Abstract
The mesolimbic reward pathway is implicated in stress-related psychiatric disorders and is a potential target of plasticity underlying the stress resistance produced by repeated voluntary exercise. It is unknown, however, whether rats find long-term access to running wheels rewarding, or if repeated voluntary exercise reward produces plastic changes in mesolimbic reward neurocircuitry. In the current studies, young adult, male Fischer 344 rats allowed voluntary access to running wheels for 6 weeks, but not 2 weeks, found wheel running rewarding, as measured by conditioned place preference (CPP). Consistent with prior reports and the behavioral data, 6 weeks of wheel running increased ΔFosB/FosB immunoreactivity in the nucleus accumbens (Acb). In addition, semi quantitative in situ hybridization revealed that 6 weeks of wheel running, compared to sedentary housing, increased tyrosine hydroxylase (TH) mRNA levels in the ventral tegmental area (VTA), increased delta opioid receptor (DOR) mRNA levels in the Acb shell, and reduced levels of dopamine receptor (DR)-D2 mRNA in the Acb core. Results indicate that repeated voluntary exercise is rewarding and alters gene transcription in mesolimbic reward neurocircuitry. The duration-dependent effects of wheel running on CPP suggest that as the weeks of wheel running progress, the rewarding effects of a night of voluntary wheel running might linger longer into the inactive cycle thus providing stronger support for CPP. The observed plasticity could contribute to the mechanisms by which exercise reduces the incidence and severity of substance abuse disorders, changes the rewarding properties of drugs of abuse, and facilitates successful coping with stress.
Keywords: Exercise, physical activity, conditioned place preference, ventral tegmental area, nucleus accumbens, FosB
Introduction
Clinical data reveal that habitual physical activity is an effective means to prevent and treat stress-related psychiatric disorders including depression [4,13], anxiety [56,63], and substance abuse disorders [15,19,20,48,87,88]. Because mood disorders and substance abuse share high comorbidity and perhaps overlapping neural substrates [21,36,49], it is possible that common neurobiological mechanisms underlie the protective effect of physical activity against these various stress-related psychiatric disorders. The mesolimbic reward pathway, which includes dopaminergic projections from the ventral tegmental area (VTA) to the nucleus accumbens (Acb), has been implicated in both the pathophysiology and treatment of depression [62,94], anxiety [24,68,84], and substance abuse disorders [18,33]. The mesolimbic reward pathway, therefore, represents a potential target for exercise-induced neuronal plasticity which could contribute to the stress buffering effects of exercise.
Physical activity is a strong natural reward [6,9,14,47,53]. Rats and mice, for example, choose to run spontaneously on running wheels, and will learn to lever-press for access to a running wheel [7,8,47]. Rats can also develop conditioned place preference (CPP) to environments associated with the after-effects of brief wheel running bouts [53,54]. Although rats can develop CPP to brief periods of wheel running; it remains unknown whether rats can develop and sustain CPP to nightly, long-term voluntary wheel running, or if the rewarding effects of wheel running change over time.
Repeated exposure to rewarding stimuli such as drugs or natural rewards can produce plasticity in the mesolimbic reward pathway. This plasticity includes alterations in the dopaminergic system such as the D1 and D2 dopamine (DA) receptors (DR-D1 and DR-D2, respectively; [35,79,81]), and the catacholamine synthetic enzyme tyrosine hydroxylase (TH; [45,52,90,92]); as well as the opioidergic system including dynorphin [25,71,86], delta [3] and kappa [69,70,86] opioid receptors (DOR and KOR, respectively). It seems likely, then, that repeated exercise reward would also produce adaptations within the dopaminergic and opioidergic components of the reward circuitry. Indeed, altered sensitivity to drugs of abuse can occur following voluntary wheel running in rats [55,73–77], and Werme et all (2002) observed an increase in ΔFosB, a factor thought to underlie natural reward-related plasticity [60,91], in the Acb of Lewis rats following 30 days of wheel running compared to sedentary housing. Other specific plastic changes in the mesolimbic reward pathway following repeated voluntary physical activity, however, have yet to be identified.
The current studies tested the hypothesis that long-term voluntary exercise is rewarding and produces plastic changes in gene transcription of factors capable of modulating dopaminergic and opioidergic neurotransmission in the mesolimbic reward pathway. To determine if wheel running is rewarding, rats were exposed to CPP training during intermittent (every other night) voluntary running wheel access. CPP behavioral testing (probe tests) occurred following 2 or 6 weeks of wheel running and CPP training. Following the final CPP probe test at week 6, wheels were locked and rats underwent daily extinction training to determine if the strength of the CPP memory to exercise is similar to that of drugs of abuse, which typically extinguish following 4 – 8 extinction trials (e.g. [12,22,82]). To examine the consequence of repeated exercise-induced activation of the reward pathway, ΔFosB/FosB protein was quantified in the Acb using immunohistochemistry, and mRNAs for factors capable of influencing reward and neurotransmission in the mesolimbic reward pathway were measured with in situ hybridization following 6 weeks of wheel running or sedentary housing. Results indicate that voluntary exercise is rewarding and results in changes in gene transcription in the reward circuitry which could be involved in the mechanisms by which exercise reduces the incidence and severity of stress-related psychiatric disorders, including substance abuse disorders, and alters the rewarding effects of other rewarding stimuli.
Materials and Methods
Animals
Young adult, male Fischer 344 rats (Harlan SPF, Indianapolis, IN) were used in all experimental procedures. Rats were housed in a temperature (22°C) and humidity-controlled environment and were maintained on a 12:12 hour light:dark cycle (lights on 0600 to 1800). Animals acclimated to these housing conditions for one week prior to any experimental manipulation. All animals were individually housed in Nalgene Plexiglas cages (45 × 25.2 × 14.7 centimeters) with attached running wheels. Wheels were rendered immobile with metal stakes during the acclimation period for the physically active animals and during the entire duration of the experiments for sedentary rats. This control housing ensured that both sedentary and physically active animals were exposed to equal environmental complexity. Single housing was necessary in these experiments to allow quantification of wheel running activity of individual animals, as well as to avoid any competition for running wheels. Care was taken to minimize animal discomfort during all procedures. The University of Colorado Animal Care and Use Committee approved all experimental protocols. All rats had ad libitum access to food (Lab Chow) and water and were weighed weekly.
Wheel running activity
Animals were randomly assigned to either remain sedentary with locked running wheels or were allowed voluntary access to mobile running wheels for 6 weeks. At the onset of the experiments, the wheels in the cages of the physically active rats were unlocked and these rats were allowed voluntary access to their wheels. Daily wheel revolutions were recorded digitally using Vital View software (Mini Mitter, Bend, OR), and distance was calculated by multiplying wheel circumference (1.081 meters) by the number of wheel revolutions.
Conditioning apparatus
The conditioning apparatus (27.9 × 40.6 × 83.8 cm) was made of opaque black Plexiglas (1.3 cm thick) and consisted of two joined compartments. The two compartments were separated by a removable black Plexiglas divider (1.3 cm thick). Each compartment of the conditioning apparatus was made distinctive by changing the walls and floors. The first compartment contained white horizontal strips (2.5 cm wide, 2.5 cm apart) and a floor made of metal chicken wire raised 2 cm from the bottom of the apparatus. The second chamber contained white vertical stripes (2.5 cm wide, 2.5 cm apart) and a metal rod floor raised 2.5 cm from the bottom of the apparatus. The rods of the second chamber were 1 cm apart and all were parallel to the midline of the short axis of the apparatus.
CPP training procedure
The conditioning training persisted daily for 6 weeks and involved alternating exposure to each of the distinctive chambers. During a paired trial, each rat (n=8) was allowed free access to their running wheels overnight. At lights-on (0600), each rat was placed for 30 min in the side of the conditioning chambers containing vertical stripes (paired side). During an unpaired trial, wheels were rendered immobile with metal stakes immediately prior to lights-out (1800). The following morning at lights-on, each rat was placed for 30 minutes into the side of the conditioning chambers containing horizontal stripes (unpaired side). Pilot experiments revealed no baseline preference for either side of the chamber (also see Figure 2B); therefore wheel running was paired with the vertical chamber, while the absence of wheel running was paired with the horizontal chamber. Rats were returned to their home cages immediately following each conditioning trial. Paired and unpaired trials occurred on alternating nights and assured that a novelty preference would not influence behavior during the probe trials. Thus, each rat had voluntary access to a running wheel every-other night (during the night prior to the paired trial), for a 6 week duration.
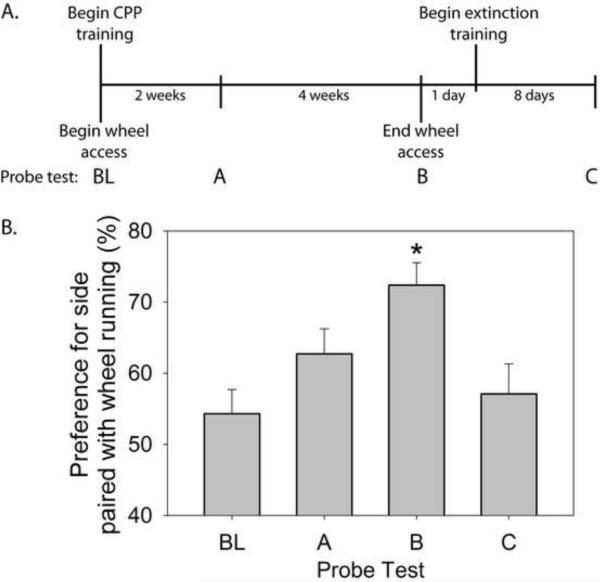
Figure 2.
