Summary
Although Δ9-tetrahydrocannabinol (THC) and other mixed CB1/CB2 receptor agonists are well established to elicit antinociceptive effects, their psychomimetic actions and potential for abuse have dampened enthusiasm for their therapeutic development. Conversely, CB2 receptor-selective agonists have been shown to reduce pain and inflammation, without eliciting apparent cannabinoid behavioral effects. In the present study, we developed a novel ethyl sulfonamide THC analog, O-3223, and compared its pharmacological effects to those of the potent, mixed CB1/CB2 receptor agonist, CP55,940, in battery of preclinical pain models. Competitive cannabinoid receptor binding experiments revealed that O-3223 was approximately 80-fold more selective for CB2 than CB1 receptors. Additionally, O-3223 behaved as full CB2 receptor agonist in [35S]GTPγS binding. O-3223 reduced nociceptive behavior in both phases of the formalin test, reduced thermal hyperalgesia in the chronic constrictive injury of the sciatic nerve (CCI) model, and reduced edema and thermal hyperalgesia elicited by intraplantar injection of LPS. These effects were blocked by pretreatment with the CB2 receptor-selective antagonist SR144528, but not by the CB1 receptor antagonist, rimonabant. Unlike CP55,940, O-3223 did not elicit acute antinociceptive effects in the hot-plate test, hypothermia, or motor disturbances, as assessed in the rotarod test. These data indicate that the CB2 receptor-selective agonist, O-3223, reduces inflammatory and neuropathic nociception, without affecting basal nociception or eliciting overt behavioral effects. Moreover, this compound can serve as a template to develop new CB2 receptor agonists with increased receptor selectivity and increased potency in treating inflammatory and neuropathic pain.
Keywords: Endogenous cannabinoid agonist, inflammation, neuropathic pain, CB1, CB2, lipopolysaccharide induced edema
Introduction
Chronic pain decreases quality of life and is comorbid with depression, anxiety, and suicidal thoughts (Braden and Sullivan, 2008). Traditional analgesic and anti-inflammatory agents, such as opiates, steroids, and non-steroidal anti-inflammatory drugs (NSAIDs) all have serious side effects, particularly at high doses taken by chronic users. Written records of the use of Cannabis sativa and various cannabis extracts to treat a variety of ailments, including pain, date back to thousands of years and interest in cannabinoids persists in modern times (Kogan and Mechoulam, 2007). The primary psychoactive constituent of marijuana, Δ9-tetrahydrocannabinol (THC), is an effective analgesic, but has limited use as a clinical therapeutic treatment because of its abuse potential and psychomimetic side effects.
THC binds to both CB1 and CB2 receptors and inhibits adenylyl cyclase through the inhibition of Gi/Go proteins (Mackie, 2006). In addition to the endogenously-produced cannabinoids, including anandamide (AEA) (Devane et al., 1992) and 2-arachidonylglycerol (2-AG) (Mechoulam et al., 1995), exogenous plant-derived and synthetic cannabinoids are also ligands for both cannabinoid receptors. Potent mixed CB1/CB2 receptor agonists, such as CP55,940, produce analgesic effects, accompanied with centrally-mediated cannabimimetic effects, including hypothermia, hypomotility, catalepsy, and THC-like subjective effects in rodents (Little et al., 1988). The CB1 receptor is expressed throughout the nervous system, including regions that regulate pain transmission, such as the periaqueductal gray, rostral ventral medulla, dorsal horn of the spinal cord, dorsal root ganglia. CB1 receptors in the central nervous system mediate the psychomimetic effects of cannabinoids, as well as the potential for abuse and dependence of THC. Although CB1 is also expressed in lung, liver, and kidney, its role in peripheral tissue is not well understood (Mackie, 2006).
In addition to the CB1 receptor, much recent work focuses on the role of the CB2 receptor in modulating nociception (Guindon and Hohmann, 2008). In healthy animals, CB2 receptors are sparsely expressed in the CNS and are predominately expressed on activated immune cells, including natural killer cells, monocytes, macrophages, dendritic cells, neutrophils, B and T cells, and microglia, at levels roughly 10–100 times that of CB1 (Galiegue et al., 1995), but are expressed at very low levels in non-activated immune cells. Thus, the CB2 receptor represents a viable target for the development of anti-inflammatory and analgesic agents that lack overt behavioral effects, and has therefore gained much recent attention, as evidenced by a rapidly growing body of research (Guindon and Hohmann, 2008).
The primary goal of the present study was to develop CB2 receptor selective compounds that selectively activate the CB2 receptor without eliciting CB1 receptor mediated cannabimimetic effects, such as locomotor inhibition and hypothermia. Here, we report that the ethyl sulfonamide THC analog, O-3223 displayed excellent selectivity and efficacy for the CB2 receptor. Accordingly, O-3223 was evaluated in a variety of murine models of pain and inflammation, including formalin injection, chronic constriction injury (CCI) of the sciatic nerve, and lipopolysaccharide-induced paw edema and hyperalgesia. In addition, CB1-specific behavioral measures were assessed to exclude possible CB1 receptor-specific effects. To examine mechanism of action, rimonabant and SR144528 were used to determine the involvement of CB1 and CB2 receptors, respectively. Finally, in addition to building a profile of O-3223, the potent, mixed CB1/CB2 agonist CP55,940 was included as a comparison throughout the battery of tests presented herein.
Methods
Subjects
Male Swiss Albino imprinting control region (ICR) mice (Harlan Laboratories, Indianapolis, IN) that weighed between 20 and 30 g served as subjects. All subjects were housed four or five animals per cage in a temperature-controlled (20–22°C), AAALAC-accredited facility. Food and water were available ad libitum. The Virginia Commonwealth University Institutional Animal Care and Use Committee approved all animal protocols.
Drugs and Chemicals
O-3223 Synthesis
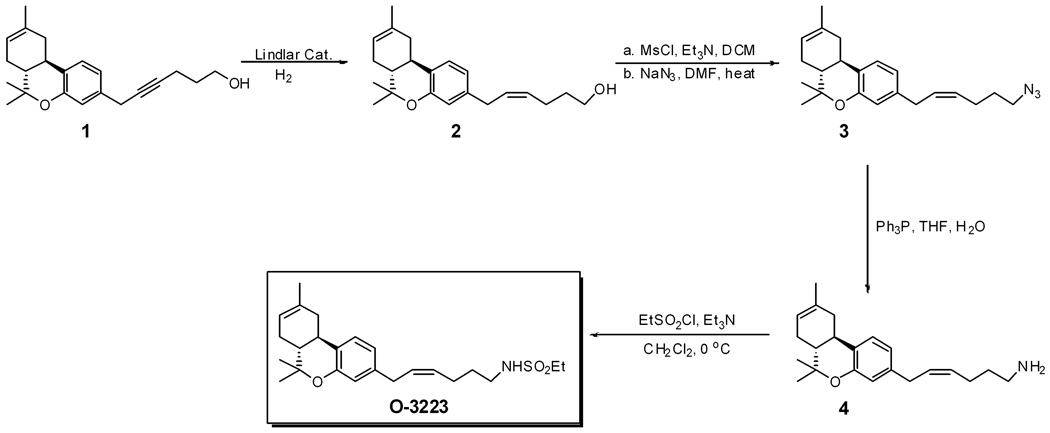
O-3223 was synthesized using a versatile methodology to synthesize 1-desoxy-Δ8-THC sulfonamide analogs with acetylene group in the C-2' position in the side chain, as described previously (Sun et al., 2004). O-3223 was synthesized from 1 (Figure 1) which was prepared per published procedure (Sun et al., 2004). Partial reduction of the alkyne 1 over Lindlar catalyst afforded the desired cis-alkene analog 2. Treatment of 2 with MsCl followed by treatment of the mesylate with NaN3/DMF converted it into the azide 3, which was then reduced to the amine 4. O-3223 was synthesized from 4 by treatment with ethane sulfonyl chloride (Crocker et al., 1999). All compounds showed appropriate 1HNMRs (Jeol Eclipse 300 MHz) and were characterized on the basis of their 1HNMRs, TLC, and elemental analyses.
Figure 1.
O-3223 synthesis pathway.
