Abstract
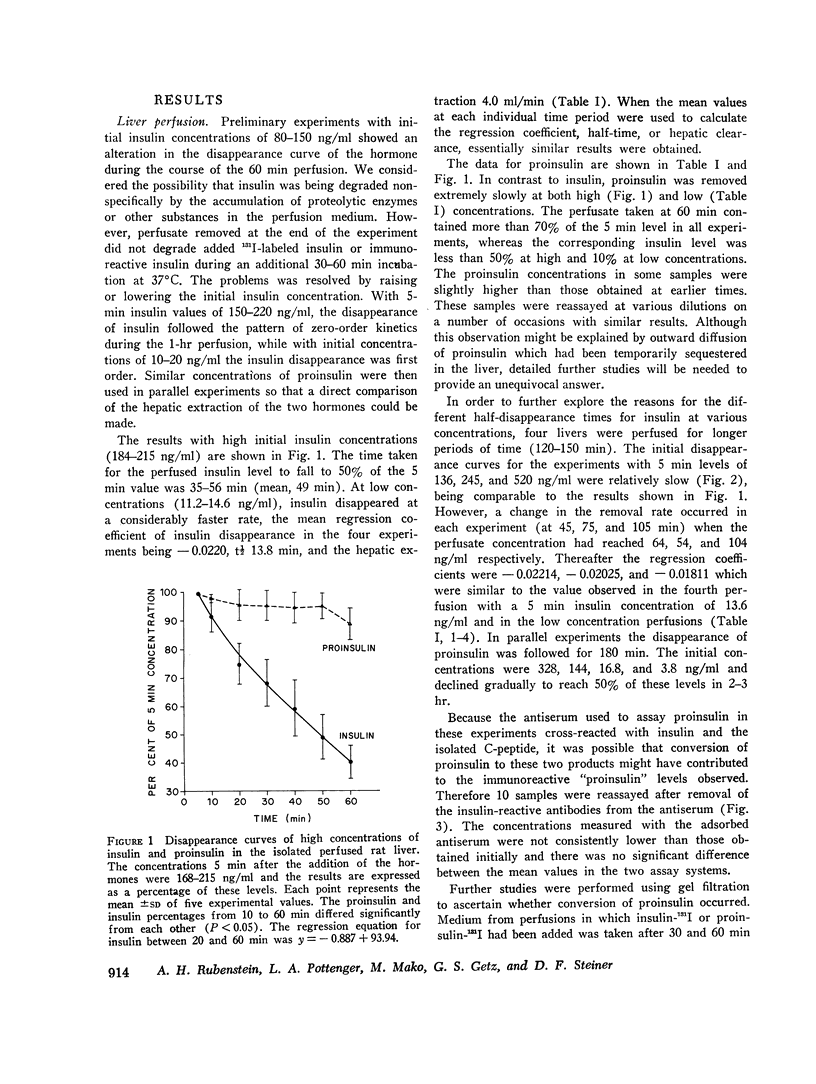
The removal of bovine proinsulin by the isolated perfused rat liver has been studied and the results compared with the removal of insulin. At high concentrations of insulin (> 180 ng/ml) the removal process was saturated and the t½ varied between 35 and 56 min. With low initial insulin levels the disappearance followed first-order kinetics, the mean regression coefficient being — 0.022, t½ 13.8 min, and the hepatic extraction 4.0 ml/min. The results with proinsulin were in striking contrast to these findings. At both high and low concentrations the hepatic removal of proinsulin was considerably slower, averaging 10-15 times less than that of insulin. Specific immunoassay techniques and gel filtration of samples taken from perfusions to which both labeled and unlabeled proinsulin had been added did not show conversion to either insulin or the C-peptide.
