Summary
Recent developments in biosensor technology allow point-of-use reporting of salivary alpha amylase (sAA) levels while approaching the precision and accuracy of conventional laboratory-based testing. We deployed a portable prototype sAA biosensor in 54 healthy, male dental students during a low stress baseline and during final exams. At baseline, participants completed the Brief Symptom Inventory (BSI). At baseline and the exam week, participants provided saliva samples at 10 AM, 1 PM, and 5 PM, and rated concurrent subjective distress. Although subjective distress was higher during exams compared to baseline, sAA levels did not differ between baseline and exams. Higher sAA levels were related to higher concurrent subjective distress, and higher depressive and social isolation symptoms on the BSI were related to lower sAA during exams. Results from this study, in combination with previous validation data, suggest that the sAA biosensor is a promising tool for point-of-use measures of exposure to stress.
Keywords: salivary alpha amylase, salivary diagnostics, biomarker, stress, biosensor, point of care measurement
1. Introduction
Stressful life events are associated with a range of human diseases and constitute a tremendous public health burden. Although the nature of stressors may vary, they have the common ability to challenge or exceed the individual s adaptive capacities, contributing to psychological, behavioral, and physiological changes that may place the individual at increased risk for physical and psychiatric illness. Clinical observations and self-reports of stressful event exposure, appraisals, and distress ratings are useful, but limited by well-known reliability and validity issues (Monroe, 2008). Moreover, reliable and objective psychological evaluations such as interviews are time and resource intensive, and can be impractical in field research (e.g., combat zones, large-scale population studies) and in clinical settings (e.g., acute care). Thus, identifying biomarkers that reflect exposure to stressful events, map onto stress-responsive peripheral systems, and predict risk for psychopathology (e.g., posttraumatic stress disorder, major depressive disorder, substance abuse) are a priority for next-generation research (Kraemer, Schultz, & Arndt, 2002).
The primary peripheral systems that are activated in response to psychological stressors are the hypothalamic-pituitary-adrenal (HPA) axis and the autonomic nervous system, which includes both the sympathetic and parasympathetic nervous systems (McEwen, 1998). While the HPA axis, and its primary peripheral biomarker, cortisol, has received significant empirical attention in stress research (Dickerson & Kemeny, 2004; Miller, Chen, & Zhou, 2007), autonomic responses, by virtue of their rapid response and effects on numerous biological systems, also play key roles in the adverse consequences of stress exposure (Ulrich-Lai & Herman, 2009). However, beyond labor-intensive electrophysiology measures (impedance cardiography, heart rate variability, electrodermal responses) (Cacioppo, Tassinary, & Berntson, 2007) and expensive catecholamine assays (Baum & Grunberg, 1997), the development of measurement strategies using peripheral biomarkers reflecting autonomic activity has lagged. More recently, salivary alpha amylase (sAA) has emerged as a surrogate marker of sympathetic nervous system activity (Nater & Rohleder, 2009; Rohleder & Nater, 2009). However, the clinical and research utility of this putative stress biomarker has been restricted by the availability of simple, point-of-use methods for measuring sAA levels in a range of patients and environments.
Emerging miniaturization technologies (such as "lab on a chip") are leading to low-cost, portable, and automated saliva-based biomarker analyses. One such example is the biosensor prototypes that utilize miniaturized optical platform and inexpensive, colorimetric test strips to rapidly detect and quantify salivary alpha amylase (Yamaguchi et al., 2006; Yamaguchi et al., 2004). We recently demonstrated in the same sample used in this study that a refined version of this salivary biosensor shows excellent comparability with conventional, laboratory-based methods for quantifying proteins (Shetty et al., 2010). Having established the performance characteristics (e.g., precision, accuracy, reliability, reproducibility) of the biosensor, we explored the feasibility and ecological validity of biosensor-measured sAA in assessing stressful event exposures and related emotional responses in an academic examination model of brief, naturalistic stressful events (Stowell, 2003).
Our interest in sAA was based on converging lines of evidence suggesting that sAA is a marker of sympathetic activation, including studies of acute laboratory stressors and pharmacological blockade (Nater & Rohleder, 2009). Some of the precursor studies to establish sAA as a possible stress biomarker of exposure to stressful life events involved academic examinations (Bosch et al., 1998; Bosch et al., 1996). For example, Bosch et al. (1996) reported higher sAA levels 30 minutes before an academic examination compared to two control days after the exam. The elevated sAA levels notwithstanding, these studies focused on acute elevations during specific points in time, rather than elevations across the day. Beyond indicating stressful life event exposures, it is plausible that sAA levels, given their association with sympathetic activation, may also co-occur with emotional responses to stressful events.
Complicating the relationship between sAA biomarkers and stressful events is the perspective that the stressors may manifest as elevated or lower levels of stress biomarkers (McEwen, 1998). On one hand, individuals who experience repeated stressful events may show a lack of adaptation to stressors, and thus show elevated responses to stress (Kirschbaum et al., 1995). On the other hand, repeated exposures to stressful events may lead to hyporesponsive stress systems (Chida & Hamer, 2008). Although research on chronic stress exposure and cardiovascular reactivity suggests elevated responses to stress in chronically stressed individuals, there is no clear evidence of hyper- or hyporesponsiveness related to chronic stress in measures of sympathetic activity (Chida & Hamer, 2008).
