Abstract
Lymphangioleiomyomatosis (LAM), a rare cystic lung disease with multi-organ involvement, occurs primarily in women of childbearing age. LAM can present sporadically or in association with tuberous sclerosis complex (TSC). Loss of lung function in patients with LAM can be attributed to the dysregulated growth of LAM cells, with dysfunctional TSC1 or TSC2 genes, which encode hamartin and tuberin, respectively, leading to hyperactivation of the mammalian target of rapamycin (mTOR). LAM cells are smooth muscle-like cells that express melanoma antigens such as gp100, a splice variant of the Pmel17 gene. Tuberin and hamartin form heterodimers that act as negative regulators of mTOR. Lack of TSC2 function, as occurs in LAM cells, leads to the production of the chemokine CCL2/monocyte chemotactic protein 1 (MCP-1), which increases LAM cell mobility. Although many chemokines and their receptors could influence LAM cell mobilization, we propose that a positive-feedback loop is generated when dysfunctional TSC2 is present in LAM cells. We identified a group of chemokine receptors that is expressed in LAM cells and differs from those on smooth muscle and melanoma cells (Malme-3M). Chemokines have been implicated in tumor metastasis, and our data suggest a role for chemokines in LAM cell mobilization and thereby in the pathogenesis of LAM.
Keywords: cell motility, chemokines, chemokine receptors, cystic lung disease, lymphangioleiomyomatosis, metastasis, mammalian target of rapamycin, smooth muscle cells, tuberous sclerosis complex
I. INTRODUCTION
Lymphangioleiomyomatosis (LAM) is a rare cystic lung disease that is primarily found in women of childbearing age. LAM presents with progressive dyspnea, wheezing, cough, recurrent pneumothoraces, chylothorax, abdominal hemorrhage, involvement of the axial lymphatics (e.g., lymphangioleiomyomas), and abdominal tumors (e.g., renal angiomyolipomas).1,2 LAM occurs sporadically or in association with tuberous sclerosis complex (TSC), an autosomal dominant syndrome of variable penetrance, which is characterized by hamartoma-like tumor growths (e.g., facial angiofibroma, ungual fibroma, shagreen patch, renal angiomyolipoma, and pulmonary LAM nodules) and neurological disorders.3 These hamartomatous tumors are believed to be of mesenchymal origin and are classified as perivascular epithelioid cell neoplasms.4
LAM cells are phenotypically smooth muscle-like cells that express melanoma antigens such as gp100, CD63/LAMP-3, Melan-A, and MART1, and have abnormalities in the TSC1 or TSC2 genes.5 Loss of TSC gene function results in hyperactivation of the mammalian target of rapamycin (mTOR).6 LAM is believed to involve the migration of LAM cells between organs, and therefore we designated these events as a metastatic-like process. There is evidence that LAM cells disseminate by lymphatic and hematogenous but not transcoelomic routes. After single-lung transplantation, LAM cells from the recipient were shown to colonize the transplanted lung, suggesting a metastatic process.7,8 Consistent with lymphatic and hematogeneous spread, LAM cells can be detected in blood, urine, expectorated chyle, and pleural and abdominal chylous fluids.9
Metastatic cells are capable of translocation to target sites. Cell motility can be directed by gradients of chemokines that interact with specific receptors on the plasma membrane of tumor cells.10 Metastatic cells migrate to specific sites distant from the primary tumor growth and “home” to an appropriate environment described as “soil,” which appears to be identified by specific soluble chemoattractants produced by cells at the metastatic site.11,12
Chemokines could be produced in response to multiple factors. Infections are one of the best-characterized processes in the recruitment and homing of immune cells, as are inflammation and tissue injury. Chronic inflammation is a characteristic of many cancerous processes that lead to the activation of pathways involving nuclear factor κB participation in the transcription of chemokines that attract cancer cells to sites of metastasis.10,12
To understand the molecular events that lead to LAM cell growth and dissemination, we investigated the potential role of chemokines and their receptors in the spread of LAM cells.
