Abstract
Oxidized and nitrated nucleotides including 8-oxogunanine and 8-nitroguanine derivatives such as 8-nitroguanosine 3',5'-cyclic monophosphate were generated by reactive nitrogen oxides and reactive oxygen species in cultured cells and in tissues. 8-oxoguanine and 8-nitroguanine in DNA and RNA are potentially mutagenic, and the former also induces cell death. Some derivative, 8-nitroguanosine 3',5'-cyclic monophosphate a major nitrated guanine nucleotide, was identified as a novel second messenger. Surprisingly, the amount of 8-nitroguanosine 3',5'-cyclic monophosphate generated was found to be higher than that of guanosine 3',5'-cyclic monophosphate in cells expressing inducible nitric oxide synthase. More important, 8-nitroguanosine 3',5'-cyclic monophosphate is electrophilic and reacted efficiently with sulfhydryls of proteins to produce a novel posttranslational modification (named S-guanylation) via guanosine 3',5'-cyclic monophosphate adduction. For example, 8-nitroguanosine 3',5'-cyclic monophosphate-induced S-guanylation of Kelch-like ECH-associated protein 1 led to NF-E2-related factor activation and induction of antioxidant enzymes. 8-nitroguanosine 3',5'-cyclic monophosphate may thus protect cells against oxidative stress-related cytotoxicity. Therefore, although chemically modified nucleotides produced via oxidative and nitrative stress are regarded simply as endogenous mutagens, the endogenous nucleotides stored in cells per se may serve functionally as a sensing mechanism for reactive nitrogen oxides and oxygen species to induce cellular adaptive responses to oxidative stress.
Keywords: oxidative stress, adaptive response, ROS signaling, nucleotide sensing, electrophilic signaling
Introduction
Nucleotides stored in the cells not only serve as substrates for nucleic acid biosynthesis but also participate in the energy metabolism and signal transduction. Nitric oxide (NO) is a gaseous free radical that is synthesized by nitric oxide synthases (NOSs).(1) NO plays important roles in the regulation of diverse physiological phenomena such as vascular and neuronal signal transduction, host defense, and cell death regulation.(2–5) Signal transduction by NO primarily involves a nucleotide signal molecule, guanosine 3',5'-cyclic monophosphate (cGMP), generated by soluble guanylate cyclase (sGC) from guanosine triphosphate (GTP).(6) cGMP thus formed binds to allosteric regulatory domains of target proteins, including protein kinases, ion channels, and phosphodiesterases, with various downstream biological consequences that allow cells to adapt to changes and stresses occurring under different environmental conditions and metabolic demands.(6)
Excess production of NO has been suggested to be a cause of diverse pathophysiological conditions, such as inflammation, neurodegenerative and cardiovascular diseases, and cancer.(7–12) These detrimental effects of NO are attributed to reactive nitrogen oxide species (RNOS), including nitrogen dioxide (NO2) and peroxynitrite (ONOO−), which are formed by the reaction of NO with molecular oxygen(13) and reactive oxygen species (ROS) such as the superoxide anion radical O2•−.(14) Peroxynitrite is thought to act as a strong oxidizing and nitrating agent in different pathophysiological situations.(15–20) Other RNOS may also contribute to pathophysiological conditions. Such RNOS include nitryl chloride (NO2Cl), which is formed from nitrite (NO2−) and hypochlorous acid (HOCl),(21,22) and NO2, which is generated by oxidation of NO with molecular oxygen(23) or by catalysis of peroxidases such as myeloperoxidase (MPO) and eosinophil peroxidase using hydrogen peroxide (H2O2) and nitrite as substrates.(24,25) When production of RNOS exceeds the cellular antioxidant capacity, these molecules can cause nitrative and oxidative damage of nucleic acids, proteins, lipids, and carbohydrates by nitrosation, nitration, and oxidation reactions. RNOS are known to have a strong potential to oxidize and nitrate nucleic acids at the level of their base structures, e.g., guanine and adenine. During the past several years, oxidized and nitrated guanine derivatives, including 8-oxoguanine and 8-nitroguanine, were identified in diverse cultured cells, in tissues and organs from humans with cancer or degenerative diseases, and in different organisms with viral pneumonia, cancer, and other inflammatory conditions.(11,26–36) Not only the mutagenic potential but also the redox-active property of 8-nitroguanine derivatives suggested that guanine nitration may have significant biological effects yet to be identified.(26,34) In fact, we recently discovered a nitrated cyclic nucleotide, 8-nitroguanosine 3',5'-cyclic monophosphate (8-nitro-cGMP), that was produced in cells expressing inducible NOS (iNOS).(35) 8-nitro-cGMP is an extremely potent signaling molecule in biological systems because of its dual nature in signal transduction, i.e., in the canonical NO/cGMP pathway and in noncanonical electrophilic signaling.(35) Among the nitrated guanine derivatives studied, 8-nitro-cGMP possessed the strongest redox-active and electrophilic properties.(35,37) Because of its electrophilic behavior, 8-nitro-cGMP reacts effectively with highly nucleophilic sulfhydryl groups of certain cysteine (Cys) residues and formed a protein-S-cGMP adduct via a unique posttranslational modification named S-guanylation. In addition, some particular biological effects, e.g., cell death induction, rather than mutagenic potential are now well recognized to be caused by 8-oxoguanine accumulated in the nucleotide pool in the cells.(38) Here, we will review current knowledge about endogenous nucleotides chemically modified via oxidative/nitrative stress with respect to their formation and biological significance.