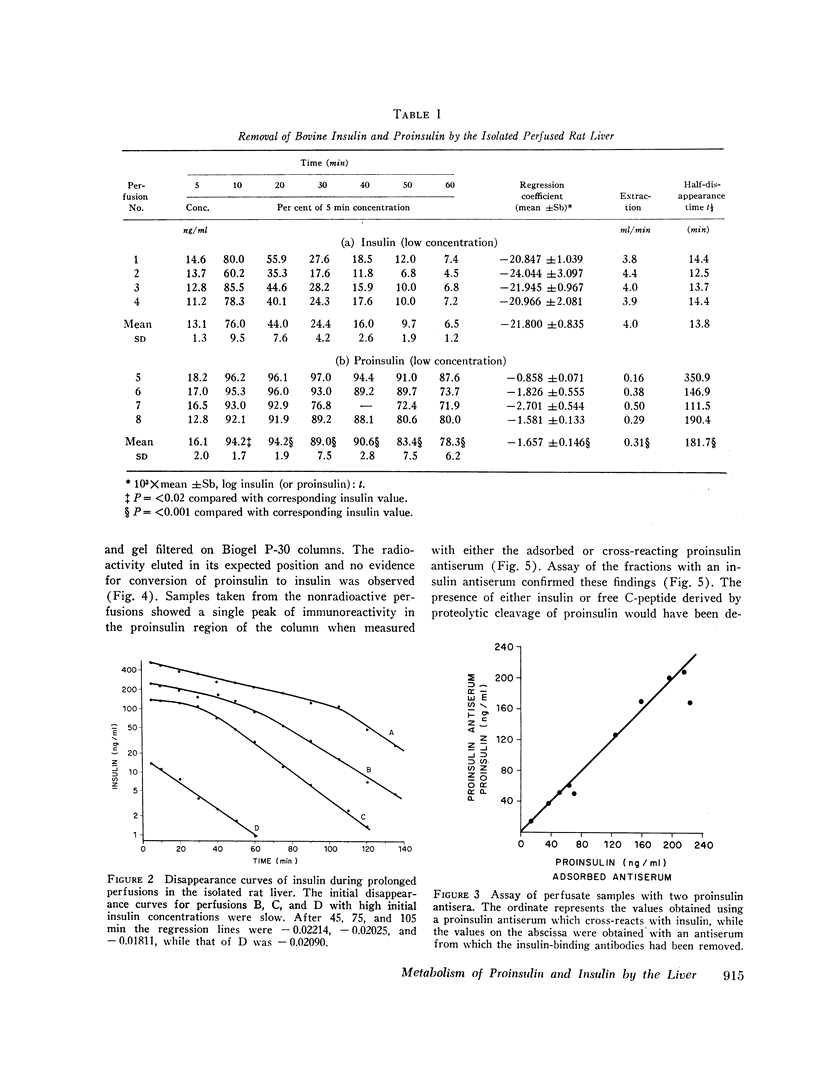
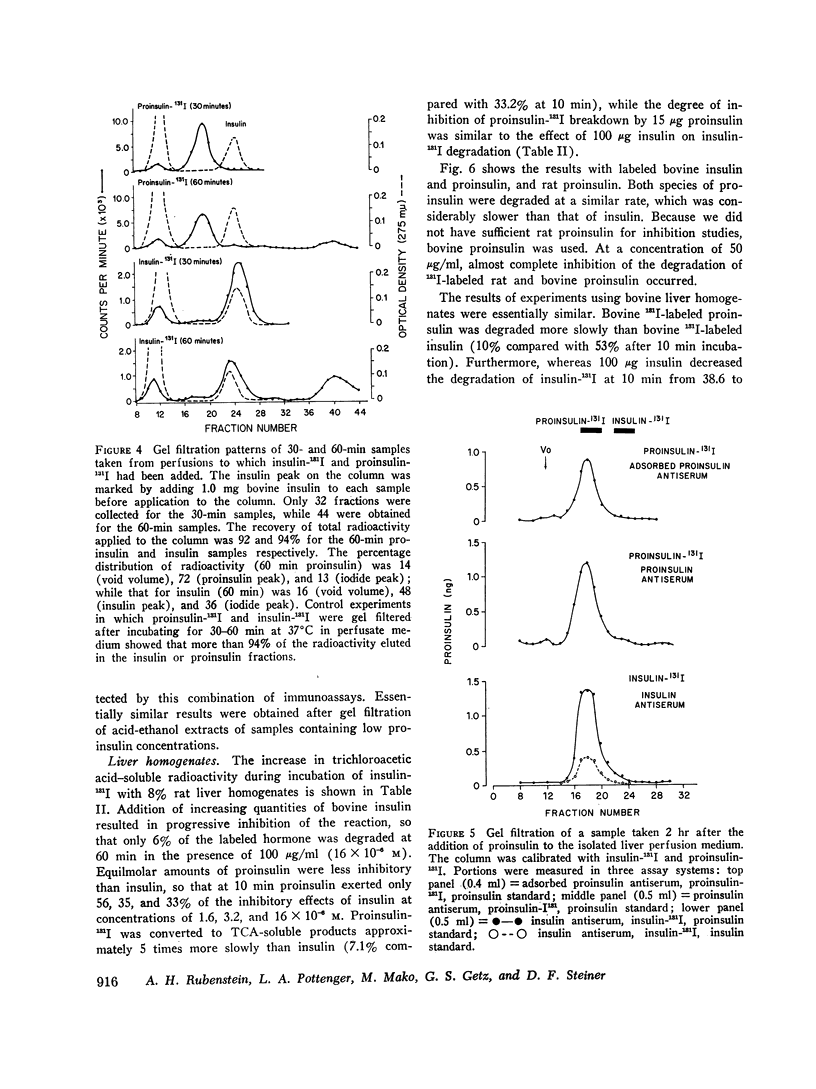
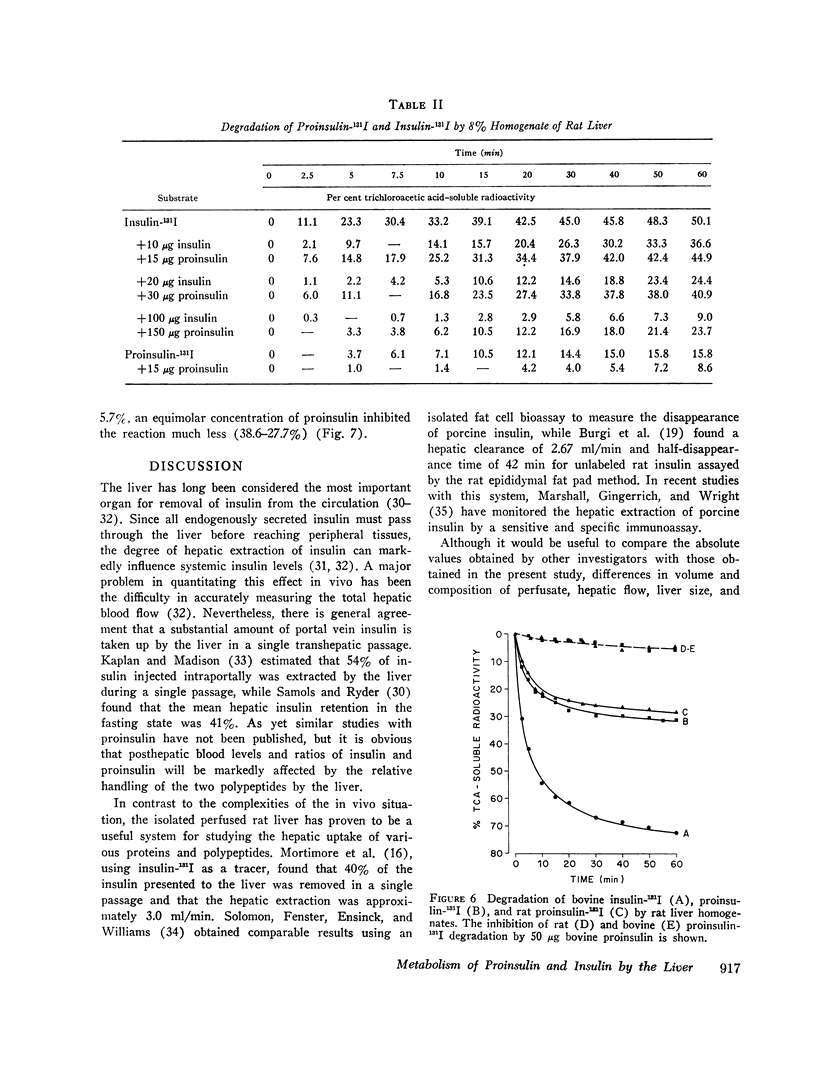
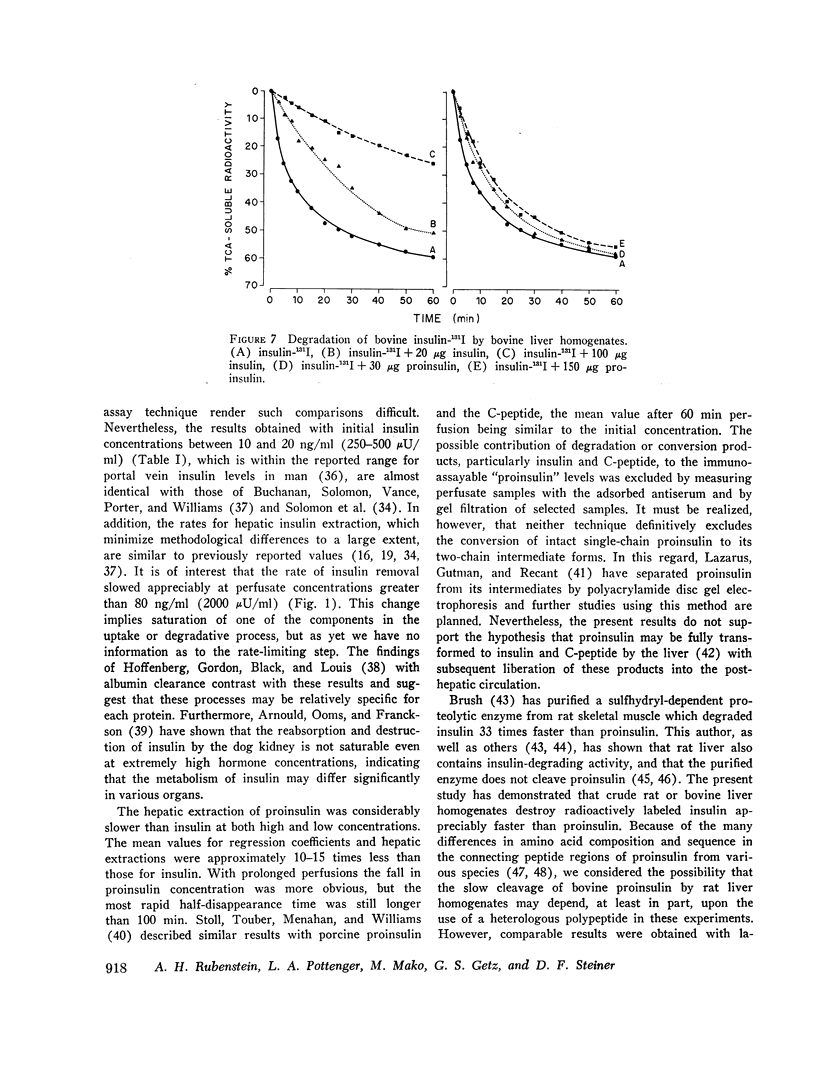
Bovine and rat 131I-labeled proinsulins were degraded more slowly than bovine insulin-131I by bovine and rat liver homogenates. Both proinsulin and insulin inhibited the degradation of insulin-131I, equimolar quantities of proinsulin being 2-5 times less effective than insulin.