Naturalistic studies of neuroendocrine stress markers usually require participants to report mood at various points during the day (Saxbe, 2008). While numerous studies have examined relationships between other salivary analytes and mood (e.g., cortisol; Jacobs et al., 2007) few have focused specifically on the relationships between emotional responses and sAA. Nater and colleagues (2007) reported no associations between self-reported stress and sAA levels over the course of the day. However, they noted that their measures of self-reported stress asked participants to retrospectively report stress over the past hour, rather than ask participants how they are feeling at the current moment. At the same time, greater self-reported chronic stress on a measure administered at baseline was related to elevated sAA levels.
In this study, we used a cohort of healthy young adults to examine temporal changes in direct, biosensor-reported sAA values and their relationship to self-reported psychological distress in the context of a naturalistic stressor (e.g. academic examinations). Our aims were: a) to replicate and extend previous findings about sAA levels, using conventional assays, under high and low academic stress; b) examine relationships between momentary self-reported distress and sAA; and c) examine relationships between baseline psychological distress and sAA. Based on previous work (Bosch et al., 1996), we expected elevated sAA levels during exam week compared to early in the academic session. Also, we expected greater self-reported recent psychological symptoms and momentary distress ratings to be related to elevated sAA levels, and larger increases in sAA over the course of the day.
2. Methods
2.1. Participants
Healthy, male, dental students (n = 54) were recruited at the UCLA School of Dentistry. Females were excluded for this phase of sAA biosensor development to minimize heterogeneity related to potential gender differences in sympathetic nervous system responses to stress (Lundberg, 2005). A short screening questionnaire was used to select participants who were at least 18 years of age, free from psychotropic medication, steroids or drug abuse, and without transitory illnesses or chronic conditions that might interfere with biomarker evaluation. Written informed consent was obtained from all the participants after a full description of the study as approved by the UCLA Institutional Review Board. Health behaviors (e.g., caffeine, tobacco, or medication use) and transient illnesses (e.g., cold or flu) were assessed before, during and after each sampling session to verify concurrent health status.
2.2. Procedures
Prior to scheduling of biomarker sampling days (all weekdays), participants were also screened for minor influenza, fever, cold or other transitory illnesses that might interfere with readings and rescheduled if necessary. At the initial interview, which took place during the beginning of the academic quarter (baseline), participants were provided a biosensor unit and conventional saliva collection packets, instructed in their use by trained interviewers, and given written instructions for completion of home sampling on the following day. Demographic and psychosocial instruments were administered and the home self-report checklist logs were introduced. Participants were instructed to record momentary distress and drowsiness/sleepiness ratings at each saliva collection time, the actual time of each collection, time of awakening, bedtime and mealtimes, as well as alcohol use, caffeine consumption, nicotine use and any medication taken during the sampling day. Participants demonstrated their understanding of the collection procedures by collecting their own saliva using the conventional swab and the biosensor unit at the first interview. All initial interviews were conducted between 9 AM and 11 AM.
On the day after home sampling, participants returned the biosensor, conventional saliva swabs and self-report logs. The logs were reviewed and participants completed a Health Behavior Questionnaire (described below) with the interviewer. Any discrepancies (e.g., sampling times or eating times prior to saliva collection) were clarified in this brief interview.
The same procedures were conducted during the high stress (academic exam) period except that demographic and psychosocial instruments were not repeated. Participants were reminded of procedures for saliva collection and logs, provided written instructions, a biosensor unit and conventional swab supplies, and demonstrated competence with collection procedures by sampling their own saliva with the biosensor and conventional swab. As before, participants completed a Health Behavior Questionnaire with the interviewer on the day after the home sampling. On the day of the exam, participants first exam typically started at 8 or 9 AM, and participants took between 1–3 exams during the exam day, which was typically a Tuesday, Wednesday, or Thursday of exam week.
2.3. Symptom measures
Recent psychological distress at baseline was assessed in an initial interview via the Brief Symptom Inventory (BSI) (Derogatis and Melisaratos, 1983), a 53-item symptom inventory with nine subscales termed anxiety, depression, somatization, hostility, phobic anxiety, paranoid ideation, psychoticism, obsessive-compulsive and interpersonal sensitivity. Respondents were presented with “a list of problems people sometimes have” and asked to rate how much that problem has distressed or bothered them during the past 7 days on a scale from 0 (not at all) to 4 (extremely). In addition to subscales, the BSI yields a Global Severity index which combines information about the numbers of symptoms and intensity of distress across all dimensions. Convergent validity for the BSI is good, with both clinical and non-clinical norms available. The subscales and global indices had good internal consistency (ranging from .71 – .85) and test-retest reliability (ranging from .68 – .91) (Derogatis, 1993). In this sample, internal consistency for the global severity index was .94.