(A) Experimental timeline for the conditioned place preference (CPP) experiment (Exp 1). Following a baseline preference (probe) test (BL), rats (n = 8) had alternating nightly access to running wheels or locked wheels for 6 weeks. During CPP training, wheel running was paired (30 minutes) with one side of the chamber and the absence of wheel running was paired with the opposite side on alternating mornings. Ten minute CPP probe tests occurred following 2 (probe test A) or 6 (probed test B) weeks of CPP training. Following probe test B at the end of the 6th week of wheel running, wheels were locked and rats underwent extinction training during which rats were exposed to the paired side, in the absence of running, daily for 30 minutes / day. A probe test occurred 24 hr following the final extinction training trial (probe test C). (B) Preference (± standard error of measurement) for the side of the CPP chamber paired with wheel running observed during the 10 minute probe tests. *p < .05 compared to all other groups.
CPP behavioral testing
CPP testing (probe trials) occurred prior to the start of the experiment to assess baseline preference, and again at week 2 and week 6 following the start of the CPP training. During probe trials, the divider separating the two chambers was removed, and each rat was given free access to both conditioning chambers for 10 minutes. Each probe trial began by placing the rat along the midline of the conditioning apparatus. Each test session was monitored with a digital video recorder (Canon HV20 High Definition Camcorder) and the amount of time spent in each chamber was determined by later scoring of the video. A rat was considered to be in a chamber only when all four paws were in that chamber. Behavioral testing occurred between 0600 and 0700 and was conducted by an observer blind to the treatment conditions of the animals. Each rat was tested in the same conditioning apparatus where training occurred. All conditioning procedures and behavioral testing occurred in the same room where the animals were housed.
CPP extinction training
Following the third probe trial at week 6, all the wheels were rendered permanently immobile. Rats underwent extinction training consisting of daily 30 min presentations to the side of the conditioning apparatus previously paired with wheel running (the vertical side). Training sessions occurred between 0600 and 0700. A 10 min probe trial identical to those described above occurred 24 hr following the final extinction training session.
Immunohistochemistry
A second group of animals naïve to behavioral CPP testing were used for detection of ΔFosB/FosB immunoreactivity in the Acb. These rats remained sedentary with locked wheels (n = 8) or had voluntary (daily) access to in-cage running wheels (n = 8). These rats had continuous free access to running wheels rather than alternating-daily access as in the above experiment in order to conform to the majority of prior studies investigating the neurochemical and behavioral effects of daily voluntary wheel running (e.g. [11,16,27,38–40]). After 6 weeks of wheel running or sedentary conditions, rats were deeply anesthetized with sodium pentobarbital (Nembutal, 0.003ml/g) between 2 – 3 hr after lights-out. Rats were sacrificed 2 – 3 hr after lights out so acute wheel running, which peaks within 2 hr after the start of the active cycle ([32] and Fig 1B), would have the greatest potential effect on FosB expression [66]. Rats were perfused transcardially with 100 ml of cold physiological saline, followed by 400–500 ml of 4% paraformaldehyde (PF) in 0.1 M phosphate buffer (PB). Following perfusion, brains were extracted, post-fixed in 4% PF for 1 hr, transferred to PB containing 0.1% sodium azide and 30% sucrose, and stored at 4oC until sectioning. After rapid freezing in isopentane and dry ice (−40°C), 35 μm coronal sections of the brain were sliced on a cryostat (CM 1850, Leica Microsystems, Nussloch, Germany) at −20°C and tissue was placed in PB containing 0.1% sodium azide. Tissue was stored at 4°C until staining.
Figure 1.
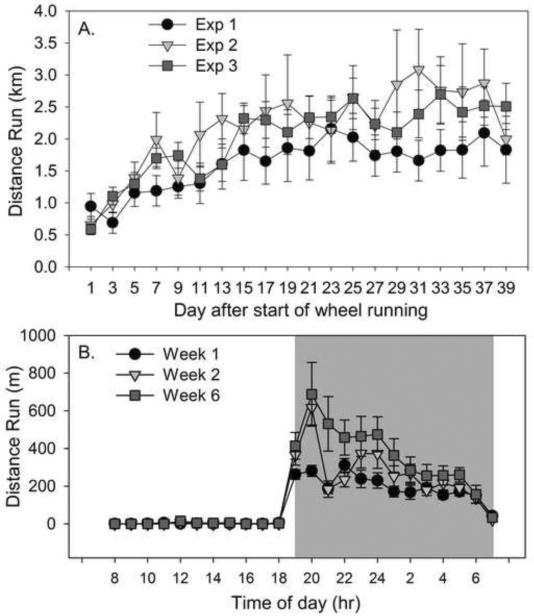
(A) Mean distance (km) run each day by adult male Fisher 344 rats allowed voluntary access to running wheels. Rats included in the first experiment (Exp 1) were used for CPP behavioral testing and had voluntary access to running wheels every other night. Rats in experiment 2 (Exp 2) and experiment 3 (Exp 3) were used for immunohistochemistry and in situ hybridization, respectively, and had nightly voluntary access to running wheels. (B) Mean hourly distance run (m) during the first, second, and sixth week of voluntary wheel access. Values represent group means ± standard error of measurement.
Floating, 35-μm sections of the rostral to caudal aspects of the Acb were rinsed 3 times for 10 minutes each in 0.01 M PBS followed by a 40 min incubation in 0.3% hydrogen peroxide. Tissue was then incubated at 4°C for 48 hr in blocking solution containing 0.1% sodium azide, 0.5% Triton X-100, 5% normal goat serum, and polyclonal rabbit anti-ΔFosB/FosB IgG (SC-48; Santa Cruz Antibodies, Santa Cruz, CA) at a dilution of 1:500. This antibody is raised against an N-terminal region of FosB and ΔFosB and it recognizes a 32–37-kDa protein corresponding to the molecular weight of ΔFosB-like proteins and full-length FosB [2,66]. ΔFosB/FosB was assessed in this experiment because, unlike c-Fos which induces rapidly and transiently following cell stimulation, ΔFosB immunoreactivity remains elevated for days after repeated stimulation [66,80]. ΔFosB, the truncated form of FosB, is unique among the Fos family proteins because it gradually accumulates in response to chronic stimuli, such as repeated drug treatment [67], stress [66], or wheel running [93], and can persist for long periods of time because of its high stability [29,44,61].
Incubation in primary antibody was followed by another series of washes in PBS after which the tissue was incubated at room temperature for 2 hr in blocking solution containing a 1:300 dilution of biotinylated goat anti-rabbit IgG (Jackson ImmunoResearch, West Grove, PA). Tissue was then incubated with avidin-biotinhorseradish peroxidase complexes (ABC; Vectastain Elite ABC kit, Vector Laboratories, Burlingame, CA) in PBS containing 0.5% Triton X-100 for 2 hr. After washes with PB, tissue was placed in a solution containing 3,3'-diaminobenzidene (DAB), ammonium chloride, cobalt chloride, nickel ammonium sulfate, and glucose oxidase in PB for 10 min. The peroxidase reaction was started by addition of glucose solution and reacted for 15–20 min, yielding a dark brown/black product. The reaction was stopped by PBS washes.
To eliminate inter-assay variability, tissues from all animals were processed simultaneously using two, 25-well circular staining nets (Brain Research Laboratories, Newton, MA, USA). ΔFosB/FosB-labeled sections were mounted onto gelatin-coated, glass slides and air-dried overnight. Slide-mounted sections were dehydrated in a series of alcohols, rinsed in Xylene, and cover-slipped with DPX. Each Acb section was assessed by an observer blind to the treatment of the animals for the number of ΔFosB/FosB immunoreactive cells (small brown/black nuclei) as in our prior work [37,39,40]. Three sections from each level of the Acb (Table 1; based on the Rat Brain in Stereotaxic Coordinates by Paxinos and Watson, 1998) were selected from each subject for analysis. Equivalent areas (boundaries are depicted in Figure 3A) were sampled from each section. The values obtained from the 3 sections were averaged to yield the cell counts for that level.
Table 1.
Brain area and coordinates of factors examined in physically active and sedentary rats
| Brain area | Factors examined | Levels and coordinates | Subdivisions |
|---|---|---|---|
| Ventral Tegmental Area | TH mRNA DR-D2 mRNA |
Rostral (−5.20 to −5.3 mm post. bregma) | Rostral |
| Mid (−5.6 to −5.8 mm post) | Mid | ||
| Caudal (−6.04 to −6.30 mm post.) | Caudal | ||
|
| |||
| Nucleus Accumbens | ΔFosB/FosB protein DR-D1 mRNA DR-D2 mRNA Dynorphin mRNA KOR mRNA DOR mRNA |
Rostral (2.70 to 2.20 mm ant.) | Core |
| Mid (1.70 to 1.60 mm ant.) | Shell | ||
| Caudal (1.20 to 1.00 mm ant.) | |||
Figure 3.
