Abstract
The role of R-α-lipoic acid as a cofactor (lipoyllysine) in mitochondrial energy metabolism is well established. Lipoic acid non-covalently bound and exogenously administered to cells or supplemented in the diet is a potent modulator of the cell’s redox status. The diversity of beneficial effects of lipoic acid in a variety of tissues can be mechanistically viewed in terms of thiol/disulfide exchange reactions that modulate the environment’s redox and energy status. Lipoic acid-driven thiol/disulfide exchange reactions appear critical for the modulation of proteins involved in cell signaling and transcription factors. This review emphasizes the effects of lipoic acid on PI3K and AMPK signaling and related transcriptional pathways that are integrated by PGC-1α, a critical regulator of energy homoestasis. The effects of lipoic acid on the neuronal energy-redox axis are largely reviewed in terms of their outcomes for aging and age-related neurodegenerative diseases.
Keywords: lipoic acid, dihydrolipoic acid, energy, redox, AMPK, insulin, mitochondria, PGC1α
Introduction
Lipoic acid (1,2-dithiolane-3-pentanoic acid)—first isolated and chemically identified in 1951 by Lester Reed and colleagues(1)—occurs in the R- and S-enantiomeric structures, only the R-form being essential in biological systems. The discovery of lipoic acid led to an unprecedented interest in basic research because of its role as a coenzyme in energy metabolism and the non-covalently bound form as a modulator of the cell’s redox status. The biochemistry, physiology, and pharmacokinetics of lipoic acid as well as its effects on several disease states have been extensively reviewed (see Ref. 2, 3); the diversity of effects of lipoic acid in a variety of tissues can be viewed within the realm of antioxidant activity, metal chelation, transcriptional responses —related to inflammation and induction of phase II enzymes—, and cell signaling responses, especially in terms of cardiovascular function and glucose metabolism.(2,3) These effects of lipoic acid can be mechanistically accounted for in terms of thiol/disulfide exchange reactions that modulate the environment’s redox and energy status (Fig. 1). The energy and redox components are integrated into an energy–redox axis; hence, on a mechanistic basis, lipoic acid co-regulates both components in the several subcellular compartments. R-Lipoic acid—as a micronutrient and a therapeutic agent—stimulated interest in clinical research because of its therapeutic implications for the metabolic syndrome,(4) diabetic polyneuropathies,(5) and neurodegenerative diseases (with emphasis on Alzheimer’s disease).(6)
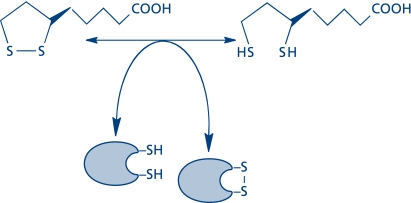
Fig. 1.
Thiol/disulfide exchange by lipoic acid is the basis for its modulation of the cell’s energy and redox status.
The Cell’s Redox Status
The redox environment of a linked set of redox couples—as found in biological fluids, organelles, cells, or tissues—is defined as the summation of the products of the reduction potential and reducing capacity of the linked redox couples.(7) Quantification of thioredoxin, glutathione/glutathione disulfide (GSH/GSSG), and cysteine/cystine redox couples—termed redox control nodes(8)— brings new dimensions to redox systems biology; assessment of these major cellular thiol/disulfide systems in different cellular compartments indicated that individual signaling and control events occur through discrete redox pathways, thereby leading to a new definition of oxidative stress as a disruption of redox signaling and control.(9)
Lipoic acid—either as a dietary supplement or a therapeutic agent—modulates distinct redox circuits because of its ability to equilibrate between different subcellular compartments as well as extracellularly. As such, lipoic acid is a critical component of the antioxidant network because of its ability to regenerate other antioxidants, such as vitamins E and C, increase intracellular GSH levels, and provide redox regulation of proteins and transcription factors.(10)
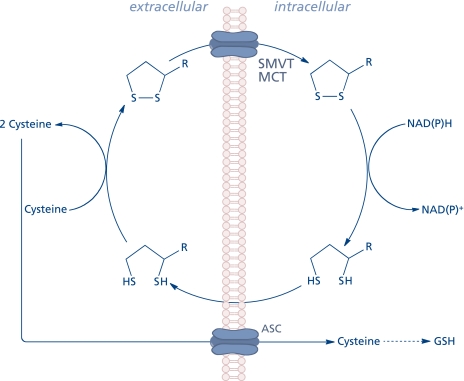
The extracellular thiol/disulfide redox environment (determined by the cysteine/cystine couple) has been reported to modulate cell proliferation, apoptosis, cell adhesion molecules, and pro-inflammatory signaling.(11) Lipoic acid may modulate the extracellular redox state inasmuch as dihydrolipoic acid is involved in the reduction of cystine to cysteine, thus facilitating rapid uptake of the latter into the cell through the ASC transport system and, consequently, its availability to stimulate GSH synthesis (Fig. 2).(12,13) Cellular transport of lipoic acid occurs probably by several systems, such as the medium chain fatty acid transporter,(14) a Na+-dependent vitamin(15) transport system,(16) and a H+-linked monocarboxylate transporter for intestinal uptake.(17) The cellular reduction of lipoic acid to dihydrolipoic acid is accomplished by NAD(P)H-driven enzymes, thioredoxin reductase, lipoamide dehydrogenase, and glutathione reductase. Erythrocytes take up and reduce lipoic acid by glucose metabolism; subsequently, dihydrolipoic acid is released to the extracellular milieu, thus reflecting the activity of disulfide reductases.(14) This phenomenon was observed in several cell types;(18) 3T3-L1 adipocytes, however, possess a low capacity to reduce lipoic acid and most of the intracellular effects in these cells are due to its pro-oxidant function.(19,20)
Fig. 2.
Cellular uptake and release of lipoic acid and modification of the extracellular redox state.