The mixed CB1 and CB2 receptor agonist CP55,940, CB1 receptor antagonist rimonabant (SR141716A) and CB2 receptor antagonist SR144528 were provided by the National Institute on Drug Abuse (Rockville, MD). The synthetic glucocorticoid hormone, dexamethasone, was purchased from Sigma-Aldrich (St. Louis, MO). Each of these compounds was dissolved in a 1:1 mixture of absolute ethanol and alkamuls-620 (Rhone-Poulenc, Princeton, NJ), and diluted with saline to a final ratio of 1:1:18 (ethanol:alkamuls:saline). The 3 mg/kg doses of rimonabant and SR144528 were chosen based on pilot data and previous reports (Kinsey et al., 2009;Lichtman et al., 2004). Morphine sulphate (NIDA; Rockville, MD), gabapentin (Cayman Chemical, Ann Arbor, MI), and naloxone hydrochloride (Sigma-Aldrich, St. Louis, MO) were dissolved in 0.9% saline. All drugs were given through the intraperitoneal (i.p.) route of administration, in a volume of 10 µl/g body weight.
[35S]GTPγS (1250 Ci/ mmol) was purchased from New England Nuclear Group (Boston,MA), GTPγS from Boehringer Mannheim (New York, NY), and DMEM/F-12 from Fischer Scientific (Pittsburg, PA). Whatman GF/B glass fiber filters were purchased from Fisher Scientific (Pittsburg, PA). All other chemicals were purchased from Sigma (St. Louis, MO).
[35S]GTPγS Binding and [3H]CP55,940 Binding
Membrane Preparations
Human Embryonic Kidney Cells (HEK293) expressing either the human CB1 or CB2 receptor were cultured at 37°C in a humidified chamber with an atmosphere of 95% and 5% CO2. The culture medium was a 50:50 mixture of DMEM and Ham F-12 containing 100 U/ml penicillin, 100 µg/ml streptomycin, 0.25 mg/ml G418 (CB2) or hygromycin (CB1) and 5% fetal calf serum. Cells were harvested by replacement of the media with cold phosphate-buffered saline containing 0.4% EDTA followed by agitation. Membranes were prepared by homogenization of cells in 50 mM Tris-HCl, 3 mM MgCl2, 1 mM EGTA, pH 7.4 (Assay Buffer A), centrifugation at 50,000 × g for 10 minutes at 4°C, and resuspension in the same buffer at 1.5 mg/ml and stored at −80°C until use.
[3H]CP55,940 Binding
Membranes were diluted with Assay Buffer A. Reactions containing membrane (10 µg protein; HEK293 cells) were incubated with 1 nM [3H]CP55,940 and varying concentrations of test compounds in Assay Buffer A containing 0.5% BSA. Non-specific binding was measured in the presence and absence of 5 µM unlabeled SR141716A (CB1) or 10 µM unlabeled WIN55,212-2 (CB2). The assay was incubated for 90 min at 30°C, and terminated by rapid filtration under vacuum through Whatman GF/B glass fiber filters that were pre-soaked in Tris buffer containing 5 g/L BSA (Tris-BSA), followed by five washes with cold Tris-BSA. Bound radioactivity was determined by liquid scintillation spectrophotometry at 45% efficiency for 3H.
[35S]GTPγS Binding
Membrane samples were thawed on ice, centrifuged at 50,000 × g for 10 minutes at 4°C, and resuspended in Assay Buffer B (50 mM Tris-HCl (pH 7.4), 3 mM MgCl2, 0.2 mM EGTA, and 100 mM NaCl). Membranes (10 µg protein) were incubated for 1.5 hr at 30°C in Assay Buffer containing 10 µM GDP, 0.1 nM [35S]GTPγS, 0.1% bovine serum albumin and various concentrations of drug. Nonspecific binding was measured in the presence of 20 µM unlabeled GTPγS, and basal [35S]GTPγS binding was determined in the absence of agonist. Reactions were terminated by rapid vacuum filtration through GF/B glass fiber filters, and bound radioactivity was measured by liquid scintillation spectrophotometry at 95% efficiency for 35S. Net-stimulated [35S]GTPγS binding is defined as agonist-stimulated binding minus basal [35S]GTPγS binding. Percentage of maximal stimulation is defined as [net-stimulated [35S]GTPγS binding by 0-3223/net-stimulated [35S]GTPγS binding by CP55,940] x 100%. All O-3223 data were normalized as the percentage of maximal stimulation produced by 20 nM CP,55,940. Data were reported as the means ± SEM of at least three experiments, each performed in triplicate.
Motor coordination (Rotarod test)
In order to measure motor coordination, a wooden rod with a 6 cm diameter was partitioned into three compartments by circular metal discs (28 cm diameter) at 18-cm intervals. The rod was attached to a motor and rotated at a rate of 4 rpm. Naive mice were trained until they could remain on the rotarod for 3 min. Animals that failed to meet this criterion within three trials were removed from the study. Mice were placed on the rotarod 30 minutes post injection and tested for 5 min. Latency to fall from the rotarod was recorded as the dependent variable. Percent motor impairment was calculated as follows: IP (%) = [1-(test time/5) X 100]. Separate groups of mice were used for each of the different time points.
Hot-Plate Test
Mice were placed into a 10-cm wide Plexiglas cylinder on a hot plate (Thermojust Apparatus) maintained at 56.5°C. Two control latencies at least 10 min apart were determined for each mouse. Antinociceptive response was calculated as percentage of maximum possible effect (% MPE, where %MPE = [(test- control)/(20-control) × 100]. The reaction time was scored when the animal jumped or licked its paw. Eight mice per dose were injected (s.c.) with CP55,940 or O-3223 and tested at various times thereafter to establish a time course.
Formalin Test
The formalin test measures antinociception and is divided into two phases, based on behavioral responses to an intraplantar injection of dilute formalin. The first phase (0–5 min) purportedly reflects the acute nociceptive response, and the second phase (20–45 min) reflects a delayed, inflammatory pain response (Tjolsen et al., 1992). The test was carried out in an open Plexiglas cage, with a mirror placed under the floor to allow an unobstructed view of the paws. Mice were allowed to acclimate for 15 min in the test cage before formalin injection. CP55940, O-3223, or vehicle was injected i.p. 60 min prior to the injection of formalin. Rimonabant (3 mg/kg i.p.) or SR144528 (3 mg/kg i.p.) was administered 30 min prior to O-3223 or CP55,940. In a separate group of mice, morphine was injected 15 min before formalin. Naloxone (1 mg/kg i.p.) or saline was injected 10 min before morphine treatment. Each mouse was injected with 20 µl of 2.5% formalin into the intraplantar region of the right hindpaw. The amount of time spent licking the injected paw was recorded over each phase.
Chronic constriction injury (CCI)
Surgery
Mice were anesthetized with pentobarbital (35 mg/kg, i.p.). An incision was made just below the hipbone, parallel to the sciatic nerve. The right common sciatic nerve was exposed at the level proximal to the sciatic trifurcation, and a nerve segment 3–5mm long was separated from surrounding connective tissue. Three tight ligatures of 6–0 silk suture, spaced 1.0–1.5mm apart, were made around the nerve (Kinsey et al., 2009;Lichtman et al., 2004). Muscles were closed with suture thread and the skin closed with surgical staples. This procedure resulted in chronic constrictive injury of the ligated nerve. In sham-operated controls, an identical surgical incision was performed on the same side, except that the sciatic nerve was not ligated. After surgery, mice were allowed to recover in a warmed cage on clean paper towels and then returned to their home cage after regaining consciousness. Surgical staples were removed three days after surgery.
Plantar stimulator test
Ten days after CCI surgery, mice were placed in clear plastic chambers (7 cm × 9 cm × 10 cm) on an elevated glass surface and allowed to acclimatize to their environment for at least one hour before testing. CP55940, O-3223, gabapentin, or vehicle was injected i.p. 60 min prior to testing. Rimonabant (3 mg/kg i.p.) or SR144528 (3 mg/kg i.p.) was administered 30 min prior to O-3223 or CP55,940. A radiant heat source was directed at the plantar surface of each hind paw, in the area immediately proximal to the toes. The paw withdrawal latency was defined as the time from the onset of radiant heat to withdrawal of the animal’s hind paw (Lichtman et al., 2004). Withdrawal thresholds were measured in the same animal on both each hind paw, ipsilateral and contralateral to the ligated nerve. Results were expressed as ΔPWL (s) = ipsilateral latency - contralateral latency.