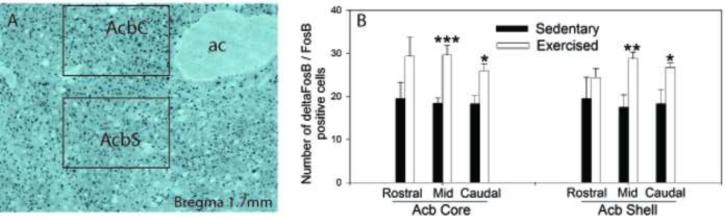
(A) Photomicrograph (10X magnification) of ΔFosB/FosB immunoreactivity in the nucleus accumbens (Acb) of a sedentary rat. Boxes represent areas analyzed. AcbC, nucleus accumbens core; AcbS, nucleus accumbens shell; ac, anterior commisure. (B) Number of ΔFosB/FosB positive cells in the Acb core and shell following 6 weeks of voluntary wheel running (exercised; n = 7) or sedentary housing with a locked wheel (n = 8). Values represent mean number of positive cells ± standard error of measurement. * p < .05, ** p < .01, *** p<.001 with respect to Sedentary group at that level.
In situ hybridization
A third group of animals were again housed with locked wheels (n = 9) or had voluntary access to running wheels (n = 10) for 6 weeks. Following 6 weeks of sedentary or wheel running conditions, rats were killed by rapid decapitation between 0600–0900. Brains were removed, frozen rapidly in isopentene and dry ice (−40 to −50°C), and stored at −80°C until sliced into 10 μm coronal sections on a cryostat. Brain slices through the rostral – caudal extent of the VTA and Acb [64] were thaw-mounted directly onto polylysine-coated slides and stored at −80°C until processing for semi-quantitative single-labeled radioactive in situ hybridization following our published procedures [23,38]. Briefly, sections were fixed in 4% paraformaldehyde for 1 hr, acetylated in 0.1 M triethanolamine containing 0.25% acetic anhydride (10 minutes), and dehydrated in graded alcohol. Complementary (c)RNA riboprobes (courtesy of Stanley Watson, University of Michigan, Ann Arbor) complementary to TH (300 bp), DR-D1 (460 bp), DR-D2 (495 bp), dynorphin (2963 bp), KOR (700 bp), and DOR (983 bp) were prepared from cDNA subclones in transcription vectors and labeled with [S-35]UTP (Amersham Biosciences, Piscataway, NJ), using standard transcription methods. Riboprobes were diluted in 50% hybridization buffer containing 50% formamide, 10% dextran sulfate, 2X saline sodium citrate (SSC), 50 mM PBS at pH 7.4, 1X Denhardt's solution, and 0.1 mg/ml yeast tRNA. Brain sections representing the rostral to caudal extent of the VTA and Acb were hybridized with the probe overnight (55°C). The next day, sections were washed in 2X SSC, treated with RNase A (200 μg/ml) for one hr at 37°C, and washed to a final stringency of 0.1× SSC at 65°C for one hr. Dehydrated, air-dried sections were exposed to X-ray film (Biomax-MR; Eastman Kodak, Rochester, NY) for 1 to 2 weeks. For each probe, slides (each containing four brain sections) from all rats exposed were processed in a single experiment to allow for direct comparisons. Control experiments with “sense” probes indicated that the labeling observed with the “antisense” probes was specific (data not shown).
Image analysis for in situ hybridization
Levels of TH, DR-D1, DR-D2, dynorphin, KOR, and DOR mRNAs were analyzed by computer-assisted optical densitometry. Brain section images were captured digitally (CCD camera, model XC-77; Sony, Tokyo, Japan), and the relative optical density of the x-ray film was determined using Scion Image Version 4.0 (Scion, Frederick, Maryland). A macro was written that enabled signal above background to be determined automatically. For each section, a background sample was taken over an area of white matter, and the signal threshold was calculated as mean gray value of background + 3.5 standard deviations. The section was automatically density-sliced at this value, so that only pixels with gray values above these criteria were included in the analysis. Results are expressed as mean integrated density, which reflects both the signal intensity and the number of pixels above assigned background (mean signal above background X number of pixels above background). Each subject's mean integrated density of a particular cRNA probe at a given level represents the average of three slices chosen for analysis. Templates for each region were made to assure that equivalent areas were analyzed between animals. Each animal's mean integrated density of a particular cRNA probe at a given level represents the average of three VTA slices chosen for analysis at that appropriate level. For analysis of each cRNA probe in the Acb, three sections were chosen from each subject for analysis at the rostral, mid, and caudal levels of analysis (Table 1).
Statistical analysis
Group differences in body weight were analyzed with repeated measures analysis of variance (ANOVA). Alternating average nightly running distance for rats used in each study were compared to one another using repeated measures ANOVA. Average hourly running distance during weeks 1, 2, and 6 were also compared with repeated measures ANOVA. CPP data for each probe test were expressed as a % preference for the side of the chamber paired with wheel running by the following formula: (time spent in the paired side / total time spent in both sides) X 100. Preference scores were compared with repeated measures ANOVA followed by paired t tests for individual comparisons. Group differences in TH, DR-D1, DR-D2, dynorphin, KOR, and DOR mRNA levels were analyzed by one-way ANOVA. One-way ANOVA was also used to analyze group differences in ΔFosB/FosB immunoreactivity in the Acb. Fisher's protected least significant difference (PLSD) post-hoc analyses were performed when required. Correlational analyses were performed on average weekly distance run to the preference score at week 6, number of ΔFosB/FosB immunoreactive cells in the Acb, and levels of individual mRNAs examined. Alpha was set at p ≤ .05 for each analysis and correlation.
Results
Voluntary exercise behavior
Average alternating nightly running distance for rats used in the three experiments is shown in Figure 1A. Average daily running distance of rats used in all experiments increased over time (F (19,437) = 9.22; p < 0.0001). Running behavior did not differ between experiments (F (2,23) = 1.16; p > 0.05). Thus, even though the rats used for CPP testing only had access to their wheels every other night, running behavior closely resembled that of the rats in the other experiments, who had daily, voluntary, access to their wheels (Figure 1A). Figure 1B shows the average hourly distance run during the first, second, and final week of running for rats used for the CPP testing (experiment 1). Running distance peaked shortly after lights-out (1900) and slowly dissipated over time until lights-on (0700). Repeated measures ANOVA revealed a significant main effect of week (F (2, 21) = 5.43; p = 0.01), time of day (F (23, 483) = 43.9; p < 0.0001), and a significant interaction between week of running and time of day (F (46, 483) = 3.56; p < 0.0001). Post hoc analyses revealed that the amount of running during the sleep cycle was negligible and did not differ between groups. For most of the active cycle, however, average hourly distance run during week 6 was greater than during week 1 (from 20:00 hr until 05:00 hr). The average hourly distance run during week 6 only differed from week 2 during 22:00 hr and 23:00 hr. Running distance during the first and second week were similar except for during the peak hour of running at 20:00 hr, when rats ran more during the second week of running compared to the first.
Experiment 1: CPP behavioral testing
Body weights of rats used for the CPP testing increased steadily over the duration of the experiment. Rats weighed 201 ± 2 g at the start of the experiment and 272 ± 5.83 g at the end of the 6 week running period. Running behavior is shown in Figure 1A. Average weekly running distance was not correlated to the preference score at the week 6 time point (r2 = 0.094; p > 0.05).
Experimental timeline appears in Figure 2A. Figure 2B shows the preference for the vertical (paired) side of the CPP chamber during the probe tests. Rats spent equal amounts of time in each chamber during the 10 minute baseline (BL) preference test which took place prior to wheel running and CPP training. Rats developed a preference to the paired side over time. Repeated measures ANOVA resulted in a significant effect of time on CPP (F (3,21) = 6.56; p < 0.01). Two weeks of wheel running did not result in a significant increase in preference for the side of the CPP chamber paired with wheel running. During the probe test at week 6 (probe test B), however, rats displayed a preference for the paired side compared to both the baseline (t = −4.16; p < 0.05) and the 2 week preference tests (t = −2.49; p < 0.05). Wheels were locked following the probe test at the end of week 6 and extinction training started the next day. A probe test following 8 extinction trials (probe test C) revealed that CPP was extinguished following 8 extinction trials. Preference for the vertical (paired) side of the conditioning apparatus during probe test C differed from the preference observed during probe test B (t = 3.5; p < .05), but did not differ from BL preference or the preference observed following 2 weeks of wheel running / CPP training (probe test A).
Experiment 2: ΔFosB/FosB protein expression in the nucleus accumbens
Sedentary (106.375 ± .78 g) and physically active (106.143 ± 1.68 g) rats weighed the same amount prior to the time the physically active rats were allowed access to their wheels. Rats in both groups gained weight over the course of the experiment, but physically active rats gained less weight than their sedentary counterparts (F (1, 14) = 5.89; p < 0.05). At the end of the experiment, sedentary rats weighed 234.25 ± 4.57 g and physically active rats weighed 223.375 ± 3.55 g. Running behavior for Experiment 2 is shown in Figure 1A. No significant correlations were found between average weekly distance run and ΔFosB/FosB immunoreactivity in the rostral (r2 = 0.612), mid (r2 = 0.568), or caudal (r2 = 0.146) Acb core; or rostral (r2 = 0.344), mid (r2 = 0.709), or caudal Acb shell (r2 = 0.085).
Acb tissue from one physically active rat was damaged during processing, yielding final group sizes of n = 8 (sedentary) and n = 7 (physically active). Consistent with prior reports [93], wheel running increased ΔFosB/FosB immunoreactivity in the Acb. Figure 3A shows a photomicrograph of Acb ΔFosB/FosB immunoreactivity. ANOVA revealed a reliable main effect of activity in total ΔFosB/FosB immunoreactivity in the mid core (F (1, 13) = 20.036; p < 0.0006), mid shell (F (1, 13) = 10.935; p < 0.006), caudal core (F (1, 13) = 9.000; p < 0.01), and caudal shell (F (1, 13) = 4.959; p = 0.04) aspects of the Acb (Figure 3B).