Momentary subjective distress ratings were assessed with a single item (“Please describe how much emotional distress you are feeling right now on a scale from 0 [no distress] to 10 [the worst distress you have ever experienced]) and were recorded at every saliva collection.
The Health Behaviors Questionnaire (HBQ) (Unpublished instrument, Glover) assessed behaviors occurring in the 24 hours prior to biomarker collection that have demonstrated influence on neuroendocrine levels (Hibel et al., 2006; Rohleder and Kirschbaum, 2006). The HBQ asks for the type, quantity and time since last caffeine drinks, cigarettes, alcoholic beverages, meals, prescribed and recreational medication, aerobic and anerobic exercise, sleep (hours and perceived quality), and illness symptoms (fever, cold, flu, headache, etc.).
2.4. Saliva collection
After brief training, participants collected unstimulated whole saliva with the biosensor (for more details about the biosensor, see Shetty et al., 2010). Participants were instructed not to chew or otherwise manipulate their tongues or mouth while collecting saliva. The biosensor consists of disposable colorimetric test strips and a hand held reader that utilizes a miniaturized optical platform to rapidly detect and quantify salivary alpha-amylase. Inputted algorithms normalize variations in ambient temperature and salivary pH. The small test strip is placed under the tongue for 15 seconds and then removed and inserted into the sensor unit. The biosensor provides a read out of the sAA level within approximately 15 seconds.
As we describe in more detail elsewhere (Shetty et al., 2010), the biosensor employed in this study is a refined version that incorporated multiple improvements over previous versions (e.g., Yamaguchi et al., 2004; Yamaguchi et al., 2006). Briefly, the technical improvements included: An upgraded collector pad that minimizes the effect of salivary flow rate by consistently collecting 23 μl of saliva when saturated; extended dynamic range with better linearity through modifications to the substrate-impregnated reagent paper; a new optical sensor and revised algorithms for determining the standard curve; incorporation of a miniature thermosensor for normalizing variations in ambient room temperature; pH adjustment to normalize variations in salivary pH; and additional design features (including date/time stamp and USB connector) to make the biosensor field deployable.
Participants were instructed not to eat or drink for 1 hour prior to any saliva collection and to rinse their mouth with clear water immediately before each collection. Telephone reminders prior to the initial interview, confirmation of last eating and drinking times at the time of each in-person saliva collection, and logs of all eating and drinking times on the day of the home saliva collections were used to remind and verify compliance.
At each sampling time, participants collected saliva via the biosensor. On the home-sampling day, participants collected saliva at three time points (10 a.m., 1 p.m., and 5 p.m.), with a window of ± 60 minutes. The first sampling time was chosen to avoid the wake-up decrease in SAA (Nater et al., 2007), whereas the remaining times were chosen so that participants could follow instructions to avoid any eating/drinking, physical activity or substance use within 1 hour prior to each collection time. Concurrent ratings of momentary subjective distress were collected at each of the three sample times at both sessions. To aid with sampling compliance, participants were given digital wristwatches during the sampling day that sounded an audible alarm during the sampling times. In addition, the biosensor displayed a time-date stamp during every measurement, which was recorded by participants and cross-checked with the times programmed into the digital wristwatches.
2.5 Statistical analyses
Unless otherwise specified, statistical tests involving sAA used log(x+1) transformed data. sAA was measured at three time points during two occasions (baseline, exam day) nested within individuals. For illustrative purposes, we used Pearson correlations to assess relationships between continuously-scaled measures, the Pearson-Filon statistic using the Fisher r-to-z-transform to compare correlations that were themselves correlated across time points (Raghunathan, Rosenthal, & Rubin, 1996), and mixed-effects longitudinal models that treated time as a categorical factor (10am, 1pm, or 5pm) to test changes in single variables over time. A covariance structure for the univariate longitudinal models employing a different compound-symmetry structure within each stress session and random subject-specific intercepts had the best fit of all attempted structures based on having the lowest Akaike s Information Criterion value. Inferences for fixed effects of time of day and session (baseline vs. exam) were assessed with linear contrasts (type 3 effects).
To model the entire data structure and formally test our hypotheses, we constructed multivariate longitudinal models using both sAA and subjective distress ratings as outcome measures in the same model. Specifically, joint longitudinal models were fit with Kroeneker-product covariance structures corresponding to separate inter- and intra-marker correlations across time (for a useful tutorial on fitting such models in SAS, see Gao, Thompson, Xiong, & Miller, 2006). These models, fit separately for each stress session, estimate a mean of each marker at each of the three time points and a within-subject covariance structure describing the correlation of each marker over time and the correlation between markers over time. A full description of the model is described in the Appendix. All longitudinal models were fit using PROC MIXED in the SAS software, Version 9.1 (SAS Institute Inc.).