II. LAM CELLS AND LAM LUNG NODULE
The cells responsible in LAM are termed “LAM cells,” spindle- and epithelioid–shaped smooth muscle-like cells that contain dysfunctional TSC2 or TSC1 genes and form part of lung nodular structures, which express melanoma as well as smooth muscle cell antigens.2,5 Whereas LAM cells of both phenotypes synthesize smooth muscle-cell proteins (e.g., smooth muscle α-actin, vimentin, desmin), the epithelioid cells appear to produce gp100, a premelanosomal protein product of alternatively spliced Pmel17 transcripts. MART-1, CD63, and PNL2 are all melanosomal proteins controlled by microphthalmia transcription factor and produced in a group of pathological mesenchymal-derived cells characterized as perivascular epithelioid cells.13 Nodular LAM structures are covered with hyperplastic type II pneumocytes and contain mast cells and mast cell products (e.g., chymase). Cells lining lymphatic channels within the nodules react with antibodies against lymphatic endothelial cells.14
The roles of TSC1/2 have been defined previously5: TSC2 gene on chromosome 16p13.3 and TSC1 gene on chromosome 9q34 encode tuberin and hamartin, respectively. Tuberin and hamartin form heterodimeric complexes that negatively regulate the mTOR serine-threonine kinase. Multimeric complexes containing mTOR termed mTORC1 and mTORC2 are central to cell growth, proliferation, gene transcription, and protein synthesis.15 mTORC1 is a multiprotein complex comprised of five proteins sensitive to rapamycin: mTOR, Raptor (regulatory-associated protein of mTOR), mLST8/GβL (mammalian lethal with Sec 13 protein 8), PRAS40 (proline-rich AKT substrate 40 kDa), and Deptor (DEP-domain-containing mTOR-interacting protein). The mTORC2 complex is less sensitive to rapamycin and contains six proteins: mTOR, Rictor (rapamycin-insensitive companion of mTOR), mSIN1 (mammalian stress-activated protein kinase interacting protein 1), Protor-1 (protein observed with Rictor-1), mLST8, and Deptor.16 Components of TORC1 (i.e., Raptor), and TORC2 (i.e., Rictor) regulate the different functions attributed to mTOR.15 mTORC1 activity is largely regulated by the GTPase Rheb (Ras homolog enriched in brain), which is a substrate for the GTPase-activating function of tuberin (Fig. 1).17–19
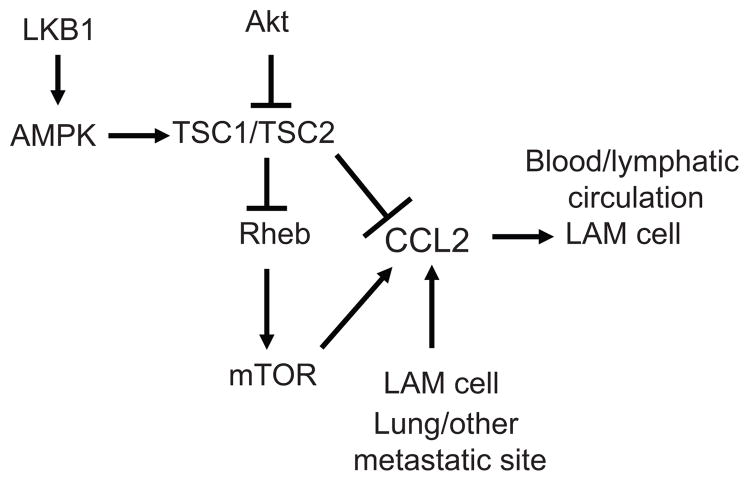
FIGURE 1.
Regulation of LAM cells by a CCL2/MCP-1 positive-feedback loop. TSC2 is a negative regulator of mTOR through conversion of Rheb-GTP to Rheb-GDP. Akt negatively regulates tuberin, which is also a substrate for the LKB1 substrate AMP-dependent kinase. Mutations that lead to dysfunctional TSC2 cause hyperactivation of mTOR. Lack of TSC2 function results in up-regulation of CCL2 production. In addition, CCL2 enhances LAM cell motility and is produced by LAM cells, by other cell types within the lung, or by extra-pulmonary cells, resulting in a positive-feedback loop regulating LAM cell motility.
Akt-dependent signaling regulates mTOR, and thereby phosphorylation of S6K1, S6K2, and eIF4E. Phosphorylation of tuberin by Akt causes its inactivation and disassembly of the TSC1/TSC2 complex18; AMP-dependent protein kinase regulated by the tumor suppressor LKB1 also modulates the function of the TSC1/TSC2 complex6 (Fig. 1).