Experiment 3: Plasticity in the mesolimbic reward pathway
Sedentary (127.78 ± 1.66 g) and physically active (121.9 ± 2.3 g) rats weighed similar amounts prior to the time the physically active rats were allowed voluntary access to their wheels. Rats in both groups gained weight over the course of the experiment and physical activity had no significant effect on body weight gain. At the end of the experiment, sedentary rats weighed 255.11 ± 4.36 g and physically active rats weighed 238.9 ± 5.38 g. Running behavior for Experiment 3 is shown in Figure 1A. No significant correlations were found between average weekly distance run and levels of any of the mRNAs investigated.
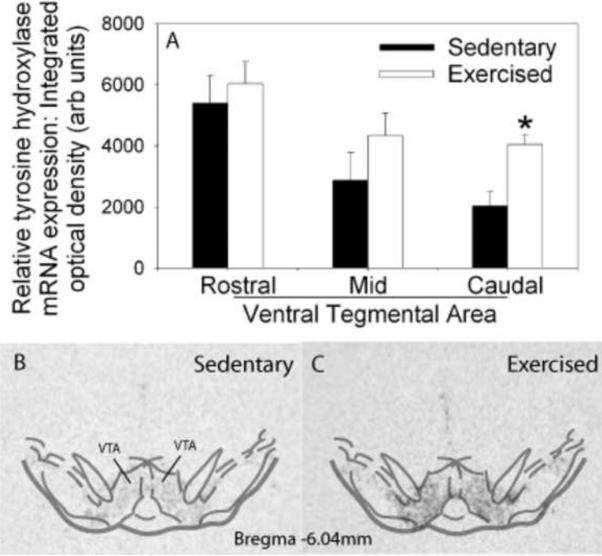
Six weeks of wheel running, compared to sedentary housing, resulted in significant changes in gene transcription of factors capable of modulating reward and dopaminergic neurotransmission in the mesolimbic reward pathway. Compared to sedentary rats, animals allowed access to running wheels for 6 weeks demonstrated an increase in TH mRNA in the VTA (Figure 4A). ANOVA revealed a reliable main effect of activity in the caudal (F (1,17) = 12.721; p < 0.002), but not rostral or mid aspects of the VTA. Representative autoradiographs from a sedentary and physically active rat showing TH mRNA expression in the VTA are shown in Figures 4B and 4C, respectively.
Figure 4.
(A) Relative levels of tyrosine hydroxylase (TH) messenger ribonucleic acid (mRNA) in the rostral to caudal subregions of the ventral tegmental area (VTA) of sedentary rats housed with a locked wheel (Sedentary; n = 9) or rats allowed access to a running wheel for 6 weeks (Exercised; n=10). Values represent mean integrated density ± standard error of measurement. * p < .01 with respect to Sedentary. Representative autoradiographs showing in situ hybridization for TH mRNA in the VTA of sedentary (B) and exercised (C) rats.
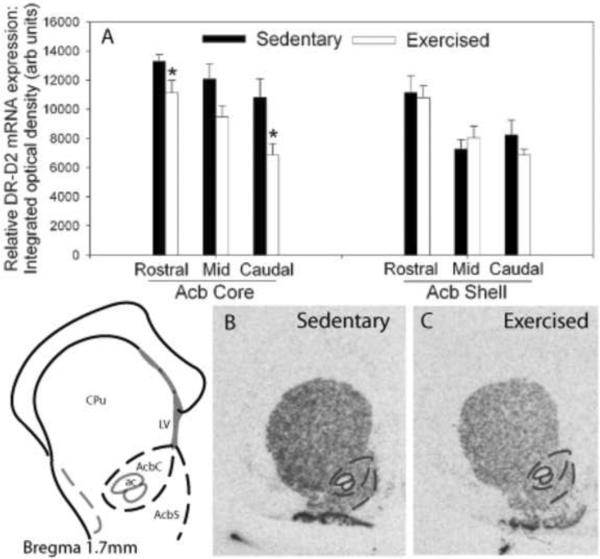
As shown in Figure 5A, 6 weeks of wheel running, compared to sedentary housing, reduced DR-D2 mRNA levels in the Acb. ANOVA revealed a reliable main effect of activity in the rostral (F (1,17) = 4.481; p < 0.05) and caudal core (F (1,17) = 7.332; p < 0.01), and a trend for a reduction in the mid core (F (1,17) = 4.094; p < 0.06) subregions of the Acb. Wheel running had no effect on DR-D2 mRNA levels in the Acb shell. Figures 5A and 5B show representative autoradiographs of DR-D2 mRNA in the Acb of a sedentary and physically active rat, respectively.
Figure 5.
(A) Relative levels of dopamine receptor (DR)-D2 messenger ribonucleic acid (mRNA) in the rostral to caudal subregions of the nucleus accumbens (Acb) of animals allowed access to a running wheel for 6 weeks (Exercised; n=10) or housed under sedentary, locked wheel, conditions (Sedentary; n=9). Values represent mean integrated density ± standard error of measurement. * p<.05 with respect to Sedentary. Representative autoradiographs showing in situ hybridization for DR-D2 mRNA in the Acb of sedentary (B) and exercised (C) rats. CPu, caudate putamen.
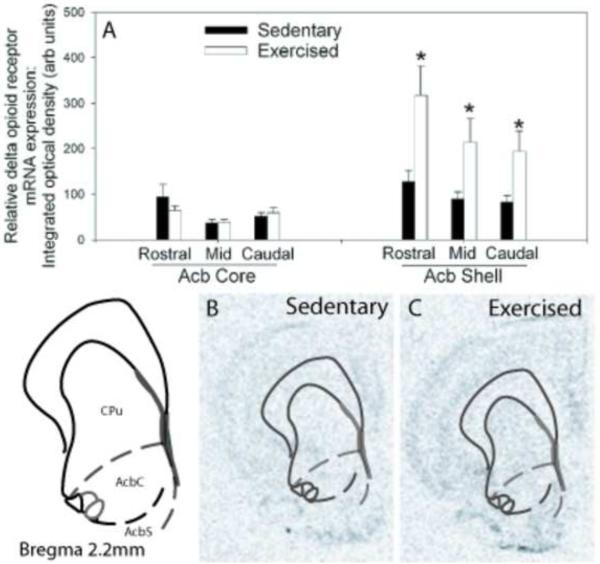
Figure 6.
(A) Relative levels of delta opioid receptor (DOR) messenger ribonucleic acid (mRNA) in the rostral to caudal subregions of the nucleus accumbens (Acb) of sedentary rats (Sedentary; n = 9) or rats allowed voluntary access to running wheels for 6 weeks (Exercised; n=10). Values represent mean integrated density ± standard error of measurement. * p<.05 with respect to Sedentary. Representative autoradiographs showing in situ hybridization for DOR mRNA in the Acb of sedentary (B) and exercised (C) rats. CPu, caudate putamen.
Figure 6A shows the results of 6 weeks of wheel running on DOR mRNA levels in the Acb. Six weeks of wheel running increased DOR mRNA expression in the Acb. ANOVA revealed a reliable main effect of activity in the rostral (F (1,17) = 6.993; p < 0.02), mid (F (1,17) = 4.746; p < 0.04), and caudal shell (F (1,17) = 5.230; p < 0.04), subregions of the Acb. Wheel running had no effect on DOR mRNA levels in the Acb core. Figures 6B and C are representative radioautographs of DOR mRNA in the Acb of a sedentary and physically active rat, respectively.
No group differences were observed for levels of DR-D2 mRNA in the VTA, DR-D1 mRNA in the Acb, dynorphin mRNA in the Acb, or KOR mRNA in the rostral – mid Acb (Table 2). Wheel running compared to sedentary housing, however, did increase KOR mRNA in the caudal aspect of the Acb core (F (1, 17) = 6.821; p < 0.02; Table 2).
Table 2.
Average densities ± standard error of the mean (S.E.M.) for mRNA levels of various factors in the mesolimbic reward pathway. *p< .05
| Factor | Brain area examined | Subdivision | Physically active average density ± S.E.M. (arbitrary units) | Sedentary average density ± S.E.M. (arbitrary units) |
|---|---|---|---|---|
| DR-D2 mRNA | VTA | Rostral | 5736 ± 525 | 6171 ± 788 |
| Mid | 4045 ± 248 | 4314 ± 64 | ||
| Caudal | 2150 ± 180 | 1774 ± 145 | ||
| DR-D1 mRNA | Acb | Rostral Core | 10401 ± 462 | 9997 ± 662 |
| Rostral Shell | 13993 ± 662 | 14966 ± 964 | ||
| Mid Core | 8184 ± 685 | 7632 ± 596 | ||
| Mid Shell | 13871 ± 1334 | 13636 ± 1055 | ||
| Caudal Core | 8889 ± 832 | 8059 ± 1196 | ||
| Caudal Shell | 8458 ± 1043 | 7178 ± 518 | ||
| Dynorphin mRNA | Acb | Rostral Core | 817 ± 282 | 1064 ± 323 |
| Rostral Shell | 872 ± 259 | 1510 ± 402 | ||
| Mid Core | 686 ± 197 | 296 ± 126 | ||
| Mid Shell | 1577 ± 485 | 577 ± 154 | ||
| Caudal Core | 608 ± 189 | 251 ± 53 | ||
| Caudal Shell | 1025 ± 276 | 681 ± 213 | ||
| KOR mRNA | Acb | Rostral Core | 247 ± 48 | 247 ± 50 |
| Rostral Shell | 741 ± 206 | 718 ± 155 | ||
| Mid Core | 254 ± 20 | 206 ± 51 | ||
| Mid Shell | 653 ± 119 | 474 ± 94 | ||
| Caudal Core | 218 ± 50 | 75 ± 15 *p < .05 | ||
| Caudal Shell | 319 ± 54 | 292 ± 109 |
Discussion
Long-term voluntary wheel running is rewarding
The present data are consistent with prior reports that rats can develop CPP to the after-effects of wheel running. The difference between past work and the current experiment is that in all prior studies of which we are aware, rats had limited access to their wheels and conditioning occurred immediately following brief bouts of wheel access [9,53,54]. In contrast, conditioning in the current experiment occurred following wheel access for the entire active cycle. Additionally, rats in prior experiments were either food restricted [53,54], which can increase voluntary running behavior [43], or trained to run using operant conditioning [9]; whereas rats used here had voluntary access to their wheels. The current study, therefore, is the first to demonstrate that rats can develop CPP to the rewarding effects of exercise that linger following a night of voluntary wheel access.