3. Results
3.1. Participants and health behaviors
Participants (N=54) were young, adult males (Mean age = 24, SD = 2.2, range = 20–30) who were mostly white (44.4%) or Asian Pacific Islanders (48.1%). None reported smoking, drug use or alcohol consumption at either session. Occurrence of other potential confounds (e.g., caffeine consumption, aerobic and anaerobic exercise) was low. During baseline, no participants reported caffeine or medication use within 60 min of taking a biosensor sample. During exams, no participants reported medication use within 60 min of taking a sample. For other behaviors (snacking, exercise), at any given timepoint across sessions, between one to four participants (less than 10% of the sample) reported engaging in those behaviors within 60 min of a sample. Several interviews were rescheduled due to transient illness (cold, flu, and headache).
3.2. Saliva collection compliance
The 54 participants provided 292 biosensor sAA measures. Compliance was defined as taking the saliva sample within 60 minutes ± the scheduled sampling time based on the self-report logs. Based on these criteria, the compliance rate was 91.3%. To determine whether session and time of day influenced compliance, we constructed a series of mixed effect models that incorporated a random-intercept term to account for subject specific effects. Noncompliance rates did not significantly differ between sessions (baseline: 9.8%, exams: 7.5%, p = ns) or time of day (10am: 9.3%, 1pm: 10.3%, 5pm: 6.3%, p = .ns). Similarly, the mean absolute discrepancy between ideal and actual sampling time did not differ between session (baseline: 30 min, exam: 27 min, p = ns) or between time of day (M10 AM = 29 min, M1 PM = 31 min, M5 PM = 26 min, p = ns). Finally, sAA levels in non-compliant samples and compliant samples did not significantly differ from each other, and the degree of non-compliance was unrelated to sAA levels.
3.3. sAA and subjective distress within-day and between-sessions
Figure 1 summarizes the sAA and subjective distress ratings readings at each time point, showing increases in sAA over time during the exam session, and higher subjective distress during exams compared to baseline. Linear mixed-effect models were fit to characterize the mean and correlation structure of the sAA measurements across time, shown in Table 1. Contrary to our hypothesis, there was no significant main effect of session (baseline vs. exam) on sAA. There was no significant time of day effect on sAA at baseline. In contrast, sAA showed a significant increase over time during the exam day, with elevated values at 5 PM compared to 10 AM, (p =.007). However, this apparent difference in time of day effect did not result in a significant interaction between session and time of day. The models included separate compound symmetry covariance structures (available on request from the authors)within each stress session coupled with a random intercept term for each subject to allow within-subject correlation across stress sessions. The correlation between measurements taken from the same participant on the same day was similar at baseline (r = .56) and exams (r = .52), with an attenuated correlation between observations taken on the same participant during different stress sessions (r =.38). Interestingly, the residual (random error) variance was higher during baseline compared to the exam day (.28 vs. .17, respectively). Covariates for demographic characteristics (age and ethnicity) did not significantly alter the model for sAA and were not included in subsequent analyses.
Figure 1.
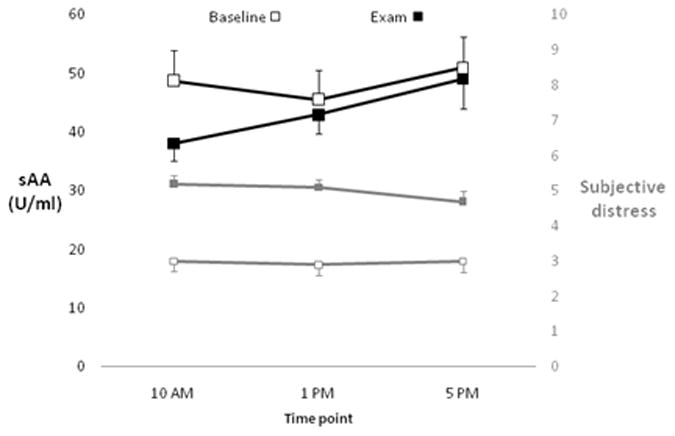
sAA and subjective distress over time, within each session. Black squares and the left y-axis represent sAA levels, and grey squares and the right y-axis represent subjective distress. Open squares represent baseline, closed squares represent exams. Error bars represent standard error of the mean.
Table 1.
Fixed-effects linear contrasts for modeling sAA between- and within- sessions
| Effect | sAA | Subjective distress | ||
|---|---|---|---|---|
| F | p | F | p | |
| Session | 0.00 | .99 | 44.00 | <.0001 |
| Baseline: Time of day | 0.17 | .84 | 0.09 | .91 |
| Exam: Time of day | 3.72 | .03 | 1.15 | .32 |
| Time of day × Session | 1.18 | .31 | 0.97 | .38 |
Note. sAA: df for Time of day and Session effects = (2,235), and df for Time of day × Session interaction = (1, 235). Subjective distress: df for Time of day and Session effects = (2, 249), and df for Time of day × Session interaction = (1, 249).
As expected, the model for subjective distress indicated greater distress during exams compared to baseline (p<.0001), but did not suggest any significant time-of-day effects (Table 1). A correlation structure similar to sAA was seen in the model fit to subjective distress (between measurement correlations at baseline, (r = .52), and exam, (r = .41); no correlation between sessions r = .07). Unlike sAA, residual variance was lower during baseline compared to the exams (1.81 vs. 2.68, respectively).