R-(+)-lipoic acid (as lipoyllisine) is present in both plant and animal tissues only in small amounts, thus its bioavailability is low;(21) however, lipoic acid is now available as a nutritional supplement: the human plasma pharmacokinetics of R-(+)-lipoic acid (administered as a sodium salt to healthy individuals) revealed that R-(+)-lipoic acid displayed high plasma maximum concentration and area under the concentration versus time curve values; the study reported negligible unbound R-(+)-lipoic acid at the highest achievable plasma concentrations.(22)
The intracellular redox status is usually determined by the GSH/GSSG, thioredoxinreduced/thioredoxinoxidized, and cysteine/cystine couples(8) and their ability to reversibly modulate cysteine- and methionine moieties in proteins. The participation of R-lipoic acid in thiol/disulfide exchange is the basis for its redox modulation of cell signaling and transcription: NFκB-, MAPK-, and PI3K/Akt signaling as well as transcription factors.(23–29)
Lipoic acid and redox control of glucose uptake and metabolism
Extensive evidence suggests that lipoic acid has potential therapeutic value in lowering glucose levels in diabetic conditions and that the intracellular redox status plays a role in the modulation of insulin action (insulin resistance). Mechanistic studies on the effects of lipoic acid on the redox status of insulin-responsive cells revealed that lipoic acid stimulated glucose uptake by affecting components of the insulin signaling pathway. The signaling networks of insulin receptors entail binding of insulin to the receptor followed by autophosphorylation of the intracellular tyrosine kinase domain of the β-subunits and activation of signaling pathways that may be considered in three sequential nodes(30) encompassing the insulin receptor substrate (IRS1/2/3/4), PI3K, and Akt (also known as PKB). PI3K/Akt activity was shown to be necessary for the translocation of glucose transporter-4 (GLUT4) from an intracellular pool to the plasma membrane.(31–33) In a comprehensive series of studies it was found that lipoic acid augmented tyrosine phosphorylation and the activity of components of insulin signaling: insulin receptor, insulin receptor substrate-1, PI3K (type I), Akt1, and p38(34,35) (Fig. 4). The authors concluded that lipoic acid stimulated glucose uptake upon translocation and regulation of the intrinsic activity of GLUT4, an effect that might be mediated by p38 MAPK.(34) (Akt phosphorylates 160 kDa AS160, facilitating its dissociation from the GLUT4 storage vesicle and preventing inactivation of Rab-GTP). R-α-lipoic acid and oxidized isoforms are effective in stimulating glucose transport in differentiated 3T3-L1 adipocytes by a mechanism entailing changes in the intracellular redox status (but not changes in the GSH levels); lipoic acid also facilitated the autophosphorylation of the insulin receptor by a mechanism that may involve oxidation of the cysteine residues in the α- and β-subunits.(19) These effects of lipoic acid are in agreement with an alteration of the thiol reactivity of redox components of the insulin pathway caused by a thiol/disulfide exchange mechanism (Fig. 3). The inhibition of protein tyrosine phosphatase 1B activity by lipoic acid was also associated with a decrease in thiol reactivity of the enzyme.(20) Lipoic acid inhibits differentiation of 3T3-L1 pre-adipocytes by activation of JNK and ERK pathways and, in turn, transcription factors,(24) a different mechanism by which lipoic acid increases glucose uptake (i.e., activation of the insulin receptor/Akt pathway).
Fig. 3.
Thiol/disulfide exchange as the basis for the activation/inhibition of cell signaling and transcription.
The stress-activated MAPK, JNK, plays a central role in the progression of insulin resistance and diabetic neuropathies;(36,37) a likely mechanism entails the phosphorylation of the insulin receptor substrate-1 serine 307 and, as a consequence, inhibition of the insulin-promoted tyrosine phosphorylation of IRS-1.(38) Lipoic acid was shown to inhibit the JNK pathway and IRS-1 serine phosphorylation, thereby improving insulin sensitivity. Although the exact mechanism by which lipoic acid inhibits the JNK pathway remains unclear, these effects place lipoic acid at the cross-road of insulin- and JNK signaling favoring glucose uptake and metabolism, thus ameliorating insulin resistance. A plausible mechanism suggests that lipoic acid-mediated induction of heat shock proteins and the subsequent inhibition of JNK and IKKβ.(39) In L6 muscle cells, lipoic acid prevented the activation of JNK triggered by either anisomycin or TNF-α.(39)
In hepatocytes, active Akt (Akt phosphorylated at Ser473), decreases as a function of age, whereas basal Akt phosphorylated at Thr308 remained unchanged; lipoic acid partially recovered Akt activation(40) and, as observed also in 3T3-L1 adipocytes, lipoic acid inhibited the phosphatase activities of PTEN and PP2A.
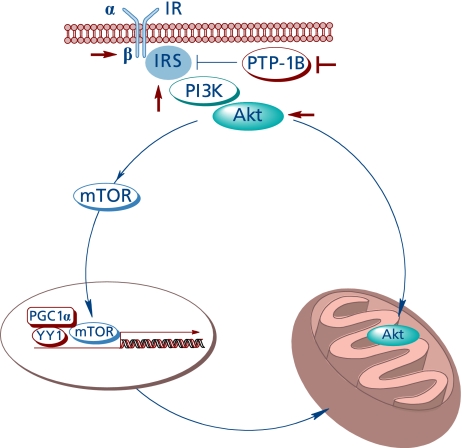
Full activation of Akt is a complex process entailing different pathways;(41) Akt activation affects mitochondrial bioenergetics by at least two pathways (Fig. 4).
Fig. 4.
The insulin-like effect of lipoic acid and Akt-dependent stimulation of mitochondrial function.
First, it was shown that Akt translocates to the mitochondrion of several cell types upon stimulation with insulin, insulin-like growth factor-1, or heat stress, where the phosphorylation targets identified were the β-subunit of ATPase and GSK3β; phosphorylation of the latter at a serine residue leads to its inactivation;(42) in unstimulated cells, heat shock protein-90 is responsible for Akt accumulation in the mitochondrion.(43) Mitochondrion-targeted Akt also protected neuroblastoma cells from apoptosis.(44) Whether or not the insulin-like effects of lipoic acid facilitate the translocation of Akt to mitochondria remains to be investigated.
