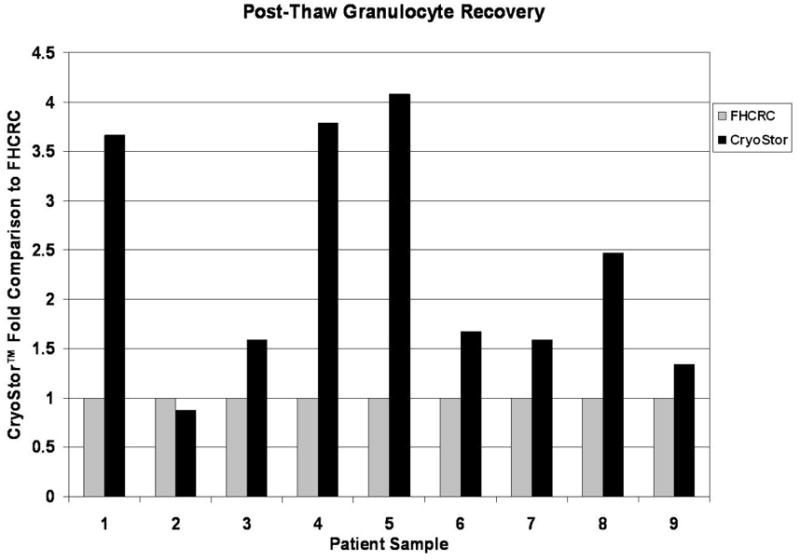
Post-thaw recovery of granulocytes following cryopreservation with CryoStor or FHCRC standard. Granulocyte recovery was performed as described and paired samples from each patient were plotted as a fold comparison to the FHCRC standard. Individual paired samples are shown.
Average fold improvement using CS10 compared with FHCRC standard
Results were determined from 9 PBSC units and displayed as a fold comparison of FHCRC standard to CS10 for TNC, total CD34+, viable CD34+, CFU and BFU, and granulocyte recovery. The average fold increase observed for each of the parameters is displayed in Figure 4. TNC recovery was essentially identical for both cryopreservation solutions. CS10 resulted in a 1.24 fold increase in BFU cell recovery, but the difference was not found to be statistically significant (p value = 0.1195; Figure 5; Table 1). All other parameters including viable CD34+, CFU-GM, and viable granulocyte recovery were significantly improved by using CS10.
Discussion
PBSC have become increasingly popular as a source of hematopoietic stem cells for transplant in the treatment of hematologic and nonhematologic disease, and cryopreservation of those cells is a critical methodology required for nearly all autologous and many allogeneic hematopoietic stem cell transplantation protocols. Numerous reports have demonstrated that the most important predictor of engraftment following autologous stem cell transplantation is the CD34+ cell dose [2–7, 10]. Thus, one primary objective of PBSC apheresis is to collect an adequate number of CD34+ cells necessary to support rapid hematopoietic reconstitution after the high-dose treatment, with acceptable doses ranging from 2–5 × 106 cells/kg [5–6]. Multiple apheresis procedures are generally necessary to achieve this targeted CD34+ cell requirement.
Typical cryopreservation/thawing techniques result in significant cell loss and lower viability/functionality of the critical CD34+ cells needed for rapid engraftment [10, 12]. Currently, no single cryopreservation solution is universally utilized; significant variations exist between different transplant centers [1]. These differences in media formulations likely contribute to variable levels of viability post-thaw, and significant total cell and, most importantly, viable CD34+ cell losses. [4–6, 9–11]. Thus, new approaches for PBSC cryopreservation need to be explored, with an emphasis on improving stem/progenitor cell recovery and functionality. In this report the use of a novel intracellular-like cryopreservation solution (CryoStor™) was compared to the standard FHCRC formulation containing 5% HSA and 10% DMSO in Normosol-R for cryopreservation of PBSC. The results demonstrate that CryoStor™ CS10, a protein-and serum-free solution at a final DMSO concentration of 5%, gave significantly higher post-thaw recoveries of viable CD34+ cells, CFU-GM, and granulocytes compared with the FHCRC standard applied solution. While CryoStor™ has been shown to enhance cryopreservation and post-thaw recoveries in a variety of cell and tissue types including but not limited to cord blood, dendritic cells, PBMC, liver cells, neuronal cells, stem cells, T-cells, and engineered tissues, this is the first report to demonstrate improved efficacy with PBSC. Furthermore, it is important to note that the CryoStor was diluted 1:1 with the cell suspension (to match the standard FHCRC approach), thereby reducing by half the critical components within the carrier solution along with the DMSO.