LPS-induced edema and hyperalgesia
Acute inflammation of mice hind paws was induced according to published methods (Kanaan et al., 1996;Pattipati et al., 2010). Briefly, lipopolysaccharide (LPS) from Escherichia coli 026:B6 (Sigma, St. Louis, MO) was dissolved in saline and injected into the intraplantar (ipl.) region of the left hind paw using a 30-gauge needle. For experiments assessing anti-hyperalgesia in the hot plate test, mice were administered a single dose 23 post LPS injection and tested at 24 h post LPS injection. Rimonabant (3 mg/kg, i.p.) or SR144528 (3 mg/kg, i.p.) was administered 10 min prior to O-3223 or CP55,940.
In the experiment measuring edema, the right hind paw received 50 µl of saline as a control, and the ensuing inflammatory reaction was monitored as a function of paw swelling. The thickness of each paw was measured both prior to and 24 h following LPS injection using electronic digital calipers (Traceable Calipers, Friendswood, TX) and expressed to the nearest ± 0.01 mm. To estimate LPS-induced edema, O-3223 (10 mg/kg, i.p.), CP55,940 (1 mg/kg, i.p.), dexamethasone (2 mg/kg; i.p.), or vehicle was administered 1 h prior to LPS injection, as well as 6 and 23 h post LPS injection for measurement of paw thickness at 24 h. Conversely, hyperalgesia was induced by administering LPS (25 µg in 50 µl of saline) into both hind paws, and nociceptive behavior was assessed 24 h later in the hot plate test. The plate was maintained at 52.0°C because this temperature was previously demonstrated to elicit similar nociceptive latencies between FAAH (−/−) and (+/+) mice, (Cravatt et al., 2001). The latency for each mouse to display one of the following five nociceptive behaviors was scored during a 30 s observation period: 1) jump (i.e., all four paws are off the surface of the hot plate), 2) licking of a hind paw, 3) shaking of a hind paw, 4) lifting of a hind paw and spreading of the phalanxes, or 5) rapid repeated lifting of the hind paws.
Statistical analyses
Behavioral data are presented as mean ± S.E.M. for the different treatments and time points. Statistical analysis of all behavioral studies was performed using between subjects ANOVA, except for the time course experiments, which used a repeated-measures ANOVA.Follow up comparisons were made using Dunnett’s or Scheffe’s test, where appropriate. Body temperature and dexamethasone effects in the LPS assay were compared using paired and unpaired t tests, respectively. All differences were considered significant at p < 0.05. For GTPγS assays, nonlinear regression analysis was conducted by iterative fitting using JMP (SAS for Macintosh). Nonspecific [35S]GTPγS binding was subtracted from all data. Basal [35S]GTPγS binding is defined as specific [35S]GTPγS binding in the absence of drug. Net-stimulated [35S]GTPγS binding is defined as [35S]GTPγS binding in the presence of drug minus basal. Percent maximal stimulation is expressed as (net stimulated [35S]GTPγS binding by O-3223 / net stimulated [35S]GTPγS binding by 20 nM CP55,940 [CB2] or 600 nM WIN55,212-2 [CB1]) x 100%. Data are reported as the means ± SEM of at least three experiments, each performed in triplicate
Results
O-3223 selectively binds to CB2 receptor
Competition binding experiments against [3H]CP55,940 revealed that O-3223 bound to human CB1 and CB2 receptors in stably transfected Human Embryonic Kidney Cells (HEK293) cells with respective Ki values ± SEM of 1155 ± 66.8 nM and 14.7 ± 1.5 nM. Therefore, the ratio of CB1/CB2 receptor Ki values was 79, indicating that O-3223 binds with relatively high selectivity to CB2 receptors.
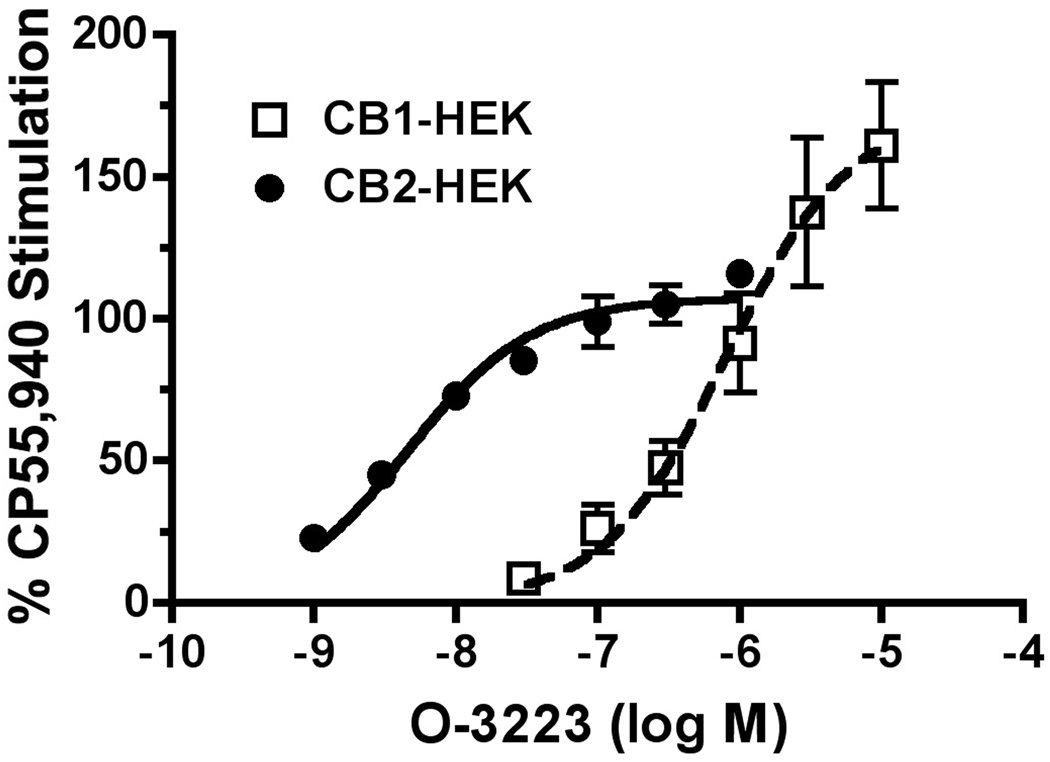
In order to determine the functional activity of O-3223 at cannabinoid receptors, the [35S]GTPγS assay was used to measure G-protein activation in HEK293 cell lines stably expressing either the human CB1 or CB2 receptor. O-3223 was about 136-fold more potent in stimulating G-protein activation in the CB2 expressing cell model, as compared with CB1 transfected HEK293 cells (Figure 2). The EC50 ± SEM values of O-3223 at CB1 and CB2 receptors were 643 ± 97 nM and 4.7 ± 0.5 nM, respectively. Thus, the ratio of the CB1/CB2 receptor EC50values was 137. However, O-3223 was equally efficacious activating both cannabinoid receptor subtypes, producing a maximal stimulation of approximately 70% of that produced by a standard full agonist
Figure 2.
O-3223 mediated stimulation of [35S]GTPγS binding in membranes from HEK cells stably expressing the human CB1 or CB2 receptor. Membranes were incubated with 10 µM GDP, 0.5 nM [35S]GTPγS and varying concentrations of O-3223 or 20 nM CP55,940, as described in Methods. Net-stimulated [35S]GTPγS binding by CP55,940 in CB2-HEK293 cells was 62.1 ± 2.85 fmol/mg and in CB1-HEK cells was 40.2 ± 7.02 fmol/mg. . Data are reported as the means ± SEM of at least three experiments, each performed in triplicate.