Second, Akt is a positive regulator of the mammalian target of rapamycin (mTOR)(45) by mechanisms entailing the Akt-mediated phosphorylation and inhibition of TSC1 or the Akt-mediated inhibition of AMPK.(46) mTOR regulates the transcription of several genes and regulates mitochondrial activity, i.e., controls mitochondrial gene expression by modulation of YY1-PGC-1α.(47)
The Cell’s Energy Status
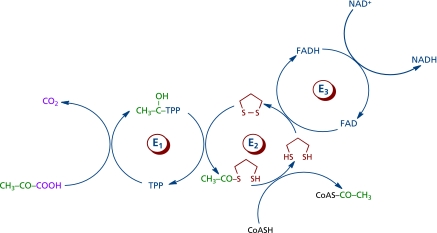
Lipoic acid is an essential cofactor for the E2 component of α-ketoacid dehydrogenase complexes, exclusively located in mitochondria, e.g., the pyruvate dehydrogenase (PDH)-, α-ketoglutarate dehydrogenase (KGDH)-, and branched chain α-ketoacid dehydrogenase (BCKDH) complexes. The former catalyzes the oxidative carboxylation of pyruvate and plays a fundamental role in carbohydrate metabolism and bioenergetics (Fig. 5), for PDH bridges anaerobic and aerobic energy metabolism, and it is the entry point of carbohydrates into the tricarboxylic acid cycle as acetyl-CoA. The latter is a regulatory control point in the tricarboxylic acid cycle; the activities of both PDH and KGDH is substantially decreased during aging and in neurodegenerative disorders.(48–50) Lipoic acid is reduced to dihydrolipoic acid by dihydrolipoamide dehydrogenase, the E3 component of PDH and KGDH. PDH activity is regulated by products, nucleotides, and reversible phosphorylation; lipoic acid supplementation increases PDH activity in hepatocyte mitochondria and it inhibits the pyruvate dehydrogenase kinase (PDK),(51,52) hence leading to a lower phosphorylation (and inactivation) of PDH. The mechanism of PDK inactivation by lipoic acid is not known yet.(51) 4-Hydroxynonenal (HNE) inhibited rather specifically KGDH, which accounted for the inhibitory effects of HNE on mitochondrial respiration;(53,54) the inactivation of KGDH (and PDH) was ascribed to the electrophilic attack of HNE on the reduced lipoyl moiety covalently bound to E2 component of the complex). Lipoic acid at the E2 of KGDH is glutathionylated upon treatment of mitochondria with H2O2; glutathionylation of the lipoiyl moiety is reversible and appears to serve as a transient protection against electrophilic attack by HNE.(55)
Fig. 5.
Pyruvate dehydrogenase-catalyzed oxidative decarboxylation of pyruvate. The lipoyl moiety is shown in red. E1, a-ketoacid decarboxylase; E2, dihydrolipoyl transcetilase; E3, dihydrolipoyl dehydrogenase.
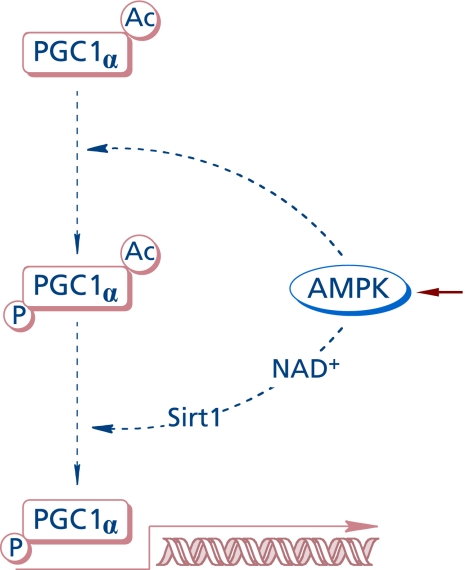
AMP-activated protein kinase (AMPK) is a sensitive cellular energy sensor(56) that supports ATP-generating catabolic pathways and decreases ATP-consuming anabolic processes by post-translational modifications and modulation of gene transcription (Fig. 6). AMPK consists of a catalytic (α) and two regulatory (β and γ) subunits, the γ subunit being the center of allosteric regulation (stimulated by AMP). Enzyme activation requires phosphorylation of a threonine residue by LKB1 or elevation of intracellular Ca++ via CaMKK. The effects of lipoic acid on AMPK differ depending on whether its action is on peripheral tissues or the hypothalamus(4) (AMPK in hypothalamic neurons integrates signals related to body’s energy metabolism). The different roles of AMPK in neurons have been critically reviewed:(57) depending on the experimental model, AMPK may function in a neuroprotective role or be harmful for neuronal survival or act as an autophagy mediator. AMPK is involved in transcriptional pathways that control mitochondrial function through PGC-1α.(58) The phosphorylation of PGC-1α protein(59) by AMPK at Thr177 and Ser538 appears to be a requirement for the induction of the PGC-1α promoter. Also, activation of AMPK was shown to enhance NAD+ levels in muscle cells and induce Sirt1-mediated PGC-1α deacetylation; apparently, PGC1α phosphorylation by AMPK facilitates the subsequent deacetylation by Sirt1.(60) The energy status of the cell is also related to activity of sirtuins,(61) which—among others—can deacetylase PGC-1α, by means of which Sirt1 controls mitochondrial biogenesis and function.
Fig. 6.
AMPK-dependent transcriptional pathway for PGC-1a activation.