The process of cryopreservation involves a compilation of perturbations and biological stresses that can damage cells during the entire cryopreservation procedure (pre-freeze cooling, controlled rate freezing, maintaining cells in the frozen state, thawing and rewarming). These stresses include osmotic/ionic imbalances, metabolic imbalances, free radical accumulation, dehydration, membrane damage, extra- and intra-cellular ice formation; each of these can lead to decreased post-preservation outcomes [13–15]. Historically, cryopreservation formulations, consisting of a vehicle solution, serum, additional proteins, and a cryoprotectant (ex. DMSO), have focused just on physical parameters. These formulations do not take into account the multiplicity of cellular changes and generally rely strictly upon the cryoprotectant (DMSO, ethylene glycol, glycerol) as a means to protect cells from intracellular-ice formation during the preservation process [14]. The intracellular-like (water-based and balanced for hypothermic temperatures) CryoStor formulations were developed to optimize preservation by more appropriately maintaining a homeostatic state of cells at low temperatures and minimizing damage and stress without the addition of serum or animal proteins. The intracellular-like formulations allow for increased cell recovery and function while decreasing toxic DMSO concentrations [14, 16]. Results observed in previous studies demonstrate the collective efficacy of the CryoStor solutions in multiple cell and tissue types [11, 16–17]. In the present study, improved cell recoveries were obtained with a 5% final concentration of DMSO which represents a two-fold reduction compared to the FHCRC 10% DMSO standard cryopreservation medium. It should be noted that the objective of this study was to investigate and compare the efficacy of the CryoStor solutions to the FHCRC standard cryopreservation medium containing 10% DMSO. The results of this study support the conclusions of others that a lower concentration of DMSO (5%) may be as effective as 10% for preserving cells [18]. Additional studies would be required to provide a direct comparison to a FHCRC cryopreservation medium containing 5% DMSO. Interestingly though, the use of CryoStor with 5% DMSO (CryoStor™ CS5) diluted to a 2.5% final DMSO concentration was not as effective as the CS10 as shown in Figure 1, but did yield comparable viable CD34+ cells and CFU to the 10% DMSO standard (data not shown). Depending on the users requirements (DMSO conc. vs. overall recovery), the option to use lower DMSO concentrations may be important. The results reported in this study are consistent with previously published results on cord blood cryopreservation [11].
Additional analysis of the data revealed a considerable increase in the total number of granulocytes recovered post-thaw as described in Figures 4 and 5. The results are preliminary, but are important to note given the impact and utility of granulocytes to engraftment [19–20]. Granulocytes are known to be highly susceptible to osmotic stress and are traditionally difficult to cryopreserve [21–22]. The osmotic stress that occurs with standard addition and removal of cryopreservation solutions coupled with the exposure to freezing temperatures can result in a high degree of volume changes to the cell (22). The novel design of the CryoStor™ solution and the ability to better maintain ionic balance and reduce osmotic cell stress during the cryopreservation process may account for the improved preservation and post-thaw outcomes compared with the FHCRC standard methods. While exciting, additional studies will need to be conducted to determine the mechanisms that contribute to the enhanced cell recovery and, if the better recovery is consistent with improved function and post-thaw engraftment.