3.4. Relationships with baseline psychological distress and momentary subjective distress
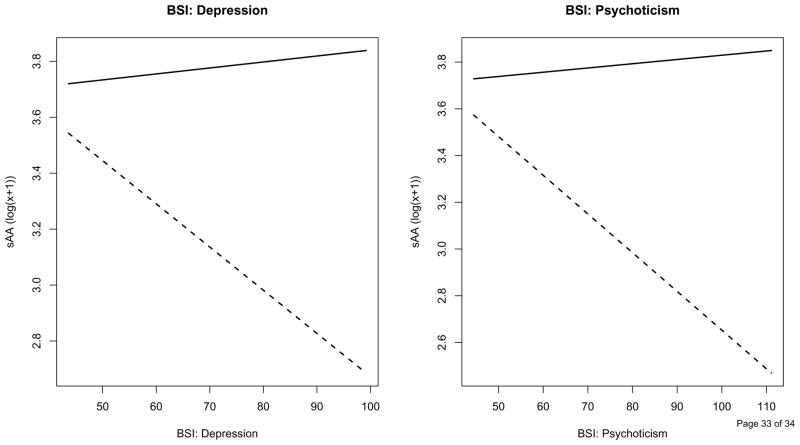
Baseline psychological distress was assessed using the BSI (descriptive statistics shown in Table 2). We examined relationships between sAA averaged across the day, within each session, and BSI subscales. sAA values at baseline were not significantly correlated with BSI subscales. However, higher scores on the depression (r = −.29, p = .04) and psychoticism (r = −.32, p = .02) subscales were related to lower sAA during the exam day.
Table 2.
Descriptive statistics for Brief Symptom Inventory subscales
| Subscale | Mean | SD | Maximum |
|---|---|---|---|
| Anxiety | 0.39 | 0.40 | 1.50 |
| Depression | 0.28 | 0.41 | 1.83 |
| Hostility | 0.43 | 0.57 | 3.60 |
| Interpersonal Sensitivity | 0.40 | 0.48 | 2.25 |
| Obsession-Compulsion | 0.80 | 0.61 | 2.17 |
| Paranoid Ideation | 0.37 | 0.53 | 2.80 |
| Phobic Anxiety | 0.10 | 0.21 | 1.00 |
| Psychoticism | 0.27 | 0.36 | 1.80 |
| Somatization | 0.25 | 0.32 | 1.50 |
Note. Minimum scores for all scales were 0.
Table 3 presents the correlations between biosensor sAA and subjective distress ratings at each measurement time. Interestingly, the relationship between sAA and subjective distress was larger in magnitude at baseline compared to the exam day. Examining within-subject means over the 6 observations (3 time points over 2 sessions) for sAA and subjective distress indicated that higher sAA was related to higher subjective distress (r = .36, p = .009). Although the relationship between sAA and subjective distress appeared larger at baseline (r = .40, p =.0052) compared to exams (r = .22, p = .12), the correlations were not significantly different between the two time points (p = .54).
Table 3.
Correlations between sAA and subjective distress
| Measure, session, timepoint | sAA |
|||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Exam | |||||||
| 10 AM | 1 PM | 5 PM | 10 AM | 1 PM | 5 PM | |||
| Subjective distress | Baseline | 10 AM | .33* | .29* | .29 | .16 | .13 | −.07 |
| 1 PM | .20 | .35* | .35* | .00 | .06 | .06 | ||
| 5 PM | .08 | .13 | .50** | −.14 | −.07 | .05 | ||
| Exam | 10 AM | .22 | .19 | .22 | .29* | .20 | .10 | |
| 1 PM | .04 | .06 | .14 | .05 | .22 | .04 | ||
| 5 PM | .06 | .20 | .19 | .00 | .20 | .20 | ||
Note. Values in bold and along the diagonal are correlations between concurrent measurements
p < .05,
p < .01,
p < .001.
The results of the basic correlational analyses between sAA and subjective distress were corroborated with bivariate longitudinal models fit jointly to sAA and subjective distress. These models assumed a common across-time correlation structure for each marker compounded with an unstructured correlation between markers. Concurrent measurements of sAA and subjective distress were significantly correlated during baseline (ρ = 0.39, p = 0.0001) and exams (ρ =0.25, p=0.006).
In our final model, we examined relationships between psychological distress at baseline (as reported on the BSI) and sAA during both sessions while accounting for subjective distress. Subjective distress values averaged across the three collection times were included as a fixed-effect predictor of sAA. Higher subjective distress (averaged across the day) was significantly associated with higher sAA (p = .0085). Including mean subjective distress did not alter results from the unadjusted model, as the adjusted model yielded no other significant time or session effects. The models that follow included subjective distress averaged across the day as a fixed effect. Each model included session, a single BSI subscale, and the interaction between session and the BSI subscale. The significant positive association between sAA and subjective distress persisted throughout all of these adjusted models. Baseline depression and psychoticism, exhibited significant interactions (all p s < .05) with session such that the relationship between distress and sAA differed between sessions; the remaining seven subscales failed to show significant relationships with sAA. Specifically, as shown in Figure 2, higher depression (p =.0041) and psychoticism (p = .0004) scores at baseline were significantly related to lower sAA levels during exams, but were unrelated to sAA levels at baseline.