PGC-1α is a transcriptional regulator of mitochondrial function and biogenesis and, as such, a critical regulator of energy homeostasis and integrates several transcriptional pathways driven by mTOR (Fig. 4), AMPK, and Sirt1 (Fig. 6).(62) Lipoic acid was reported to increase energy metabolism and mitochondrial biogenesis in the skeletal muscle of aged mice by increasing the phosphorylation of AMPK at Thr172 and expression of PGC-1α.(63) In this report, lipoic acid also increased the expression of GLUT4 (as observed in other cell types), but it decreased the phosphorylation of mTOR at Ser2448.(63) As in the case of Akt-driven signaling, AMPK phosphorylates AS160, thereby facilitating its dissociation from the glucose transporter vesicle and preventing the inactivation of Rab-GTP. It remains to be investigated whether or not AMPK is a preferential pathway of lipoic acid for the transcriptional activation of PGC-1α (opposite to mTOR) in tissues other than skeletal muscle.
It is well established that aging is associated with a loss of mitochondrial function and insulin resistance. In brain, there is an increased activation of JNK (bisphosphorylation) with age as well as its translocation to mitochondria, thereby blunting the activity of pyruvate dehydrogenase.(48,49) In muscle, AMPK activity—of significance in the regulation of energy metabolism and maintenance of energy homeostasis through PGC1α—is also reduced as a function of age;(64) whether its activity also decreases with age in neurons remains to be determined.
Lipoic Acid and the Neuronal Redox-Energy Axis
Pharmacokinetic studies on the distribution of orally administered R-, and S-lipoic acid from the systemic circulation into rat brain tissues (with consideration of the transport efficiency across the brain blood barrier) showed blood endogenous levels of 0.05–0.27 µM and brain levels of 0–0.024 µM after either a single or chronic oral dosing at 50 mg/kg;(65) the authors concluded that lipoic acid does not readily cross the blood-brain barrier, thereby questioning a direct effect of lipoic acid in the central nervous system.(65) Despite this, there is a myriad of reports on the effects of lipoic acid on improving mitochondrial function in aging and neurodegenerative disorders. However, few studies have investigated the mechanistic implications of lipoic acid in terms of PI3K/Akt- and MAPK-driven signaling and transcription (as in peripheral tissues referred to above).
It is widely accepted that loss of mitochondrial function may be an underlying event in brain aging and neurodegenerative disorders, such as Alzheimer’s, Parkinson’s, and Huntington’s diseases. Loss of mitochondrial function entails a reduction of the energy-transducing systems partly due to oxidative/nitrative damage. From this perspective, exogenously administered lipoic acid has been considered a mitochondrial nutrient. Mitochondrial dysfunction inherent in the pathogenesis of neurodegenerative diseases is aggravated by downregulation of cytosolic glutaredoxin-1 (which helps maintain mitochondrial integrity in terms of VDAC redox status) and is recovered by lipoic acid.(66)
The properties of lipoic acid that help improve age-associated loss of cognitive function are elevation of cofactors of defective mitochondrial enzymes, such as PDH and KGDH, protection of enzymes against oxidative stress, and enhancement of antioxidant defense systems through the activation of phase II enzymes and an increase in mitochondrial biogenesis.(67) The amount and activity of PDH and KGDH are decreased in Alzheimer’s disease,(68) a finding that might be partly explained by the higher susceptibility of mitochondria in Alzheimer’s disease to autophagy.(69) PDH activity is decreased in post-mortem tissues from Alzheimer’s dementia and vascular dementia, but R-lipoic acid (not S-lipoic acid) appears to stimulate PDH activity only in vascular dementia.(70)
In aged rats, spatial and temporary memory loss was associated with loss of brain mitochondrial function as well as RNA/DNA oxidation in hippocampus; these effects were partially reversed upon feeding animals with a combination of lipoic acid and acetyl-L-carnitine.(67) Age-related changes in synaptic function (in terms of impairment of long-term potentiation (LTP) and glutamate release) were reversed by dietary supplementation with lipoic acid (entailing restoration of IL-1β and tocopherol levels to values of young rats).(71)
Chronic administration of lipoic acid partially restored the age-associated loss of mitochondrial function to the level of young rats (in terms of activity of complex I, IV, and V) and improved oxidative stress markers.(72) A combination of lipoic acid and acetyl-L-carnitine was suggested to delay the loss of mitochondrial function associated with aging, restored mitochondrial ultrastructural changes, and increase mitochondrial biogenesis in the hippocampus.(73) Chronic administration of lipoic acid decreased biomarkers of oxidative stress in young and old control mice and a transgenic mouse model overexpressing the amyloid-β protein precursor without having an impact on end-point amyloid-β load; however, this reduction in oxidative stress was not correlated with an improvement on cognitive behavior (Y-maze performance).(74) Conversely, a combination of acetyl-L-carnitine and lipoic acid partially improved spatial and temporal memory in an ApoE4 transgenic mouse model.(75) Dietary supplementation with a combination of several micronutrients—among them lipoic acid—improved cognitive performance in ApoE-deficient mice.(76) Lipoic acid also improved survival in two transgenic mouse models of Huntington’s disease.(77) Interestingly, dichloroacetate—an inhibitor of pyruvate dehydrogenase kinase, which phosphorylates and inactivates PDH—also increased survival in these two transgenic models of Huntington’s disease showing improved motor function and decreased striatal neuron atrophy.(78) Hence, the protective effect of lipoic acid on these models of Huntington’s disease could be partly due to its inhibition of pyruvate dehydrogenase kinase (as reported in(52)). A recent study concluded that short-term supplementation with lipoic acid and acetyl-L-carnitine is insufficient to improve cognition in aged dogs, and that the beneficial effects of the full spectrum diet arose from either the cellular antioxidants alone or their interaction with lipoic acid and acetyl-L-carnitine.(79)
Lipoic acid protected cortical neurons against amyloid-β or H2O2-induced cytotoxicity and also induced the expression of Akt (and the downstream Akt signaling pathway).(26) Pretreatment of cortical neurons with lipoic acid (and in combination with acetyl-L-carnitine) results in the activation of the PI3K and ERK1/2 pathways and the inherent neuronal survival;(80) lipoic acid also protected against 4-hydroxy-2-nonenal (HNE)-mediated oxidative modifications in cortical neurons.(80)
Concluding Remarks
R-α-Lipoic acid, a cofactor for four enzyme complexes exclusively located in mitochondria, is essential for energy production and the regulation of carbohydrate and protein metabolism. Lipoic acid is synthesized in vivo and it is almost entirely covalently bound to the E2 component of three α-ketodehydrogenase complexes and the glycine cleavage system. Hence, it would be expected that only trace amounts are available from dietary sources. However, when lipoic acid is supplemented in the diet, it is readily absorbed and present in all cell compartments and extracellular fluids where it acts as a redox modulator and antioxidant par excellence.
