Abstract
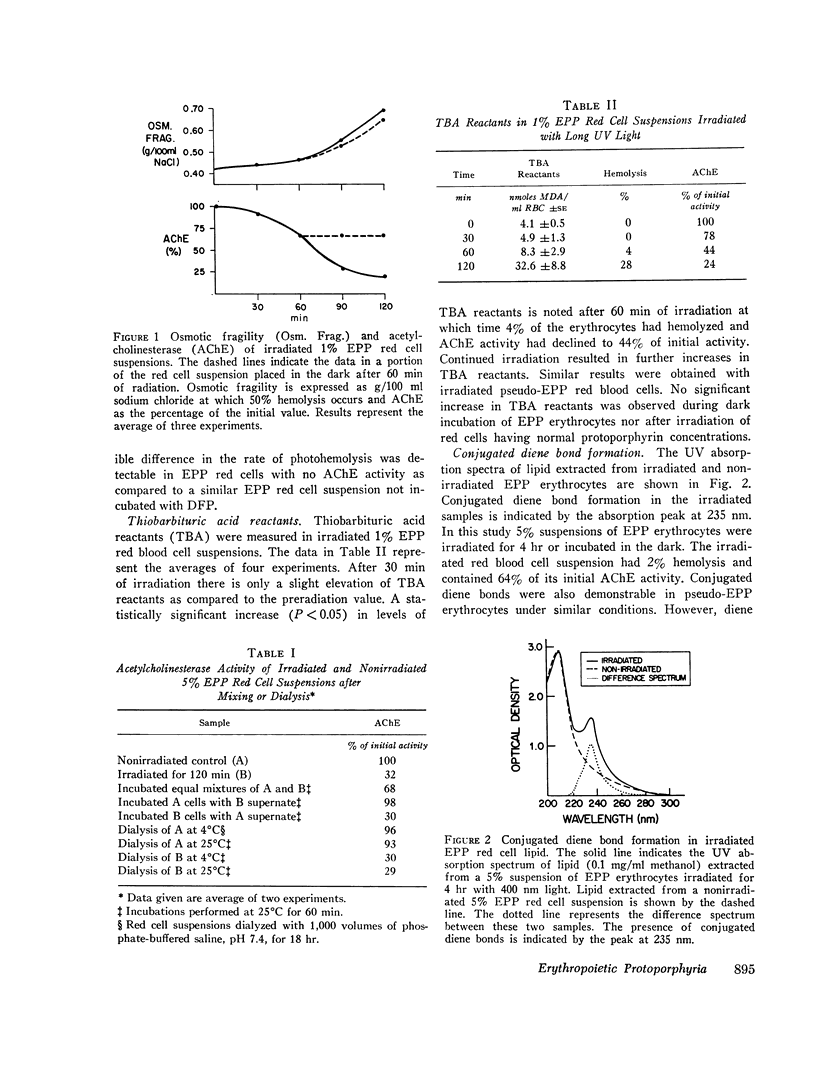
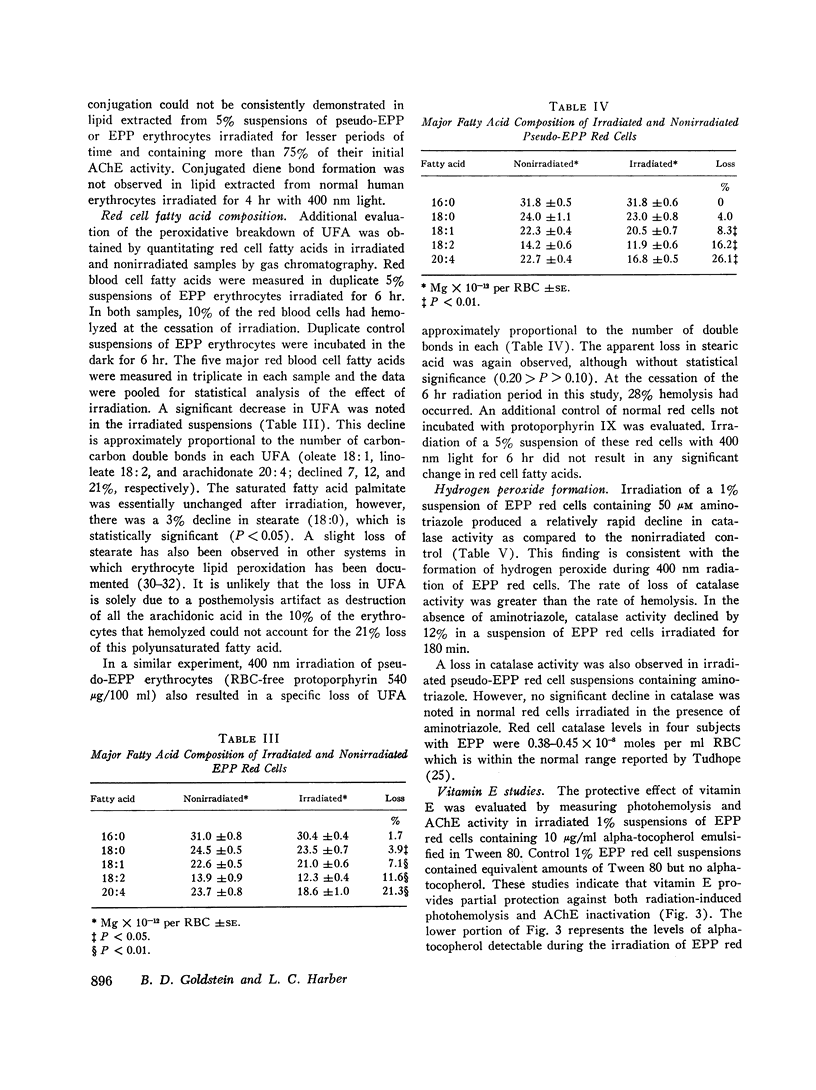
The mechanism by which long wavelength ultraviolet light hemolyzes red cells obtained from patients with erythropoietic protoporphyria (EPP) was investigated. Previous studies had suggested that irradiation of these red cells with wavelengths of light capable of eliciting dermatological manifestations led to oxygen-dependent colloid osmotic hemolysis through the formation of peroxides. In the present report, lipid peroxidation during in vitro irradiation of EPP red cells with long ultraviolet light was demonstrated by: (a) the formation of 2-thiobarbituric acid reactants; (b) the presence of conjugated diene bonds in red cell lipid; and (c) the selective loss of unsaturated fatty acids proportional to the number of carbon-carbon double bonds in each. Irradiation of EPP red cells was also shown to result in the formation of hydrogen peroxide.
Before photohemolysis there was a decline in cell membrane sulfhydryl groups and a loss in activity of the cell membrane enzyme acetylcholinesterase. These parameters provide further evidence suggesting that the cell membrane is a primary site of the photohemolytic effect of long ultraviolet light in EPP red cells.