CPP to the side of the conditioning apparatus paired with wheel running was extinguished following 8, 30 min extinction trials. Since the only extinction probe test occurred following 8 extinction training sessions, it is unknown how many days the preference to the paired side would persist following wheel lock. That CPP memory was extinguished following 8 extinction trials is not surprising, however, as extinction to both drug-induced CPP [12,22,82] and CPP to other natural rewards [51,85] can also be observed following 8 or fewer extinction sessions. Thus, the strength of the CPP memory to exercise reward seems to be comparable to other rewarding stimuli. The fact that CPP extinguished supports the interpretation that the significant CPP to the side of the conditioning chamber paired with wheel running observed at week 6 is due to an association between that side of the chamber and the lingering rewarding effects of wheel running, and not to a bias that could have inadvertently developed to the vertically-striped (paired) side over time. In fact, rats spent equivalent amounts of time in both sides of the conditioning apparatus during the extinction probe trial; similar to what was observed during baseline testing. The possibility, however, that a bias to the vertical side of the CPP developed over time regardless of wheel running cannot be ruled out.
Interestingly, 2 weeks of wheel running did not produce significant CPP. Even though average daily distance run during week 2 was less than during week 6, the time-dependent effect of wheel running on CPP is unlikely due to a simple matter of increasing distance run, because; similar to prior reports [9], nightly running distance was not correlated to CPP. These data do not imply that rats do not find short-term voluntary wheel running rewarding, or that 8 pairings of the after-effects of running with the CPP chamber is an insufficient number to support significant CPP. Indeed, F344 rats are motivated to run spontaneously, and Lett et al. (2000) reported significant CPP after only 6 days of once-daily pairings of the after effects of brief (2 hr) wheel running bouts with the CCP chamber, albeit in food deprived rats. Thus, if conditioning had occurred immediately following peak activity levels (2 hr after lights out; Figure 1B), significant CPP may have been observed at the 2 week time point. Instead, the current data support the novel interpretation that as the duration of voluntary wheel access progresses, the rewarding effects of voluntary exercise that support CPP linger longer past the peak of wheel running. The observation that the rewarding effects of exercise persist for hours following the activity peak is similar to that of Smith and Yancey (2003) who reported that the endogenous opioid-dependent antinociception produced by long-term voluntary exercise can also persist for hours after the end of the active cycle. What is especially intriguing is that the persistence of the lingering rewarding effects of voluntary exercise could depend upon the duration of prior wheel access. This effect might be at least partly explained by the slight shift in activity patterns during week 6 compared to week 2. Although the peak of running behavior occurred at 20:00 hr during both weeks 2 and 6 of wheel access, rats ran a greater distance closer to the start of the active cycle as the weeks of running progressed (Figure 1B).
Of note is that paired trials were alternated with unpaired trials during CPP training. In other words, after-effects of wheel running were paired with one side of the CPP chamber during paired trials, and on alternating days, after-effects of the absence of a running wheel were paired with the opposite side of the chamber (an unpaired trial). This design was necessary to avoid a novelty bias during probe trials. There is, however, evidence that animals that are expecting to exercise find the absence of the ability to exercise aversive [58]. Although this effect was probably limited by the fact that rats were accustomed to the alternating schedule of wheel access from the start of the experiment, it is possible that the contrast between the rewarding effects of running and the aversive effects of denied access to the wheel was required to support CPP. In fact, it is feasible that the aversive effect of a lack of an expected running wheel grew over time and thus contributed to the time-dependent effect of wheel running on CPP. Regardless of the interpretation, the data suggest that rats find repeated voluntary wheel running rewarding.
Exercise-induced neuroplasticity in the mesolimbic reward pathway
Six weeks of voluntary wheel running elicited an increase in ΔFosB/FosB immunoreactivity in the Acb. Because the antibody used does not discriminate between ΔFosB and full length FosB, the increase in immunoreactivity could represent either an accumulation of ΔFosB due to repeated exercise-induced activity in the reward pathway, or a state-dependent increase in FosB elicited by wheel running immediately preceding sacrifice. Because FosB induction can habituate following repeated homotypic stimulation [66,80], it is more likely that the increase in ΔFosB/FosB-like immunoreactivity observed represents accumulation of ΔFosB-related antigens. The current observation of increased ΔFosB/FosB in the Acb following wheel running is consistent with prior reports that repeated access to other natural rewards such as sucrose and sexual behavior [91], as well as drugs of abuse [59,60,67], all increase ΔFosB-related antigens in the Acb core and shell. Additionally, Werme et al. (2002) reported an increase in ΔFosB in the Acb core, but not shell, following 30 days of wheel running in Lewis rats. The reason for the discrepancy in ΔFosB expression in the Acb shell between the current data and those of Werme et al. (2002) could lay in the strain of animal used, the time of day of sacrifice, or the duration of wheel running prior to sacrifice. The current observation of increased ΔFosB/FosB expression in the Acb of F344 rats is consistent with the hypothesis [93] that ΔFosB regulates wheel running behavior, which increases over time in this strain (Figure 1 and [37–39]).
Six weeks of wheel running altered gene transcription for several factors involved in reward and dopaminergic neurotransmission in the mesolimbic reward pathway. In the VTA, levels of TH mRNA were elevated in physically active, compared to sedentary, rats. The increase in TH mRNA in the VTA following 6 weeks of voluntary exercise is similar to reported effects of repeated administration of several drugs of abuse [45,52,90,92]. Wheel running has also been reported to increase TH mRNA in substantia nigra [34] and the locus coeruleus [31]. Thus, along with reports that wheel running increases DA and norepinephrine content in several brain regions [28,78], these cumulative data suggest that voluntary exercise can increase the synthetic capacity of catecholamines throughout the brain.
Voluntary exercise also produced specific neuronal plasticity in the Acb. Six weeks of wheel running reduced DR-D2 mRNA in the Acb core, but not Acb shell or VTA, increased DOR mRNA in the Acb shell but not core, and increased KOR mRNA in the caudal aspect of the Acb core. The observed differences in mRNA levels between sedentary and physically active rats could represent stable changes in gene expression in the mesolimbic reward pathway as a consequence of repeated exposure to voluntary exercise. Because of these regions' involvement in motor activity [17,30,83] as well as reward, it is impossible to determine whether the observed changes are due to the rewarding effects of exercise or are simply consequences of motor activity per se. The observation that running distance did not correlate with the CPP behavior or any of the mRNA changes suggests that the neural plasticity in the mesolimbic pathway is not due to movement or activity per se. Instead, reaching a certain threshold of running behavior may be sufficient for wheel running to elicit rewarding effects and alterations in gene expression in the mesolimbic reward pathway. Regardless of the cause of the observed changes in mRNA levels (be it repeated reward or activity), the effects of these changes could certainly impact neurotransmission in the mesolimbic reward pathway and thus have important consequences on reward- or activity-related behavior.
Due to the complex interplay between opioidergic and DA systems in the Acb [1,26,42], how the potential changes in Acb gene expression could functionally influence the mesolimbic pathway is not obvious. DR-D2 are Gi-coupled receptors located on GABA-expressing neurons in the Acb. Thus, a reduction of DR-D2 expression in the Acb following physical activity could alter Acb neurotransmission via GABAergic disinhibition. Furthermore, because DOR activation can facilitate DA release in the Acb [42,65], an increase in DOR expression in the Acb following voluntary exercise could, along with the increase in DA synthetic capacity, contribute to an enhancement of Acb DA neurotransmission.
Although an understanding of whether the plastic changes produced by repeated voluntary exercise reward are similar to those elicited by other natural rewards or drugs of abuse would be useful, such a comparison is difficult due to the inconsistencies in the literature regarding the effects of rewarding stimuli on plasticity in the mesolimbic reward pathway. For example, although repeated sucrose consumption [79], repeated morphine [35,86], and cocaine [81] have all been reported to decrease DR-D2 mRNA in the rodent Acb similar to the observed effect of wheel running, opposite effects have also been reported [5,50,57]. Inconsistencies could be due to a variety of factors including differences in duration of administration, type of drug, dose, or route of administration. Additional research will be necessary in order to reveal whether the observed changes in gene transcription following repeated voluntary exercise include changes common to many rewarding stimuli, or represent unique effects specific to exercise. Regardless of the commonalities between voluntary exercise and other rewarding stimuli, the observed neuronal plasticity elicited by repeated wheel running could alter opioidergic or DA neurotransmission in the mesolimbic reward pathway. Such changes could account for the altered sensitivity to drugs of abuse often observed following repeated voluntary wheel running [10,41,46,52,72,81,89].
