Abstract
Metastasis, the process by which cells spread from the primary tumor to a distant site to form secondary tumors, is still not fully understood. Although histological techniques have provided important information, they give only a static image and thus compromise interpretation of this dynamic process. New advances in intravital microscopy (IVM), such as two-photon microscopy, imaging chambers, and multicolor and fluorescent resonance energy transfer imaging, have recently been used to visualize the behavior of single metastasizing cells at subcellular resolution over several days, yielding new and unexpected insights into this process. For example, IVM studies showed that tumor cells can switch between multiple invasion strategies in response to various densities of extracellular matrix. Moreover, other IVM studies showed that tumor cell migration and blood entry take place not only at the invasive front, but also within the tumor mass at tumor-associated vessels that lack an intact basement membrane. In this Commentary, we will give an overview of the recent advances in high-resolution IVM techniques and discuss some of the latest insights in the metastasis field obtained with IVM.
Keywords: Intravital imaging, Intravital microscopy, Cancer, Invasion, Metastasis
Introduction
Complete surgical resection of the primary tumor is still one of the most efficient therapies for cancer. Unfortunately, cancer can progress to a stage at which tumor cells leave the primary tumor and spread to a distant organ to form a secondary tumor, a process referred to as metastasis. Complications caused by metastases are the major cause of cancer-related death, but this process is not fully understood.
Initially, it was thought that spread of tumor cells and subsequent metastasis formation are late events in tumor progression; however, more recently it was realized that it might instead be an early event (Hüsemann et al., 2008; Klein, 2009). In either case, tumor cells have to acquire certain traits that allow them to escape from the primary tumor site and home in on and colonize a secondary site (Fig. 1). These gained properties, such as survival, invasiveness and motility, allow tumor cells to move into the surrounding tissue, where they enter blood or lymph vessels (Talmadge and Fidler, 2010; Wyckoff et al., 2000; Wyckoff et al., 2007). Once in circulation, tumor cells are transported to a secondary site, where they can grow out to form metastatic foci or become dormant (Chambers et al., 2002; Chambers et al., 1995; Nguyen et al., 2009; Talmadge and Fidler, 2010). For these colonization events to take place, tumor cells need to be able to respond to chemoattractants and growth factors, and survive in the new environment. The requirement for these traits might vary during tumor progression or among different tumor types, and the acquisition of these traits does not follow a particular order (Chiang and Massague, 2008; Nguyen et al., 2009). It is worth noting that only a small fraction of the cells present in the primary tumor have the necessary characteristics to escape from the primary site and colonize a secondary site, which renders metastasis an inefficient process (Chambers et al., 2002). To investigate these dynamic processes underlying metastasis, most studies rely on techniques that are only able to provide a static view, such as histochemistry, visual inspection of tumor size and end-stage measurements (e.g. the number of metastatic foci). In addition, these techniques analyze large numbers of cells, which obscures the signaling properties and activities of individual cells. This results in loss of crucial information concerning the adaptive properties of the few tumor cells that spread and form metastases.
Fig. 1.
IVM of individual steps of metastasis. The schematic illustrations aim to provide a simplified overview of the metastatic process. To metastasize, tumor cells (green) have to escape from the primary tumor and colonize a distant site. During this process, cells employ traits, such as invasiveness, motility, attachment, chemosensing and survival, that allow them to detach from the primary tumor, invade the interstitial matrix (purple), overcome the barrier of the BM (blue), enter the blood (red), be transported to a distant site, exit the blood and finally grow out to form metastatic foci. IVM can be used to image metastatic processes, as illustrated by the IVM images of tumor cells (green), type I collagen (purple) and blood (red). IVM images A and B represent different time points of invasion of a polyoma middle T (PyMT) mammary tumor. IVM image C shows the tumor cells present in a vessel that collects blood from a C26 colorectal tumor and is one time point of supplementary material Movie 1. Scale bar: 10 μm.
Recent advances in optical methods have made it possible to visualize the metastatic process at a subcellular resolution in real time in vivo. By the 19th century, microscopes were being used to image tissue in living animals (e.g. Wagner, 1839), a technique referred to as intravital microscopy (IVM). In these early days, most IVM studies could only examine the vasculature and the microcirculation, because the optics available at that time and lack of contrast limited the visualization of other tissues. In the 1950s, the visualization of metastasis was pioneered in a rabbit ear chamber (Wood, 1958). Major breakthroughs in this field occurred in the 1990s, when intravital imaging techniques improved considerably and genetic tumor models of rodents that expressed fluorescent proteins (FPs) became available. Since then, IVM has evolved into an important tool for investigating the processes underlying cancer and metastasis (see IVM images in Fig. 1) (Chishima et al., 1997; Farina et al., 1998; MacDonald et al., 1992; Naumov et al., 1999).
Recently, a number of new IVM techniques have become available with different properties in relation to imaging depth, resolution, timescale and applications (Table 1) [for an extensive review on in vivo optical microscopy methods, see Ntziachristos (Ntziachristos, 2010)]. Techniques that are based on fluorescence microscopy provide (sub)cellular resolution and have successfully been used to image single cells in living organisms in their natural environment, such as in fruit flies, zebrafish, nematodes and small rodents (Fouquet et al., 2009; Ghosh and Hope, 2010; Le Dévédec et al., 2010; Stoletov et al., 2010). In this Commentary, we will focus on high-resolution IVM techniques used in small rodents (rats and mice), as they are the most widely used model organisms to study cancer. We will describe the advances in high-resolution IVM techniques that have made it possible to directly visualize the various steps of metastasis at cellular resolution (see Fig. 1). We will also discuss the new insights into the process of tumor cell invasion that have been obtained with such IVM studies.
Table 1.
Overview of different types of intravital imaging
Advances in IVM
High-resolution IVM techniques
There are several high-resolution IVM techniques, including optical frequency domain imaging (OFDI), spinning disk confocal microscopy and multiphoton microscopy (Table 1). In OFDI, an optical beam is focused into the tissue and reflected light is detected after interference with a reference beam. The amplitude and phase of the reflected signal as a function of time are then used to derive the optical scattering properties and thereby the tissue structure at different depths. OFDI has been used to study angiogenesis, lymphangiogenesis and tissue viability (Vakoc et al., 2009). The advantage of this technique is that it enables the imaging of substantial volumes of tissue over prolonged periods without the need for contrast agents.
At present, a number of mouse tumor models are available in which various FPs are expressed. For the imaging of tumors that express FPs, spinning disk confocal microscopy and multiphoton microscopy have been the IVM techniques of choice. In spinning disk confocal microscopy, visible light is used to excite FPs. Here, emitted out-of-focus light (outside the optical section) is eliminated by multiple pinholes, resulting in increased Z-resolution and contrast enhancement, which makes subcellular structures more easily visible. A spinning disk confocal microscope can acquire high-resolution images at high speed and low phototoxicity, therefore allowing the long-term intravital imaging of tumors (up to 30 hours) (Egeblad et al., 2008; Lohela and Werb, 2010). The downside of spinning disk microscopy is that the visible light used for excitation is scattered in the tissue, which limits its imaging depth to 100 μm. By contrast, the short pulses of infrared (IR) light used in multiphoton microscopy, with a typical wavelength range of 800–1000 nm, penetrate the tissue more than tenfold deeper compared with spinning disk microscopy (see Box 1) (Theer et al., 2003). An additional advantage of IR excitation is its ability to create a second harmonic generation (SHG) signal from non-centrosymmetric structures with highly ordered repeats, such as collagen type I fibers, an extracellular matrix component (Campagnola et al., 2002). In contrast to fluorescence microscopy, SHG does not involve the excitation of fluorophores and hence the signal does not suffer from photobleaching (for reviews, see Helmchen and Denk, 2005; Mohler et al., 2003; Zipfel et al., 2003b). SHG microscopy has been used to study the role of fibrillar collagen in tissues and organs, including skin, gut and breast (e.g. Wolf et al., 2009).
Box 1. Multiphoton microscopy.
Multiphoton excitation is based on the (almost) simultaneous absorption of two low-energy IR photons, whose combined energies induce fluorescence in the visible range (see Figure, panel A). Simultaneous absorption of two photons by a fluorophore is an extremely rare event and multiphoton excitation therefore requires high excitation powers (i.e. a dense photon flux). IR lasers achieve this by condensing all photons over a time span of 12.5 ns into a 100 fs pulse (thus concentrating the photon density 1.25 × 105 times). Although the overall power is low (i.e. low number of photons per second), the photon density per pulse is sufficiently high to cause efficient fluorophore excitation in the focal plane (see Figure, panel B). Beyond the focal plane, however, the photon density is too low for simultaneous absorption by a fluorophore to occur (see Figure, panel B) (Dunn and Young, 2006; Helmchen and Denk, 2005; Zipfel et al., 2003b). Therefore, in contrast to confocal microscopy, excitation is strictly confined to the focal plane, thus avoiding the need for a pinhole to delete out-of-focus light (see Figure, panel B). This greatly enhances the detection efficiency, as the in-focus emitted light can be detected close to the objective, without the need to travel back through the scanner optical path (with loss of tissue-scattered emission at the pinhole and further losses at each filter) (Centonze and White, 1998). Furthermore, deep tissue penetration, reduced excitation of surrounding tissue, increased detection and low-energy IR light give the additional advantage of low phototoxicity and low photobleaching (Eggeling et al., 2005).