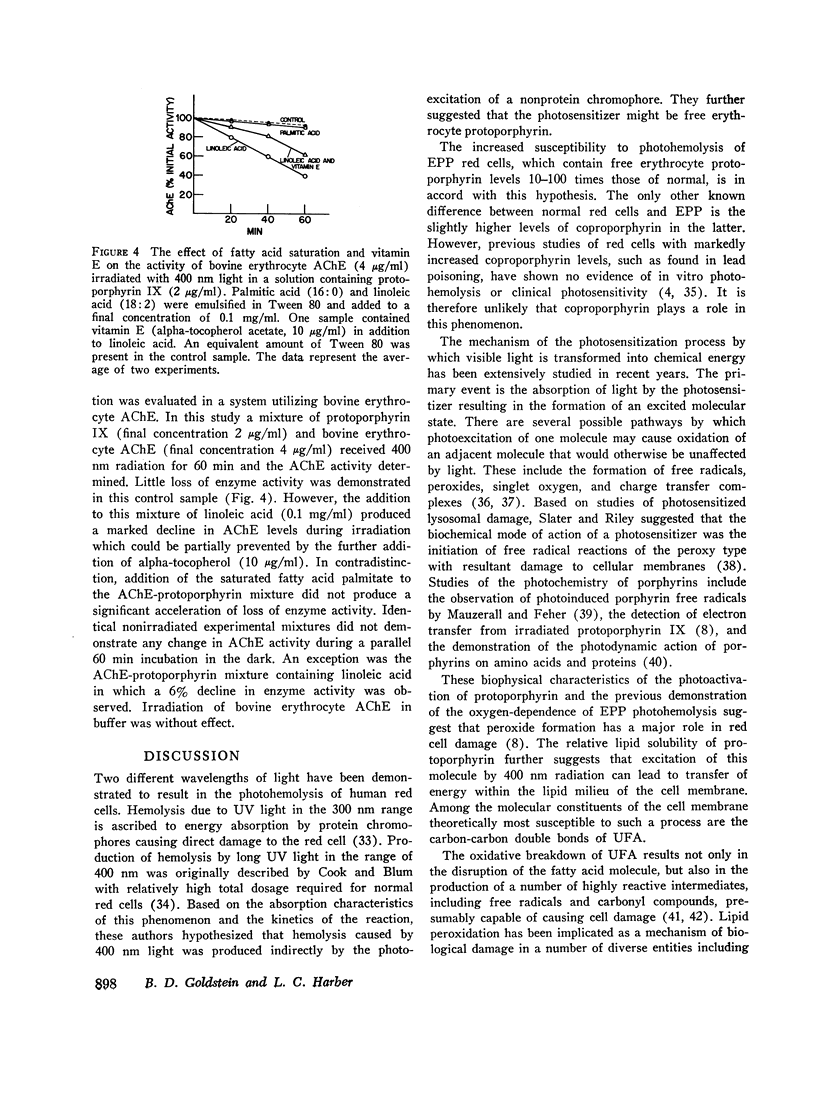
Further evaluation of the radiation-induced inactivation of EPP red cell acetylcholinesterase was performed by radiating mixtures containing bovine erythrocyte acetylcholinesterase and protoporphyrin IX. These studies revealed that the rate of decline in enzyme activity is accelerated by the addition of linoleic acid, an unsaturated fatty acid, but not by palmitic acid, a saturated fatty acid.
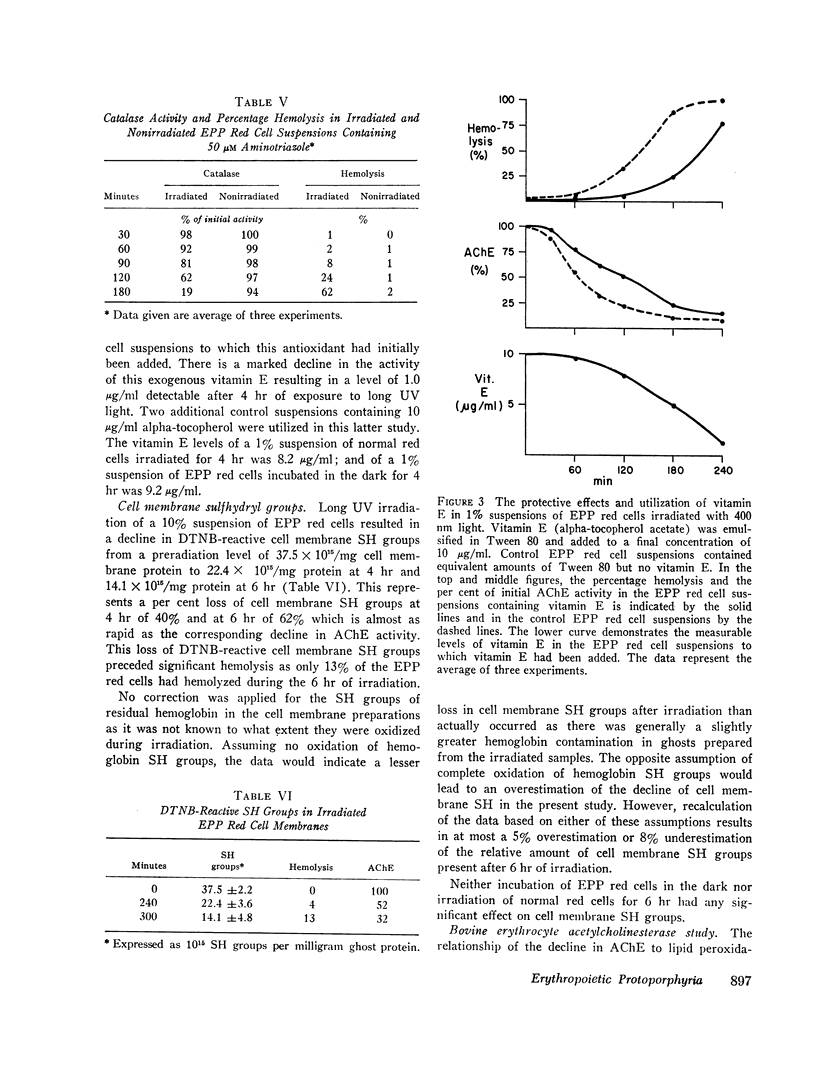
Partial protection against both photohemolysis and acetylcholinesterase decline is provided by alpha-to-copherol. This lipid antioxidant loses its activity during the irradiation of EPP red cells suggesting that it is utilized in this process.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAKER N., WILSON L. Inhibition of tumor glycolysis by hydrogen peroxide formed from autoxidation of unsaturated fatty acids. Biochem Biophys Res Commun. 1963 Apr 2;11:60–64. doi: 10.1016/0006-291x(63)90028-7. [DOI] [PubMed] [Google Scholar]
- BIERI J. G., TEETS L., BELAVADY B., ANDREWS E. L. SERUM VITAMIN E LEVELS IN A NORMAL ADULT POPULATION IN THE WASHINGTON, D. C., AREA. Proc Soc Exp Biol Med. 1964 Oct;117:131–133. doi: 10.3181/00379727-117-29515. [DOI] [PubMed] [Google Scholar]
- Balchum O. J., O'Brien J. S., Goldstein B. D. Ozone and unsaturated fatty acids. Arch Environ Health. 1971 Jan;22(1):32–34. doi: 10.1080/00039896.1971.10665811. [DOI] [PubMed] [Google Scholar]
- COHEN G., HOCHSTEIN P. GENERATION OF HYDROGEN PEROXIDE IN ERYTHROCYTES BY HEMOLYTIC AGENTS. Biochemistry. 1964 Jul;3:895–900. doi: 10.1021/bi00895a006. [DOI] [PubMed] [Google Scholar]
- COHEN G., HOCHSTEIN P. GLUTATHIONE PEROXIDASE: THE PRIMARY AGENT FOR THE ELIMINATION OF HYDROGEN PEROXIDE IN ERYTHROCYTES. Biochemistry. 1963 Nov-Dec;2:1420–1428. doi: 10.1021/bi00906a038. [DOI] [PubMed] [Google Scholar]
- COOK J. S., BLUM H. F. Dose relationships and oxygen dependence in ultraviolet and photodynamic hemolysis. J Cell Comp Physiol. 1959 Feb;53:41–60. doi: 10.1002/jcp.1030530106. [DOI] [PubMed] [Google Scholar]
- COOK J. S. Some characteristics of hemolysis by ultraviolet light. J Cell Physiol. 1956 Feb;47(1):55–84. doi: 10.1002/jcp.1030470105. [DOI] [PubMed] [Google Scholar]