Cryopreservation solutions are generally classified as excipients within a cell therapy process. Often, what is utilized as the cryopreservation cocktail is a vehicle solution (i.e. saline or plasma) with a cryoprotectant (i.e. DMSO), each of which may have regulatory approval for separate uses, but is still an off-label use of each component for a non-approved application (cryopreservation). In practice, this “home-brew” cryopreservation cocktail is rarely subjected to final testing for sterility, endotoxin, or efficacy as other reagents might be required. As such, the incorporation of a pre-formulated complete fully-defined serum-free and protein-free cryopreservation solution, manufactured to cGMP standards, subjected to testing standards, and incorporating an intracellular-like composition of salts and sugars, offers several positive considerations from a quality/regulatory perspective as a material within a cell therapy process. Furthermore, following the completion of a small animal safety study, the results demonstrated that intravenous injection of the CryoStor solution presented no safety risk within the parameters tested (results unpublished). The injection volumes significantly exceeded typical human dose equivalents. The studies were performed to mimic human stem cell clinical applications. In short, the use of a cGMP produced product provides a level of consistency in formulation and efficacy that is likely missing from the standard “home-brew”.
In conclusion, the results from this study demonstrate that use of an intracellular-like preservation solution (CryoStor) can considerably improve the recovery of total viable CD34+ cells and subsequent colony formation from cryopreserved PBSC products compared to the standard, well recognized FHCRC cryopreservation medium. It remains to be seen through further investigation whether this increased recovery might translate into improved engraftment. In addition, this study demonstrates that the use of CryoStor without serum or serum-derived proteins can be a suitable alternative to traditional cryopreservation cocktails that utilize serum and serum-derived proteins in the extracellular-like freezing media. The elimination of serum (animal and human) and serum-derived proteins would be a favorable consideration for potential clinical cell therapy products. Finally, these improved recoveries were obtained with CryoStor™ CS10 diluted 1:1 with Normosol-R, which resulted in ½ the typical amount of DMSO in the final product. Lower DMSO concentration may also reduce the incidence and severity of infusional toxicity sometimes observed with the FHCRC formulation (as well as other traditional cryopreservation cocktails). The CryoStor™ solution offers an enhanced approach for the cryopreservation of PBSC and also provides certain positive quality and regulatory considerations.
References
- 1.Berz D, McCormack EM, Winer ES, et al. Cryopreservation of hematopoietic stem cells. Am J Hematol. 2007;82:463–472. doi: 10.1002/ajh.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jansen J, Thompson JM, Dugan MJ, et al. Peripheral blood progenitor cell transplantation. Therapeutic Apher. 2002;6(1):5–14. doi: 10.1046/j.1526-0968.2002.00392.x. [DOI] [PubMed] [Google Scholar]
- 3.Shpall EJ, Jones RB, Franklin WA, Archer PG, Curiel T, Bitter M, Claman H, Bearman S, Stemmer S, Purdy M, Myers S, Hami L, Taffs S, Heimfeld S, Hallagan J, Berenson RJ. Transplantation of enriched CD34-positive autologous marrow into breast cancer patients following high-dose chemotherapy: Influence of CD34+ peripheral blood progenitors and growth factors on engraftment. J Clin Oncol. 1994;12:28–36. doi: 10.1200/JCO.1994.12.1.28. [DOI] [PubMed] [Google Scholar]
- 4.Rowley SD, MacLeod B, Heimfeld S, Holmberg L, Bensinger WI. Severe central nervous system toxicity associated with the infusion of cryopreserved PBSC components. Cytotherapy. 1999;1:311–317. [PubMed] [Google Scholar]
- 5.Rowley SD, Feng Z, Holmberg L, Heimfeld S. Post-thaw removal of dimethysulfoxide does not completely abrogate infusional toxicity or the need for pre-infusion histamine blockade. Cytotherapy. 1999;6:439–446. doi: 10.1080/0032472031000141303. [DOI] [PubMed] [Google Scholar]