Box 1 Fig. Two-photon versus single-photon excitation.
The combination of high-resolution acquisition of fluorescent tissues and deep tissue penetration has made multiphoton IVM the technique of choice for most research groups that exploit intravital imaging for tumor cell biology (for more details, see Box 1).
Advanced detection methods for simultaneous imaging of multiple cell types and molecules
Using multicolor IVM, different cell types and molecules can be visualized simultaneously, which has made it possible to study the interaction between different cell types and molecules in real time (Fig. 2A) (Bajénoff et al., 2006; Boissonnas et al., 2007; Castellino et al., 2006; Egeblad et al., 2008; Egen et al., 2008; Farina et al., 1998; Goswami et al., 2005; Lohela and Werb, 2010; Massberg et al., 2007; Wyckoff et al., 2007). Since the introduction of the green fluorescent protein (GFP) (Chalfie et al., 1994; Heim et al., 2005; Shimomura et al., 1962) and its color variants, cyan fluorescent protein (CFP), yellow fluorescent protein (YFP) and red fluorescent protein (RFP) (Giepmans et al., 2006), together with the more recently discovered FPs [e.g. photoswitchable proteins (Piatkevich and Verkhusha, 2009)], several cell types can now be genetically labeled and visualized in tumors using, for example, CFP and YFP (e.g. Sahai et al., 2005). In addition, tumor cells labeled with an FP can also be imaged at the same time as cells that are labeled with fluorescent dyes [e.g. dextran-labeled macrophages (Fig. 2)]. A specific way to label immune cells (often lymphocytes) is to isolate them and load them with fluorescent tracker dyes before transferring them back into the recipient animal. The disadvantage to this approach is, however, that the fluorescently labeled cells will constitute only a small fraction of the population compared with their endogenous, non-labeled counterparts. A simple but functional approach to accomplish contrast between different cell types and tissues is intravenous or subcutaneous injection of cell-type-specific fluorescent antibodies or other targeted tracers [e.g. for lymphatics, see Alexander et al. (Alexander et al., 2008) and McElroy et al. (McElroy et al., 2008); for Gr1+ myeloid cells, see Egeblad et al. (Egeblad et al., 2008)]. Nevertheless, it should be noted that these signals are short lived, whereas FP signals persist. However, a major disadvantage of FPs is the difficulty in translating their use into patients. A general problem with multicolor IVM is that the excitation (particularly in multiphoton excitation) and emission spectra of most FPs and dyes overlap, and some FPs and dyes can only be successfully distinguished when complicated algorithms are used (Jalink and van Rheenen, 2009). Alternatively, FPs and dyes can be discriminated based on their unique fluorescence lifetime (the average time an excited fluorophore is in its excited state), which can be measured with fluorescence lifetime imaging microscopy (FLIM) (see Box 2) (Bastiaens and Squire, 1999). Using FLIM increases the number of different fluorophores and thus cells that can be imaged simultaneously.
Fig. 2.
Multicolor and FRET IVM. Caspase-3 activity and cell–cell interactions can be imaged using multicolor and FRET IVM. (A) Schematic representation (left) and corresponding multicolor IVM image (zoom and overview on the right) of a colorectal C26 tumor. Merged images of Dendra2-expressing tumor cells (green), 70 kDa Texas-Red-labeled macrophages (red) and type I collagen (purple; SHG) illustrate the interaction between macrophages and tumor cells. (B) Cartoon of a caspase-3 FRET biosensor to measure caspase-3 activity during apoptosis induction. Here, CFP (C) and YFP (Y) are in close proximity, resulting in efficient FRET. When caspase-3 is activated in the cell by induction of apoptosis, the sensor is cleaved, leading to increased distance between CFP and YFP with a subsequent loss of FRET. This loss is observed as increased CFP fluorescence and decreased YFP fluorescence. (C) The IVM image shows a large overview of the skin of a mouse in which single keratinocytes are transfected by DNA tattooing with the caspase-3 FRET biosensor and a chemical-sensitive inducer of apoptosis. The arrows point to examples of cells that are transfected with the caspase-3 sensor, in which the apoptosis status can be imaged using FRET. (D) Using the system shown in B, IVM images of CFP and YFP are acquired upon CFP excitation and FRET is expressed as a ratio of YFP over CFP (see color bar on the right). A striking drop in FRET is observed upon chemical induction of apoptosis (lower row of images), illustrating the measurement of signaling events in vivo. All scale bars: 10 μm.
Box 2. Fluorescence lifetime imaging microscopy to distinguish spectrally overlapping fluorophores.
FLIM produces images that are based on the fluorescence lifetime of a fluorophore, rather than on its intensity and color (Gerritsen et al., 2009; Wouters et al., 2001). A major advantage of the FLIM technology is that fluorophores that have overlapping emission spectra (see Figure, panel A) can be distinguished, a feature that is not possible with an intensity image. When fluorophores are excited with a femtosecond IR pulse, the fluorescence will decay exponentially with a rate that is determined by the fluorescence lifetime, which is close to zero for SHG (see Figure, panel B) and around 2 ns for Dendra2 (see Figure, panel C). In every pixel of a FLIM image, the fluorescence decay curve is measured and used to calculate the fluorescence lifetime. Many pixels of the image contain both fluorophores, and therefore will have an average lifetime that depends on the fraction of each fluorophore that is present in that pixel (see Figure, panel D). A FLIM image can be visualized by color coding the fluorescence lifetime (see color bar next to the FLIM image). In the FLIM image, fluorophores with distinct fluorescence lifetimes, such as SHG and Dendra2, will be color coded differently and can therefore be distinguished.
Box 2 Fig. Fluorescence lifetime imaging.
In addition to discriminating spectral overlap between fluorophores, FLIM is also used to distinguish between the autofluorescence of various tissue types (see Box 2). Autofluorescence is the intrinsic property of cells to emit fluorescence upon excitation and is caused by its endogenous constituents, such as tryptophan, pyridinic coenzymes (i.e. NADPH), flavin coenzymes, melanin and elastin, which all have fluorescent properties [e.g. Monici and El-Gewely, 2005; Zipfel et al., 2003a]. Because most of the autofluorescent tissue components exhibit comparable excitation and emission spectra, the resulting fluorescence levels are similar and cannot be used to distinguish tissues. However, as the lifetime of autofluorescence differs between different cell types, autofluorescence lifetime measurements have successfully been used to distinguish healthy tissues from tumor tissues (Galletly et al., 2008; Provenzano et al., 2009).
Intravital imaging of proteins, proteases, signaling pathways and cellular processes in vivo
Imaging of molecular processes in living mice has been achieved by multiphoton IVM of FP fusion proteins and injectable dyes. High-resolution IVM of cells that express a fusion of FP with a receptor [e.g. ErbB2 (Dennis et al., 2007)], an adhesion molecule [e.g. E-cadherin (Serrel et al., 2009)], or an actin- or myosin-binding protein (Philippar et al., 2008; Wyckoff et al., 2006) has been used to study intracellular signaling in single tumor cells. In addition to FP fusion proteins, many biosensors that are based on measuring a change in fluorescent resonance energy transfer (FRET) (see Box 2) can be used to study intracellular signaling in vivo. The number of available biosensors is rapidly increasing, and specific FRET biosensors are already available to measure the levels of second messengers, protein–protein interactions or the activation status of proteins (e.g. Rho) [for a review on biosensors, see Morris (Morris, 2010) and VanEngelenburg and Palmer (VanEngelenburg and Palmer, 2008)]. However, as the field of FRET biosensors is still an emerging area, only a few research groups have thus far used this powerful technique in vivo. For example, FRET biosensors have been used to measure the proteolytic activity of calpain in muscles (Stockholm et al., 2005), changes in calcium concentration and myosin light-chain kinase activity in arteries (Zhang et al., 2010), and the induction of apoptosis in the skin (Bins et al., 2007), colorectal carcinomas and lymphomas (Breart et al., 2008; Keese et al., 2010).
In particular, the apoptosis sensor has been of interest for tumor biology, because tumor cells have to overcome many apoptotic signals during several steps of metastasis. The FRET apoptosis sensor consists of a CFP that is separated from a YFP by a caspase-3 recognition sequence (Fig. 2B). Caspase-3 activity cleaves CFP from YFP, leading to a loss of FRET efficiency. Indeed, when we induced apoptosis in single keratinocytes in the skin of living mice, a caspase-3-mediated loss of FRET efficiency is observed, which shows that caspase-3 activity is a good gauge of apoptosis induction in vivo (Fig. 2C,D) (Bins et al., 2007). Keese and colleagues used FRET IVM of this biosensor to study the induction of apoptosis resistance upon chemotherapy (Keese et al., 2007; Keese et al., 2010). Moreover, Breart and colleagues used this biosensor to study apoptosis of tumor cells by cytotoxic T lymphocytes (CTLs) and showed that a CTL kills, on average, one tumor cell every 6 hours (Breart et al., 2008).