Figure 2.
Estimated regression lines from models examining the relationship between baseline psychological distress reported on the Depression and Psychoticism subscales of the BSI and sAA. Slopes represent the relationship between baseline psychological distress and sAA at baseline (solid line) and exam (dashed line), adjusting for time of day and subjective distress ratings (averaged across the day).
4.1. Discussion
This study demonstrates that self-reported measures of distress, including concurrent measures, are related to biosensor measures of sAA. Regardless of time of day, and even the presence of background stressful events (exams), higher ratings of self-reported distress were related to higher sAA levels. Moreover, this study demonstrates that the sAA biosensor may be operated in naturalistic settings to obtain multiple measures throughout the day. In concert with our data from the same sample showing excellent comparability of the sAA biosensor with standard clinical chemistry assay techniques (Shetty et al., 2010), biosensor measurement is a promising point-of-use tool for quantifying exposures and responses to psychological stress.
Our results indicating greater sAA related to greater self-reported distress is in line with other work that demonstrated elevated sAA during stressful or aversive events (Nater & Rohleder, 2009). However, in previous work, Nater and colleagues found that higher momentary ratings of positive mood and calm, but not stress levels, were related to higher momentary sAA levels (Nater, Rohleder, Schlotz, Ehlert, & Kirschbaum, 2007). The authors noted that overall stress levels were low in their study sample, whereas our sample consisted of dental students who were in class around 6–7 hr per day, even during baseline. Moreover, in the Nater et al. (2007) study, participants provided mood ratings that were intended to summarize mood over the past hour, whereas participants in our sample were asked to rate subjective distress at the current moment. Retrospective ratings that ask participants to aggregate subjective experience over a long period of time (i.e., an hour) may not adequately correspond to sAA levels, which are more reflective of momentary fluctuations in mood over shorter periods of time (i.e., several minutes).
Based on previous work, we hypothesized that participants would show elevated sAA levels during a day of academic examinations compared to baseline (Bosch, et al., 1998; Bosch, et al., 1996). Contrary to our hypotheses, sAA levels did not differ between baseline and exams. Although sAA levels increased across the exam day, and showed no significant change across the baseline day, the critical test of whether the pattern of change differed between sessions was not significant. Moreover, baseline psychological symptoms were not related to greater sAA levels at baseline; interestingly, greater baseline psychological symptoms were related to lower sAA levels during the exam day, a finding conflicting with our initial premise.
Design limitations may have contributed to our counterintuitive findings. We collected sAA at 10 AM, 1 PM, and 5 PM; during the exam day, the samples often corresponded to periods during or just after academic examinations. Previous work showing elevated sAA during academic examination days actually sampled sAA 30 min prior to the beginning of an exam (Bosch, et al., 1996), or shortly before and after a brief oral academic examination (Schoofs, Hartmann, & Wolf, 2008). In contrast, our first sample, obtained around 10 AM, often occurred 1 – 2 h after the beginning of participants first exam. Thus, rather than capturing acute sAA elevations that occur when anticipating the examination, our 10 AM sample may have captured sAA recovery returning to baseline. Indeed, other studies using salivary analytes, such as salivary immunoglobulin A (Bosch, de Geus, Ring, & Nieuw Amerongen, 2004) have determined that sample timing relative to the initiation of an academic exam influences the pattern of results. Moreover, more integrated measures of sympathetic activity across the day may reveal a pattern more consistent with our hypotheses. For example, a recent intervention study showed pronounced elevations in 15-hr urinary epinephrine collected between the day prior to the exam to the morning of the exam in the control group (Sherman, Bunyan, Creswell, & Jaremka, 2009). Our study sampled sAA three times during the day, and a more comprehensive assessment of sAA activity during the day, consisting of more sampling time points (e.g., Nater et al., 2007) may be necessary to capture the full profile of sAA in response to repeated stressors (exams) during the day.
Beyond study design, our results may reflect the influence of cumulative psychological stress on the neuroendocrine system. The allostatic load framework proposes that repeated exposure to stressful events may lead to hypoactivation of stress-responsive systems (McEwen, 1998). Hypoactivation is consistent with our finding that individuals reporting high levels of psychological distress, notably depression and social isolation (which is assessed by the BSI “psychoticism” subscale), showed lower sAA levels during exams. Blunted HPA axis responses to stress in individuals with a history of recent psychological distress or diminished psychosocial resources have been observed in previous work, including a meta-analysis of 30 years of research (Chida & Hamer, 2008). However, blunted sympathetic responses to stress in the context of psychological distress or diminished resources have not been consistently observed in the literature (Chida & Hamer, 2008).