III. MOLECULAR ASPECTS OF LAM CELL METASTASIS
Identification of phenotypically and genotypically similar cells in kidneys, lymphatics, and lungs of LAM patients suggest that LAM cells disseminate in a metastatic-like process.20 Because LAM cells are able to invade transplanted lungs, it was suggested that hematogenous and lymphatic routes are used for their dissemination.3–9,20,21 Cells that metastasize by lymphatic and hematogenous routes appear to begin with detachment from the primary locus and invade the local tissue stroma, followed by penetration into local blood or lymphatic vessels and transit to arrest points after surviving the circulatory system. Arrested cells penetrate the parenchyma at a metastatic site, adapt to the environment, and proliferate.22 All of these events are required for metastatic cell proliferation at a distant site, but the intercellular communication among these cells and receptive tissues plus additional cues (e.g., chemokines) to mobilize and anchor cells at metastatic sites are also important.23,24
IV. CHEMOKINES AND CHEMOKINE RECEPTORS
Chemokines were initially identified as molecules that induce the migration of leukocytes and are produced by immune cells that respond to inflammatory responses.25 The lung responds to environmental insults by recruiting inflammatory cells that secrete chemokines that attract other cells to the lung; thus, chemokines may have roles in both innate and adaptive immunity. Chemokines also participate in organ homeostasis and in cancerous processes.10 Many cells of non-immune origin not only have chemokine receptors, but also synthesize and secrete chemokines.26
Four subfamilies of chemokines (C, CC, CXC, and CX3C) are defined by the location of cysteine in their primary structure. There are at least 53 chemokines, secreted proteins of 66 to 111 amino acids, except for CXCL16 and CX3CL1, which are membrane-bound proteins.26 Genes that encode chemokines are present on different human chromosomes, with clusters of chemokine genes on chromosomes 4q21.1, 17q11.2, and 17q12. Chemokines are also classified into functional subfamilies,27 including: inflammatory chemokines, which participate in innate immunity (e.g. CXCL16, CXCL1), extravasation (CX3CR1), and adaptive immunity (e.g., CCL2, CCL6, CCL27, CCL28); and homeostatic chemokines, which participate in hematopoiesis (CXCL12), follicular activities (CXCL13), T lymphopoiesis (CCL19 and CCL21), and T-cell-denditric cell interaction (CCL18).
Chemokine receptors are seven-transmembrane-domain, G protein-coupled receptors of the Gi2 family and members of the rhodopsin-like seven-transmembrane superfamily. Binding of chemokines to their receptors causes conformational changes and, in some cases, receptor dimerization, which affects guanine nucleotide exchange on the alpha subunit of the G-protein complex (Gαβγ), leading to the formation of Gα-GTP and its dissociation from G-βγ. The individual units activate specific signaling pathways in which small GTPases such as Rho, Cdc42, and Rac affect cell motility. Gβγ subunits activate phospholipase C, resulting in the production of inositol triphosphate and diacylglycerol.26 Thus, the multiple effects of chemokines could lead to LAM cell invasiveness and migration due to the activation of the GTPases Rac1 and Rho. Notably, the Rho protein is also regulated by the TSC2 gene product tuberin.28,29
V. LAM AND CHEMOKINES
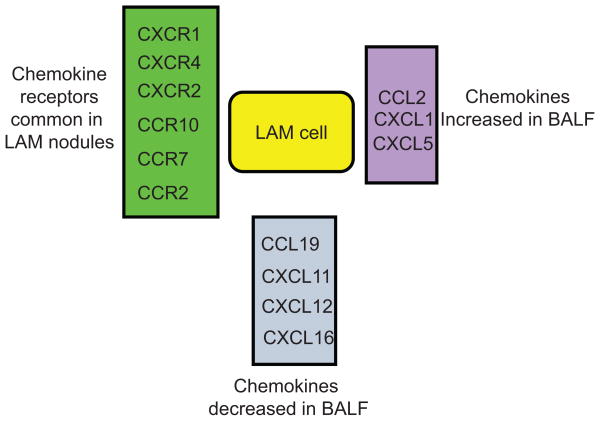
Concentrations of CCL2 (MCP-1), CXCL1 (GRO1), and CXCL5 (ENA-78) in bronchoalveolar lavage fluid from patients with LAM were higher than in that from healthy volunteers, suggesting that these chemokines could be involved in the recruitment of LAM cells to the lung30 (Fig. 2). Levels of CCL2/MCP-1 were higher in brochoalveolar lavage fluid from LAM patients than in that from healthy volunteers.
FIGURE 2.
Chemokine and chemokine receptors differentially expressed in LAM cells and bronchoalveolar lavage fluid.
Polymorphisms of the CCL2/MCP-1 gene were more frequent in patients with LAM than in healthy volunteers, and were correlated with rates of decline in lung function. Frequencies of two polymorphisms in the promoter of the CCL2/MCP-1 gene were compared in LAM patients and age- and sex-matched healthy volunteers, and differed significantly in the two groups. The frequency of AA at positions −2578 and −2136 was greater in LAM patients than in volunteers, and more detailed analysis suggested that the CCL2/MCP-1 gene and protein may be genetic modifiers in the development of LAM.