Many synthetic fluorescent probes have been developed to study the intracellular and extracellular of activity of proteases such as matrix metalloproteinases (MMPs) and cathepsins in tumors. The injectable probes that have been used to measure protease activity in tumors include autoquenched protease substrates that fluoresce upon cleavage (Weissleder et al., 1999), proteolytic beacons that lose FRET upon cleavage (e.g. McIntyre et al., 2010; Scherer et al., 2008) and fluorescently labeled activatable cell-penetrating peptides (ACCPs) that translocate into cells upon cleavage (Olson et al., 2009; Olson et al., 2010). The disadvantage of these probes is that chemical knowledge is required to synthesize them, although recent commercialization of some of these probes has overcome this problem. It is also important to note that phagocytic cells, such as macrophages, might take up some of the probes despite not having any proteolytic activity, which complicates the interpretation of high-resolution IMV images. Nevertheless, IVM of fluorescently tagged proteins, and of genetic and synthetic fluorescent probes has become a great tool to visualize protein activity, signaling pathways and cellular processes during several steps of metastasis.
Long-term intravital imaging over several days
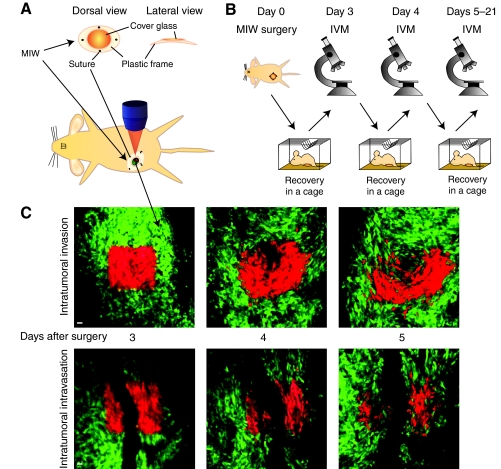
IVM has contributed to the visualization of the behavior of tumor cells in living mice. However, most IVM studies only monitor short-term movement of cancer cells because they use tumor dissection, which effectively limits experimental observations to a few hours after surgery [typically around 4 hours and after optimization up to 24 hours (Egeblad et al., 2008)]. Given the small number of tumor cells that display high motility and subsequently migrate and invade over periods that last up to days, long-term IVM techniques are needed to provide valuable information regarding these processes. By the 1990s, Lehr and co-workers had developed the dorsal skinfold chamber, which is surgically implanted on the back of a mouse, allowing multiple tumor-imaging sessions over several days (Alexander et al., 2008; Brown et al., 2001; Lehr et al., 1993). Since then, orthotopic chambers (i.e. in their natural environment) have been developed, including the so-called ‘mammary imaging window’ (MIW) for mammary tumors that we developed (Fig. 3A,B) (Gligorijevic et al., 2009; Kedrin et al., 2008; Shan et al., 2003) and the ‘cranial imaging window’ for brain tumors (Yuan et al., 1994). These imaging windows have made it possible to visualize a number of metastatic processes over several days, including the formation of new blood vessels (Abdul-Karim et al., 2003; Farhadi et al., 2005; Fukumura et al., 2010), tumor cell invasion and intravasation (Fig. 3C) (Kedrin et al., 2008), and tumor cell colonization at a distant site (Kienast et al., 2010).
Fig. 3.
IVM over several days using an MIW. Experimental setup for repeated IVM imaging of live mice over several days using an MIW. (A) Schematic illustration of an MIW consisting of a plastic frame and a cover glass (top image), which is surgically placed on a mammary tumor (lower image). (B) After implantation of the MIW at day 0, the mouse recovers for two days. This is followed by repeated IVM sessions in the subsequent days. (C) IVM images of a Dendra2-expressing colorectal C26 tumor, in which green represents the non-switched Dendra2 and red the switched Dendra2. At day 3, tumor cells that are present either close to (upper images) or surrounding (lower images) a blood vessel are photolabeled in a square region by photoswitching Dendra2 from green to red using violet illumination. Note that the combination of using a MIW and photomarking of tumor cells allows imaging regions to be retraced in subsequent imaging sessions, and thus the motility and intravasation to be visualized. Also note the high intratumoral motility (top images) and the loss of red-shifted tumor cells by intravasation (lower images). Scale bars: 10 μm.
Tracking of individual tumor cells over several imaging sessions
To enable long-term tracking of individual cells, reference points such as vascular and extracellular matrix (ECM) structures in healthy tissues have been used to enable retracing of the previously imaged area over several imaging sessions (Bins et al., 2007; Hayashi et al., 2007; Kimura et al., 2010; Perentes et al., 2009; Yamauchi et al., 2006; Yang et al., 2002). However, when tumorigenic tissues are observed in which the topology changes dynamically, these initial reference points might change and cannot be used for retracing. To be able to track the dynamics of areas of interest, tumor cells have been photomarked using photoconvertable fluorophores, such as Kaede (Ando et al., 2002) and Dendra2 (Gurskaya et al., 2006). These fluorophores have absorption and emission spectra that are significantly red shifted by violet light. For example, we have used this property of Dendra2 to mark regions of interest in a tumor over several days with single-cell precision (Fig. 3C) (Gligorijevic et al., 2009; Kedrin et al., 2008) and were thus able to track a large population of cells over multiple imaging sessions.
Taken together, we are now able to detect fluorescence at high resolution, deep inside living animals. By combining imaging chambers and photomarking of regions of interest in the tumor, single tumor cells can be tracked and visualized over several days. By using FRET IVM, dynamic changes in subcellular signaling events that are required for metastasis can be imaged over time, whereas multicolor IVM allows the visualization of cell–cell and cell–molecule interactions. These recent advances in intravital imaging open the field to new and exciting discoveries.
Important new insights into tumor cell biology
Recent IVM studies using the above-described techniques visualized how tumor cells exit the tumor and spread to a secondary site, generating new important insights into this process. For example, it is generally believed that cells at the tumor border have to invade the surrounding tissue, where they traverse physical barriers of the ECM and endothelial cells before they enter the circulation to be transported to a secondary site. Interestingly, IVM studies show a much more dynamic picture, in which cells switch between different invasion strategies to exit the primary tumor as a reaction to physical ECM barriers or intracellular signaling, as discussed below.
Hematogenous versus lymphatic tumor cell spread
Tumor cells can exit the primary site by entering either lymphatic vessels or blood vessels. Interestingly, IVM of lymphatic vessels, which drain labeled macromolecules, shows that lymphatic vessels inside hyperplastic lesions and the tumor mass are non-functional or even absent (Hagendoorn et al., 2006; Padera et al., 2002). By contrast, regions at the tumor margin contain functional lymphatic vessels and thus might be the starting point for lymphatic metastasis (Padera et al., 2002). Cells that metastasize through the lymphatic system will first be transported to the draining lymph node (Fig. 4A). From there, the tumor cells flow through efferent lymphatic vessels to the heart and into the blood system and, consequently, are transported to distant sites where they can form metastatic foci (Fig. 4A). Although the presence of tumor cells in regional lymph nodes is recognized as a prognostic factor for breast cancer and other cancers (Hartveit, 1979; Hartveit, 1984; Sleeman and Thiele, 2009; Tanis et al., 2001), it remains elusive whether tumor cells metastasize from the lymph nodes or whether their presence in lymph nodes merely reflects their intrinsic invasiveness (Das and Skobe, 2008). Therefore, it is unknown whether tumor cells use the lymphatic route, the hematogenous (blood) route, or indeed both, to metastasize to other organs.
Fig. 4.
Single moving tumor cells of MTLn3 tumors use the hematogenous route to metastasize to other organs. (A) Simplified schematic overview of spreading routes from a breast tumor to the lung (L). At the primary tumor site, tumor cells can enter the blood either directly (blue route) or through lymphatic vessels (purple route), which drain into the blood at a merging point located just before the heart (H), from where the blood is then transported into the lung. Using IVM of the invasive MTLn3 tumor model, it has been shown that cells move either as single cells, which leave the primary tumor using the hematogenous route, or as collective chains using the lymphatic route. (B) Inhibition of TFGβ signaling leads to inhibition of invasion by single moving cells and a subsequent drop in the number of tumor cells in the heart, whereas the collective movement to lymphatic vessels remains unaltered. These experiments show that, in this invasive mammary carcinoma model, tumor cells use the hematogenous (blood) route to metastasize to other organs.
To address this question, IVM has been used to visualize the signaling pathways that are required for the entry of tumor cells into lymphatic or blood vessels (Farina et al., 1998; Giampieri et al., 2009; Hagendoorn et al., 2006; Hoffman, 2009; Kedrin et al., 2008; Wyckoff et al., 2007). For this purpose, Gaimpieri and colleagues engineered MTLn3 mammary tumor cells to express Smad2 fused to GFP, which translocates to the nucleus in response to transforming growth factor β (TGFβ) (Giampieri et al., 2009), a well-studied inducer of tumor cell motility (Leivonen and Kähäri, 2007). Using high-resolution IVM of MTLn3 mammary tumors, they showed that TGFβ signaling is active in cells that move as single units, but not in those that move cohesively as cell chains. (Giampieri et al., 2009; Giampieri et al., 2010) (Fig. 4A). In addition, they showed that single moving cells enter the blood vessels, whereas collectively moving cells enter lymphatic vessels. As the motility of single cells entering the blood vessels is TGFβ dependent, blood entry, but not lymphatic entry, is blocked upon TGFβ inhibition (Giampieri et al., 2009). Interestingly, the number of tumor cells drained from the heart, which corresponds to the added effects of hematogenous and lymphatic spread (see merging point in Fig. 4A), also decreases upon inhibition of TGFβ signaling (Giampieri et al., 2009). This suggests that tumor cells in these tumor models use the hematogenous route to metastasize to other organs, as TGFβ signaling is only required for the entry of tumor cells into blood vessels.