These results indicate significant differences in the capacity of the liver to remove and degrade insulin and proinsulin. The low hepatic extraction of proinsulin may account for its prolonged half-life in vivo and contribute to its relatively high plasma concentration in the fasting state. Furthermore this finding will have to be taken into account in the interpretation of changes in the proinsulin:insulin ratios in peripheral blood in a variety of metabolich situations.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnould Y., Ooms H. A., Franckson J. R. Analysis of urinary excretion of insulin in the normal dog. Arch Int Pharmacodyn Ther. 1967 Jun;167(2):480–482. [PubMed] [Google Scholar]
- BUERGI H., SCHWARZ K., KOPETZ K., FROESCH E. R. FATE OF RAT INSULIN IN RAT-LIVER PERFUSION STUDIED BY ADIPOSE-TISSUE ASSAY. Lancet. 1963 Aug 17;2(7303):314–316. doi: 10.1016/s0140-6736(63)92987-8. [DOI] [PubMed] [Google Scholar]
- Blackard W. G., Nelson N. C. Portal and peripheral vein immunoreactive insulin concentrations before and after glucose infusion. Diabetes. 1970 May;19(5):302–306. doi: 10.2337/diab.19.5.302. [DOI] [PubMed] [Google Scholar]
- Brush J. S. Purification and characterizatoion of a protease with specificity for insulin from rat muscle. Diabetes. 1971 Mar;20(3):140–145. [PubMed] [Google Scholar]
- Buchanan K. D., Solomon S. S., Vance J. E., Porter H. P., Williams R. H. Glucagon clearance by the isolated perfused rat liver. Proc Soc Exp Biol Med. 1968 Jun;128(2):620–623. doi: 10.3181/00379727-128-33081. [DOI] [PubMed] [Google Scholar]
- Challoner D. R. Degradation of porcine insulin and proinsulin by rat adipose tissue. Diabetes. 1971 May;20(5):276–281. doi: 10.2337/diab.20.5.276. [DOI] [PubMed] [Google Scholar]
- Chance R. E., Ellis R. M., Bromer W. W. Porcine proinsulin: characterization and amino acid sequence. Science. 1968 Jul 12;161(3837):165–167. doi: 10.1126/science.161.3837.165. [DOI] [PubMed] [Google Scholar]
- Clark J. L., Steiner D. F. Insulin biosynthesis in the rat: demonstration of two proinsulins. Proc Natl Acad Sci U S A. 1969 Jan;62(1):278–285. doi: 10.1073/pnas.62.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVOREN P. R. The isolation of insulin from a single cat pancreas. Biochim Biophys Acta. 1962 Sep 10;63:150–153. doi: 10.1016/0006-3002(62)90347-5. [DOI] [PubMed] [Google Scholar]
- Exton J. H., Park C. R. Control of gluconeogenesis in liver. I. General features of gluconeogenesis in the perfused livers of rats. J Biol Chem. 1967 Jun 10;242(11):2622–2636. [PubMed] [Google Scholar]
- Freychet P., Roth J., Neville D. M., Jr Monoiodoinsulin: demonstration of its biological activity and binding to fat cells and liver membranes. Biochem Biophys Res Commun. 1971 Apr 16;43(2):400–408. doi: 10.1016/0006-291x(71)90767-4. [DOI] [PubMed] [Google Scholar]
- Gliemann J., Sorensen H. H. Assay of insulin-like activity by the isolated fat cell method. IV. The biological activity of proinsulin. Diabetologia. 1970 Oct;6(5):499–504. doi: 10.1007/BF01211891. [DOI] [PubMed] [Google Scholar]