CCL2/MCP-1 selectively attracted cells with dysfunctional TSC2.30 CCL2/MCP-1 was further shown to be associated with LAM nodules in about 70% of patients. As part of a potential feed-forward pathway, TSC2 regulated CCL2/MCP-1 production in human skin and mouse cells31–33. CCL2 was overexpressed in human cells grown from TSC skin, periungualfibroma, and angiofibroma.31 Overexpression of MCP-1 in mouse Tsc2−/− cells was mTOR dependent and resulted from a loss of tuberin function.32,33 High levels of CCL2/MCP-1 were reduced in both human and rodent cells lacking tuberin function, but reconstitution of cells with tuberin or treatment with rapamycin to abrogate mTOR activity decreased the production of CCL2/MCP-1. These data also suggest that TSC2 could down-regulate CCL2/MCP-1 production directly or indirectly by the action of mTOR. Altogether, it is proposed that CCL2/MCP-1 is involved in LAM cell dissemination by a paracrine feedback loop (Fig. 1).
VI. LAM AND CHEMOKINE RECEPTORS
LAM cells within lung lesions displayed a unique group of chemokine receptors, including CCR2, CXCR4, CCR7, and CCR10, which allowed grouping of the LAM samples from smooth muscle or melanoma cells.30 Global gene-expression analysis of microdissected LAM lung cells of patients with sporadic LAM identified a set of transcripts that distinguished lung LAM cells from smooth muscle and melanoma cells (GEO database GSE12027). Some chemokines were differentially expressed and may participate in attracting and anchoring cells to sites of metastasis.
Among the receptors most frequently found in diverse cancers are CXCR4, CCR7, CCR4, CCR10, and CXCR7.34 Metastatic breast cancer cells express the chemokine receptors CXCR4 and CCR7, whereas malignant melanoma cells express CCR10, CXCR4, and CCR7.12 Smooth muscle-like LAM cells in more than 50% of patients reacted with antibodies against the chemokine receptors CCR2, CCR7, CCR10, CXCR2, CXCR4, and CXCR1,30 which supports the conclusion that cancer cells exhibit a characteristic profile of chemokines and their receptors.12 Thus, cells can to some extent be identified by their chemokine/chemokine receptor molecular signatures, which seem to direct metastasis to specific organs. Although it is not clear why a single tumor cell has multiple chemokine receptors, the observation that chemokine receptors are able to homo- and heterodimerize to produce specific effects suggests this as a mode of chemokine/chemokine receptor action in LAM cells.35,36
We determined which chemokine receptors were more common in LAM lung nodules (Fig. 2), and found that the receptor and ligand found most often in LAM nodules and known to be involved in cancer are CXCR4 and its ligand SDF-1 (CCL12).37 This receptor has been identified in at least 23 types of cancer, including breast and ovarian cancers and melanoma.10,38 CXCR4 and CXCR3 are functional receptors in melanoma cells and are involved in the activation of the MAPKs p44/42 and p38 pathways.39 We did not detect high levels of CCL12 in the bronchoalveolar lavage fluid of LAM patients, but a large percentage of LAM cells expressed CXCR4,30 and CXCR4 mRNA levels were high in LAM cells from lung nodules.30 We were unable to define the role of the CCL12-CXCR4 pathway in LAM cells, but angiomyolipoma cells appear to respond to CXCL12 by activating AKT and p42/44.40
Some cells in LAM nodules express receptors such as CXCR6, CXCR5, CCR8, CCR5, CCR4, CCR3, and CCR1.31 CXCR6 and its ligand CCL16 are present in several cancers including liver, prostate, colon, breast, ovary, glioblastoma, lung, lymphoma, melanoma, and renal cell carcinoma, but not in thyroid or head and neck cancers.41 CXCL16 may be relevant to LAM because it stimulates secretion of IL-8 and IL-6, activation of Akt, p70S6, and initiation factor 4E, thereby affecting cell proliferation and invasion.42 Thus, activation of some pathways in LAM lesions could result from signaling cross-talk.
CCR2 and CCR10 are among the receptors found most frequently in LAM lungs. CCR10 has been identified as a receptor involved in melanoma metastasis.26,43 CCR2 is clearly involved in breast cancer, glioma, lung cancer, melanoma, and prostate cancer.26 We were unable to show that CCR2 is a receptor that distinguishes LAM cells from melanoma or smooth muscle cells, but both CCR2 and CCR10 bind CCL2/MCP-1, which is one of the chemokines most elevated in LAM.
VII. MECHANISMS OF DYSREGULATION OF CHEMOKINES AND THEIR RECEPTORS
Multiple factors could contribute to CCL2/MCP-1 levels in individuals with LAM, and the effects apparently differ from those in other interstitial lung diseases. Mechanisms that lead to dysregulated expression of chemokine and chemokine receptors in LAM patients are not established. It is known that infections, inflammatory responses, and hypoxia via co-receptors (e.g., HER2) can enhance the levels of chemokine receptors.44 Indeed, LAM lungs exhibit bronchiolitis, which could be a response to inflammation.45,46 In addition, the presence of hyperplastic type II pneumocytes surrounding the LAM cells might suggest an association with underlying inflammation or LAM cell products that could affect their proliferation.