O-3223 lacks CB1 receptor cannabimimetic effects
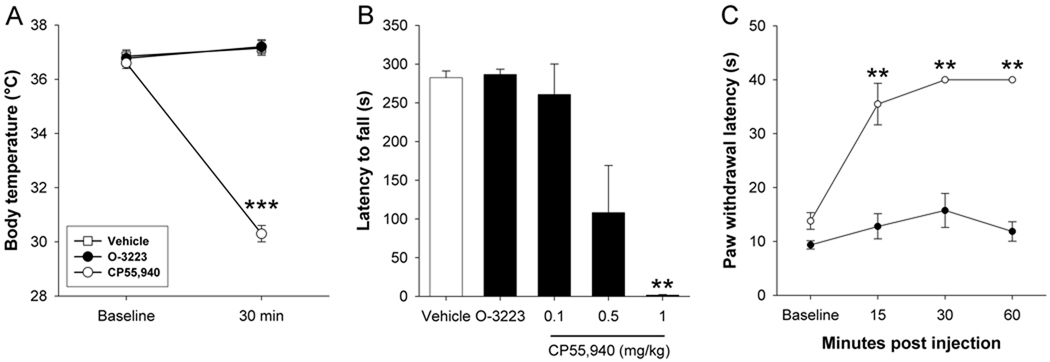
Hypothermia is a common effect of CB1 receptor activation in mice. Body temperature was unaffected by 50 mg/kg O-3223 (p = 0.34), although 1.0 mg/kg CP55,940, the global CB1/CB2 cannabinoid receptor agonist, induced hypothermia [t(4) = 32.6; p < 0.0001]. Similarly, motor coordination, as assessed on the rotarod test, was unaffected by 50mg/kg O-3223 [p = 0.71], although CP55,940 dose-dependently reduced the latency to fall from the rotarod [F(3,15) = 7.43; p < 0.01], indicating impaired motor coordination (Figure 3B). Whereas CP55,940 elicited a dose-dependent antinociceptive effects in the hot plate test, an acute thermal pain assay (F(3,15) = 41.5; p < 0.0001; Figure 3C), 50 mg/kg O-3223 did not significantly affect thermal nociception (p = 0.09).
Figure 3.
The CB2 selective agonist O-3223 (50 mg/kg) did not elicit CB1 mediated effects on body temperature, motor coordination, or nociception, whereas the non-selective cannabinoid receptor agonist CP55,940 (1 mg/kg) caused hypothermia, decreased motor coordination, and antinociception. (A) Thirty min after administration, CP55,940 significantly decreased body temperature and (B) impaired motor coordination, as measured on the Rotarod test, whereas O-3223 had no effect in either test. (C) CP55,940 induced significant antinociception in the hot plate test. O-3223 had no effect in the hot plate test. Data are presented as mean ± S.E.M. (n = 5–6). **p < 0.01, ***p < 0.001 vs. baseline; †† p < 0.01 vs. vehicle
Comparison between O-3223 and CP55,940 in the formalin test
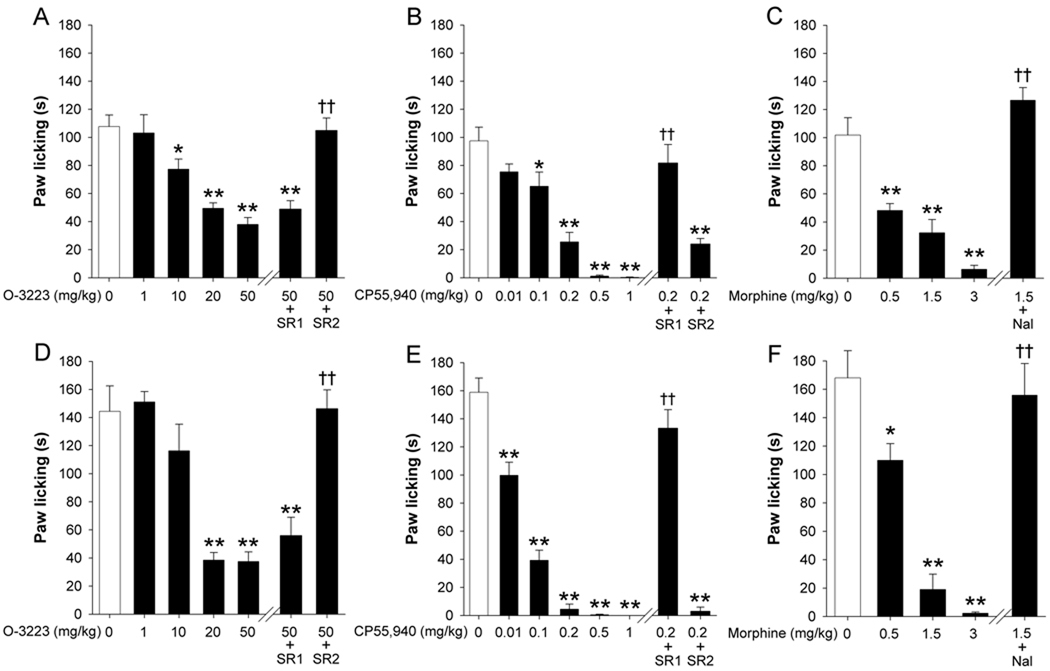
In the formalin test, both O-3223 and CP55,940 dose-dependently attenuated behavioral signs of nociception (Figure 4). In the first phase of the formalin test (0–5 min post injection), O-3223 significantly attenuated paw licking in a dose-dependent manner [F(4,20) = 16.8; p < 0.0001; Figure 4A]. The antinociceptive effects of O-3223 were unaffected by pretreatment with 3 mg/kg rimonabant (p = 0.95); however 3 mg/kg SR144528 fully blocked this effect (p < 0.0001). Similarly, CP55,940 significantly attenuated paw licking in a dose-dependent manner [F(5,25) = 35.6; p < 0.0001; Figure 4B]. Whereas pretreatment with rimonabant fully blocked the antinociceptive effects of CP55,940 (p < 0.05), SR144528 had no effect (p = 0.43). Morphine significantly attenuated paw licking in a dose-dependent manner [F(3,20) = 23.59; p < 0.0001; Figure 4C], and this effect was blocked by pretreatment with naloxone (p < 0.0001).
Figure 4.
Comparison of the antinociceptive effects among the CB2 receptor selective agonist O-3223 (A and D) the mixed CB1/CB2 cannabinoid receptor agonist CP55,940 (B and E), and morphine (C and F) in phase 1 and 2 of the formalin test. O-3223 dose-dependently attenuated paw licking during both phases of the formalin test (A and D). Pretreatment with the CB1 receptor antagonist, rimonabant (SR1; 3 mg/kg) did not reverse this attenuation in paw licking, whereas pretreatment with the CB2 receptor antagonist SR144528 (SR2; 3 mg/kg) fully blocked the effects of O-3223. CP55,940 dose-dependently attenuated paw licking during both phases (B and E). Pretreatment with SR1, but not SR blocked this antinociceptive effect. Morphine dose-dependently reduced nociceptive behavior (C and F), which was antagonized by naloxone hydrochloride (Nal; 1 mg/kg). Data are presented as mean ± S.E.M. (n = 6). **p < 0.01, ***p < 0.001 vs. vehicle; †† p < 0.01, ††† p < 0.001 vs. O-3223 (50 mg/kg), CP55,940 (0.2 mg/kg), or morphine (1.5 mg/kg).
In the second phase of the formalin test (20–45 min post injection), O-3223 significantly attenuated paw licking in a dose-dependent manner [F(4,20) = 20.4; p < 0.0001; Figure D]. This antinociceptive effect was unaffected by pretreatment with rimonabant (p = 0.26), but was fully blocked by SR144528 (p < 0.001). Similarly, CP55,940 significantly attenuated paw licking [F(5,25) = 101; p < 0.0001; Figure E]. However, pretreatment with rimonabant fully blocked the antinociceptive effect (p < 0.01), whereas pretreatment with SR144528 did not significantly affect paw licking behavior (p = 0.21). Morphine significantly attenuated paw licking in a dose-dependent manner [F(3,20) = 27.72; p < 0.0001; Figure 4F], and this effect was prevented by naloxone injection (p < 0.001).