Although known for more than 60 years to have potent effects in biological systems, lipoic acid studies have been hampered by the inability to detect accurately its presence in tissue samples. A study of the plasma pharmacokinetics of R-(+)-lipoic acid revealed that maximum concentrations were reached within ~30 min of administration and had a short half life when administered as sodium R-(+)-lipoate to healthy human subjects. Therefore, due to its very rapid metabolism, precise sampling times are required to establish an association of lipoic acid concentration with function in cells and tissues. Nevertheless, the redox modulating action of lipoic acid on signaling and transcription exhibits remarkable promise. Future studies are warranted to elucidate the therapeutic effects of exogenous lipoic acid during aging and age-related diseases, with emphasis on Alzheimer’s disease, of special interest to the co-authors of this review.
Acknowledgments
Supported by grants from NIA (AG016718) and the California Tobacco-Related Disease Research Program (17RT-0171).
Abbreviations
- AMPK
AMP-activated protein kinase
- ASC
alanine, serine, cysteine transporter
- CaMKK
calcium/calmodulin-dependent protein
- AS160
Akt substrate of 160 kDa
- HNE
4-hydroxy-2-nonenal
- IRS
insulin receptor substrate
- KGDH
α-ketoglutarate dehydrogenase
- LKB1
liver kinase B1
- mTOR
mammalian target of rapamycin
- PDH
pyruvate dehydrogenase
- PDK
pyruvate dehydrogenase kinase
- PGC-1α
Peroxisome proliferator-activated receptor γ (PPARγ) coactivator-1α
- PI3K
phosphotidylinositide 3-kinase
- PP2A
protein phosphatase 2A
- PTEN
phosphatase and tensin homologue
References
- 1.Reed LJ, De BB, Gunsalus IC, Hornberger CS., Jr Crystalline alpha-lipoic acid; a catalytic agent associated with pyruvate dehydrogenase. Science. 1951;114:93–94. doi: 10.1126/science.114.2952.93. [DOI] [PubMed] [Google Scholar]
- 2.Patel MS, Packer L, editors. Lipoid acid. energy production, antioxidant activity, and health effects. Boca Raton: CRC Press, Taylor & Francis Group; 2008. [Google Scholar]
- 3.Shay KP, Moreau RF, Smith EJ, Smith AR, Hagen TM. Alpha-lipoic acid as a dietary supplement: molecular mechanisms and therapeutic potential. Biochim Biophys Acta. 2009;1790:1149–1160. doi: 10.1016/j.bbagen.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koh EH, Cho EH, Kim MS, Park JY, Lee KU. Effects of alpha-lipoic acid on AMP-activated protein kinase in different tissues: therapeutic implications for the metabolic syndrome. In: Patel MS, Packer L, editors. Lipoic Acid: Energy Production, Antioxidant Activity, and Health Effects. Boca Raton, Florida: CRC Press/Taylor & Francis Group; 2008. pp. 495–519. [Google Scholar]
- 5.Ziegler D. Painful diabetic neuropathy: advantage of novel drugs over old drugs? Diabetes Care. 2009;32(Suppl 2):S414–S419. doi: 10.2337/dc09-S350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maczurek AE, Krautwald M, Steele ML, et al. Lipoic acid as a novel treatment for mild cognitive impairment and early-stage Alzheimer’s disease. In: Packer L, Sies H, Eggersdorfer M, Cadenas E, editors. Micronutrients and Brain Health. Boca Raton, Florida: CRC Press/Taylor & Francis Group; 2009. pp. 133–147. [Google Scholar]
- 7.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 8.Kemp M, Go YM, Jones DP. Nonequilibrium thermodynamics of thiol/disulfide redox systems: A perspective on redox systems biology. Free Radic Biol Med. 2008;44:921–937. doi: 10.1016/j.freeradbiomed.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones DP. Redefining oxidative stress. Antioxid Redox Signal. 2006;8:1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- 10.Packer L, Witt EH, Tritschler HJ. Alpha-Lipoic acid as a biological antioxidant. Free Radic Biol Med. 1995;19:227–250. doi: 10.1016/0891-5849(95)00017-r. [DOI] [PubMed] [Google Scholar]
- 11.Go YM, Jones DP. Redox clamp model for study of extracellular thiols and disulfides in redox signaling. Methods Enzymol. 2010;474:165–179. doi: 10.1016/S0076-6879(10)74010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han D, Handelman G, Marcocci L, et al. Lipoic acid increases de novo synthesis of cellular glutathione by improving cystine utilization. Biofactors. 1997;6:321–338. doi: 10.1002/biof.5520060303. [DOI] [PubMed] [Google Scholar]
- 13.Han D, Tritschler HJ, Packer L. Alpha-lipoic acid increases intracellular glutathione in a human T-lymphocyte Jurkat cell line. Biochem Biophys Res Commun. 1995;207:258–264. doi: 10.1006/bbrc.1995.1181. [DOI] [PubMed] [Google Scholar]
- 14.May JM, Qu ZC, Nelson DJ. Uptake and reduction of alpha-lipoic acid by human erythrocytes. Clin Biochem. 2007;40:1135–1142. doi: 10.1016/j.clinbiochem.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Machlin LJ, Bendich A. Free radical tissue damage: protective role of antioxidant nutrients. FASEB J. 1987;1:441–445. [PubMed] [Google Scholar]
- 16.Balamurugan K, Vaziri ND, Said HM. Biotin uptake by human proximal tubular epithelial cells: cellular and molecular aspects. Am J Physiol Renal Physiol. 2005;288:F823–F831. doi: 10.1152/ajprenal.00375.2004. [DOI] [PubMed] [Google Scholar]