The levels of CCL2 are regulated by multiple factors under conditions including menstruation and early pregnancy.47 These findings could be important to female LAM patients because of the putative role of estrogens in LAM pathogenesis. In support of this notion, 17-β-estradiol increased metastasis in both male and female mice engrafted with Tsc2−/− cells derived from Eker rats.48 Although all of these findings appear congruent with the pathogenesis of pulmonary LAM, an effect of estrogens on LAM cannot be deduced because of the use of non-human cell lines. The fact that estrogens regulate synthesis of chemokines could be a mechanism for enhanced metastasis in women with sporadic LAM.
The effects of LAM cells on adjacent stromal cells are not clearly established. We found, however, that CXCL5/Gro-1, which has been implicated in tumor progression by causing senescence of tumor-associated fibroblasts,49,50 was elevated in bronchoalveolar lavage fluid from LAM patients.
VIII. OTHER ASPECTS OF LAM CELLS
Metastatic growth involves adhesion molecules (e.g., cadherins, integrins, immunoglobulins, proteoglycans, CD44), and proteolytic enzymes such as metalloproteases (MMPs) (e.g., MMP-1, MMP-2, MMP-3, MMP-7, MMP-9). The proteoglycan CD44 receptor for hyaluronan is present on LAM cells.51 CD44, encoded in 20 exons on chromosome 11, is present in many different types of cells. The splice variant CD44v6, which has been involved in the metastasis of various cancers, is present on LAM cells.51 Thus, proteins implicated in metastatic pathways are associated with LAM cells, conferring metastatic potential. CD44 binds osteopontin and is cleaved by metalloproteinases.52 LAM nodules contain MMP-2, MMP-9, MMP1, and MMP activators (MT1-MMP) and inhibitors (TIMPs).53,54 The presence of CD44v6 and MMPs support molecular aspects of LAM metastasis. In addition to providing a metastatic phenotype to LAM cells, CD44 could be involved in the metabolism of chemokines by interacting with chemokine scavenger receptors.
IX. SUMMARY
Chemokines such as those found at high levels in bronchoalveolar lavage fluid from LAM patients could be important in metastasis and modify the LAM microenvironment to affect cell-stroma interactions. LAM cell mobilization could be favored by the presence of specific chemokines and their receptors, making both ligand and receptor potential therapeutic targets. The selective attraction of LAM cells by CCL2/MCP-1 and overexpression of CCL2/MCP-1 by cells lacking the tumor suppressor tuberin lead us to propose a paracrine feedback loop of LAM cell motility in which this chemokine has a major role (Fig. 1).
ABBREVIATIONS
- Deptor
DEP-domain-containing mTOR interacting protein
- LAM
lymphangioleiomyomatosis
- mTOR
mammalian target of rapamycin
- mLST8/GβL
mammalian lethal with Sec 13 protein 8
- mSIN1
mammalian stress-activated protein kinase interacting protein 1
- MCP-1
monocyte chemotactic protein 1
- MMP
matrix metalloprotease
- PRAS40
proline-rich AKT substrate 40 kDa
- Protor-1
protein observed with Rictor-1
- Raptor
regulatory-associated protein of mTOR
- Rheb
Ras homolog enriched in brain
- Rictor
rapamycin-insensitive companion of mTOR
- TSC
tuberous sclerosis complex
References
- 1.Ryu JH, Moss J, Beck GJ, Lee JC, Brown KK, Chapman JT, Finlay GA, Olson EJ, Ruoss SJ, Maurer JR, Raffin TA, Peavy HH, McCarthy K, Taveira-Dasilva A, McCormack FX, Avila NA, Decastro RM, Jacobs SS, Stylianou M, Fanburg BL NHLBI LAM Registry Group. The nhlbi lymphangioleiomyomatosis registry: Characteristics of 230 patients at enrollment. Am J Respir Crit Care Med. 2006;173:105–11. doi: 10.1164/rccm.200409-1298OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taveira-DaSilva AM, Steagall WK, Moss J. Lymphangioleiomyomatosis. Cancer Control. 2006;13:276–85. doi: 10.1177/107327480601300405. [DOI] [PubMed] [Google Scholar]
- 3.Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med. 2006;355:1345–1356. doi: 10.1056/NEJMra055323. [DOI] [PubMed] [Google Scholar]