Comparison between O-3223 and CP55,940 in the CCI model of neuropathic pain
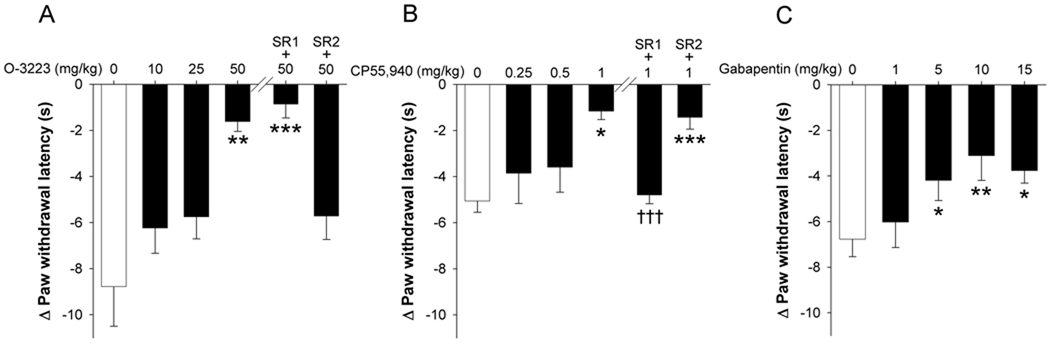
In the chronic constriction injury model of neuropathic pain, both O-3223 and CP55,940 attenuated thermal hyperalgesia in a dose-dependent manner (Figure 5). Ten days after CCI surgery, paw withdrawal thresholds were significantly reduced in the paw ipsilateral to nerve injury, as compared with the paw contralateral to nerve injury [t(7) = 9.92; p < 0.0001]. Acute O-3223 significantly attenuated sciatic nerve injury-induced hyperalgesia [F(3,28) = 6.7; p < 0.01; Figure 5A]. This effect was effectively blocked by pretreatment with SR144528 (p < 0.001), but not by rimonabant (p = 0.97), indicating a CB2 receptor specific mechanism of action. Acute CP55,940 also significantly attenuated CCI-induced hyperalgesia [F(3,20) = 3.5; p < 0.05; Figure 5B]. The anti-hyperalgesic effects of CP55,940 were effectively blocked by rimonabant (p < 0.0001), but not SR144528 (p = 0.98), indicating a CB1 receptor specific mechanism of action. Gabapentin significantly attenuated CCI-induced hyperalgesia [F(4,20) = 6.11; p < 0.01; Figure 5C]. Mean (±SEM) paw withdrawal threshold in the contralateral, non-surgery paws was 11.2(0.6) seconds and did not differ across drug treatments [F(3,28) = 14.5; p = 0.22]. Sham operated mice did not develop hyperalgesia [t(30) = 0.15; p = 0.88]. Similarly, 50 mg/kg O-3223 had no effect on paw withdrawal latency in sham operated mice (p = 0.95).
Figure 5.
O-3223 and CP55,940 attenuated chronic constriction injury (CCI)-induced hyperalgesia. Ten days after chronic constriction injury, mice were pretreated with O-3223 or CP55,940, then tested one hour later in the Hargreaves plantar stimulator test. (A) O-3223 significantly attenuated CCI-induced hyperalgesia, and this effect was blocked by pretreatment with the CB2 receptor antagonist, SR144528 (SR2; 3 mg/kg), although the CB1 receptor antagonist, rimonabant (SR1; 3 mg/kg) had no effect. (B) CP55,940 significantly attenuated CCI-induced hyperalgesia, although this effect was blocked by pretreatment with rimonabant, but not SR144528. (C) Gabapentin significantly reduced CCI-induced hyperalgesia. Data are presented as mean ± S.E.M. of paw withdrawal latency, standardized to the contralateral, non-surgery paw (n = 6–7). *p < 0.05, **p < 0.01, ***p < 0.001 vs. vehicle; †††p < 0.001 vs.CP55,940 (1.0mg/kg).
Comparison between O-3223 and CP55,940 in the in the LPS model of inflammation and inflammatory hyperalgesia
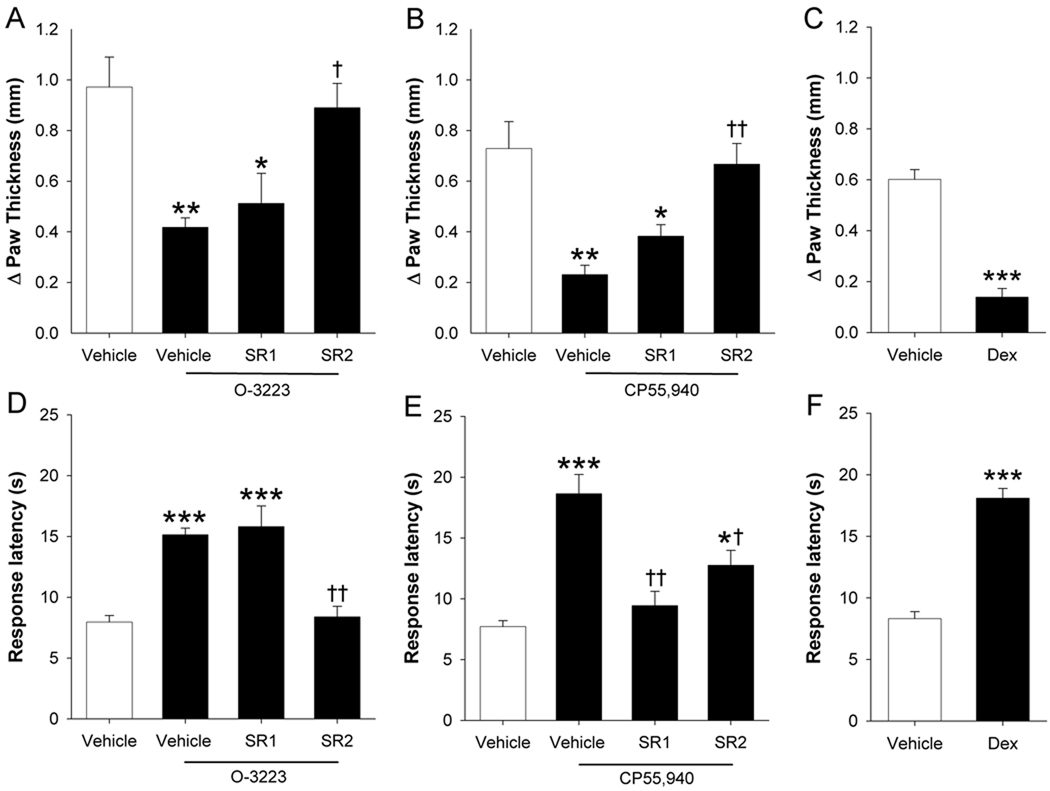
Mice were given intraplantar injection of the gram negative bacterial endotoxin LPS, then tested for edema and hyperalgesia 24 h later. LPS treatment caused a significant increase in paw thickness (t(6) = 7.3; p < 0.001). O-3223 injected 1 h before, as well as 6 and 23 h after LPS significantly decreased the development of LPS-induced paw edema [F(3,20) = 7.7; p < 0.01; Figure 6A). The anti-edematous effects of O-3223 were completely blocked by SR144528 pretreatment (p < 0.05), but not by rimonabant (p = 0.93). Similarly, repeated administration of CP55,940 significantly reduced LPS-induced paw edema (F(3,24) = 10.4; p < 0.0001; Figure 6B). In contrast to the results of CP55,940 in the other behavioral assays of nociception, the anti-edematous effects of CP55,940 were completely blocked by SR144528 pretreatment (p < 0.01), but not rimonabant (p = 0.55).
Figure 6.
LPS-induced edema and hyperalgesia were reduced by O-3223 and CP55,940. Mice were given an intraplantar injection of LPS, and paw edema and hyperalgesia were assessed 24 hours later. (A) Repeated injections of O-3223 (10 mg/kg) at 1 h before, as well as 6 and 23 h after LPS significantly attenuated LPS-induced paw edema. This anti-edematous effect was blocked by pretreatment with the CB2 receptor antagonist, SR144528 (SR2; 3 mg/kg), but not by the CB1 receptor antagonist, rimonabant (SR1; 3 mg/kg). (B) Repeated injection of CP55,940 (1 mg/kg) also significantly attenuated LPS-induced paw edema, and this effect was blocked by pretreatment with SR144528, but not by rimonabant. (C) Repeated injections of the synthetic glucocorticoid hormone, dexamethasone (Dex; 2 mg/kg) significantly attenuated LPS-induced paw edema. (D) A single injection of O-3223 at 23 h after LPS significantly attenuated LPS-induced hyperalgesia. This effect was fully blocked by pretreatment with SR144528, but not by rimonabant. The respective mean ± SEM hot plate latencies before LPS administration and at 22.5 h (just before O-3223 administration) were 15.2 ± 0.4 and 7.9 ± 0.3 s. (E) Acute CP55,940 also significantly attenuated LPS-induced hyperalgesia, and this effect was partially blocked by pretreatment with SR144528, and fully blocked by rimonabant. The respective mean ± SEM hot plate latencies before LPS administration and at 22.5 h (just before CP55,940 administration) were 15.9 ± 0.6 and 8.6 ± 0.4 s. (F) Repeated injections of Dex (2 mg/kg) significantly prevented the development of LPS-induced thermal hyperalgesia. Data are presented as mean ± S.E.M. (n=6–8). *p < 0.05, **p < 0.01, ***p < 0.001 vs. vehicle; † p < 0.05, †† p < 0.01 vs. O-3223 or CP55,940.