These studies clearly illustrate that IVM of signaling pathways can provide crucial information regarding the spread of tumor cells, but future research is necessary to determine whether these observations are also true for other tumor models.
The role of proteolysis in invasion
The proteolytic activity of peptidases is important for several aspects of metastasis, including tumor growth, apoptosis, angiogenesis and invasion (Kessenbrock et al., 2010). The roles of peptidases, such as MMPs, include remodeling of the ECM and cleavage of numerous other substrates, such as growth-factor-binding proteins, cell adhesion molecules, receptor tyrosine kinases and other proteinases (Egeblad and Werb, 2002; Kessenbrock et al., 2010). Many of these roles are thought to be crucial for tumor cells to be able to overcome the physical barriers of dense ECM layers when they invade surrounding tissues and enter the blood (Christofori, 2006; Rowe and Weiss, 2008; Tanjore and Kalluri, 2006). These ECM layers include a three-dimensional fibrillar meshwork of interstitial tissues and the two-dimensional sheet of the basement membrane (BM), which underlies epithelial and endothelial tissues (Fig. 1) (Kalluri, 2003; LeBleu et al., 2007; Rowe and Weiss, 2009). The BM is thought to act as a particularly effective physical barrier owing to its small pore size (~50 nm). However, it is not clear whether the breakdown of these ECM layers is always required for tumor cell invasion and intravasation.
IVM experiments show that proteolytic activity supports the collective invasion of tumor cells (e.g. Friedl and Gilmour, 2009; Giampieri et al., 2009). For example, cancer-associated fibroblasts have been found to remodel the interstitial ECM meshwork, through both peptidase- and force-mediated matrix remodeling. This forms tracks through the matrix, which enable tumor cells to collectively move into the surrounding tissue (Gaggioli et al., 2007; Giampieri et al., 2009). Macrophages have been shown to assist in the entry of tumor cells into the circulation by producing chemokines and peptidases, leading to BM breakdown and tumor cell motility (Goswami et al., 2005; Wyckoff et al., 2007). Alternatively, tumor cells might degrade the endothelial BM themselves. In vitro, many tumor cells can form actin-rich structures, the so-called invadopodia, which locally degrade the ECM (Poincloux et al., 2009; Stylli et al., 2008). However, a corresponding function or even the very existence of such invadopodia in vivo has not yet been established. With the recent advances in high-resolution IVM techniques and protease probes, these questions might be answered soon.
Interestingly, using IVM, several laboratories have now established that ECM degradation is not always required for tumor cell invasion through dense ECM layers. For instance, Wolf et al. showed that, upon inhibition of MMPs, tumor cells can still invade connective collagen matrices by switching from a mysenchymal mode of migration to an amoeboid mode of migration (Wolf et al., 2003). Both in vitro and in vivo experiments have shown that mysenchymal migration is characterized by secretion of proteases and an elongated polarized cell morphology, whereas amoeboid movement is characterized by a non-polarized flexible round morphology of the cell and does not require any proteolytic activity (Wolf et al., 2003; Wolf et al., 2007; Wyckoff et al., 2007; Sahai and Marshall, 2003). The amoeboid mode of migration enables cells to squeeze through small gaps by forming bleb-like structures, thereby pushing these fibers out of the way without the requirement for proteolytic activity (Pinner and Sahai, 2008), allowing them to migrate within mammary tumors with high velocity (Wyckoff et al., 2006; Wyckoff et al., 2007). Although amoeboid movement does not depend on ECM layer breakdown, it might be facilitated by other peptidase-dependent processes, such as the activation of chemokines. Nevertheless, these data illustrate that a tumor cell can adapt its invasion strategy, that is, its mode of migration, to the surrounding microenvironment.
Invasion and intravasation within the tumor mass
In static histological samples, the tumor cells that are located in the surrounding tissue close to the invasive front of the tumor are identified as motile tumor cells. As the presence of these cells in the surrounding tissue correlates with poor prognosis, it is generally thought that tumor cells escape the tumor by invading the tumor stroma (Christofori, 2006; Rowe and Weiss, 2008; Tanjore and Kalluri, 2006). However, the contribution of motile cells within the tumor mass, which also enter the vessels, has either not been studied or simply been overlooked, as the motility and intravasation of this population of cells cannot be visualized in static histological images.
With the recent high-resolution IVM studies in mice, intratumoral motility and intravasation can now be directly visualized in real time (Fig. 5) (Farina et al., 1998; Giampieri et al., 2009; Kedrin et al., 2008; Wyckoff et al., 2000). In an elegant study using multicolor spinning disk microscopy, Egeblad and co-workers showed that stromal cells and leukocytes (including T cells, dendritic cells, myeloid cells and fibroblasts) exhibit higher motility at the tumor stroma of mammary tumors than within the tumor mass (Egeblad et al., 2008; Lohela and Werb, 2010). However, the motility of tumor cells has not been visualized in this study. The extent of intratumoral motility became clear when we tracked tumor cells through MIWs over long periods of time. By photomarking a subset of tumor cells, we observed intratumoral cell movement and entry into the vessels within the tumor mass (Fig. 3C) (Gligorijevic et al., 2009; Kedrin et al., 2008). Importantly, in contrast to tumor cells present in the tumor stroma, invading and intravasating tumor cells within the tumor mass no longer encounter physical barriers, such as the dense epithelial and endothelial BMs. Vessels that are embedded within the tumor mass, referred to as tumor-associated vessels, are less organized and less solid compared with normal vasculature (Fukumura et al., 2010; Jain, 1988). In fact, owing to the lack of an intact BM and irregularly organized endothelial cells and pericytes, these vessels have been shown to be leakier than vessels of healthy tissues (Baluk et al., 2003; Chang et al., 2000; Fukumura et al., 2010; Jain, 2003; Kessenbrock et al., 2010). Therefore, tumor-associated vessels within the tumor mass are more easily accessible to tumor cells, thereby alleviating the intravasation process (see cartoon in Fig. 5).
Fig. 5.
Direct entry of tumor cells into tumor-associated vessels as shown by IVM. Tumor cell migration and intravasation take place not only at the invasive front, but also deep within the tumor mass. The upper row of IVM images, in which the mouse invasive lobular carcinoma tumor cells are in green and the SHG signal is in purple, shows high motility within the tumor mass. The arrows point to the position of motile cells at time (t) zero (see also supplementary material Movie 3). The row of images below shows maximum projections of Z-stacks with a total depth of 50 μm. In the IVM images, green represents C26 colorectal tumor cells and purple represents SHG. This time series of the maximum projections shows the disappearance of a tumor cell, which is no longer present in the Z-stack because it enters a tumor-associated vessel and is transported out of sight by blood (see circle; supplementary material Movie 2). The cartoon shows a simplified model of leaky tumor-associated vessels within tumors, which lack an intact BM and through which tumor cells can enter the blood. Scale bars: 10 μm.
IVM also shows that, in addition to active entry of cells into tumor-associated blood vessels, tumor cells can also accidentally enter the blood stream when they are positioned close to a poorly structured tumor-associated vessel that collapses upon elevated interstitial fluid pressure caused by proliferating cancer cells. As a result of the collapse, big clumps of cells from the surrounding tissue shed into the lumen of the collapsed vessel and are transported through the vasculature (Baluk et al., 2003; Chang et al., 2000; Fukumura et al., 2010; Jain, 2003; Kessenbrock et al., 2010).
Taken together, IVM has shown that both active and inactive entry of tumor cells into the vasculature occurs within the tumor mass, implying that tumor cell migration and intravasation do not only take place at the invasive front. Therefore, intratumoral motility and intravasation should be considered an important escape route for tumor cells. This idea also explains the observation that many patients who have metastatic outgrowth show no BM breakdown and therefore no invasion at the invasive front at the primary tumor site; this was initially explained by a resynthesis of removed BM (Dingemans, 1988).
Conclusions and future perspectives
In this Commentary, we have focused on the advances in IVM and have given examples of its usefulness in studying the metastatic process. So far, most of the advances have resulted in the ability to image the earlier steps of metastasis, including migration, invasion and intravasation. Only a small number of studies have attempted to image cells in organs that are prone to metastasis, such as the lungs (Kimura et al., 2010), bone marrow (Sipkins et al., 2005), lymph nodes (Mempel et al., 2004) and spleen (Grayson et al., 2001). Unfortunately, these experiments rely on surgical dissection to expose the imaging site, which hampers long-term imaging and therefore the visualization of colonization and dormancy. The use of the cranial imaging window has overcome these problems for the brain (Kienast et al., 2010) and, to expand this advance to other organs, the next generation of imaging chambers should aim to visualize the spleen, liver and lymph nodes. IVM of colonization will help to answer many unresolved questions, such as why tumor cells colonize a specific organ without the formation of colonies in other organs, how long tumor cells stay dormant and what factors can reactivate them (e.g. angiogenesis, growth factors).