- CROSBY W. H., FURTH F. W. A modification of the benzidine method for measurement of hemoglobin in plasma and urine. Blood. 1956 Apr;11(4):380–383. [PubMed] [Google Scholar]
- Cohen G., Somerson N. L. Catalase-aminotriazole method for measuring secretion of hydrogen peroxide by microorganisms. J Bacteriol. 1969 May;98(2):543–546. doi: 10.1128/jb.98.2.543-546.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Dodge J. T., Cohen G., Kayden H. J., Phillips G. B. Peroxidative hemolysis of red blood cells from patients with abetalipoproteinemia (acanthocytosis). J Clin Invest. 1967 Mar;46(3):357–368. doi: 10.1172/JCI105537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge J. T., Phillips G. B. Autoxidation as a cause of altered lipid distribution in extracts from human red cells. J Lipid Res. 1966 May;7(3):387–395. [PubMed] [Google Scholar]
- ELLMAN G. L., COURTNEY K. D., ANDRES V., Jr, FEATHER-STONE R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961 Jul;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Foote C. S. Mechanisms of photosensitized oxidation. There are several different types of photosensitized oxidation which may be important in biological systems. Science. 1968 Nov 29;162(3857):963–970. doi: 10.1126/science.162.3857.963. [DOI] [PubMed] [Google Scholar]
- Goldstein B. D., Balchum O. J. Effect of ozone on lipid peroxidation in the red blood cell. Proc Soc Exp Biol Med. 1967 Nov;126(2):356–358. doi: 10.3181/00379727-126-32444. [DOI] [PubMed] [Google Scholar]
- Goldstein B. D., Lodi C., Collinson C., Balchum O. J. Ozone and lipid peroxidation. Arch Environ Health. 1969 Apr;18(4):631–635. doi: 10.1080/00039896.1969.10665464. [DOI] [PubMed] [Google Scholar]
- HARBER L. C., FLEISCHER A. S., BAER R. L. ERYTHROPOIETIC PROTOPORPHYRIA AND PHOTOHEMOLYSIS. JAMA. 1964 Jul 20;189:191–194. doi: 10.1001/jama.1964.03070030013004. [DOI] [PubMed] [Google Scholar]
- HARMAN D. Atherosclerosis: a hypothesis concerning the initiating steps in pathogenesis. J Gerontol. 1957 Apr;12(2):199–202. doi: 10.1093/geronj/12.2.199. [DOI] [PubMed] [Google Scholar]
- HORWITT M. K., HARVEY C. C., DUNCAN G. D., WILSON W. C. Effects of limited tocopherol intake in man with relationships to erythrocyte hemolysis and lipid oxidations. Am J Clin Nutr. 1956 Jul-Aug;4(4):408–419. doi: 10.1093/ajcn/4.4.408. [DOI] [PubMed] [Google Scholar]