- 4.Folpe AL, Kwiatkowski DJ. Perivascular epithelioid cell neoplasms: pathology and pathogenesis. Hum Pathol. 2010;41:1–15. doi: 10.1016/j.humpath.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 5.Carsillo T, Astrinidis A, Henske EP. Mutations in the tuberous sclerosis complex gene tsc2 are a cause of sporadic pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci U S A. 2000;97:6085–90. doi: 10.1073/pnas.97.11.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang J, Manning BD. The tsc1-tsc2 complex: a molecular switchboard controlling cell growth. Biochem J. 2008;412:179–90. doi: 10.1042/BJ20080281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bittmann I, Dose TB, Muller C, Dienemann H, Vogelmeier C, Lohrs U. Lymphangioleiomyomatosis: recurrence after single lung transplantation. Hum Pathol. 1997;28:1420–23. doi: 10.1016/s0046-8177(97)90233-1. [DOI] [PubMed] [Google Scholar]
- 8.Bittmann I, Rolf B, Amann G, Lohrs U. Recurrence of lymphangioleiomyomatosis after single lung transplantation: new insights into pathogenesis. Hum Pathol. 2003;34:95–8. doi: 10.1053/hupa.2003.50. [DOI] [PubMed] [Google Scholar]
- 9.Crooks DM, Pacheco-Rodriguez G, DeCastro RM, McCoy JP, Jr, Wang JA, Kumaki F, Darling T, Moss J. Molecular and genetic analysis of disseminated neoplastic cells in lymphangioleiomyomatosis. Proc Natl Acad Sci U S A. 2004;101:17462–7. doi: 10.1073/pnas.0407971101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zlotnik A. Chemokines and cancer. Int J Cancer. 2006;119:2026–9. doi: 10.1002/ijc.22024. [DOI] [PubMed] [Google Scholar]
- 11.Fidler IJ, Poste G. The ‘seed and soil’ hypothesis revisited. Lancet Oncol. 2008;9:808. doi: 10.1016/S1470-2045(08)70201-8. [DOI] [PubMed] [Google Scholar]
- 12.Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verástegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–6. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 13.Martignoni G, Pea M, Reghellin D, Zamboni G, Bonetti F. Pecomas: The past, the present and the future. Virchows Arch. 2008;452:119–32. doi: 10.1007/s00428-007-0509-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCormack FX. Lymphangioleiomyomatosis. MedGenMed. 2006 Jan 18;8:15. [PMC free article] [PubMed] [Google Scholar]
- 15.Wullschleger S, Loewith R, Hall MN. Tor signaling in growth and metabolism. Cell. 2006;124:471–84. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 16.Huang J, Manning BD. A complex interplay between akt, tsc2 and the two mtor complexes. Biochem Soc Trans. 2009;37:217–22. doi: 10.1042/BST0370217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Gao X, Saucedo LJ, Ru B, Edgar BA, Pan D. Rheb is a direct target of the tuberous sclerosis tumour suppressor proteins. Nat Cell Biol. 2003;5:578–81. doi: 10.1038/ncb999. [DOI] [PubMed] [Google Scholar]
- 18.Inoki K, Li Y, Xu T, Guan KL. Rheb gtpase is a direct target of tsc2 gap activity and regulates mtor signaling. Genes Dev. 2003;17:1829–34. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tee AR, Manning BD, Roux PP, Cantley LC, Blenis J. Tuberous sclerosis complex gene products, tuberin and hamartin, control mtor signaling by acting as a gtpase-activating protein complex toward rheb. Curr Biol. 2003;13:1259–68. doi: 10.1016/s0960-9822(03)00506-2. [DOI] [PubMed] [Google Scholar]
- 20.Henske EP. Metastasis of benign tumor cells in tuberous sclerosis complex. Genes Chromosomes Cancer. 2003;38:376–81. doi: 10.1002/gcc.10252. [DOI] [PubMed] [Google Scholar]
- 21.Kumasaka T, Seyama K, Mitani K, Souma S, Kashiwagi S, Hebisawa A, Sato T, Kubo H, Gomi K, Shibuya K, Fukuchi Y, Suda K. Lymphangiogenesis-mediated shedding of lam cell clusters as a mechanism for dissemination in lymphangioleiomyomatosis. Am J Surg Pathol. 2005;29:1356–66. doi: 10.1097/01.pas.0000172192.25295.45. [DOI] [PubMed] [Google Scholar]
- 22.Bacac M, Stamenkovic I. Metastatic cancer cell. Annu Rev Pathol. 2008;3:221–47. doi: 10.1146/annurev.pathmechdis.3.121806.151523. [DOI] [PubMed] [Google Scholar]