Although IVM has been successful in providing new insights into the early steps of metastasis, most studies are only observational. In the next few years, it will be important to move the field from observational IVM to experiments that can also characterize the underlying molecular processes. For example, new cancer models should be imaged that have been engineered to manipulate the behavior of cancer cells by inducible expression of oncogenes or signaling proteins (Le Dévédec et al., 2010). Developments in photo-manipulation will further help the transition to experimentally driven studies, such as laser-induced release and activation of caged compounds (NPE-caged cAMP) or of transcription. Moreover, high-resolution and FRET IVM of biosensors are important tools to study molecular processes, such as cell–cell communication, intracellular signaling events and the gain of traits that are required for cells to metastasize. For example, a FRET probe for the proto-oncogenic tyrosine kinase Src (Ting et al., 2001) can be used to study the regulation of integrin–cytoskeleton interactions during cell motility, and a reactive oxygen species (ROS)-sensitive FRET probe that responds to hypoxia (Guzy et al., 2005) might be important for studying the influence of the tumor microenvironment during escape and colonization. Unfortunately, most FRET biosensors have a low signal-to-noise ratio and are not sensitive enough to be used in IVM experiments. Biosensor development is an expanding field, and the next-generation probes should aim to increase the signal-to-noise ratio and to probe for proteolytic activity, proliferation, adhesion and senescence.
Given the increasing number of genetically engineered tumor cell lines and fluorescent reporter mice, the transcriptional and differentiation state of cells that metastasize can also be studied by IVM. For example, Pinner and colleagues engineered melanoma tumor cells to express GFP driven by the Brn-2 promoter, and showed that melanoma cells can switch from a less differentiated state to a differentiated state when these cells exit the primary tumor and enter the secondary site (Pinner and Sahai, 2008). These types of IVM experiments will be able to answer long-standing questions in the field, such as whether cells can transiently switch from an epithelial to a mesenchymal phenotype (EMT) during metastasis, or whether the metastasizing tumor cells have a stem cell phenotype.
As discussed in this Commentary, IVM is an important tool to gain knowledge of tumor cell dynamics during metastasis, which will enable the development of more powerful strategies for treatment of human cancer. Although xenograft cancer models in immune-deficient mice have been used extensively for IVM, these are end-stage tumors that do not recapitulate the natural development and morphology of common human cancers (e.g. Cardiff et al., 2000). More importantly, they lack particular interactions with the microenvironment, which contains key players, such as T cells, that influence tumor development and metastasis (Condeelis and Pollard, 2006; de Visser and Coussens, 2006; DeNardo et al., 2009; Orimo and Weinberg, 2006). Novel cancer models have recently been developed and result in mice that have a spectrum of tumors, which strongly resembles human tumor development and progression in the breast (Derksen et al., 2006; Jonkers et al., 2001; Olive et al., 2004; Xu et al., 2001), pancreas (Hingorani et al., 2003), lung (Jongsma et al., 2008; Meuwissen et al., 2003) and brain (Llaguno et al., 2008; Xiao et al., 2002). Even though the incorporation of fluorophores will be technically challenging, these models will undoubtedly advance the translational aspects of future intravital imaging experiments.
Supplementary Material
Acknowledgments
We thank Johan de Rooij, Onno Kranenburg and Stephan Huveneers for critically reading this manuscript, Tom Schonewille for technical assistance, and Anko de Graaff and the Hubrecht Imaging Center for imaging support. This work was supported by VIDI fellowships 91710330 (J.v.R. and E.B.) and 91796318 (P.W.B.D.) from the Dutch Organization of Scientific Research (NWO), a grant from the Dutch Cancer Society (KWF: HUBR 2009-4621) (L.R.) and an equipment grant (175.010.2007.00) from the Dutch Organization of Scientific Research (NWO). N.V. was supported by the intramural research program of NIAID, NIH. Deposited in PMC for release after 12 months.
Footnotes
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/124/3/299/DC1
References
- Abdul-Karim M.-A., Al-Kofahi K., Brown E. B., Jain R. K., Roysam B. (2003). Automated tracing and change analysis of angiogenic vasculature from in vivo multiphoton confocal image time series. Microvasc. Res. 66, 113-125 [DOI] [PubMed] [Google Scholar]
- Alexander S., Koehl G., Hirschberg M., Geissler E., Friedl P. (2008). Dynamic imaging of cancer growth and invasion: a modified skin-fold chamber model. Histochem. Cell Biol. 130, 1147-1154 [DOI] [PubMed] [Google Scholar]
- Ando R., Hama H., Yamamoto-Hino M., Mizuno H., Miyawaki A. (2002). An optical marker based on the UV-induced green-to-red photoconversion of a fluorescent protein. Proc. Natl. Acad. Sci. USA 99, 12651-12656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajénoff M., Egen J. G., Koo L. Y., Laugier Jean P., Brau F., Glaichenhaus N., Germain R. N. (2006). Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity 25, 989-1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluk P., Morikawa S., Haskell A., Mancuso M., McDonald D. M. (2003). Abnormalities of basement membrane on blood vessels and endothelial sprouts in tumors. Am. J. Pathol. 163, 1801-1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiaens P. I. H., Squire A. (1999). Fluorescence lifetime imaging microscopy: spatial resolution of biochemical processes in the cell. Trends Cell Biol. 9, 48-52 [DOI] [PubMed] [Google Scholar]
- Bins A., van Rheenen J., Jalink K., Halstead J., Divecha N., Spencer D., Haanen J., Schumacher T. (2007). Intravital imaging of fluorescent markers and FRET probes by DNA tattooing. BMC Biotechnol. 7, 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissonnas A., Fetler L., Zeelenberg I. S., Hugues S., Amigorena S. (2007). In vivo imaging of cytotoxic T cell infiltration and elimination of a solid tumor. J. Exp. Med. 204, 345-356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breart B., Lemaître F., Celli S., Bousso P. (2008). Two-photon imaging of intratumoral CD8+ T cell cytotoxic activity during adoptive T cell therapy in mice. J. Clin. Invest. 118, 1390-1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. B., Campbell R. B., Tsuzuki Y., Xu L., Carmeliet P., Fukumura D., Jain R. K. (2001). In vivo measurement of gene expression, angiogenesis and physiological function in tumors using multiphoton laser scanning microscopy. Nat. Med. 7, 864-868 [DOI] [PubMed] [Google Scholar]
- Campagnola P. J., Millard A. C., Terasaki M., Hoppe P. E., Malone C. J., Mohler W. A. (2002). Three-dimensional high-resolution second-harmonic generation imaging of endogenous structural proteins in biological tissues. Biophys. J. 82, 493-508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardiff R., Anver M., Gusterson B., Hennighausen L., Jensen R., Merino M., Rehm S., Russo J., Tavassoli F., Wakefield L., et al. (2000). The mammary pathology of genetically engineered mice: the consensus report and recommendations from the Annapolis meeting. Oncogene 19, 968-988 [DOI] [PubMed] [Google Scholar]
- Castellino F., Huang A. Y., Altan-Bonnet G., Stoll S., Scheinecker C., Germain R. N. (2006). Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature 440, 890-895 [DOI] [PubMed] [Google Scholar]
- Centonze V. E., White J. G. (1998). Multiphoton excitation provides optical sections from deeper within scattering specimens than confocal imaging. Biophys. J. 75, 2015-2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M., Tu Y., Euskirchen G., Ward W. W., Prasher D. C. (1994). Green fluorescent protein as a marker for gene expression. Science 263, 802-805 [DOI] [PubMed] [Google Scholar]
- Chambers A. F., MacDonald I. C., Schmidt E. E., Koop S., Morris V. L., Khokha R., Groom A. C. (1995). Steps in tumor metastasis: new concepts from intravital videomicroscopy. Cancer Metastasis Rev. 14, 279-301 [DOI] [PubMed] [Google Scholar]
- Chambers A. F., Groom A. C., MacDonald I. C. (2002). Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer 2, 563-572 [DOI] [PubMed] [Google Scholar]