- 17.Takaishi N, Yoshida K, Satsu H, Shimizu M. Transepithelial transport of alpha-lipoic acid across human intestinal Caco-2 cell monolayers. J Agric Food Chem. 2007;55:5253–5259. doi: 10.1021/jf063624i. [DOI] [PubMed] [Google Scholar]
- 18.Han D, Handelman GJ, Packer L. Analysis of reduced and oxidized lipoic acid in biological samples by high-performance liquid chromatography. Meth Enzymol. 1995;251:315–325. doi: 10.1016/0076-6879(95)51134-2. [DOI] [PubMed] [Google Scholar]
- 19.Moini H, Tirosh O, Park YC, Cho KJ, Packer L. R-alpha-lipoic acid action on cell redox status, the insulin receptor, and glucose uptake in 3T3-L1 adipocytes. Arch Biochem Biophys. 2002;397:384–391. doi: 10.1006/abbi.2001.2680. [DOI] [PubMed] [Google Scholar]
- 20.Cho KJ, Moini H, Shon HK, Chung AS, Packer L. Alpha-lipoic acid decreases thiol reactivity of the insulin receptor and protein tyrosine phosphatase 1B in 3T3-L1 adipocytes. Biochem Pharmacol. 2003;66:849–858. doi: 10.1016/s0006-2952(03)00395-2. [DOI] [PubMed] [Google Scholar]
- 21.Lodge JK, Packer L. Natural sources of lipoic acid in plant and animal tissues. In: Packer L, Hiramatsu M, Yoshikawa K, editors. Antioxidant Food Supplements in Human Health. New York: Academic Press; 1999. pp. 121–134. [Google Scholar]
- 22.Carlson DA, Smith AR, Fischer SJ, Young KL, Packer L. The plasma pharmacokinetics of R-(+)-lipoic acid administered as sodium R-(+)-lipoate to healthy human subjects. Altern Med Rev. 2007;12:343–351. [PubMed] [Google Scholar]
- 23.Suzuki YJ, Aggarwal BB, Packer L. Alpha-lipoic acid is a potent inhibitor of NF-kappa B activation in human T cells. Biochem Biophys Res Commun. 1992;189:1709–1715. doi: 10.1016/0006-291x(92)90275-p. [DOI] [PubMed] [Google Scholar]
- 24.Cho KJ, Moon HE, Moini H, Packer L, Yoon DY, Chung AS. Alpha-lipoic acid inhibits adipocyte differentiation by regulating pro-adipogenic transcription factors via mitogen-activated protein kinase pathways. J Biol Chem. 2003;278:34823–34833. doi: 10.1074/jbc.M210747200. [DOI] [PubMed] [Google Scholar]
- 25.Müller C, Dünschede F, Koch E, Vollmar AM, Kiemer AK. Alpha-lipoic acid preconditioning reduces ischemia-reperfusion injury of the rat liver via the PI3-kinase/Akt pathway. Am J Physiol Gastrointest Liver Physiol. 2003;285:G769–G778. doi: 10.1152/ajpgi.00009.2003. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L, Xing GQ, Barker JL, et al. Alpha-lipoic acid protects rat cortical neurons against cell death induced by amyloid and hydrogen peroxide through the Akt signalling pathway. Neuroscience Lett. 2001;312:125–128. doi: 10.1016/s0304-3940(01)02205-4. [DOI] [PubMed] [Google Scholar]
- 27.Zhang WJ, Wei H, Hagen T, Frei B. Alpha-lipoic acid attenuates LPS-induced inflammatory responses by activating the phosphoinositide 3-kinase/Akt signaling pathway. Proc Natl Acad Sci USA. 2007;104:4077–4082. doi: 10.1073/pnas.0700305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang WJ, Bird KE, McMillen TS, LeBoeuf RC, Hagen TM, Frei B. Dietary alpha-lipoic acid supplementation inhibits atherosclerotic lesion development in apolipoprotein E-deficient and apolipoprotein E/low-density lipoprotein receptor-deficient mice. Circulation. 2008;117:421–428. doi: 10.1161/CIRCULATIONAHA.107.725275. [DOI] [PubMed] [Google Scholar]
- 29.Zhang DD. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev. 2006;38:769–789. doi: 10.1080/03602530600971974. [DOI] [PubMed] [Google Scholar]
- 30.Khan CR, Suzuki R. Insulin action in the brain and the pathogenesis of Alzheimer’s disease. In: Craft S, Christen Y, editors. Diabetes, Insulin, and Alzheimer’s Disease. Heidelberg: Springer; 2010. pp. 1–20. [Google Scholar]
- 31.Luo RZ, Beniac DR, Fernandes A, Yip CC, Ottensmeyer FP. Quaternary structure of the insulin-insulin receptor complex. Science. 1999;285:1077–1080. doi: 10.1126/science.285.5430.1077. [DOI] [PubMed] [Google Scholar]
- 32.Ueki K, Yamamoto-Honda R, Kaburagi Y, et al. Potential role of protein kinase B in insulin-induced glucose transport, glycogen synthesis, and protein synthesis. J Biol Chem. 1998;273:5315–5322. doi: 10.1074/jbc.273.9.5315. [DOI] [PubMed] [Google Scholar]
- 33.Czech MP, Corvera S. Signaling mechanisms that regulate glucose transport. J Biol Chem. 1999;274:1865–1868. doi: 10.1074/jbc.274.4.1865. [DOI] [PubMed] [Google Scholar]
- 34.Konrad D, Somwar R, Sweeney G, et al. The antihyperglycemic drug alpha-lipoic acid stimulates glucose uptake via both GLUT4 translocation and GLUT4 activation: potential role of p38 mitogen-activated protein kinase in GLUT4 activation. Diabetes. 2001;50:1464–1471. doi: 10.2337/diabetes.50.6.1464. [DOI] [PubMed] [Google Scholar]
- 35.Yaworsky K, Somwar R, Ramlal T, Tritschler HJ, Klip A. Engagement of the insulin-sensitive pathway in the stimulation of glucose transport by alpha-lipoic acid in 3T3-L1 adipocytes. Diabetologia. 2000;43:294–303. doi: 10.1007/s001250050047. [DOI] [PubMed] [Google Scholar]
- 36.Hirosumi J, Tuncman G, Chang L, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 37.Qiao LY, Goldberg JL, Russell JC, Sun XJ. Identification of enhanced serine kinase activity in insulin resistance. J Biol Chem. 1999;274:10625–10632. doi: 10.1074/jbc.274.15.10625. [DOI] [PubMed] [Google Scholar]