A portable biosensor offers several advantages to studying salivary biomarkers compared to conventional collection and assay methods. Conventional methods require 1–2 min to collect sufficient saliva samples through either passive drool or swabs. In contrast, saliva collection can be accomplished in 10 sec using the biosensor, and much smaller saliva quantities are required to quantify sAA (20–30 μl). Shorter collection times may be more advantageous in studies using ecological momentary assessment (Shiffman, Stone, & Hufford, 2008), where participant self-reports of momentary mood and other experiences may be disrupted by taking significant time to collect biological samples. Moreover, smaller quantities of saliva can eliminate missing data due to inadequate sample volumes; indeed, in our study no biosensor sAA values were lost due to inadequate readings in this study. Conventional laboratory-based assay methods involve the multiple steps of sample acquisition, labeling, freezing, transportation, processing (e.g., centrifugation of the sample, sorting, aliquotting, loading into analyzer), analysis and results reporting. Each step can be a potential quality failure point. The costs associated with expensive analytical equipment and testing supplies, sample acquisition and transport supplies, as well as all the labor costs incurred across the total process can be significant impediments. Finally, rapid quantification offers significant advantages for investigators, such as the ability to get rapid feedback on the reliability and validity of biomarker sampling protocols in the field and in the laboratory. In sum, biosensor collection can reduce participant burden, missing data, and speed the development of reliable collection protocols. The ability to readily measure salivary stress biomarkers at the site of patient care increases the likelihood that the data will be utilized by the care provider to inform clinical decision making or provide appropriate referrals.
This study has several limitations which may be improved upon in future work. Similar to previous academic examination studies (e.g., (Bosch, et al., 1996) the order of baseline and exams was not counterbalanced. The novelty of the biosensor may have affected data collection at baseline. Indeed, sampling compliance was worse, and perhaps as a consequence, within-participant (residual) variance in sAA was larger during the baseline session compared to exams. Although sampling compliance rates were similar compared to previous studies of naturalistic cortisol and sAA collection (Nater, et al., 2007), our window for defining compliance was wider compared to previous studies. Future iterations of the biosensor could build in technology to improve compliance, such as procedures to prevent participants from taking samples outside a predefined sampling window. This study limited participation to men, although a recent summary of the current literature suggests that there are no sex differences in basal sAA activity, sAA responses to stress, and that sAA levels do not vary by menstrual phase or oral contraceptive use (Rohleder & Nater, 2009). Finally, although examinations occurred throughout the week, sAA was only collected during one day. Given that sAA reflects short-term sympathetic nervous system responses, more than three samples per day, and sampling on multiple days may be a more ideal sampling method in the future.
In summary, this study provides preliminary evidence of the utility of a portable sAA biosensor in naturalistic studies, and that sAA levels are related to concurrently measured psychological distress. Biosensor measurement is a promising technology for assessing biomarkers in the field, and with further development in assay technology may incorporate additional salivary biomarkers, such as cortisol or DHEA-S. Combined with other methodological and technological innovations in studying exposure to stress (http://www.gei.nih.gov/exposurebiology/program/tools.asp), biosensor enabled point-of-use measurement may become an important component of next-generation, multi-method approaches to studying exposures to stress.
Appendix. Joint longitudinal models for sAA and subjective distress
The following statement of the model is for a single individual, usually designated with the subscript i which we omit below for ease of presentation.
where Y1 and Y2 are both 3x1 vectors corresponding respectively to sAA and subjective distress readings at the three time points (10 AM, 1 PM, 5 PM), X1 and X2 are 3 × 3 design matrices corresponding to different mean values at each time point for each marker, β1 and β2 are 3 × 1 vectors corresponding to the mean differences between time points for each marker, and ε ~ N(0,Σ) where
with B corresponding to the between-marker covariance structure and W corresponding to the within-marker structure, which is assumed the same for both markers. We fit unstructured models to both B and W. Our parameter of primary interest in this model is b12 (or the correlation ) which corresponds to the relationship between concurrently-recorded sAA and subjective distress.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baum A, Grunberg N. Measurement of stress hormones. In: Cohen S, Kessler RC, editors. Measuring stress: A guide for health and social scientists. New York: Oxford University Press; 1997. pp. 175–192. [Google Scholar]
- Bosch JA, Brand HS, Ligtenberg AJM, Bermond B, Hoogstraten J, Nieuw Amgerongen AV. The response of salivary protein levels and S-IgA to an academic examination are associated with daily stress. Journal of Psychophysiology. 1998;12(4):384–391. [Google Scholar]