- Chang Y. S., di Tomaso E., McDonald D. M., Jones R., Jain R. K., Munn L. L. (2000). Mosaic blood vessels in tumors: frequency of cancer cells in contact with flowing blood. Proc. Natl. Acad. Sci. USA 97, 14608-14613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang A. C., Massague J. (2008). Molecular basis of metastasis. N. Eng. J. Med. 359, 2814-2823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chishima T., Miyagi Y., Wang X., Yamaoka H., Shimada H., Moossa A. R., Hoffman R. M. (1997). Cancer invasion and micrometastasis visualized in live tissue by green fluorescent protein expression. Cancer Res. 57, 2042-2047 [PubMed] [Google Scholar]
- Christofori G. (2006). New signals from the invasive front. Nature 441, 444-450 [DOI] [PubMed] [Google Scholar]
- Condeelis J., Pollard J. W. (2006). Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 124, 263-266 [DOI] [PubMed] [Google Scholar]
- Das S., Skobe M. (2008). Lymphatic vessel activation in cancer. Ann. N. Y. Acad. Sci. 1131, 235-241 [DOI] [PubMed] [Google Scholar]
- de Visser K., Coussens L. (2006). The inflammatory tumor microenvironment and Its impact on cancer development. Contrib. Microbiol. 13, 118-137 [DOI] [PubMed] [Google Scholar]
- DeNardo D. G., Barreto J. B., Andreu P., Vasquez L., Tawfik D., Kolhatkar N., Coussens L. M. (2009). CD4+ T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell 16, 91-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M. S., Jin H., Dugger D., Yang R., McFarland L., Ogasawara A., Williams S., Cole M. J., Ross S., Schwall R. (2007). Imaging tumors with an albumin-binding Fab, a novel tumor-targeting agent. Cancer Res. 67, 254-261 [DOI] [PubMed] [Google Scholar]
- Derksen P. W. B., Liu X., Saridin F., van der Gulden H., Zevenhoven J., Evers B., van Beijnum J. R., Griffioen A. W., Vink J., Krimpenfort P., et al. (2006). Somatic inactivation of E-cadherin and p53 in mice leads to metastatic lobular mammary carcinoma through induction of anoikis resistance and angiogenesis. Cancer Cell 10, 437-449 [DOI] [PubMed] [Google Scholar]
- Dingemans K. (1988). What's new in the ultrastructure of tumor invasion in vivo? Pathol. Res. Pract. 183, 792-808 [DOI] [PubMed] [Google Scholar]
- Dunn K. W., Young P. A. (2006). Principles of multiphoton microscopy. Nephron Exp. Nephrol. 103, e33-e40 [DOI] [PubMed] [Google Scholar]
- Egeblad M., Werb Z. (2002). New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer 2, 161-174 [DOI] [PubMed] [Google Scholar]
- Egeblad M., Ewald A. J., Askautrud H. A., Truitt M. L., Welm B. E., Bainbridge E., Peeters G., Krummel M. F., Werb Z. (2008). Visualizing stromal cell dynamics in different tumor microenvironments by spinning disk confocal microscopy. Dis. Model. Mech. 1, 155-167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egen J. G., Rothfuchs A. G., Feng C. G., Winter N., Sher A., Germain R. N. (2008). Macrophage and T cell dynamics during the development and disintegration of mycobacterial granulomas. Immunity 28, 271-284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggeling C., Volkmer A., Seidel C. A. M. (2005). Molecular photobleaching kinetics of rhodamine 6G by one- and two-photon induced confocal fluorescence microscopy. ChemPhysChem 6, 791-804 [DOI] [PubMed] [Google Scholar]
- Farhadi M. R., Capelle H. H., Erber R., Ullrich A., Vajkoczy P. (2005). Combined inhibition of vascular endothelial growth factor and platelet-derived growth factor signaling: effects on the angiogenesis, microcirculation, and growth of orthotopic malignant gliomas. J. Neurosurg. 102, 363-370 [DOI] [PubMed] [Google Scholar]
- Farina K. L., Wyckoff J. B., Rivera J., Lee H., Segall J. E., Condeelis J. S., Jones J. G. (1998). Cell motility of tumor cells visualized in living intact primary tumors using green fluorescent protein. Cancer Res. 58, 2528-2532 [PubMed] [Google Scholar]
- Fouquet W., Owald D., Wichmann C., Mertel S., Depner H., Dyba M., Hallermann S., Kittel R. J., Eimer S., Sigrist S. J. (2009). Maturation of active zone assembly by Drosophila Bruchpilot. J. Cell Biol. 186, 129-145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl P., Gilmour D. (2009). Collective cell migration in morphogenesis, regeneration and cancer. Nat. Rev. Mol. Cell Biol. 10, 445-457 [DOI] [PubMed] [Google Scholar]
- Fukumura D., Duda D. G., Munn L. L., Jain R. K. (2010). Tumor microvasculature and microenvironment: novel insights through intravital imaging in pre-clinical models. Microcirculation 17, 206-225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaggioli C., Hooper S., Hidalgo-Carcedo C., Grosse R., Marshall J. F., Harrington K., Sahai E. (2007). Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat. Cell Biol. 9, 1392-1400 [DOI] [PubMed] [Google Scholar]
- Galletly N., McGinty J., Dunsby C., Teixeira F., Requejo-Isidro J., Munro I., Elson D., Neil M., Chu A., French P., et al. (2008). Fluorescence lifetime imaging distinguishes basal cell carcinoma from surrounding uninvolved skin. Br. J. Dermatol. 159, 152-161 [DOI] [PubMed] [Google Scholar]
- Gerritsen H., Agronskaia A., Bader A., Esposito A. (2009). Time Domain FLIM; Theory, Instrumentation. In FRET and FLIM Techniques, vol. 33 (ed. Gadella T. W. J.), pp. 95-132 Amsterdam: Elsevier Science; [Google Scholar]
- Ghosh S. R., Hope I. A. (2010). Determination of the mobility of novel and established Caenorhabditis elegans sarcomeric proteins in vivo. Eur. J. Cell Biol. 89, 437-448 [DOI] [PubMed] [Google Scholar]
- Giampieri S., Manning C., Hooper S., Jones L., Hill C. S., Sahai E. (2009). Localized and reversible TGF-beta signalling switches breast cancer cells from cohesive to single cell motility. Nat. Cell Biol. 11, 1287-1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giampieri S., Pinner S., Sahai E. (2010). Intravital imaging illuminates transforming growth factor {beta} signaling switches during metastasis. Cancer Res. 70, 3435-3439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giepmans B. N. G., Adams S. R., Ellisman M. H., Tsien R. Y. (2006). The fluorescent toolbox for assessing protein location and function. Science 312, 217-224 [DOI] [PubMed] [Google Scholar]
- Gligorijevic B., Kedrin D., Segall J., Condeelis J., van Rheenen J. (2009). Dendra2 photoswitching through the mammary imaging window. J. Vis. Exp. 28, 1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami S., Sahai E., Wyckoff J. B., Cammer M., Cox D., Pixley F. J., Stanley E. R., Segall J. E., Condeelis J. S. (2005). Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res. 65, 5278-5283 [DOI] [PubMed] [Google Scholar]
- Grayson M. H., Chaplin D. D., Karl I. E., Hotchkiss R. S. (2001). Confocal fluorescent intravital microscopy of the murine spleen. J. Immunol. Methods 256, 55-63 [DOI] [PubMed] [Google Scholar]
- Gurskaya N. G., Verkhusha V. V., Shcheglov A. S., Staroverov D. B., Chepurnykh T. V., Fradkov A. F., Lukyanov S., Lukyanov K. A. (2006). Engineering of a monomeric green-to-red photoactivatable fluorescent protein induced by blue light. Nat. Biotechnol. 24, 461-465 [DOI] [PubMed] [Google Scholar]
- Guzy R. D., Hoyos B., Robin E., Chen H., Liu L., Mansfield K. D., Simon M. C., Hammerling U., Schumacker P. T. (2005). Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 1, 401-408 [DOI] [PubMed] [Google Scholar]
- Hagendoorn J., Tong R., Fukumura D., Lin Q., Lobo J., Padera T. P., Xu L., Kucherlapati R., Jain R. K. (2006). Onset of abnormal blood and lymphatic vessel function and interstitial hypertension in early stages of carcinogenesis. Cancer Res. 66, 3360-3364 [DOI] [PubMed] [Google Scholar]
- Hartveit F. (1979). Paranodal vascular spread in breast cancer with axillary node involvement. J. Pathol. 127, 111-114 [DOI] [PubMed] [Google Scholar]
- Hartveit F. (1984). Paranodal tumour in breast cancer: extranodal extension versus vascular spread. J. Pathol. 144, 253-256 [DOI] [PubMed] [Google Scholar]
- Hayashi K., Jiang P., Yamauchi K., Yamamoto N., Tsuchiya H., Tomita K., Moossa A. R., Bouvet M., Hoffman R. M. (2007). Real-time imaging of tumor-cell shedding and trafficking in lymphatic channels. Cancer Res. 67, 8223-8228 [DOI] [PubMed] [Google Scholar]
- Heim R. C., Cubitt A. B., Tsien R. Y. (1995). Improved green fluorescence. Nature 373, 663-664 [DOI] [PubMed] [Google Scholar]
- Helmchen F., Denk W. (2005). Deep tissue two-photon microscopy. Nat. Methods 2, 932-940 [DOI] [PubMed] [Google Scholar]
- Hingorani S. R., Petricoin E. F., Maitra A., Rajapakse V., King C., Jacobetz M. A., Ross S., Conrads T. P., Veenstra T. D., Hitt B. A., et al. (2003). Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 4, 437-450 [DOI] [PubMed] [Google Scholar]
- Hoffman R. (2009). Imaging cancer dynamics in vivo at the tumor and cellular level with fluorescent proteins. Clin. Exp. Metastasis 26, 345-355 [DOI] [PubMed] [Google Scholar]