- 6.Rowley SD, Feng Z, Chen L, Holmberg L, Heimfeld S, MacLeod B, Bensinger WI. A randomized phase III clinical trial of autologous blood stem cell transplantation comparing cryopreservation using dimethylsulfoxide versus dimethylsulfoxide with hydroxyethylstarch. Bone Marrow Transplant. 2003;31:1043–1051. doi: 10.1038/sj.bmt.1704030. [DOI] [PubMed] [Google Scholar]
- 7.Reddy RL. Mobilization and collection of peripheral blood progenitor cells for transplantation. Transfus Apher Sci. 2005;32:63–72. doi: 10.1016/j.transci.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Barrett J. Improving outcome of allogeneic stem cell transplantation by immunomodulation of the early post-transplant environment. Curr Opin Immunol. 2006;18:592–598. doi: 10.1016/j.coi.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Sartor M, Antonenas V, Garvin F, et al. Recovery of viable CD34+ cells from cryopreserved hemopoietic progenitor cell products. Bone Marrow Trans. 2005;36:199–204. doi: 10.1038/sj.bmt.1705009. [DOI] [PubMed] [Google Scholar]
- 10.Frey NV, Lazarus HM, Goldstein SC. Has allogeneic stem cell cryopreservation been given the ‘cold shoulder’? An analysis of the pros and cons of using frozen versus fresh stem cell products in allogeneic stem cell transplantation. Bone Marrow Trans. 2006;38:399–405. doi: 10.1038/sj.bmt.1705462. [DOI] [PubMed] [Google Scholar]
- 11.Stylianou J, Vowels M, Hadfield K. Novel cryoprotectant significantly improves the post-thaw recovery and quality of HSC from CB. Cytotherapy. 2006;8(1):57–61. doi: 10.1080/14653240500501021. [DOI] [PubMed] [Google Scholar]
- 12.Parkins MD, Bahlis N, Brown C, et al. Overnight storage of autologous stem cell apheresis products before cryopreservation does not adversely impact early or long-term engraftment following transplantation. Bone Marrow Trans. 2006;38:609–614. doi: 10.1038/sj.bmt.1705501. [DOI] [PubMed] [Google Scholar]
- 13.Mazur P. Freezing and low-temperature storage of freezing of living cells: Mechanisms and implications. Am J Physiol. 1984;247:C125–C142. doi: 10.1152/ajpcell.1984.247.3.C125. [DOI] [PubMed] [Google Scholar]
- 14.Baust JG. Biology of cell survival in the cold: The basis for biopreservation of tissues and organs. In: Baust JG, Baust JM, editors. Advances in Biopreservation. Florida: Taylor and Francis; 2007. pp. 1–14. [Google Scholar]
- 15.Taylor MJ. Biology of cell survival in the cold: The basis for biopreservation of tissues and organs. In: Baust JG, Baust JM, editors. Advances in Biopreservation. Florida: Taylor and Francis; 2007. pp. 15–62. [Google Scholar]
- 16.Van Buskirk RG, Snyder KK, Baust JG, et al. Cryopreservation: It’s not just about cell yield. BioProcess Intl. 2005;3(4):64–72. [Google Scholar]
- 17.Baust JM, Van Buskirk RG, Baust JG. Modulation of the cryopreservation cap: elevated survival with reduced dimethyl sulfoxide concentration. Cryobiology. 2002;45:97–108. doi: 10.1016/s0011-2240(02)00100-1. [DOI] [PubMed] [Google Scholar]
- 18.Liseth K, Abrahamsen JF, Bjorsvik S, et al. The viability of cryopreserved PBPC depends on the DMSO concentration and the concentration of nucleated cells in the graft. Cytotherapy. 2005;7(4):328–333. doi: 10.1080/14653240500238251. [DOI] [PubMed] [Google Scholar]
- 19.Hubel K, Elin R, Gaviria JM, et al. Effective storage of granulocytes collected by centrifugation leukapheresis from donors stimulated with granulocyte-colony-stimulating factor. Transfusion. 45:1876–1879. doi: 10.1111/j.1537-2995.2005.00636.x. [DOI] [PubMed] [Google Scholar]
- 20.Yang H, Acker JP, Cabuhat M, et al. Association of post-thaw viable CD34+ cells and CFU-GM with time to hematopoietic engraftment. Bone Marrow Trans. 2005;35(9):881–887. doi: 10.1038/sj.bmt.1704926. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi T, Inada S, Pommier CG, et al. Osmotic stress and the freeze-thaw cycle cause shedding of Fc and C3b receptors by human polymorphonuclear leukocytes. J Immunol. 1985;134(6):4062–4068. [PubMed] [Google Scholar]
- 22.Armitage J, Mazur P. Osmotic tolerance of human granulocytes. Am J Physiol Cell Physiol. 1984;247:C373–C381. doi: 10.1152/ajpcell.1984.247.5.C373. [DOI] [PubMed] [Google Scholar]