- 38.Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307) J Biol Chem. 2000;275:9047–9054. doi: 10.1074/jbc.275.12.9047. [DOI] [PubMed] [Google Scholar]
- 39.Gupte AA, Bomhoff GL, Morris JK, Gorres BK, Geiger PC. Lipoic acid increases heat shock protein expression and inhibits stress kinase activation to improve insulin signaling in skeletal muscle from high-fat-fed rats. J Appl Physiol. 2009;106:1425–1434. doi: 10.1152/japplphysiol.91210.2008. [DOI] [PubMed] [Google Scholar]
- 40.Shay KP, Hagen TM. Age-associated impairment of Akt phosphorylation in primary rat hepatocytes is remediated by alpha-lipoic acid through PI3 kinase, PTEN, and PP2A. Biogerontology. 2009;10:443–456. doi: 10.1007/s10522-008-9187-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scheid MP, Woodgett JR. PKB/AKT: functional insights from genetic models. Nat Rev Mol Cell Biol. 2001;2:760–768. doi: 10.1038/35096067. [DOI] [PubMed] [Google Scholar]
- 42.Bijur GN, Jope RS. Rapid accumulation of Akt in mitochondria following phosphatidylinositol 3-kinase activation. J Neurochem. 2003;87:1427–1435. doi: 10.1046/j.1471-4159.2003.02113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barksdale KA, Bijur GN. The basal flux of Akt in the mitochondria is mediated by heat shock protein 90. J Neurochem. 2009;108:1289–1299. doi: 10.1111/j.1471-4159.2009.05878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mookherjee P, Quintanilla R, Roh MS, Zmijewska AA, Jope RS, Johnson GV. Mitochondrial-targeted active Akt protects SH-SY5Y neuroblastoma cells from staurosporine-induced apoptotic cell death. J Cell Biochem. 2007;102:196–210. doi: 10.1002/jcb.21287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 46.Hahn-Windgassen A, Nogueira V, Chen CC, Skeen JE, Sonenberg N, Hay N. Akt activates the mammalian target of rapamycin by regulating cellular ATP level and AMPK activity. J Biol Chem. 2005;280:32081–32089. doi: 10.1074/jbc.M502876200. [DOI] [PubMed] [Google Scholar]
- 47.Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, Puigserver P. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature. 2007;450:736–740. doi: 10.1038/nature06322. [DOI] [PubMed] [Google Scholar]
- 48.Zhou Q, Lam PY, Han D, Cadenas E. c-Jun N-terminal kinase regulates mitochondrial bioenergetics by modulating pyruvate dehydrogenase activity in primary cortical neurons. J Neurochem. 104:325–335. doi: 10.1111/j.1471-4159.2007.04957.x. [DOI] [PubMed] [Google Scholar]
- 49.Zhou Q, Lam PY, Han D, Cadenas E. Activation of c-Jun-N-terminal kinase and decline of mitochondrial pyruvate dehydrogenase activity during brain aging. FEBS Lett. 2009;583:1132–1140. doi: 10.1016/j.febslet.2009.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gibson GE, Blass JP, Beal MF, Bunik V. The alpha-ketoglutarate dehydrogenase complex: a mediator between mitochondria and oxidative stress in neurodegeneration. Mol Neurobiol. 2004;31:43–63. doi: 10.1385/MN:31:1-3:043. [DOI] [PubMed] [Google Scholar]
- 51.Korotchkina LG, Patel MS. Lipoic Acid: Energy Production, Antioxidant Atcivity, and Health Effects. Boca Raton: CRC Press; 2008. Pyruvate dehydrogenase complex regulation and lipoic acid; pp. 149–165. [Google Scholar]
- 52.Korotchkina LG, Sidhu S, Patel MS. R-lipoic acid inhibits mammalian pyruvate dehydrogenase kinase. Free Radic Res. 2004;38:1083–1092. doi: 10.1080/10715760400004168. [DOI] [PubMed] [Google Scholar]
- 53.Humphries K, Yoo Y, Szweda LI. Inhibition of NADH-linked mitochondrial respiration by 4-hydroxy-2-nonenal. Biochemistry. 1998;37:552–557. doi: 10.1021/bi971958i. [DOI] [PubMed] [Google Scholar]
- 54.Humphries KM, Szweda LI. Selective inactivation of alpha-ketoglutarate dehydrogenase and pyruvate dehydrogenase: reaction of lipoic acid with 4-hydroxy-2-nonenal. Biochemistry. 1998;37:15835–15841. doi: 10.1021/bi981512h. [DOI] [PubMed] [Google Scholar]
- 55.Applegate MA, Humphries KM, Szweda LI. Reversible inhibition of alpha-ketoglutarate dehydrogenase by hydrogen peroxide: glutathionylation and protection of lipoic acid. Biochemistry. 2008;47:473–478. doi: 10.1021/bi7017464. [DOI] [PubMed] [Google Scholar]
- 56.Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 57.Poels J, Spasić MR, Callaerts P, Norga KK. Expanding roles for AMP-activated protein kinase in neuronal survival and autophagy. Bioessays. 2009;31:944–952. doi: 10.1002/bies.200900003. [DOI] [PubMed] [Google Scholar]