Intraplantar LPS injection also caused a significant increase in hyperalgesia, as shown by decreased hot plate latencies compared to mice given intraplantar injections of saline [t(7) = −11.2; p < 0.001]. A single injection of O-3223 given 23 h after LPS completely reversed LPS-induced thermal hyperalgesia [F(3,28) = 16.9p < 0.01; Figure 6D). These anti-hyperalgesic effects of O-3223 were completely blocked by SR144528 pretreatment (p < 0.05), but not by rimonabant (p = 0.93). CP55,940 also completely reversed LPS-induced thermal hyperalgesia [F(3,28) = 16.4; p < 0.0001; Figure 6E). The anti-hyperalgesic effects of this compound were completely blocked by rimonabant pretreatment (p < 0.0001) and partially reduced by SR144528 (p < 0.05). As shown previously, the synthetic glucocorticoid dexamethasone attenuated LPS-induced paw edema [t(14) = 9.1; p < 0.0001; Figure 6C] and hyperalgesia [t(14) = 10.04; p < 0.0001; Figure 6F].
Discussion
In the present study, we developed a novel ethyl sulfonamide THC analog, O-3223 that selectively binds to and activates CB2 receptors. In addition, this compound reduced nociceptive behavior in neuropathic and inflammatory mouse models of pain. The effects of O-3223 were compared to the potent, mixed CB1/CB2 receptor agonist, CP55,940 in battery of preclinical models of pain, including the tail withdrawal test, hot plate test, formalin test, chronic constrictive injury of the sciatic nerve (CCI) model of neuropathic pain, and LPS model of inflammatory pain. O-3223 bound selectively to CB2 receptors in transfected HEK cells and elicited anti-inflammatory and anti-hyperalgesic effects after systemic administration in variety of pain models. Importantly, O-3223 did not cause apparent CB1 receptor-mediated side effects, such as hypothermia, or impaired motor coordination, as measured in the rotarod test.
O-3223 elicited differential effects depending on the nociceptive assay. While it did not produce significant antinociceptive effects in tail immersion or hot plate tests (Figure 3A and 3C), it had partial efficacy in the formalin test (Figure 4A and 4D), and elicited a nearly full antihyperalgesic effect to thermal nociceptive stimuli in the CCI model (Figure 5A). Additionally, three injections of O-3223 (10 mg/kg) given across a 24 h period attenuated the edematous effects caused by intraplantar injections of LPS (Figure 6A), with similar efficacy as CP55,940 as well as the fully efficacious CB1/CB2 receptor agonist WIN55,212-2 and URB597, a fatty acid amide (FAAH) inhibitor (Naidu et al., 2010). In contrast, an acute injection of O-3233 completely reversed LPS-induced thermal hyperalgesia (Figure 6D). The observations that the anti-hyperalgesic and anti-edematous effects of O-3223were fully blocked by a CB2 receptor antagonist, not a CB1 receptor antagonist, and the lack of overt CB1 cannabimimetic effects, indicate that this compound elicits functional activity through the activation of the CB1 receptor at the doses tested.
As shown previously, CP55,940 produced antinociceptive effects through the CB1 receptor (Little et al., 1988). These data are consistent with a recent report that CP55,940 attenuated pain in a range of models, including complete Freund’s adjuvant injection, tail immersion in hot water, and spinal nerve ligation-induced tactile allodynia (Sain et al., 2009). These effects were absent in CB1(−/−) mice, but not CB2(−/−) mice, indicating that CP55,940 blocks pain primarily via a CB1 receptor-selective mechanism of action (Sain et al., 2009). In line with these data, in the present study the anti-hyperalgesic effects of CP55,940 were prevented by pretreatment with the CB1 receptor antagonist rimonabant, whereas the CB2 receptor antagonist SR144528 had no effect. Of note, CP55,940 reduced LPS-induced paw edema through a CB2 receptor mechanism of action, although the concomitant thermal hyperalgesia was reduced via a CB1 receptor mechanism of action.
Most studies evaluating the antinociceptive and anti-inflammatory effects of cannabinoids have used nonselective, global cannabinoid receptor agonists, coupled with pharmacological antagonism or genetic deletion of either the CB1 or CB2 receptor. However, an emerging body of research indicates that CB2 receptor agonists, including AM1241, GW405833, JWH133, A-796260, and A-836339, are effective in a variety of preclinical pain models, particularly in models of inflammatory pain. In rats, systemic AM1241 attenuated carrageenan-induced hyperalgesia and mechanical allodynia via a CB2, but not CB1, receptor mechanism (Gutierrez et al., 2007;Nackley et al., 2003). However, the antinociceptive effects of AM1241 were also blocked by pretreatment with the opioid antagonist naloxone, indicating that the antinociceptive effects of AM1241 may not be mediated by the cannabinoid system alone (Ibrahim et al., 2005;Yao et al., 2008), though more recently, Rahn et al. (2010) did not find an opioid component of AM1241induced-antinociception. A different CB2 selective agonist, GW405833 significantly attenuated carrageenan-induced edema and decreased weight bearing, and both effects were blocked with SR144528, suggesting a CB2 receptor-selective mechanism of action (Clayton et al., 2002). A recent report showed that JWH133, a CB2 selective agonist, as well as the phytocannabinoid (E)-BCP ((E)-beta-caryophyllene) significantly reduced carrageenan-induced edema in, CB2(+/+) mice, but not CB2(−/−) mice (Gertsch et al., 2008). In the complete Freund’s adjuvant (CFA) assay, a chronic inflammatory pain model, systemic GW405833 dose-dependently reduced CFA-induced hyperalgesia and allodynia in CB2(+/+) mice, but not in CB2(−/−) mice (Valenzano et al., 2005). Similarly, the CB2 receptor-selective agonists A-796260 and A-836339 reduced CFA-induced thermal hyperalgesia, and this antinociceptive effect was blocked by pretreatment with SR144528, but not the opioid antagonist naloxone (Yao et al., 2009;Yao et al., 2008).
Although not as well documented as inflammatory models, some evidence suggests that the CB2 receptor plays a role in neuropathic pain and may be targeted to reduce pain associated with nerve injury. For example, CB2 receptor mRNA is upregulated in non-neuronal lumbar tissue following CCI (Zhang et al., 2003). Human dorsal root ganglia neurons cultured with the selective CB2 agonists GW842166 and GW833972 were less responsive to capsaicin-induced Ca2+ influx, and this effect was reversed with the CB2 antagonist GW818646X (Anand et al., 2008). In mice, partial nerve ligation-induced mechanical allodynia was attenuated by intrathecal administration of JWH133 in wild type mice, but this effect did not occur in CB2 knockout mice (Yamamoto et al., 2008). Similarly, the CB2 receptor-selective agonists A-796260 and A-836339 dose-dependently attenuated CCI-induced allodynia in rats (Yao et al., 2009;Yao et al., 2008). Of particular interest, unlike repeated morphine treatment, mice did not become tolerant to repeated administration of A-796260 or A-836339 (Yao et al., 2009;Yao et al., 2008).
Endocannabinoids also appear to inhibit neuropathic pain via the CB2 receptor. Blocking degradation of anandamide with the irreversible FAAH inhibitor URB597 blocked CCI-induced thermal hyperalgesia and mechanical allodynia (Kinsey et al., 2009;Russo et al., 2007). Pretreatment with either rimonabant or SR144528 prevented the antinociceptive effects of URB597 (Russo et al., 2007), as well as those of the reversible FAAH inhibitor, OL-135 (Kinsey et al., 2009). On the other hand, inhibition of MAGL, the primary catabolic enzyme of the endocannabinoid 2-AG, attenuated CCI-induced mechanical and cold allodynia in mice via a CB1 receptor-specific mechanism of action (Kinsey et al., 2009). FAAH deficient mice also exhibited an anti-inflammatory phenotype in the carrageenan paw edema model, which was mediated by a CB2 receptor mechanism of action (Lichtman et al., 2004). Whether any of these effects are driven by CB2 expression on nociceptors or on glial cells remains to be elucidated. In the brain, quinolinic acid infusion caused increased damage in CB2 receptor deficient mice, as compared with wild type mice (Palazuelos et al., 2009). The microglia-inhibiting compound minocycline blocked this effect, indicating that receptors expressed on microglia may be neuroprotective following a neuroinflammatory response to insult (Palazuelos et al., 2009). These data support the development of CB2 selective agonists for preventing and treating neuropathic pain, along with concomitant functional loss.