- 23.Poste G, Fidler IJ. The pathogenesis of cancer metastasis. Nature. 1980;283:139–46. doi: 10.1038/283139a0. [DOI] [PubMed] [Google Scholar]
- 24.Hart IR, Fidler IJ. Role of organ selectivity in the determination of metastatic patterns of b16 melanoma. Cancer Res. 1980;40:2281–7. [PubMed] [Google Scholar]
- 25.Rollins BJ. Chemokines. Blood. 1997;90:909–28. [PubMed] [Google Scholar]
- 26.O’Hayre M, Salanga CL, Handel TM, Allen SJ. Chemokines and cancer: migration, intracellular signalling and intercellular communication in the microenvironment. Biochem J. 2008;409:635–49. doi: 10.1042/BJ20071493. [DOI] [PubMed] [Google Scholar]
- 27.Moser B, Wolf M, Walz A, Loetscher P. Chemokines: multiple levels of leukocyte migration control. Trends Immunol. 2004;25:75–84. doi: 10.1016/j.it.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 28.Goncharova EA, Goncharov DA, Lim PN, Noonan D, Krymskaya VP. Modulation of cell migration and invasiveness by tumor suppressor tsc2 in lymphangioleiomyomatosis. Am J Respir Cell Mol Biol. 2006;34:473–80. doi: 10.1165/rcmb.2005-0374OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goncharova E, Goncharov D, Noonan D, Krymskaya VP. Tsc2 modulates actin cytoskeleton and focal adhesion through tsc1-binding domain and the rac1 gtpase. J Cell Biol. 2004;167:1171–82. doi: 10.1083/jcb.200405130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pacheco-Rodriguez G, Kumaki F, Steagall WK, Zhang Y, Ikeda Y, Lin JP, Billings EM, Moss J. Chemokine-enhanced chemotaxis of lymphangioleiomyomatosis cells with mutations in the tumor suppressor tsc2 gene. J Immunol. 2009;182:1270–7. doi: 10.4049/jimmunol.182.3.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li S, Takeuchi F, Wang JA, Fuller C, Pacheco-Rodriguez G, Moss J, Darling TN. Mcp-1 overexpressed in tuberous sclerosis lesions acts as a paracrine factor for tumor development. J Exp Med. 2005;202:617–24. doi: 10.1084/jem.20042469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Govindarajan B, Brat DJ, Csete M, Martin WD, Murad E, Litani K, Cohen C, Cerimele F, Nunnelley M, Lefkove B, Yamamoto T, Lee C, Arbiser JL. Transgenic expression of dominant negative tuberin through a strong constitutive promoter results in a tissue-specific tuberous sclerosis phenotype in the skin and brain. J Biol Chem. 2005;280:5870–4. doi: 10.1074/jbc.M411768200. [DOI] [PubMed] [Google Scholar]
- 33.Weichhart T, Costantino G, Poglitsch M, Rosner M, Zeyda M, Stuhlmeier KM, Kolbe T, Stulnig TM, Hörl WH, Hengstschläger M, Müller M, Säemann MD. The tsc-mtor signaling pathway regulates the innate inflammatory response. Immunity. 2008;29:565–77. doi: 10.1016/j.immuni.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 34.Wu X, Lee VC, Chevalier E, Hwang ST. Chemokine receptors as targets for cancer therapy. Curr Pharm Des. 2009;15:742–57. doi: 10.2174/138161209787582165. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez-Frade JM, Mellado M, Martinez AC. Chemokine receptor dimerization: two are better than one. Trends Immunol. 2001;22:612–7. doi: 10.1016/s1471-4906(01)02036-1. [DOI] [PubMed] [Google Scholar]
- 36.Wang J, Norcross M. Dimerization of chemokine receptors in living cells: key to receptor function and novel targets for therapy. Drug Discov Today. 2008;13:625–32. doi: 10.1016/j.drudis.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 37.Balkwill F. The significance of cancer cell expression of the chemokine receptor cxcr4. Semin Cancer Biol. 2004;14:171–9. doi: 10.1016/j.semcancer.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 38.Raman D, Baugher PJ, Thu YM, Richmond A. Role of chemokines in tumor growth. Cancer Lett. 2007;256:137–65. doi: 10.1016/j.canlet.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robledo MM, Bartolome RA, Longo N, Rodriguez-Frade JM, Mellado M, Longo I, van Muijen GN, Sanchez-Mateos P, Teixido J. Expression of functional chemokine receptors cxcr3 and cxcr4 on human melanoma cells. J Biol Chem. 2001;276:45098–105. doi: 10.1074/jbc.M106912200. [DOI] [PubMed] [Google Scholar]