- 58.Lee WJ, Kim M, Park HS, et al. AMPK activation increases fatty acid oxidation in skeletal muscle by activating PPARalpha and PGC-1. Biochem Biophys Res Commun. 2006;340:291–295. doi: 10.1016/j.bbrc.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 59.Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci USA. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cantó C, Gerhart-Hines Z, Feige JN, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haigis MC, Guarente LP. Mammalian sirtuins—emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20:2913–2921. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- 62.Jeninga EH, Schoonjans K, Auwerx J. Reversible acetylation of PGC-1: connecting energy sensors and effectors to guarantee metabolic flexibility. Oncogene. 2010;29:4617–4624. doi: 10.1038/onc.2010.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Y, Li X, Guo Y, Chan L, Guan X. Alpha-Lipoic acid increases energy expenditure by enhancing adenosine monophosphate-activated protein kinase-peroxisome proliferator-activated receptor-gamma coactivator-1alpha signaling in the skeletal muscle of aged mice. Metabolism. 2010;59:967–976. doi: 10.1016/j.metabol.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reznick RM, Zong H, Li J, et al. Aging-associated reductions in AMP-activated protein kinase activity and mitochondrial biogenesis. Cell Metab. 2007;5:151–156. doi: 10.1016/j.cmet.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chng HT, New LS, Neo AH, Goh CW, Browne ER, Chan EC. Distribution study of orally administered lipoic acid in rat brain tissues. Brain Res. 2009;1251:80–86. doi: 10.1016/j.brainres.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 66.Saeed U, Durgadoss L, Valli RK, Joshi DC, Joshi PG, Ravindranath V. Knockdown of cytosolic glutaredoxin 1 leads to loss of mitochondrial membrane potential: implication in neurodegenerative diseases. PLoS One. 2008;3:e2459. doi: 10.1371/journal.pone.0002459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu J. The effects and mechanisms of mitochondrial nutrient alpha-lipoic acid on improving age-associated mitochondrial and cognitive dysfunction: an overview. Neurochem Res. 2008;33:194–203. doi: 10.1007/s11064-007-9403-0. [DOI] [PubMed] [Google Scholar]
- 68.Hirai K, Aliev G, Nunomura A, et al. Mitochondrial abnormalities in Alzheimer’s disease. J Neurosci. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moreira PI, Siedlak SL, Wang X, et al. Autophagocytosis of mitochondria is prominent in Alzheimer disease. J Neuropathol Exp Neurol. 2007;66:525–532. doi: 10.1097/01.jnen.0000240476.73532.b0. [DOI] [PubMed] [Google Scholar]
- 70.Frölich L, Götz ME, Weinmüller M, et al. (r)-, but not (s)-alpha lipoic acid stimulates deficient brain pyruvate dehydrogenase complex in vascular dementia, but not in Alzheimer dementia. J Neural Transm. 2004;111:295–310. doi: 10.1007/s00702-003-0043-5. [DOI] [PubMed] [Google Scholar]
- 71.McGahon BM, Martin DS, Horrobin DF, Lynch MA. Age-related changes in LTP and antioxidant defenses are reversed by an alpha-lipoic acid-enriched diet. Neurobiol Aging. 1999;20:655–664. doi: 10.1016/s0197-4580(99)00050-0. [DOI] [PubMed] [Google Scholar]
- 72.Long J, Gao F, Tong L, Cotman CW, Ames BN, Liu J. Mitochondrial decay in the brains of old rats: ameliorating effect of alpha-lipoic acid and acetyl-L-carnitine. Neurochem Res. 2009;34:755–763. doi: 10.1007/s11064-008-9850-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aliev G, Liu J, Shenk JC, et al. Neuronal mitochondrial amelioration by feeding acetyl-L-carnitine and lipoic acid to aged rats. J Cell Mol Med. 2009;13:320–333. doi: 10.1111/j.1582-4934.2008.00324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Siedlak SL, Casadesus G, Webber KM, et al. Chronic antioxidant therapy reduces oxidative stress in a mouse model of Alzheimer’s disease. Free Radic Res. 2009;43:156–164. doi: 10.1080/10715760802644694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shenk JC, Liu J, Fischbach K, et al. The effect of acetyl-L-carnitine and R-alpha-lipoic acid treatment in ApoE4 mouse as a model of human Alzheimer’s disease. J Neurol Sci. 2009;283:199–206. doi: 10.1016/j.jns.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Suchy J, Chan A, Shea TB. Dietary supplementation with a combination of alpha-lipoic acid, acetyl-L-carnitine, glycerophosphocoline, docosahexaenoic acid, and phosphatidylserine reduces oxidative damage to murine brain and improves cognitive performance. Nutr Res. 2009;29:70–74. doi: 10.1016/j.nutres.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 77.Andreassen OA, Ferrante RJ, Dedeoglu A, Beal MF. Lipoic acid improves survival in transgenic mouse models of Huntington’s disease. Neuroreport. 2001;12:3371–3373. doi: 10.1097/00001756-200110290-00044. [DOI] [PubMed] [Google Scholar]
- 78.Andreassen OA, Ferrante RJ, Huang HM, et al. Dichloroacetate exerts therapeutic effects in transgenic mouse models of Huntington’s disease. Ann Neurol. 2001;50:112–117. doi: 10.1002/ana.1085. [DOI] [PubMed] [Google Scholar]
- 79.Christie LA, Opii WO, Head E, Araujo JA, de Rivera C, Milgram NW, Cotman CW. Short-term supplementation with acetyl-L-carnitine and lipoic acid alters plasma protein carbonyl levels but does not improve cognition in aged beagles. Exp Gerontol. 2009;44:752–759. doi: 10.1016/j.exger.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Abdul HM, Butterfield DA. Involvement of PI3K/PKG/ERK1/2 signaling pathways in cortical neurons to trigger protection by cotreatment of acetyl-L-carnitine and alpha-lipoic acid against HNE-mediated oxidative stress and neurotoxicity: implications for Alzheimer’s disease. Free Radic Biol Med. 2007;42:371–384. doi: 10.1016/j.freeradbiomed.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]