In acute analgesic nociceptive models, such as the hot plate test, CB2 receptor agonists have led to mixed results. The CB2 receptor agonists HU-308 (Hanus et al., 1999) and GW405833 (Valenzano et al., 2005) are not analgesic in acute thermal nociception models that lack an inflammatory component. However, the CB2 specific agonist AM1241 has been reported to increase withdrawal latencies in the plantar stimulator test, and this antinociception was prevented by the CB2 antagonist AM630, but not by the CB1 antagonist AM251 (Malan et al., 2001). The same group later repeated this experiment in CB1 and CB2 receptor knockout mice and confirmed the CB2 receptor mechanism of action (Ibrahim et al., 2006). However, in vitro data suggest that AM1241 can act as an agonist or antagonist for CB2, indicating that test conditions may direct receptor interactions with AM1241 (Yao et al., 2006). In the present study, although O-3223 reduced inflammatory nociception in the LPS and formalin tests, it had no effect on withdrawal latency in the hot plate test.
Until somewhat recently, CB2 receptors were thought to be restricted exclusively to peripheral tissues, such as immune cells. However, with the development of higher affinity antibodies, CB2 has now been identified within the brain stem (Van Sickle et al., 2005). Although CB2 is expressed within peripheral and central nervous tissues, receptor expression has not conclusively been shown on neurons. Although not typically expressed at high levels in healthy tissues, CB2 expression increased in injured and diseased neural tissue in humans (Wotherspoon et al., 2005). Concordantly, rats subjected to CCI showed increased CB2 receptor expression in lumbar spinal cord, as compared with controls, although this upregulated receptor expression may have been limited to resident microglia (Zhang et al., 2003). As it pertains to pain, the specific mechanism of action through which CB2 modulates nociception is not well understood. Peripheral nerve injury causes an increase in receptor expression for the proinflammatory cytokine, TNF-α, in the dorsal root ganglia (Ohtori et al., 2004). Given that production of proinflammatory cytokines, including TNF-α, contributes to pathogenesis following nerve injury (Watkins et al., 2007), and that activation of CB2 has anti-inflammatory effects (Cabral and Griffin-Thomas, 2009), the concomitant increase in CB2 receptor expression in damaged tissue may act as a feedback loop, preventing exaggerated damage from activated glial cells. Whether CB2 receptor binding directly affects peripheral nociceptors or supraspinal systems, current evidence clearly points to CB2 inflammatory modulation, which indirectly affects nociception.
Highly selective CB2 receptor agonists offer important gains in the development of novel analgesics, especially for the treatment of pain with an inflammatory etiology. These compounds offer fewer undesirable CB1 dependent psychomimetic side effects, along with concomitantly lowered abuse liability, as compared with currently available global cannabinoid agonists. The results of the present study support the idea that the CB2 receptor is a viable target for the development of additional, highly selective agonists for use as anti-inflammatory therapeutics that lack undesirable behavioral effects. The data presented herein indicate that the novel ethyl sulfonamide THC analog, O-3223, has significant anti-inflammatory and antinociceptive effects in vivo, but does not cause any of the CB1 receptor-specific behavioral effects observed with the global cannabinoid agonist, CP55,940. Thus, this compound can serve as a pharmacophore to develop CB2 receptors with increased antinociceptive potency.
Acknowledgements
The authors thank Dr. Mary Abood for generously providing additional HEK293 cells, Dr. Tie Han for his excellent technical assistance, and the National Institute on Drug Abuse for financial support (R01DA003672, R01DA005488, and DA007027-32). This paper is dedicated to the memory of our dear friend and mentor, Dr. Billy R. Martin, whose wisdom and insight guided this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Steven G. Kinsey, Department of Pharmacology and Toxicology, Virginia Commonwealth University, Richmond, VA 23298 USA
Anu Mahadevan, Organix, Inc., 240 Salem St., Woburn, MA 01801 USA.
Bingjun Zhao, Organix, Inc., 240 Salem St., Woburn, MA 01801 USA.
Hang Sun, Organix, Inc., 240 Salem St., Woburn, MA 01801 USA.
Pattipati S. Naidu, Department of Pharmacology and Toxicology, Virginia Commonwealth University, Richmond, VA 23298 USA
Raj K. Razdan, Organix, Inc., 240 Salem St., Woburn, MA 01801 USA
Dana E. Selley, Department of Pharmacology and Toxicology, Virginia Commonwealth University, Richmond, VA 23298 USA
M. Imad Damaj, Department of Pharmacology and Toxicology, Virginia Commonwealth University, Richmond, VA 23298 USA.
Aron H. Lichtman, Department of Pharmacology and Toxicology, Virginia Commonwealth University, Richmond, VA 23298 USA
References
- Anand U, Otto WR, Sanchez-Herrera D, Facer P, Yiangou Y, Korchev Y, Birch R, Benham C, Bountra C, Chessell IP, Anand P. Cannabinoid receptor CB2 localisation and agonist-mediated inhibition of capsaicin responses in human sensory neurons. Pain. 2008;138:667–680. doi: 10.1016/j.pain.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Braden JB, Sullivan MD. Suicidal thoughts and behavior among adults with self-reported pain conditions in the national comorbidity survey replication. J Pain. 2008;9:1106–1115. doi: 10.1016/j.jpain.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral GA, Griffin-Thomas L. Emerging role of the cannabinoid receptor CB2 in immune regulation: therapeutic prospects for neuroinflammation. Expert Rev Mol Med. 2009;11:e3. doi: 10.1017/S1462399409000957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton N, Marshall FH, Bountra C, O’Shaughnessy CT. CB1 and CB2 cannabinoid receptors are implicated in inflammatory pain. Pain. 2002;96:253–260. doi: 10.1016/S0304-3959(01)00454-7. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, Lichtman AH. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci U S A. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker PJ, Saha B, Ryan WJ, Wiley JL, Martin BR, Ross RA, Pertwee RG, Razdan RK. Development of agonists, partial agonists and antagonists in the Delta(8)-tetrahydrocannabinol series. Tetrahedron. 1999;55:13907–13926. [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, Bouaboula M, Shire D, Le Fur G, Casellas P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- Gertsch J, Leonti M, Raduner S, Racz I, Chen JZ, Xie XQ, Altmann KH, Karsak M, Zimmer A. Beta-caryophyllene is a dietary cannabinoid. Proc Natl Acad Sci U S A. 2008;105:9099–9104. doi: 10.1073/pnas.0803601105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon J, Hohmann AG. Cannabinoid CB2 receptors: a therapeutic target for the treatment of inflammatory and neuropathic pain. Br J Pharmacol. 2008;153:319–334. doi: 10.1038/sj.bjp.0707531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez T, Farthing JN, Zvonok AM, Makriyannis A, Hohmann AG. Activation of peripheral cannabinoid CB1 and CB2 receptors suppresses the maintenance of inflammatory nociception: a comparative analysis. Br J Pharmacol. 2007;150:153–163. doi: 10.1038/sj.bjp.0706984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanus L, Breuer A, Tchilibon S, Shiloah S, Goldenberg D, Horowitz M, Pertwee RG, Ross RA, Mechoulam R, Fride E. HU-308: a specific agonist for CB(2), a peripheral cannabinoid receptor. Proc Natl Acad Sci U S A. 1999;96:14228–14233. doi: 10.1073/pnas.96.25.14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim MM, Porreca F, Lai J, Albrecht PJ, Rice FL, Khodorova A, Davar G, Makriyannis A, Vanderah TW, Mata HP, Malan TP., Jr CB2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. Proc Natl Acad Sci U S A. 2005;102:3093–3098. doi: 10.1073/pnas.0409888102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim MM, Rude ML, Stagg NJ, Mata HP, Lai J, Vanderah TW, Porreca F, Buckley NE, Makriyannis A, Malan TP., Jr CB2 cannabinoid receptor mediation of antinociception. Pain. 2006;122:36–42. doi: 10.1016/j.pain.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Kanaan SA, Saade NE, Haddad JJ, Abdelnoor AM, Atweh SF, Jabbur SJ, Safieh-Garabedian B. Endotoxin-induced local inflammation and hyperalgesia in rats and mice: a new model for inflammatory pain. Pain. 1996;66:373–379. doi: 10.1016/0304-3959(96)03068-0. [DOI] [PubMed] [Google Scholar]