In conclusion, the current results support the idea that long-term, repeated voluntary exercise produces a rewarding state that persists following exercise cessation, and elicits plastic changes in the mesolimbic reward pathway. Neuroplasticity in the reward pathway following voluntary exercise could contribute to the beneficial effects of exercise which are related to DA neurotransmission including reduced incidence and severity of substance abuse disorders and facilitation of successful stress coping.
Research Highlights.
Rats find repeated voluntary exercise rewarding
Rewarding effects of exercise grow over time
Voluntary exercise alters gene transcription in the mesolimbic reward neurocircuitry
Acknowledgments
Support: R01 MH 068283 (MF)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Akil H, Owens C, Gutstein H, Taylor L, Curran E, Watson S. Endogenous opioids: overview and current issues. Drug Alcohol Depend. 1998;51:127–40. doi: 10.1016/s0376-8716(98)00071-4. [DOI] [PubMed] [Google Scholar]
- [2].Andersson M, Westin JE, Cenci MA. Time course of striatal DeltaFosB-like immunoreactivity and prodynorphin mRNA levels after discontinuation of chronic dopaminomimetic treatment. Eur J Neurosci. 2003;17:661–6. doi: 10.1046/j.1460-9568.2003.02469.x. [DOI] [PubMed] [Google Scholar]
- [3].Azaryan AV, Coughlin LJ, Buzas B, Clock BJ, Cox BM. Effect of chronic cocaine treatment on mu- and delta-opioid receptor mRNA levels in dopaminergically innervated brain regions. J Neurochem. 1996;66:443–8. doi: 10.1046/j.1471-4159.1996.66020443.x. [DOI] [PubMed] [Google Scholar]
- [4].Babyak M, Blumenthal JA, Herman S, Khatri P, Doraiswamy M, Moore K, Craighead WE, Baldewicz TT, Krishnan KR. Exercise treatment for major depression: maintenance of therapeutic benefit at 10 months. Psychosom Med. 2000;62:633–8. doi: 10.1097/00006842-200009000-00006. [DOI] [PubMed] [Google Scholar]
- [5].Bahk JY, Li S, Park MS, Kim MO. Dopamine D1 and D2 receptor mRNA up-regulation in the caudate-putamen and nucleus accumbens of rat brains by smoking. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:1095–104. doi: 10.1016/s0278-5846(02)00243-9. [DOI] [PubMed] [Google Scholar]
- [6].Belke TW. Running and responding reinforced by the opportunity to run: effect of reinforcer duration. J Exp Anal Behav. 1997;67:337–51. doi: 10.1901/jeab.1997.67-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Belke TW. Studies of wheel-running reinforcement: parameters of Herrnstein's (1970) response-strength equation vary with schedule order. J Exp Anal Behav. 2000;73:319–31. doi: 10.1901/jeab.2000.73-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Belke TW. Varying wheel-running reinforcer duration within a session: effect on the revolution-postreinforcement pause relation. J Exp Anal Behav. 2000;73:225–39. doi: 10.1901/jeab.2000.73-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Belke TW, Wagner JP. The reinforcing property and the rewarding aftereffect of wheel running in rats: a combination of two paradigms. Behav Processes. 2005;68:165–72. doi: 10.1016/j.beproc.2004.12.006. [DOI] [PubMed] [Google Scholar]
- [10].Bello NT, Lucas LR, Hajnal A. Repeated sucrose access influences dopamine D2 receptor density in the striatum. Neuroreport. 2002;13:1575–8. doi: 10.1097/00001756-200208270-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Berchtold NC, Chinn G, Chou M, Kesslak JP, Cotman CW. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience. 2005;133:853–61. doi: 10.1016/j.neuroscience.2005.03.026. [DOI] [PubMed] [Google Scholar]
- [12].Bernardi RE, Lattal KM. A role for alpha-adrenergic receptors in extinction of conditioned fear and cocaine conditioned place preference. Behav Neurosci. 124:204–10. doi: 10.1037/a0018909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Blumenthal JA, Babyak MA, Doraiswamy PM, Watkins L, Hoffman BM, Barbour KA, Herman S, Craighead WE, Brosse AL, Waugh R, Hinderliter A, Sherwood A. Exercise and pharmacotherapy in the treatment of major depressive disorder. Psychosom Med. 2007;69:587–96. doi: 10.1097/PSY.0b013e318148c19a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Brene S, Bjornebekk A, Aberg E, Mathe AA, Olson L, Werme M. Running is rewarding and antidepressive. Physiol Behav. 2007 doi: 10.1016/j.physbeh.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Brown RA, Abrantes AM, Read JP, Marcus BH, Jakicic J, Strong DR, Oakley JR, Ramsey SE, Kahler CW, Stuart G, Dubreuil ME, Gordon AA. Aerobic exercise for alcohol recovery: rationale, program description, and preliminary findings. Behav Modif. 2009;33:220–49. doi: 10.1177/0145445508329112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Burghardt PR, Fulk LJ, Hand GA, Wilson MA. The effects of chronic treadmill and wheel running on behavior in rats. Brain Res. 2004;1019:84–96. doi: 10.1016/j.brainres.2004.05.086. [DOI] [PubMed] [Google Scholar]
- [17].Churchill L, Klitenick MA, Kalivas PW. Dopamine depletion reorganizes projections from the nucleus accumbens and ventral pallidum that mediate opioid-induced motor activity. J Neurosci. 1998;18:8074–85. doi: 10.1523/JNEUROSCI.18-19-08074.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Clay SW, Allen J, Parran T. A review of addiction. Postgrad Med. 2008;120:E01–7. doi: 10.3810/pgm.2008.07.1802. [DOI] [PubMed] [Google Scholar]
- [19].Collingwood TR, Reynolds R, Kohl HW, Smith W, Sloan S. Physical fitness effects on substance abuse risk factors and use patterns. J Drug Educ. 1991;21:73–84. doi: 10.2190/HV5J-4EYN-GPP7-Y3QG. [DOI] [PubMed] [Google Scholar]
- [20].Collingwood TR, Sunderlin J, Reynolds R, Kohl HW., 3rd Physical training as a substance abuse prevention intervention for youth. J Drug Educ. 2000;30:435–51. doi: 10.2190/RVUE-9XW7-TYRQ-EJR8. [DOI] [PubMed] [Google Scholar]
- [21].Conway KP, Compton W, Stinson FS, Grant BF. Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2006;67:247–57. doi: 10.4088/jcp.v67n0211. [DOI] [PubMed] [Google Scholar]
- [22].Crawford CA, Baella SA, Farley CM, Herbert MS, Horn LR, Campbell RH, Zavala AR. Early methylphenidate exposure enhances cocaine self-administration but not cocaine-induced conditioned place preference in young adult rats. Psychopharmacology (Berl) doi: 10.1007/s00213-010-2011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Day HE, Akil H. Differential pattern of c-fos mRNA in rat brain following central and systemic administration of interleukin-1-beta: implications for mechanism of action. Neuroendocrinology. 1996;63:207–18. doi: 10.1159/000126959. [DOI] [PubMed] [Google Scholar]
- [24].de Oliveira AR, Reimer AE, Brandao ML. Role of dopamine receptors in the ventral tegmental area in conditioned fear. Behav Brain Res. 2009;199:271–7. doi: 10.1016/j.bbr.2008.12.004. [DOI] [PubMed] [Google Scholar]
- [25].Di Benedetto M, D'Addario C, Candeletti S, Romualdi P. Chronic and acute effects of 3,4-methylenedioxy-N-methylamphetamine (`Ecstasy') administration on the dynorphinergic system in the rat brain. Neuroscience. 2006;137:187–96. doi: 10.1016/j.neuroscience.2005.09.015. [DOI] [PubMed] [Google Scholar]
- [26].Di Chiara G, Imperato A. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther. 1988;244:1067–80. [PubMed] [Google Scholar]
- [27].Dishman RK, Berthoud HR, Booth FW, Cotman CW, Edgerton VR, Fleshner MR, Gandevia SC, Gomez-Pinilla F, Greenwood BN, Hillman CH, Kramer AF, Levin BE, Moran TH, Russo-Neustadt AA, Salamone JD, Van Hoomissen JD, Wade CE, York DA, Zigmond MJ. Neurobiology of exercise. Obesity (Silver Spring) 2006;14:345–56. doi: 10.1038/oby.2006.46. [DOI] [PubMed] [Google Scholar]
- [28].Dishman RK, Warren JM, Youngstedt SD, Yoo H, Bunnell BN, Mougey EH, Meyerhoff JL, Friedmann LJ, Evans DL. Brain monoamines, exercise, and behavioral stress: animal models. Medicine and Science in Sports and Exercise. 1997;29:63–74. doi: 10.1097/00005768-199701000-00010. [DOI] [PubMed] [Google Scholar]
- [29].Doucet JP, Nakabeppu Y, Bedard PJ, Hope BT, Nestler EJ, Jasmin BJ, Chen JS, Iadarola MJ, St-Jean M, Wigle N, Blanchet P, Grondin R, Robertson GS. Chronic alterations in dopaminergic neurotransmission produce a persistent elevation of deltaFosB-like protein(s) in both the rodent and primate striatum. Eur J Neurosci. 1996;8:365–81. doi: 10.1111/j.1460-9568.1996.tb01220.x. [DOI] [PubMed] [Google Scholar]