- Hüsemann Y., Geigl J. B., Schubert F., Musiani P., Meyer M., Burghart E., Forni G., Eils R., Fehm T., Riethmüller G., et al. (2008). Systemic spread is an early step in breast cancer. Cancer Cell 13, 58-68 [DOI] [PubMed] [Google Scholar]
- Jain R. K. (1988). Determinants of tumor blood flow: a review. Cancer Res. 48, 2641-2658 [PubMed] [Google Scholar]
- Jain R. K. (2003). Molecular regulation of vessel maturation. Nat. Med. 9, 685-693 [DOI] [PubMed] [Google Scholar]
- Jalink K., van Rheenen J. (2009). Filter FRET: quantitative imaging of sensitized emission. In FRET and FLIM Techniques, vol. 33 (ed. Gadella T. W. J.), pp. 289-349 Amsterdam: Elsevier Science; [Google Scholar]
- Jongsma J., van Montfort E., Vooijs M., Zevenhoven J., Krimpenfort P., van der Valk M., van de Vijver M., Berns A. (2008). A conditional mouse model for malignant mesothelioma. Cancer Cell 13, 261-271 [DOI] [PubMed] [Google Scholar]
- Jonkers J., Meuwissen R., van der Gulden H., Peterse H., van der Valk M., Berns A. (2001). Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat. Genet. 29, 418-425 [DOI] [PubMed] [Google Scholar]
- Kalluri R. (2003). Basement membranes: structure, assembly and role in tumour angiogenesis. Nat. Rev. Cancer 3, 422-433 [DOI] [PubMed] [Google Scholar]
- Kedrin D., Gligorijevic B., Wyckoff J., Verkhusha V. V., Condeelis J., Segall J. E., van Rheenen J. (2008). Intravital imaging of metastatic behavior through a mammary imaging window. Nat. Methods 5, 1019-1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keese M., Offterdinger M., Tischer C., Girod A., Lommerse P. H., Yagublu V., Magdeburg R., Bastiaens P. I. (2007). Quantitative imaging of apoptosis commitment in colorectal tumor cells. Differentiation 75, 809-818 [DOI] [PubMed] [Google Scholar]
- Keese M., Yagublu V., Schwenke K., Post S., Bastiaens P. (2010). Fluorescence lifetime imaging microscopy of chemotherapy-induced apoptosis resistance in a syngenic mouse tumor model. Int. J. Cancer 126, 104-113 [DOI] [PubMed] [Google Scholar]
- Kessenbrock K., Plaks V., Werb Z. (2010). Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 141, 52-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienast Y., von Baumgarten L., Fuhrmann M., Klinkert W. E. F., Goldbrunner R., Herms J., Winkler F. (2010). Real-time imaging reveals the single steps of brain metastasis formation. Nat. Med. 16, 116-122 [DOI] [PubMed] [Google Scholar]
- Kimura H., Hayashi K., Yamauchi K., Yamamoto N., Tsuchiya H., Tomita K., Kishimoto H., Bouvet M., Hoffman R. M. (2010). Real-time imaging of single cancer-cell dynamics of lung metastasis. J. Cell. Biochem. 109, 58-64 [DOI] [PubMed] [Google Scholar]
- Klein C. A. (2009). Parallel progression of primary tumours and metastases. Nat. Rev. Cancer 9, 302-312 [DOI] [PubMed] [Google Scholar]
- Le Dévédec S., Lalai R., Pont C., de Bont H., van de Water B. (2010). Two-photon intravital multicolor imaging combined with inducible gene expression to distinguish metastatic behavior of breast cancer cells in vivo. Mol. Imaging Biol. doi:10.1007/s11307-010-0307-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBleu V. S., MacDonald B., Kalluri R. (2007). Structure and function of basement membranes. Exp. Biol. Med. 232, 1121-1129 [DOI] [PubMed] [Google Scholar]
- Lehr H., Leunig M., Menger M., Nolte D., Messmer K. (1993). Dorsal skinfold chamber technique for intravital microscopy in nude mice. Am. J. Pathol. 143, 1055-1062 [PMC free article] [PubMed] [Google Scholar]
- Leivonen S.-K., Kähäri V.-M. (2007). Transforming growth factor-beta signaling in cancer invasion and metastasis. Int. J. Cancer 121, 2119-2124 [DOI] [PubMed] [Google Scholar]
- Llaguno S. A., Chen J., Kwon C.-H., Parada L. F. (2008). Neural and cancer stem cells in tumor suppressor mouse models of malignant astrocytoma. Cold Spring Harb. Symp. Quant. Biol. 73, 421-426 [DOI] [PubMed] [Google Scholar]
- Lohela M., Werb Z. (2010). Intravital imaging of stromal cell dynamics in tumors. Curr. Opin. Genet. Dev. 20, 72-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald I. C., Schmidt E. E., Morris V. L., Chambers A. F., Groom A. C. (1992). Intravital videomicroscopy of the chorioallantoic microcirculation: A model system for studying metastasis. Microvasc. Res. 44, 185-199 [DOI] [PubMed] [Google Scholar]
- Massberg S., Schaerli P., Knezevic-Maramica I., Köllnberger M., Tubo N., Moseman E. A., Huff I. V., Junt T., Wagers A. J., Mazo I. B., et al. (2007). Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell 131, 994-1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy M., Bouvet M., Hoffman R. M. (2008). Color-coded fluorescent mouse models of cancer cell interactions with blood vessels and lymphatics. In Methods in Enzymology, vol. 445, pp. 27-52 New York: Academic Press; [DOI] [PubMed] [Google Scholar]
- McIntyre J. O., Scherer R. L., Matrisian L. M. (2010). Near-infrared optical proteolytic beacons for in vivo imaging of matrix metalloproteinase activity. Methods Mol. Biol. 622, 279-304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mempel T. R., Scimone M. L., Mora J. R., von Andrian U. H. (2004). In vivo imaging of leukocyte trafficking in blood vessels and tissues. Curr. Opin. Immunol. 16, 406-417 [DOI] [PubMed] [Google Scholar]
- Meuwissen R., Linn S. C., Linnoila R. I., Zevenhoven J., Mooi W. J., Berns A. (2003). Induction of small cell lung cancer by somatic inactivation of both Trp53 and Rb1 in a conditional mouse model. Cancer Cell 4, 181-189 [DOI] [PubMed] [Google Scholar]
- Mohler W., Millard A. C., Campagnola P. J. (2003). Second harmonic generation imaging of endogenous structural proteins. Methods 29, 97-109 [DOI] [PubMed] [Google Scholar]
- Monici M., El-Gewely M. R. (2005). Cell and tissue autofluorescence research and diagnostic applications. Biotechnol. Annu. Rev. 11, 227-256 [DOI] [PubMed] [Google Scholar]
- Morris M. (2010). Fluorescent biosensors of intracellular targets from genetically encoded reporters to modular polypeptide probes. Cell Biochem. Biophys. 56, 19-37 [DOI] [PubMed] [Google Scholar]
- Naumov G., Wilson S., MacDonald I., Schmidt E., Morris V., Groom A., Hoffman R., Chambers A. (1999). Cellular expression of green fluorescent protein, coupled with high-resolution in vivo videomicroscopy, to monitor steps in tumor metastasis. J. Cell Sci. 112, 1835-1842 [DOI] [PubMed] [Google Scholar]
- Nguyen D. X., Bos P. D., Massague J. (2009). Metastasis: from dissemination to organ-specific colonization. Nat. Rev. Cancer 9, 274-284 [DOI] [PubMed] [Google Scholar]
- Ntziachristos V. (2010). Going deeper than microscopy: the optical imaging frontier in biology. Nat. Methods 7, 603-614 [DOI] [PubMed] [Google Scholar]
- Olive K. P., Tuveson D. A., Ruhe Z. C., Yin B., Willis N. A., Bronson R. T., Crowley D., Jacks T. (2004). Mutant p53 gain of function in two mouse models of Li-Fraumeni Syndrome. Cell 119, 847-860 [DOI] [PubMed] [Google Scholar]
- Olson E. S., Aguilera T. A., Jiang T., Ellies L. G., Nguyen Q. T., Wong E. H., Gross L. A., Tsien R. Y. (2009). In vivo characterization of activatable cell penetrating peptides for targeting protease activity in cancer. Integr. Biol. (Camb) 1, 382-393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson E. S., Jiang T., Aguilera T. A., Nguyen Q. T., Ellies L. G., Scadeng M., Tsien R. Y. (2010). Activatable cell penetrating peptides linked to nanoparticles as dual probes for in vivo fluorescence and MR imaging of proteases. Proc. Natl. Acad. Sci. USA 107, 4311-4316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orimo A., Weinberg R. (2006). Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle 5, 1597-1601 [DOI] [PubMed] [Google Scholar]
- Padera T. P., Kadambi A., di Tomaso E., Carreira C. M., Brown E. B., Boucher Y., Choi N. C., Mathisen D., Wain J., Mark E. J., et al. (2002). Lymphatic metastasis in the absence of functional intratumor lymphatics. Science 296, 1883-1886 [DOI] [PubMed] [Google Scholar]
- Perentes J. Y., McKee T. D., Ley C. D., Mathiew H., Dawson M., Padera T. P., Munn L. L., Jain R. K., Boucher Y. (2009). In vivo imaging of extracellular matrix remodeling by tumor-associated fibroblasts. Nat. Methods 6, 143-145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippar U., Roussos E. T., Oser M., Yamaguchi H., Kim H.-D., Giampieri S., Wang Y., Goswami S., Wyckoff J. B., Lauffenburger D. A., et al. (2008). A mena invasion isoform potentiates EGF-induced carcinoma cell invasion and metastasis. Dev. Cell 15, 813-828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatkevich K. D., Verkhusha V. V. (2009). Advances in engineering of fluorescent proteins and photoactivatable proteins with red emission. Curr. Opin. Chem. Biol. 14, 23-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinner S., Sahai E. (2008). Imaging amoeboid cancer cell motility in vivo. J. Microsc. 231, 441-445 [DOI] [PubMed] [Google Scholar]