- Gorden P., Roth J. Plasma insulin: fluctuations in the "big" insulin component in man after glucose and other stimuli. J Clin Invest. 1969 Dec;48(12):2225–2234. doi: 10.1172/JCI106188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman R. A., Lazarus N. R., Penhos J. C., Fajans S., Recant L. Circulating proinsulin-like material in patients with functioning insulinomas. N Engl J Med. 1971 May 6;284(18):1003–1008. doi: 10.1056/NEJM197105062841803. [DOI] [PubMed] [Google Scholar]
- HENRY R. J., CHIAMORI N., GOLUB O. J., BERKMAN S. Revised spectrophotometric methods for the determination of glutamic-oxalacetic transaminase, glutamic-pyruvic transaminase, and lactic acid dehydrogenase. Am J Clin Pathol. 1960 Oct;34:381–398. doi: 10.1093/ajcp/34.4_ts.381. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hoffenberg R., Gordon A. H., Black E. G., Louis L. N. Plasma protein catabolism by the perfused rat liver. The effect of alteration of albumin concentration and dietary protein depletion. Biochem J. 1970 Jul;118(3):401–404. doi: 10.1042/bj1180401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa Y., Kuzuya T., Ide T., Kosaka K. Plasma insulin responses to glucose in femoral, hepatic, and pancreatic veins in dogs. Am J Physiol. 1966 Aug;211(2):442–448. doi: 10.1152/ajplegacy.1966.211.2.442. [DOI] [PubMed] [Google Scholar]
- Kaneto A., Kosaka K., Nakao K. Effects of stimulation of the vagus nerve on insulin secretion. Endocrinology. 1967 Mar;80(3):530–536. doi: 10.1210/endo-80-3-530. [DOI] [PubMed] [Google Scholar]
- Lazarus N. R., Gutman R. A., Recant L. A method for electrophoretic characterization on polyacrylamide gel of circulating insulin immunoreactive substances. Anal Biochem. 1971 Apr;40(2):241–246. doi: 10.1016/0003-2697(71)90382-4. [DOI] [PubMed] [Google Scholar]
- MORTIMORE G. E., TIETZE F., STETTEN D., Jr Metabolism of insulin-I 131; studies in isolated, perfused rat liver and hindlimb preparations. Diabetes. 1959 Jul-Aug;8(4):307–314. doi: 10.2337/diab.8.4.307. [DOI] [PubMed] [Google Scholar]
- Marshall A., Gingerich R. L., Wright P. H. Hepatic effect of sulfonylureas. Metabolism. 1970 Dec;19(12):1046–1052. doi: 10.1016/0026-0495(70)90028-4. [DOI] [PubMed] [Google Scholar]
- Melani F., Rubenstein A. H., Oyer P. E., Steiner D. F. Identification of proinsulin and C-peptide in human serum by a specific immunoassay. Proc Natl Acad Sci U S A. 1970 Sep;67(1):148–155. doi: 10.1073/pnas.67.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melani F., Rubenstein A. H., Steiner D. F. Human serum proinsulin. J Clin Invest. 1970 Mar;49(3):497–507. doi: 10.1172/JCI106259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melani F., Ryan W. G., Rubenstein A. H., Steiner D. F. Proinsulin secretion by a pancreatic beta-cell adenoma. Proinsulin and C-peptide secretion. N Engl J Med. 1970 Oct 1;283(14):713–719. doi: 10.1056/NEJM197010012831401. [DOI] [PubMed] [Google Scholar]
- Morgan C. R., Wiesman H. J. Liver insulinase activity in insulin deficient rats. Proc Soc Exp Biol Med. 1968 Mar;127(3):763–765. doi: 10.3181/00379727-127-32795. [DOI] [PubMed] [Google Scholar]
- Pottenger L. A., Getz G. S. Serum lipoprotein accumulation in the livers of orotic acid-fed rats. J Lipid Res. 1971 Jul;12(4):450–459. [PubMed] [Google Scholar]