- [30].Dreher JK, Jackson DM. Role of D1 and D2 dopamine receptors in mediating locomotor activity elicited from the nucleus accumbens of rats. Brain Res. 1989;487:267–77. doi: 10.1016/0006-8993(89)90831-7. [DOI] [PubMed] [Google Scholar]
- [31].Droste SK, Schweizer MC, Ulbricht S, Reul JM. Long-term voluntary exercise and the mouse hypothalamic-pituitary-adrenocortical axis: impact of concurrent treatment with the antidepressant drug tianeptine. J Neuroendocrinol. 2006;18:915–25. doi: 10.1111/j.1365-2826.2006.01489.x. [DOI] [PubMed] [Google Scholar]
- [32].Eikelboom R, Mills R. A microanalysis of wheel running in male and female rats. Physiol Behav. 1988;43:625–30. doi: 10.1016/0031-9384(88)90217-x. [DOI] [PubMed] [Google Scholar]
- [33].Feltenstein MW, See RE. The neurocircuitry of addiction: an overview. Br J Pharmacol. 2008;154:261–74. doi: 10.1038/bjp.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Foley TE, Fleshner M. Neuroplasticity of dopamine circuits after exercise: implications for central fatigue. Neuromolecular Med. 2008;10:67–80. doi: 10.1007/s12017-008-8032-3. [DOI] [PubMed] [Google Scholar]
- [35].Georges F, Stinus L, Bloch B, Le Moine C. Chronic morphine exposure and spontaneous withdrawal are associated with modifications of dopamine receptor and neuropeptide gene expression in the rat striatum. Eur J Neurosci. 1999;11:481–90. doi: 10.1046/j.1460-9568.1999.00462.x. [DOI] [PubMed] [Google Scholar]
- [36].Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, Pickering RP, Kaplan K. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61:807–16. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- [37].Greenwood BN, Foley TE, Burhans D, Maier SF, Fleshner M. The consequences of uncontrollable stress are sensitive to duration of prior wheel running. Brain Res. 2005;1033:164–78. doi: 10.1016/j.brainres.2004.11.037. [DOI] [PubMed] [Google Scholar]
- [38].Greenwood BN, Foley TE, Day HE, Burhans D, Brooks L, Campeau S, Fleshner M. Wheel running alters serotonin (5-HT) transporter, 5-HT(1A), 5-HT(1B), and alpha(1b)-adrenergic receptor mRNA in the rat raphe nuclei. Biol Psychiatry. 2005;57:559–68. doi: 10.1016/j.biopsych.2004.11.025. [DOI] [PubMed] [Google Scholar]
- [39].Greenwood BN, Foley TE, Day HE, Campisi J, Hammack SH, Campeau S, Maier SF, Fleshner M. Freewheel running prevents learned helplessness/behavioral depression: role of dorsal raphe serotonergic neurons. J Neurosci. 2003;23:2889–98. doi: 10.1523/JNEUROSCI.23-07-02889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Greenwood BN, Kennedy S, Smith TP, Campeau S, Day HE, Fleshner M. Voluntary freewheel running selectively modulates catecholamine content in peripheral tissue and c-Fos expression in the central sympathetic circuit following exposure to uncontrollable stress in rats. Neuroscience. 2003;120:269–81. doi: 10.1016/s0306-4522(03)00047-2. [DOI] [PubMed] [Google Scholar]
- [41].Hajnal A, Norgren R. Repeated access to sucrose augments dopamine turnover in the nucleus accumbens. Neuroreport. 2002;13:2213–6. doi: 10.1097/00001756-200212030-00010. [DOI] [PubMed] [Google Scholar]
- [42].Hipolito L, Sanchez-Catalan MJ, Zanolini I, Polache A, Granero L. Shell/core differences in mu- and delta-opioid receptor modulation of dopamine efflux in nucleus accumbens. Neuropharmacology. 2008;55:183–9. doi: 10.1016/j.neuropharm.2008.05.012. [DOI] [PubMed] [Google Scholar]
- [43].Holloszy JO. Mortality rate and longevity of food-restricted exercising male rats: a reevaluation. J Appl Physiol. 1997;82:399–403. doi: 10.1152/jappl.1997.82.2.399. [DOI] [PubMed] [Google Scholar]
- [44].Hope BT, Nye HE, Kelz MB, Self DW, Iadarola MJ, Nakabeppu Y, Duman RS, Nestler EJ. Induction of a long-lasting AP-1 complex composed of altered Fos-like proteins in brain by chronic cocaine and other chronic treatments. Neuron. 1994;13:1235–44. doi: 10.1016/0896-6273(94)90061-2. [DOI] [PubMed] [Google Scholar]
- [45].Hurd YL, Lindefors N, Brodin E, Brene S, Persson H, Ungerstedt U, Hokfelt T. Amphetamine regulation of mesolimbic dopamine/cholecystokinin neurotransmission. Brain Res. 1992;578:317–26. doi: 10.1016/0006-8993(92)90264-a. [DOI] [PubMed] [Google Scholar]
- [46].Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–98. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- [47].Iversen IH. Techniques for establishing schedules with wheel running as reinforcement in rats. J Exp Anal Behav. 1993;60:219–38. doi: 10.1901/jeab.1993.60-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Janse Van Rensburg K, Taylor A, Hodgson T, Benattayallah A. Acute exercise modulates cigarette cravings and brain activation in response to smoking-related images: an fMRI study. Psychopharmacology (Berl) 2009;203:589–98. doi: 10.1007/s00213-008-1405-3. [DOI] [PubMed] [Google Scholar]
- [49].Kessler RC, Demler O, Frank RG, Olfson M, Pincus HA, Walters EE, Wang P, Wells KB, Zaslavsky AM. Prevalence and treatment of mental disorders, 1990 to 2003. N Engl J Med. 2005;352:2515–23. doi: 10.1056/NEJMsa043266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kim MO, Lee YK, Choi WS, Kim JH, Hwang SK, Lee BJ, Kang SG, Kim K, Baik SH. Prolonged ethanol intake increases D2 dopamine receptor expression in the rat brain. Mol Cells. 1997;7:682–7. [PubMed] [Google Scholar]
- [51].La Mela I, Latagliata EC, Patrono E, Puglisi-Allegra S, Ventura R. Olfactory priming reinstates extinguished chocolate-induced conditioned place preference. Appetite. 54:237–40. doi: 10.1016/j.appet.2009.12.008. [DOI] [PubMed] [Google Scholar]
- [52].Lee YK, Park SW, Kim YK, Kim DJ, Jeong J, Myrick H, Kim YH. Effects of naltrexone on the ethanol-induced changes in the rat central dopaminergic system. Alcohol Alcohol. 2005;40:297–301. doi: 10.1093/alcalc/agh163. [DOI] [PubMed] [Google Scholar]
- [53].Lett BT, Grant VL, Byrne MJ, Koh MT. Pairings of a distinctive chamber with the aftereffect of wheel running produce conditioned place preference. Appetite. 2000;34:87–94. doi: 10.1006/appe.1999.0274. [DOI] [PubMed] [Google Scholar]
- [54].Lett BT, Grant VL, Koh MT. Naloxone attenuates the conditioned place preference induced by wheel running in rats. Physiol Behav. 2001;72:355–8. doi: 10.1016/s0031-9384(00)00427-3. [DOI] [PubMed] [Google Scholar]
- [55].Lett BT, Grant VL, Koh MT, Flynn G. Prior experience with wheel running produces crosstolerance to the rewarding effect of morphine. Pharmacol Biochem Behav. 2002;72:101–5. doi: 10.1016/s0091-3057(01)00722-5. [DOI] [PubMed] [Google Scholar]
- [56].Manger TA, Motta RW. The impact of an exercise program on posttraumatic stress disorder, anxiety, and depression. Int J Emerg Ment Health. 2005;7:49–57. [PubMed] [Google Scholar]
- [57].Mantsch JR, Yuferov V, Mathieu-Kia AM, Ho A, Kreek MJ. Effects of extended access to high versus low cocaine doses on self-administration, cocaine-induced reinstatement and brain mRNA levels in rats. Psychopharmacology (Berl) 2004;175:26–36. doi: 10.1007/s00213-004-1778-x. [DOI] [PubMed] [Google Scholar]
- [58].Mason GJ, Cooper J, Clarebrough C. Frustrations of fur-farmed mink. Nature. 2001;410:35–6. doi: 10.1038/35065157. [DOI] [PubMed] [Google Scholar]
- [59].Muller DL, Unterwald EM. D1 dopamine receptors modulate deltaFosB induction in rat striatum after intermittent morphine administration. J Pharmacol Exp Ther. 2005;314:148–54. doi: 10.1124/jpet.105.083410. [DOI] [PubMed] [Google Scholar]
- [60].Nestler EJ. Review. Transcriptional mechanisms of addiction: role of DeltaFosB. Philos Trans R Soc Lond B Biol Sci. 2008;363:3245–55. doi: 10.1098/rstb.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Nestler EJ, Barrot M, Self DW. DeltaFosB: a sustained molecular switch for addiction. Proc Natl Acad Sci U S A. 2001;98:11042–6. doi: 10.1073/pnas.191352698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Nestler EJ, Carlezon WA., Jr. The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–9. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- [63].Newman CL, Motta RW. The effects of aerobic exercise on childhood PTSD, anxiety, and depression. Int J Emerg Ment Health. 2007;9:133–58. [PubMed] [Google Scholar]