- 40.Clements D, Markwick LM, Johnson SR. The cxcr4/cxcl12 axis in lymphangioleiomyomatosis. Am J Respir Crit Care Med. 2009;179:A4350. [Google Scholar]
- 41.Darash-Yahana M, Gillespie JW, Hewitt SM, Chen YY, Maeda S, Stein I, Singh SP, Bedolla RB, Peled A, Troyer DA, Pikarsky E, Karin M, Farber JM. The chemokine cxcl16 and its receptor, cxcr6, as markers and promoters of inflammation-associated cancers. PLoS One. 2009;4:e6695. doi: 10.1371/journal.pone.0006695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang J, Lu Y, Koch AE, Zhang J, Taichman RS. Cxcr6 induces prostate cancer progression by the akt/mammalian target of rapamycin signaling pathway. Cancer Res. 2008;68:10367–76. doi: 10.1158/0008-5472.CAN-08-2780. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Murakami T, Cardones AR, Finkelstein SE, Restifo NP, Klaunberg BA, Nestle FO, Castillo SS, Dennis PA, Hwang ST. Immune evasion by murine melanoma mediated through cc chemokine receptor-10. J Exp Med. 2003;198:1337–47. doi: 10.1084/jem.20030593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li YM, Pan Y, Wei Y, Cheng X, Zhou BP, Tan M, Zhou X, Xia W, Hortobagyi GN, Yu D, Hung MC. Upregulation of cxcr4 is essential for her2-mediated tumor metastasis. Cancer Cell. 2004;6:459–69. doi: 10.1016/j.ccr.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 45.Taveira-DaSilva AM, Hedin C, Stylianou MP, Travis WD, Matsui K, Ferrans VJ, Moss J. Reversible airflow obstruction, proliferation of abnormal smooth muscle cells, and impairment of gas exchange as predictors of outcome in lymphangioleiomyomatosis. Am J Respir Crit Care Med. 2001;164:1072–6. doi: 10.1164/ajrccm.164.6.2102125. [DOI] [PubMed] [Google Scholar]
- 46.Ryu JH, Myers JL, Swensen SJ. Bronchiolar disorders. Am J Respir Crit Care Med. 2003;168:1277–92. doi: 10.1164/rccm.200301-053SO. [DOI] [PubMed] [Google Scholar]
- 47.Kayisli UA, Mahutte NG, Arici A. Uterine chemokines in reproductive physiology and pathology. Am J Reprod Immunol. 2002;47:213–21. doi: 10.1034/j.1600-0897.2002.01075.x. [DOI] [PubMed] [Google Scholar]
- 48.Yu JJ, Robb VA, Morrison TA, Ariazi EA, Karbowniczek M, Astrinidis A, Wang C, Hernandez-Cuebas L, Seeholzer LF, Nicolas E, Hensley H, Jordan VC, Walker CL, Henske EP. Estrogen promotes the survival and pulmonary metastasis of tuberin-null cells. Proc Natl Acad Sci U S A. 2009;106:2635–40. doi: 10.1073/pnas.0810790106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang G, Rosen DG, Zhang Z, Bast RC, Jr, Mills GB, Colacino JA, Mercado-Uribe I, Liu J. The chemokine growth-regulated oncogene 1 (gro-1) links ras signaling to the senescence of stromal fibroblasts and ovarian tumorigenesis. Proc Natl Acad Sci U S A. 2006;103:16472–7. doi: 10.1073/pnas.0605752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ancrile BB, O’Hayer KM, Counter CM. Oncogenic ras-induced expression of cytokines: A new target of anti-cancer therapeutics. Mol Interv. 2008;8:22–7. doi: 10.1124/mi.8.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pacheco-Rodriguez G, Steagall WK, Crooks DM, Stevens LA, Hashimoto H, Li S, Wang JA, Darling TN, Moss J. Tsc2 loss in lymphangioleiomyomatosis cells correlated with expression of cd44v6, a molecular determinant of metastasis. Cancer Res. 2007;67:10573–81. doi: 10.1158/0008-5472.CAN-07-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ponta H, Sherman L, Herrlich PA. Cd44: From adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4:33–45. doi: 10.1038/nrm1004. [DOI] [PubMed] [Google Scholar]
- 53.Matsui K, Takeda K, Yu ZX, Travis WD, Moss J, Ferrans VJ. Role for activation of matrix metalloproteinases in the pathogenesis of pulmonary lymphangioleiomyomatosis. Arch Pathol Lab Med. 2000;124:267–75. doi: 10.5858/2000-124-0267-RFAOMM. [DOI] [PubMed] [Google Scholar]
- 54.Ferrans VJ, Yu ZX, Nelson WK, Valencia JC, Tatsuguchi A, Avila NA, Riemenschn W, Matsui K, Travis WD, Moss J. Lymphangioleiomyomatosis (lam): A review of clinical and morphological features. J Nippon Med Sch. 2000;67:311–29. doi: 10.1272/jnms.67.311. [DOI] [PubMed] [Google Scholar]