- Bosch JA, Brand HS, Ligtenberg TJM, Bermond B, Hoogstraten J, Nieuw Amerongen AV. Psychological stress as a determinant of protein levels and salivary-induced aggregation of Streptococcus gordonii in human whole saliva. Psychosomatic Medicine. 1996;58(4):374–382. doi: 10.1097/00006842-199607000-00010. [DOI] [PubMed] [Google Scholar]
- Bosch JA, de Geus EE, Ring C, Nieuw Amerongen AV. Academic examinations and immunity: academic stress or examination stress? Psychosomatic Medicine. 2004;66:625–626. doi: 10.1097/01.psy.0000133254.46947.ac. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. 3. New York: Cambridge University Press; 2007. [Google Scholar]
- Chida Y, Hamer M. Chronic psychosocial factors and acute physiological responses to laboratory-induced stress in healthy populations: A quantitative review of 30 years of investigations. Psychological Bulletin. 2008;134:829–885. doi: 10.1037/a0013342. [DOI] [PubMed] [Google Scholar]
- Derogatis LR. BSI Brief Symptom Inventory, Administration, Scoring, and Procedures Manual. 4. Minneapolis, MN: National Computer Systems; 1993. [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and meta-analytic review. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Gao F, Thompson P, Xiong C, Miller JP. Analyzing multivariate longitudinal data using SAS. Paper 187–31. Paper presented at the Proceedings of the Thirty-first annual SAS Users Group International Conference.2006. [Google Scholar]
- Jacobs N, Myin-Germeys I, Derom C, Delespaul P, van Os J, Nicolson NA. A momentary assessment study of the relationship between affective and adrenocortical stress responses in daily life. Biological Psychology. 2007;74:60–66. doi: 10.1016/j.biopsycho.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Prussner JC, Stone AA, Federenko I, Gaab J, Lintz D. Persistent high cortisol responses to repeated psychological stress in a subpopulation of healthy men. Psychosomatic Medicine. 1995;57:366–372. doi: 10.1097/00006842-199509000-00009. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Schultz SK, Arndt S. Biomarkers in psychiatry: Methodological issues. American Journal of Geriatric Psychiatry. 2002;10:653–659. [PubMed] [Google Scholar]
- Lundberg U. Stress hormones in health and illness: The roles of work and gender. Psychoneuroendocrinology. 2005;30:1017–1021. doi: 10.1016/j.psyneuen.2005.03.014. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. New England Journal of Medicine. 1998;388:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin. 2007;133:25–45. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Monroe SM. Modern approaches to conceptualizing and measuring human life stress. Annual Review of Clinical Psychology. 2008;4:33–52. doi: 10.1146/annurev.clinpsy.4.022007.141207. [DOI] [PubMed] [Google Scholar]
- Nater UM, Rohleder N. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: current state of research. Psychoneuroendocrinology. 2009;34:486–496. doi: 10.1016/j.psyneuen.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Nater UM, Rohleder N, Schlotz W, Ehlert U, Kirschbaum C. Determinants of the diurnal course of salivary alpha-amylase. Psychoneuroendocrinology. 2007;32:392–401. doi: 10.1016/j.psyneuen.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Raghunathan TE, Rosenthal R, Rubin DB. Comparing correlated but nonoverlapping correlations. Psychological Methods. 1996;1:178–183. [Google Scholar]
- Rohleder N, Nater UM. Determinants of salivary alpha-amylase in humans and methodological considerations. Psychoneuroendocrinology. 2009;34:369–485. doi: 10.1016/j.psyneuen.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Saxbe DE. A field (researcher's) guide to cortisol: Tracking HPA axis functioning in everyday life. Health Psychology Review. 2008;2:163–190. [Google Scholar]
- Schoofs D, Hartmann R, Wolf OT. Neuroendocrine stress responses to an oral academic examination: No strong influence of sex, repeated participation and personality traits. Stress. 2008;11:52–61. doi: 10.1080/10253890701453943. [DOI] [PubMed] [Google Scholar]
- Sherman DK, Bunyan DP, Creswell JD, Jaremka LM. Psychological vulnerability and stress: The effects of self-affirmation on sympathetic nervous system responses to naturalistic stressors. Health Psychology. 2009;28:554–562. doi: 10.1037/a0014663. [DOI] [PubMed] [Google Scholar]
- Shetty V, Zigler C, Robles TF, Elashoff D, Yamaguchi M. Developmental validation of a point-of-care, salivary alpha-amylase biosensor. Psychoneuroendocrinology. 2010 doi: 10.1016/j.psyneuen.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Stone AA, Hufford MR. Ecological momentary assessment. Annual Review of Clinical Psychology. 2008;4:1–32. doi: 10.1146/annurev.clinpsy.3.022806.091415. [DOI] [PubMed] [Google Scholar]
- Stowell JR. Use and abuse of academic examinations in stress research. Psychosomatic Medicine. 2003;65:1055–1057. doi: 10.1097/01.psy.0000097349.84109.1f. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nature Reviews Neuroscience. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M, Deguchi M, Wakasugi J, Ono S, Takai N, Higashi T. Hand-held monitor of sympathetic nervous system using salivary amylase activity and its validation by driver fatigue assessment. Biosensors and Bioelectronics. 2006;21:1007–1014. doi: 10.1016/j.bios.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Kanemori T, Kanemaru M, Takai N, Mizuno Y, Yoshida H. Performance evaluation of salivary amylase activity monitor. Biosensors and Bioelectronics. 2004;20:491–497. doi: 10.1016/j.bios.2004.02.012. [DOI] [PubMed] [Google Scholar]