- [64].Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1998. [Google Scholar]
- [65].Pentney RJ, Gratton A. Effects of local delta and mu opioid receptor activation on basal and stimulated dopamine release in striatum and nucleus accumbens of rat: an in vivo electrochemical study. Neuroscience. 1991;45:95–102. doi: 10.1016/0306-4522(91)90106-x. [DOI] [PubMed] [Google Scholar]
- [66].Perrotti LI, Hadeishi Y, Ulery PG, Barrot M, Monteggia L, Duman RS, Nestler EJ. Induction of deltaFosB in reward-related brain structures after chronic stress. J Neurosci. 2004;24:10594–602. doi: 10.1523/JNEUROSCI.2542-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Perrotti LI, Weaver RR, Robison B, Renthal W, Maze I, Yazdani S, Elmore RG, Knapp DJ, Selley DE, Martin BR, Sim-Selley L, Bachtell RK, Self DW, Nestler EJ. Distinct patterns of DeltaFosB induction in brain by drugs of abuse. Synapse. 2008;62:358–69. doi: 10.1002/syn.20500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Pezze MA, Feldon J. Mesolimbic dopaminergic pathways in fear conditioning. Prog Neurobiol. 2004;74:301–20. doi: 10.1016/j.pneurobio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- [69].Rosin A, Lindholm S, Franck J, Georgieva J. Downregulation of kappa opioid receptor mRNA levels by chronic ethanol and repetitive cocaine in rat ventral tegmentum and nucleus accumbens. Neurosci Lett. 1999;275:1–4. doi: 10.1016/s0304-3940(99)00675-8. [DOI] [PubMed] [Google Scholar]
- [70].Rosin A, van der Ploeg I, Georgieva J. Basal and cocaine-induced opioid receptor gene expression in the rat CNS analyzed by competitive reverse transcription PCR. Brain Res. 2000;872:102–9. doi: 10.1016/s0006-8993(00)02481-1. [DOI] [PubMed] [Google Scholar]
- [71].Schlussman SD, Zhou Y, Bailey A, Ho A, Kreek MJ. Steady-dose and escalating-dose “binge” administration of cocaine alter expression of behavioral stereotypy and striatal preprodynorphin mRNA levels in rats. Brain Res Bull. 2005;67:169–75. doi: 10.1016/j.brainresbull.2005.04.018. [DOI] [PubMed] [Google Scholar]
- [72].Schmidt HD, Pierce RC. Cocaine-induced neuroadaptations in glutamate transmission: potential therapeutic targets for craving and addiction. Ann N Y Acad Sci. 1187:35–75. doi: 10.1111/j.1749-6632.2009.05144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Smith MA, Gergans SR, Iordanou JC, Lyle MA. Chronic exercise increases sensitivity to the conditioned rewarding effects of cocaine. Pharmacol Rep. 2008;60:561–5. [PMC free article] [PubMed] [Google Scholar]
- [74].Smith MA, Lyle MA. Chronic exercise decreases sensitivity to mu opioids in female rats: correlation with exercise output. Pharmacol Biochem Behav. 2006;85:12–22. doi: 10.1016/j.pbb.2006.06.020. [DOI] [PubMed] [Google Scholar]
- [75].Smith MA, McClean JM, Bryant PA. Sensitivity to the effects of a kappa opioid in rats with free access to exercise wheels: differential effects across behavioral measures. Pharmacol Biochem Behav. 2004;77:49–57. doi: 10.1016/j.pbb.2003.09.021. [DOI] [PubMed] [Google Scholar]
- [76].Smith MA, Schmidt KT, Iordanou JC, Mustroph ML. Aerobic exercise decreases the positive-reinforcing effects of cocaine. Drug Alcohol Depend. 2008;98:129–35. doi: 10.1016/j.drugalcdep.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Smith MA, Yancey DL. Sensitivity to the effects of opioids in rats with free access to exercise wheels: mu-opioid tolerance and physical dependence. Psychopharmacology (Berl) 2003;168:426–34. doi: 10.1007/s00213-003-1471-5. [DOI] [PubMed] [Google Scholar]
- [78].Soares J, Holmes PV, Renner KJ, Edwards GL, Bunnell BN, Dishman RK. Brain noradrenergic responses to footshock after chronic activity-wheel running. Behav Neurosci. 1999;113:558–66. doi: 10.1037//0735-7044.113.3.558. [DOI] [PubMed] [Google Scholar]
- [79].Spangler R, Wittkowski KM, Goddard NL, Avena NM, Hoebel BG, Leibowitz SF. Opiate-like effects of sugar on gene expression in reward areas of the rat brain. Brain Res Mol Brain Res. 2004;124:134–42. doi: 10.1016/j.molbrainres.2004.02.013. [DOI] [PubMed] [Google Scholar]
- [80].Stamp JA, Herbert J. Multiple immediate-early gene expression during physiological and endocrine adaptation to repeated stress. Neuroscience. 1999;94:1313–22. doi: 10.1016/s0306-4522(99)00368-1. [DOI] [PubMed] [Google Scholar]
- [81].Stefanski R, Ziolkowska B, Kusmider M, Mierzejewski P, Wyszogrodzka E, Kolomanska P, Dziedzicka-Wasylewska M, Przewlocki R, Kostowski W. Active versus passive cocaine administration: differences in the neuroadaptive changes in the brain dopaminergic system. Brain Res. 2007;1157:1–10. doi: 10.1016/j.brainres.2007.04.074. [DOI] [PubMed] [Google Scholar]
- [82].Sticht M, Mitsubata J, Tucci M, Leri F. Reacquisition of heroin and cocaine place preference involves a memory consolidation process sensitive to systemic and intra-ventral tegmental area naloxone. Neurobiol Learn Mem. 93:248–60. doi: 10.1016/j.nlm.2009.10.005. [DOI] [PubMed] [Google Scholar]
- [83].Swanson CJ, Kalivas PW. Regulation of locomotor activity by metabotropic glutamate receptors in the nucleus accumbens and ventral tegmental area. J Pharmacol Exp Ther. 2000;292:406–14. [PubMed] [Google Scholar]
- [84].Talalaenko AN, Abramets IA, Stakhovskii Yu V, Shekhovtsov AA, Chernikov AV, Shevchenko SL. The role of dopaminergic mechanisms on the brain in various models of anxious states. Neurosci Behav Physiol. 1994;24:284–8. doi: 10.1007/BF02362037. [DOI] [PubMed] [Google Scholar]
- [85].Trezza V, Damsteegt R, Vanderschuren LJ. Conditioned place preference induced by social play behavior: parametrics, extinction, reinstatement and disruption by methylphenidate. Eur Neuropsychopharmacol. 2009;19:659–69. doi: 10.1016/j.euroneuro.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Turchan J, Lason W, Budziszewska B, Przewlocka B. Effects of single and repeated morphine administration on the prodynorphin, proenkephalin and dopamine D2 receptor gene expression in the mouse brain. Neuropeptides. 1997;31:24–8. doi: 10.1016/s0143-4179(97)90015-9. [DOI] [PubMed] [Google Scholar]
- [87].Ussher M, Nunziata P, Cropley M, West R. Effect of a short bout of exercise on tobacco withdrawal symptoms and desire to smoke. Psychopharmacology (Berl) 2001;158:66–72. doi: 10.1007/s002130100846. [DOI] [PubMed] [Google Scholar]
- [88].Van Rensburg KJ, Taylor A, Hodgson T. The effects of acute exercise on attentional bias towards smoking-related stimuli during temporary abstinence from smoking. Addiction. 2009;104:1910–7. doi: 10.1111/j.1360-0443.2009.02692.x. [DOI] [PubMed] [Google Scholar]
- [89].Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- [90].Vrana SL, Vrana KE, Koves TR, Smith JE, Dworkin SI. Chronic cocaine administration increases CNS tyrosine hydroxylase enzyme activity and mRNA levels and tryptophan hydroxylase enzyme activity levels. J Neurochem. 1993;61:2262–8. doi: 10.1111/j.1471-4159.1993.tb07468.x. [DOI] [PubMed] [Google Scholar]
- [91].Wallace DL, Vialou V, Rios L, Carle-Florence TL, Chakravarty S, Kumar A, Graham DL, Green TA, Kirk A, Iniguez SD, Perrotti LI, Barrot M, DiLeone RJ, Nestler EJ, Bolanos-Guzman CA. The influence of DeltaFosB in the nucleus accumbens on natural reward-related behavior. J Neurosci. 2008;28:10272–7. doi: 10.1523/JNEUROSCI.1531-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Wei XL, Su RB, Wu N, Lu XQ, Zheng JQ, Li J. Agmatine inhibits morphine-induced locomotion sensitization and morphine-induced changes in striatal dopamine and metabolites in rats. Eur Neuropsychopharmacol. 2007;17:790–9. doi: 10.1016/j.euroneuro.2007.04.001. [DOI] [PubMed] [Google Scholar]
- [93].Werme M, Messer C, Olson L, Gilden L, Thoren P, Nestler EJ, Brene S. Delta FosB Regulates Wheel Running. J Neurosci. 2002;22:8133–8. doi: 10.1523/JNEUROSCI.22-18-08133.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Yadid G, Friedman A. Dynamics of the dopaminergic system as a key component to the understanding of depression. Prog Brain Res. 2008;172:265–86. doi: 10.1016/S0079-6123(08)00913-8. [DOI] [PubMed] [Google Scholar]