- Haining R. G., Hulse T. E., Labbe R. F. Photohemolysis. The comparative behavior of erythrocytes from patients with different types of porphyria. Proc Soc Exp Biol Med. 1969 Nov;132(2):625–628. doi: 10.3181/00379727-132-34274. [DOI] [PubMed] [Google Scholar]
- Hsu J., Goldstein B. D., Harber L. C. Photoreactions associated with in vitro hemolysis in erythropoietic protoporphyria. Photochem Photobiol. 1971 Jan;13(1):67–77. doi: 10.1111/j.1751-1097.1971.tb06092.x. [DOI] [PubMed] [Google Scholar]
- JACOB H. S., JANDL J. H. Effects of sulfhydryl inhibition on red blood cells. I. Mechanism of hemolysis. J Clin Invest. 1962 Apr;41:779–792. doi: 10.1172/JCI104536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jori G., Galiazzo G., Scoffone E. Photodynamic action of porphyrins on amino acids and proteins. I. Selective photooxidation of methionine in Aqueous solution. Biochemistry. 1969 Jul;8(7):2868–2875. doi: 10.1021/bi00835a026. [DOI] [PubMed] [Google Scholar]
- KANN H. E., Jr, MENGEL C. E., SMITH W., HORTON B. OXYGEN TOXICITY AND VITAMIN E. Aerosp Med. 1964 Sep;35:840–844. [PubMed] [Google Scholar]
- Kaplowitz N., Javitt N., Harber L. C. Isolation of erythrocytes with normal protoporphyrin levels in erythropoietic protoporphyria. N Engl J Med. 1968 May 16;278(20):1077–1081. doi: 10.1056/NEJM196805162782001. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAGNUS I. A., JARRETT A., PRANKERD T. A., RIMINGTON C. Erythropoietic protoporphyria. A new porphyria syndrome with solar urticaria due to protoporphyrinaemia. Lancet. 1961 Aug 26;2(7200):448–451. doi: 10.1016/s0140-6736(61)92427-8. [DOI] [PubMed] [Google Scholar]
- MAUZERALL D., FEHER G. A STUDY OF THE PHOTOINDUCED PORPHYRIN FREE RADICAL BY ELECTRON SPIN RESONANCE. Biochim Biophys Acta. 1964 Mar 30;79:430–432. [PubMed] [Google Scholar]
- METZ J., VAN RENSBURG N. J., STEVENS K., HART D. Acetylcholinesterase and the life-span of the erythrocyte. Nature. 1961 Jun 24;190:1208–1209. doi: 10.1038/1901208a0. [DOI] [PubMed] [Google Scholar]
- MORRISON W. R., SMITH L. M. PREPARATION OF FATTY ACID METHYL ESTERS AND DIMETHYLACETALS FROM LIPIDS WITH BORON FLUORIDE--METHANOL. J Lipid Res. 1964 Oct;5:600–608. [PubMed] [Google Scholar]