- Poincloux R., Lizarraga F., Chavrier P. (2009). Matrix invasion by tumour cells: a focus on MT1-MMP trafficking to invadopodia. J. Cell Sci. 122, 3015-3024 [DOI] [PubMed] [Google Scholar]
- Provenzano P., Eliceiri K., Keely P. (2009). Multiphoton microscopy and fluorescence lifetime imaging microscopy (FLIM) to monitor metastasis and the tumor microenvironment. Clin. Exp. Metastasis 26, 357-370 [DOI] [PubMed] [Google Scholar]
- Rowe R. G., Weiss S. J. (2008). Breaching the basement membrane: who, when and how? Trends Cell Biol. 18, 560-574 [DOI] [PubMed] [Google Scholar]
- Rowe R. G., Weiss S. J. (2009). Navigating ECM barriers at the invasive front: the cancer cell-stroma interface. Annu. Rev. Cell Dev. Biol. 25, 567-595 [DOI] [PubMed] [Google Scholar]
- Sahai E., Marshall C. J. (2003). Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat. Cell Biol. 5, 711-719 [DOI] [PubMed] [Google Scholar]
- Sahai E., Wyckoff J., Philippar U., Segall J., Gertler F., Condeelis J. (2005). Simultaneous imaging of GFP, CFP and collagen in tumors in vivo using multiphoton microscopy. BMC Biotechnol. 5, 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer R. L., VanSaun M. N., McIntyre O., Matrisian L. M. (2008). Optical imaging of matrix metalloproteinase-7 activity in vivo using a proteolytic nanobeacon. Mol. Imaging 7, 118-131 [PMC free article] [PubMed] [Google Scholar]
- Serrel A., Timpson P., Canel M., Schwarz J. P., Carragher N. O., Frame M. C., Brunton V. G., Anderson K. I. (2009). Real-time study of E-cadherin and membrane dynamics in living animals: implications for disease modeling and drug development. Cancer Res. 69, 2714-2719 [DOI] [PubMed] [Google Scholar]
- Shan S., Sorg B., Dewhirst M. W. (2003). A novel rodent mammary window of orthotopic breast cancer for intravital microscopy. Microvasc. Res. 65, 109-117 [DOI] [PubMed] [Google Scholar]
- Shimomura O., Johnson F. H., Saiga Y. (1962). Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. J. Cell. Comp. Physiol. 59, 223-239 [DOI] [PubMed] [Google Scholar]
- Sipkins D. A., Wei X., Wu J. W., Runnels J. M., Cote D., Means T. K., Luster A. D., Scadden D. T., Lin C. P. (2005). In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature 435, 969-973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleeman J. P., Thiele W. (2009). Tumor metastasis and the lymphatic vasculature. Int. J. Cancer 125, 2747-2756 [DOI] [PubMed] [Google Scholar]
- Stockholm D., Bartoli M., Sillon G., Bourg N., Davoust J., Richard I. (2005). Imaging calpain protease activity by multiphoton FRET in living mice. J. Mol. Biol. 346, 215-222 [DOI] [PubMed] [Google Scholar]
- Stoletov K., Kato H., Zardouzian E., Kelber J., Yang J., Shattil S., Klemke R. (2010). Visualizing extravasation dynamics of metastatic tumor cells. J. Cell Sci. 123, 2332-2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stylli S. S., Kaye A. H., Lock P. (2008). Invadopodia: At the cutting edge of tumour invasion. J. Clin. Neurosci. 15, 725-737 [DOI] [PubMed] [Google Scholar]
- Talmadge J. E., Fidler I. J. (2010). AACR Centennial Series: The Biology of Cancer Metastasis: Historical Perspective. Cancer Res. 70, 5649-5669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanis P., Nieweg O., Valdes Olmos R., Th Rutgers E., Kroon B. (2001). History of sentinel node and validation of the technique. Breast Cancer Res. 3, 109-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanjore H., Kalluri R. (2006). The role of type IV collagen and basement membranes in cancer progression and metastasis. Am. J. Pathol. 168, 715-717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theer P., Hasan M. T., Denk W. (2003). Two-photon imaging to a depth of 1000 μm in living brains by use of a Ti:Al2O3 regenerative amplifier. Opt. Lett. 28, 1022-1024 [DOI] [PubMed] [Google Scholar]
- Ting A. Y., Kain K. H., Klemke R. L., Tsien R. Y. (2001). Genetically encoded fluorescent reporters of protein tyrosine kinase activities in living cells. Proc. Natl. Acad. Sci. USA 98, 15003-15008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakoc B. J., Lanning R. M., Tyrrell J. A., Padera T. P., Bartlett L. A., Stylianopoulos T., Munn L. L., Tearney G. J., Fukumura D., Jain R. K., et al. (2009). Three-dimensional microscopy of the tumor microenvironment in vivo using optical frequency domain imaging. Nat. Med. 15, 1219-1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanEngelenburg S. B., Palmer A. E. (2008). Fluorescent biosensors of protein function. Curr. Opin. Chem. Biol. 12, 60-65 [DOI] [PubMed] [Google Scholar]
- Wagner R. (1839). Erlauterungstaflen zur physiologie und entwicklungsgeschichte. Leipzig, Germany: Leopold Voss; [Google Scholar]
- Weissleder R., Tung C.-H., Mahmood U., Bogdanov A. (1999). In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat. Biotech. 17, 375-378 [DOI] [PubMed] [Google Scholar]
- Wolf K., Mazo I., Leung H., Engelke K., von Andrian U. H., Deryugina E. I., Strongin A. Y., Bröcker E.-B., Friedl P. (2003). Compensation mechanism in tumor cell migration. J. Cell Biol. 160, 267-277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf K., Alexander S., Schacht V., Coussens L. M., von Andrian U. H., van Rheenen J., Deryugina E., Friedl P. (2009). Collagen-based cell migration models in vitro and in vivo. Semin. Cell Dev. Biol. 20, 931-941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S. J. (1958). Pathogenesis of metastasis formation observed in vivo in the rabbit ear chamber. AMA Arch. Pathol. 66, 550-568 [PubMed] [Google Scholar]
- Wouters F. S., Verveer P. J., Bastiaens P. I. H. (2001). Imaging biochemistry inside cells. Trends Cell Biol. 11, 203-211 [DOI] [PubMed] [Google Scholar]
- Wyckoff J. B., Jones J. G., Condeelis J. S., Segall J. E. (2000). A critical step in metastasis: in vivo analysis of intravasation at the primary tumor. Cancer Res. 60, 2504-2511 [PubMed] [Google Scholar]
- Wyckoff J. B., Pinner S. E., Gschmeissner S., Condeelis J. S., Sahai E. (2006). ROCK- and myosin-dependent matrix deformation enables protease-independent tumor-cell invasion in vivo. Curr. Biol. 16, 1515-1523 [DOI] [PubMed] [Google Scholar]
- Wyckoff J. B., Wang Y., Lin E. Y., Li J.-f., Goswami S., Stanley E. R., Segall J. E., Pollard J. W., Condeelis J. (2007). Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Res. 67, 2649-2656 [DOI] [PubMed] [Google Scholar]
- Xiao A., Wu H., Pandolfi P. P., Louis D. N., Van Dyke T. (2002). Astrocyte inactivation of the pRb pathway predisposes mice to malignant astrocytoma development that is accelerated by PTEN mutation. Cancer Cell 1, 157-168 [DOI] [PubMed] [Google Scholar]
- Xu X., Qiao W., Linke S. P., Cao L., Li W.-M., Furth P. A., Harris C. C., Deng C.-X. (2001). Genetic interactions between tumor suppressors Brca1 and p53 in apoptosis, cell cycle and tumorigenesis. Natl. Genet. 28, 266-271 [DOI] [PubMed] [Google Scholar]
- Yamauchi K., Yang M., Jiang P., Xu M., Yamamoto N., Tsuchiya H., Tomita K., Moossa A. R., Bouvet M., Hoffman R. M. (2006). Development of real-time subcellular dynamic multicolor imaging of cancer-cell trafficking in live mice with a variable-magnification whole-mouse imaging system. Cancer Res. 66, 4208-4214 [DOI] [PubMed] [Google Scholar]
- Yang M., Baranov E., Wang J.-W., Jiang P., Wang X., Sun F.-X., Bouvet M., Moossa A. R., Penman S., Hoffman R. M. (2002). Direct external imaging of nascent cancer, tumor progression, angiogenesis, and metastasis on internal organs in the fluorescent orthotopic model. Proc. Natl. Acad. Sci. USA 99, 3824-3829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan F., Salehi H. A., Boucher Y., Vasthare U. S., Tuma R. F., Jain R. K. (1994). Vascular permeability and microcirculation of gliomas and mammary carcinomas transplanted in rat and mouse cranial windows. Cancer Res. 54, 4564-4568 [PubMed] [Google Scholar]
- Zhang J., Chen L., Raina H., Blaustein M. P., Wier W. G. (2010). In vivo assessment of artery smooth muscle [Ca2+]i and MLCK activation in FRET-based biosensor mice. Am. J. Physiol. Heart Circ. Physiol. 299, H946-H956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel W. R., Williams R. M., Christie R., Nikitin A. Y., Hyman B. T., Webb W. W. (2003a). Live tissue intrinsic emission microscopy using multiphoton-excited native fluorescence and second harmonic generation. Proc. Natl. Acad. Sci. USA 100, 7075-7080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel W. R., Williams R. M., Webb W. W. (2003b). Nonlinear magic: multiphoton microscopy in the biosciences. Nat. Biotechnol. 21, 1369-1377 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.