- Roth J., Gorden P., Pastan I. "Big insulin": a new component of plasma insulin detected by immunoassay. Proc Natl Acad Sci U S A. 1968 Sep;61(1):138–145. doi: 10.1073/pnas.61.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein A. H., Cho S., Steiner D. F. Evidence for proinsulin in human urine and serum. Lancet. 1968 Jun 22;1(7556):1353–1355. doi: 10.1016/s0140-6736(68)92040-0. [DOI] [PubMed] [Google Scholar]
- Rubenstein A. H., Steiner D. F., Cho S., Lawrence A. M., Kirsteins L. Immunological properties of bovine proinsulin and related fractions. Diabetes. 1969 Sep;18(9):598–605. doi: 10.2337/diab.18.9.598. [DOI] [PubMed] [Google Scholar]
- Rubenstein A. H., Steiner D. F. Proinsulin. Annu Rev Med. 1971;22:1–18. doi: 10.1146/annurev.me.22.020171.000245. [DOI] [PubMed] [Google Scholar]
- Sherman B. M., Gorden P., Roth J., Freychet P. Circulating insulin: th proinsulin-like properties of "big" insulin in patients withou islet cell tumors. J Clin Invest. 1971 Apr;50(4):849–858. doi: 10.1172/JCI106556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon S. S., Fenster L. F., Ensinck J. W., Williams R. H. Clearance studies of insulin and nonsuppressible insulin-like activity (NSILA) in the rat liver. Proc Soc Exp Biol Med. 1967 Oct;126(1):166–169. doi: 10.3181/00379727-126-32392. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Cho S., Oyer P. E., Terris S., Peterson J. D., Rubenstein A. H. Isolation and characterization of proinsulin C-peptide from bovine pancreas. J Biol Chem. 1971 Mar 10;246(5):1365–1374. [PubMed] [Google Scholar]
- Steiner D. F., Hallund O., Rubenstein A., Cho S., Bayliss C. Isolation and properties of proinsulin, intermediate forms, and other minor components from crystalline bovine insulin. Diabetes. 1968 Dec;17(12):725–736. doi: 10.2337/diab.17.12.725. [DOI] [PubMed] [Google Scholar]
- Stern M. P., Farquhar J. W., Silvers A., Reaven G. M. Insulin delivery rate into plasma in normal and diabetic subjects. J Clin Invest. 1968 Sep;47(9):1947–1957. doi: 10.1172/JCI105884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll R. W., Touber J. L., Ensinck J. W., Williams R. H. Substances immunologically related to proinsulin or connecting peptide in swine plasma. Horm Metab Res. 1970 May;2(3):153–156. doi: 10.1055/s-0028-1095105. [DOI] [PubMed] [Google Scholar]
- Stoll R. W., Touber J. L., Menahan L. A., Williams R. H. Clearance of porcine insulin, proinsulin, and connecting peptide by the isolated rat liver. Proc Soc Exp Biol Med. 1970 Mar;133(3):894–896. doi: 10.3181/00379727-133-34589. [DOI] [PubMed] [Google Scholar]
- Stoll R. W., Touber J. L., Winterscheid L. C., Ensinck J. W., Williams R. H. Hypoglycemic activity and immunological half-life of porcine insulin and proinsulin in baboons and swine. Endocrinology. 1971 Mar;88(3):714–717. doi: 10.1210/endo-88-3-714. [DOI] [PubMed] [Google Scholar]
- TOMIZAWA H. H., WILLIAMS R. H. Studies on the specificity of an insulin-inactivating system of the liver. J Biol Chem. 1955 Dec;217(2):685–694. [PubMed] [Google Scholar]
- Yip C. C., Logothetopoulos J. A specific anti-proinsulin serum and the presence of proinsulin in calf serum. Proc Natl Acad Sci U S A. 1969 Feb;62(2):415–419. doi: 10.1073/pnas.62.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]