- Mengel C. E., Kann H. E., Jr Effects of in vivo hyperoxia on erythrocytes. 3. In vivo peroxidation of erythrocyte lipid. J Clin Invest. 1966 Jul;45(7):1150–1158. doi: 10.1172/JCI105421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengel C. E., Zirkle L. G., O'Malley B. W., Horton B. D. Studies of the mechanism of in vivo RBC damage by oxygen. Aerosp Med. 1965 Nov;36(11):1036–1041. [PubMed] [Google Scholar]
- NORINS A. L. Free radical formation in the skin following exposure to ultraviolet light. J Invest Dermatol. 1962 Nov;39:445–448. doi: 10.1038/jid.1962.137. [DOI] [PubMed] [Google Scholar]
- O'Brien J. S., Sampson E. L. Kinky hair disease. II. Biochemical studies. J Neuropathol Exp Neurol. 1966 Oct;25(4):523–530. doi: 10.1097/00005072-196610000-00002. [DOI] [PubMed] [Google Scholar]
- O'Malley B. W., Mengel C. E., Meriwether W. D., Zirkle L. G., Jr Inhibition of erythrocyte acetylcholinesterase by peroxides. Biochemistry. 1966 Jan;5(1):40–44. doi: 10.1021/bi00865a006. [DOI] [PubMed] [Google Scholar]
- Peterka E. S., Runge W. J., Fusaro R. M. Erythropoietic protoporphyria. III. Photohemolysis. Arch Dermatol. 1966 Sep;94(3):282–285. [PubMed] [Google Scholar]
- Phillips G. B., Dodge J. T., Rockmore C. S. Analysis of fatty acids of human red cells without lipid extraction. J Lipid Res. 1968 Mar;9(2):285–286. [PubMed] [Google Scholar]
- ROSE H. G., OKLANDER M. IMPROVED PROCEDURE FOR THE EXTRACTION OF LIPIDS FROM HUMAN ERYTHROCYTES. J Lipid Res. 1965 Jul;6:428–431. [PubMed] [Google Scholar]
- Recknagel R. O., Ghoshal A. K. Lipoperoxidation as a vector in carbon tetrachloride hepatotoxicity. Lab Invest. 1966 Jan;15(1 Pt 1):132–148. [PubMed] [Google Scholar]
- SZEINBERG A., CLEJAN L. SULFHYDRYL GROUPS IN THE RED CELLS OF NORMAL AND GLUCOSE-6-PHOSPHATE DEHYDROGENASE-DEFICIENT SUBJECTS. Biochim Biophys Acta. 1964 Dec 9;93:564–572. doi: 10.1016/0304-4165(64)90340-x. [DOI] [PubMed] [Google Scholar]
- Scholnick P., Marver H. S., Schmid R. Erythropoietic protoporphyria: evidence for multiple sites of excess protoporphyrin formation. J Clin Invest. 1971 Jan;50(1):203–207. doi: 10.1172/JCI106474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schothorst A. A., Van Steveninck J., Went L. N., Suurmond D. Protoporphyrin-induced photohemolysis in protoporphyria and in normal red blood cells. Clin Chim Acta. 1970 Apr;28(1):41–49. doi: 10.1016/0009-8981(70)90158-0. [DOI] [PubMed] [Google Scholar]