- Kinsey SG, Long JZ, O'Neal ST, Abdullah RA, Poklis JL, Boger DL, Cravatt BF, Lichtman AH. Blockade of endocannabinoid-degrading enzymes attenuates neuropathic pain. Journal of Pharmacology and Experimental Therapeutics. 2009;330:902–910. doi: 10.1124/jpet.109.155465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan NM, Mechoulam R. Cannabinoids in health and disease. Dialogues Clin Neurosci. 2007;9:413–430. doi: 10.31887/DCNS.2007.9.4/nkogan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH, Shelton CC, Advani T, Cravatt BF. Mice lacking fatty acid amide hydrolase exhibit a cannabinoid receptor-mediated phenotypic hypoalgesia. Pain. 2004;109:319–327. doi: 10.1016/j.pain.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Little PJ, Compton DR, Johnson MR, Melvin LS, Martin BR. Pharmacology and stereoselectivity of structurally novel cannabinoids in mice. J. Pharmacol. Exp. Ther. 1988;247:1046–1051. [PubMed] [Google Scholar]
- Mackie K. Cannabinoid receptors as therapeutic targets. Annu Rev Pharmacol Toxicol. 2006;46:101–122. doi: 10.1146/annurev.pharmtox.46.120604.141254. [DOI] [PubMed] [Google Scholar]
- Malan TP, Jr, Ibrahim MM, Deng H, Liu Q, Mata HP, Vanderah T, Porreca F, Makriyannis A. CB2 cannabinoid receptor-mediated peripheral antinociception. Pain. 2001;93:239–245. doi: 10.1016/S0304-3959(01)00321-9. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- Nackley AG, Makriyannis A, Hohmann AG. Selective activation of cannabinoid CB(2) receptors suppresses spinal fos protein expression and pain behavior in a rat model of inflammation. Neuroscience. 2003;119:747–757. doi: 10.1016/s0306-4522(03)00126-x. [DOI] [PubMed] [Google Scholar]
- Ohtori S, Takahashi K, Moriya H, Myers RR. TNF-alpha and TNF-alpha receptor type 1 upregulation in glia and neurons after peripheral nerve injury: studies in murine DRG and spinal cord. Spine (Phila Pa 1976) 2004;29:1082–1088. doi: 10.1097/00007632-200405150-00006. [DOI] [PubMed] [Google Scholar]
- Palazuelos J, Aguado T, Pazos MR, Julien B, Carrasco C, Resel E, Sagredo O, Benito C, Romero J, Azcoitia I, Fernandez-Ruiz J, Guzman M, Galve-Roperh I. Microglial CB2 cannabinoid receptors are neuroprotective in Huntington's disease excitotoxicity. Brain. 2009;132:3152–3164. doi: 10.1093/brain/awp239. [DOI] [PubMed] [Google Scholar]
- Pattipati SN, Kinsey SG, Guo T, Cravatt BF, Lichtman AH. Regulation of Inflammatory Pain by Inhibition of Fatty Acid Amide Hydrolase (FAAH) Journal of Pharmacology and Experimental Therapeutics. 2010;334:182–190. doi: 10.1124/jpet.109.164806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahn EJ, Zvonok AM, Makriyannis A, Hohmann AG. Antinociceptive effects of racemic AM1241 an d its chirally synthesized enantiomers: lack of dependence upon opioid receptor activation. AAPS Journal. 2010;12:147–157. doi: 10.1208/s12248-009-9170-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo R, Loverme J, La Rana G, Compton TR, Parrott J, Duranti A, Tontini A, Mor M, Tarzia G, Calignano A, Piomelli D. The fatty acid amide hydrolase inhibitor URB597 (cyclohexylcarbamic acid 3'-carbamoylbiphenyl-3-yl ester) reduces neuropathic pain after oral administration in mice. Journal of Pharmacology and Experimental Therapeutics. 2007;322:236–242. doi: 10.1124/jpet.107.119941. [DOI] [PubMed] [Google Scholar]
- Sain NM, Liang A, Kane SA, Urban MO. Antinociceptive effects of the non-selective cannabinoid receptor agonist CP 55,940 are absent in CB1(−/−) and not CB2(−/−) mice in models of acute and persistent pain. Neuropharmacology. 2009;57:235–241. doi: 10.1016/j.neuropharm.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Sun H, Mahadevan A, Razdan RK. A novel methodology for the synthesis of 1-desoxy-Delta(8)-tetrahydrocannabinol (THC) analogues. Tetrahedron Letters. 2004;45:615–617. [Google Scholar]
- Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. The formalin test: an evaluation of the method. Pain. 1992;51:5–17. doi: 10.1016/0304-3959(92)90003-T. [DOI] [PubMed] [Google Scholar]
- Valenzano KJ, Tafesse L, Lee G, Harrison JE, Boulet JM, Gottshall SL, Mark L, Pearson MS, Miller W, Shan S, Rabadi L, Rotshteyn Y, Chaffer SM, Turchin PI, Elsemore DA, Toth M, Koetzner L, Whiteside GT. Pharmacological and pharmacokinetic characterization of the cannabinoid receptor 2 agonist, GW405833, utilizing rodent models of acute and chronic pain, anxiety, ataxia and catalepsy. Neuropharmacology. 2005;48:658–672. doi: 10.1016/j.neuropharm.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison JS, Marnett LJ, Di Marzo V, Pittman QJ, Patel KD, Sharkey KA. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Ledeboer A, Wieseler-Frank J, Milligan ED, Maier SF. Norman Cousins Lecture. Glia as the "bad guys": implications for improving clinical pain control and the clinical utility of opioids. Brain Behav Immun. 2007;21:131–146. doi: 10.1016/j.bbi.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wotherspoon G, Fox A, McIntyre P, Colley S, Bevan S, Winter J. Peripheral nerve injury induces cannabinoid receptor 2 protein expression in rat sensory neurons. Neuroscience. 2005;135:235–245. doi: 10.1016/j.neuroscience.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Yamamoto W, Mikami T, Iwamura H. Involvement of central cannabinoid CB2 receptor in reducing mechanical allodynia in a mouse model of neuropathic pain. Eur J Pharmacol. 2008;583:56–61. doi: 10.1016/j.ejphar.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Yao BB, Hsieh G, Daza AV, Fan Y, Grayson GK, Garrison TR, El Kouhen O, Hooker BA, Pai M, Wensink EJ, Salyers AK, Chandran P, Zhu CZ, Zhong C, Ryther K, Gallagher ME, Chin CL, Tovcimak AE, Hradil VP, Fox GB, Dart MJ, Honore P, Meyer MD. Characterization of a cannabinoid CB2 receptor-selective agonist, A-836339 [2,2,3,3-tetramethyl-cyclopropanecarboxylic acid [3-(2-methoxy-ethyl)−4,5-dimethyl-3H-thiazol-(2Z)-ylidene]-amide], using in vitro pharmacological assays, in vivo pain models, and pharmacological magnetic resonance imaging. Journal of Pharmacology and Experimental Therapeutics. 2009;328:141–151. doi: 10.1124/jpet.108.145011. [DOI] [PubMed] [Google Scholar]
- Yao BB, Hsieh GC, Frost JM, Fan Y, Garrison TR, Daza AV, Grayson GK, Zhu CZ, Pai M, Chandran P, Salyers AK, Wensink EJ, Honore P, Sullivan JP, Dart MJ, Meyer MD. In vitro and in vivo characterization of A-796260: a selective cannabinoid CB2 receptor agonist exhibiting analgesic activity in rodent pain models. Br J Pharmacol. 2008;153:390–401. doi: 10.1038/sj.bjp.0707568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao BB, Mukherjee S, Fan Y, Garrison TR, Daza AV, Grayson GK, Hooker BA, Dart MJ, Sullivan JP, Meyer MD. In vitro pharmacological characterization of AM1241: a protean agonist at the cannabinoid CB2 receptor? Br J Pharmacol. 2006;149:145–154. doi: 10.1038/sj.bjp.0706838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Hoffert C, Vu HK, Groblewski T, Ahmad S, O’Donnell D. Induction of CB2 receptor expression in the rat spinal cord of neuropathic but not inflammatory chronic pain models. Eur J Neurosci. 2003;17:2750–2754. doi: 10.1046/j.1460-9568.2003.02704.x. [DOI] [PubMed] [Google Scholar]