- Slater T. F., Riley P. A. Photosensitization and lysosomal damage. Nature. 1966 Jan 8;209(5019):151–154. doi: 10.1038/209151a0. [DOI] [PubMed] [Google Scholar]
- Stocks J., Dormandy T. L. A direct thiobarbituric acid-reacting chromogen in human red blood cells. Clin Chim Acta. 1970 Jan;27(1):117–120. doi: 10.1016/0009-8981(70)90383-9. [DOI] [PubMed] [Google Scholar]
- Stocks J., Dormandy T. L. The autoxidation of human red cell lipids induced by hydrogen peroxide. Br J Haematol. 1971 Jan;20(1):95–111. doi: 10.1111/j.1365-2141.1971.tb00790.x. [DOI] [PubMed] [Google Scholar]
- Sutherland R. M., Stannard J. N., Weed R. I. Involvement of sulphhydryl groups in radiation damage to the human erythrocyte membrane. Int J Radiat Biol Relat Stud Phys Chem Med. 1967;12(6):551–564. doi: 10.1080/09553006714551161. [DOI] [PubMed] [Google Scholar]
- TAPPEL A. L. FREE-RADICAL LIPID PEROXIDATION DAMAGE AND ITS INHIBITION BY VITAMIN E AND SELENIUM. Fed Proc. 1965 Jan-Feb;24:73–78. [PubMed] [Google Scholar]
- TSEN C. C., COLLIER H. B. The protective action of tocopherol against hemolysis of rat erythrocytes by dialuric acid. Can J Biochem Physiol. 1960 Sep;38:957–964. [PubMed] [Google Scholar]
- Thomas H. V., Mueller P. K., Lyman R. L. Lipoperoxidation of lung lipids in rats exposed to nitrogen dioxide. Science. 1968 Feb 2;159(3814):532–534. doi: 10.1126/science.159.3814.532. [DOI] [PubMed] [Google Scholar]
- Tudhope G. R. Red cell catalase in health and in disease, with reference to the enzyme activity in anaemia. Clin Sci. 1967 Aug;33(1):165–182. [PubMed] [Google Scholar]
- WALLING C. Chemistry of the organic peroxides. Radiat Res. 1963;Suppl 3:3–16. [PubMed] [Google Scholar]
- WILLS E. D. THE EFFECT OF INORGANIC IRON ON THE THIOBARBITURIC ACID METHOD FOR THE DETERMINATION OF LIPID PEROXIDES. Biochim Biophys Acta. 1964 Aug 5;84:475–477. doi: 10.1016/0926-6542(64)90016-2. [DOI] [PubMed] [Google Scholar]
- WRANNE L. Free erythrocyte copro-and protoporphyrin. A methodoogical and clinical study. Acta Paediatr Suppl. 1960 May;49(Suppl 124):1–78. [PubMed] [Google Scholar]
- Ways P., Hanahan D. J. Characterization and quantification of red cell lipids in normal man. J Lipid Res. 1964 Jul;5(3):318–328. [PubMed] [Google Scholar]