Biological Formation of Oxidized and Nitrated Nucleotides
There is now ample evidence from a number of data indicating relatively frequent formation of 8-oxoguanine in various cells and tissues under oxidative stress.(36) ROS derived from both endogenous origins such as mitochondria, leukocytes (oxidative burst), peroxisomes (degradation of fatty acids) and cytochrome P450 system (mixed function oxidative system), as well as exogenous origins such as cigarette smoking, UV radiation, and ionizing radiation can contribute to the formation of 8-oxoguanine.(39) Epidemiological studies showed the increased formation of 8-oxoguanine as a risk factor for cancer, atherosclerosis, diabetes(40) and neurodegenerative disorders.(41) There are two pathways for the accumulation of 8-oxoguanine in DNA or RNA: one is a result of the incorporation of oxidized (deoxy)guanosine triphosphate (8-oxo-dGTP) generated in nucleotide pools while the other is a result of the direct oxidation of guanine in DNA or RNA. Recent progress in studies of the sanitization of nucleotide pools, as well as DNA repair, has revealed that the impact of oxidation of free nucleotides such as dGTP is unexpectedly large, in comparison with the direct oxidation of DNA.(38)
Similarly, in vitro and in vivo experiments have shown possible nitration of nucleic acids, more specifically guanine derivatives, that have been associated with various inflammatory conditions.(11,12,26–35) Yermilov et al.(42) found that peroxynitrite reacted with the guanine base of nucleic acids in vitro to form 8-nitroguanine. Masuda et al.(43) demonstrated that peroxynitrite mediated 8-nitroguanosine formation from RNA in vitro. Our group was the first to report in vivo evidence of guanine nitration: we found marked guanine nitration in the lungs of influenza virus-infected mice and in the lungs of patients with idiopathic pulmonary fibrosis and lung cancer, with the nitration depending on production of NO by iNOS.(26,28,30) We also observed formation of 8-nitroguanosine in mice infected with bacteria such as Salmonella typhimurium.(44) In addition, Hoki et al.(45) detected 8-nitroguanine formation in malignant fibrous histiocytoma specimens from patients. It is also interesting that formation of 8-nitroguanine was recently suggested to be linked to diabetic retinopathy, which is a major cause of blindness.(46)
Our chemical analyses using high-performance liquid chromatography-based electrochemical detection and tandem mass spectrometry (LC-MS/MS) revealed that, from among a series of 8-nitroguanine derivatives and related compounds, only a nitrated derivative of cGMP, 8-nitro-cGMP, was generated in significant amounts in cell culture models with different types of cells.(35,47) For example, by using LC-MS/MS, we identified 8-nitro-cGMP in murine macrophage RAW 264.7 cells that had been stimulated with interferon-γ and lipopolysaccharide to produce NO via iNOS. As just mentioned, infection of murine macrophages with the gram-negative bacterium Salmonella also facilitated formation of 8-nitro-cGMP, which was reported to be involved in host defense against infection.(12,35,44) Formation of 8-nitro-cGMP and 8-nitroguanine derivatives can be easily detected by means of conventional immunocytochemistry with the use of anti-8-nitro-cGMP monoclonal antibodies. It was intriguing that intracellular 8-nitro-cGMP formation and 8-nitroguanine formation had similar immunostaining profiles for time and location.(26,30,44) This may suggest that a major nitrated guanine derivatives formed in the cells is likely to be 8-nitro-cGMP rather than other nitrated nucleotides and DNA/RNA.
We recently precisely quantified the NO-dependent formation of 8-nitro-cGMP in C6 glioma cells via LC-MS/MS.(47) Treatment of cultured rat C6 glial cells with the NO donor S-nitroso-N-acetylpenicillamine (SNAP) led to a rapid and transient increase in cGMP, but the level of 8-nitro-cGMP gradually increased linearly up to a peak value comparable to that of cGMP at 24 h and declined thereafter. Markedly high levels (reaching up to 100 µM) of 8-nitro-cGMP were also evident in C6 cells that had been stimulated to express iNOS and produce excessive NO. The amount of 8-nitro-cGMP generated was much higher than that of cGMP, whose production profile slightly preceded 8-nitro-cGMP formation in the activated iNOS-expressing cells. Because of these unexpectedly large amounts of 8-nitro-cGMP, we suspected that GTP (a substrate of cGMP biosynthesis), rather than cGMP per se, may undergo guanine nitration. This idea was indeed supported by the fact that 8-nitroguanosine 5'-triphosphate (8-nitro-GTP), produced in a cell-free chemical reaction of GTP with peroxynitrite, served as an effective substrate for sGC.(47)
Mutagenesis Caused by Guanine Derivatives Chemically Modified via Oxidative and Nitrative Stress
Among the pathological effects associated with oxidative and nitrative stress, the mutagenic potential of ROS and RNOS is of great interest. RNOS such as peroxynitrite that commonly generated during infection and inflammation nonselectively affect a host’s cells and tissues. Obviously, such host defense molecules are produced to kill invading pathogens, which then suffer oxidative stress because of the host’s antimicrobial attack. It may therefore be logical to expect that mutagenesis of various microbial pathogens occurs during infections in biological systems as a result of host defense.(48)
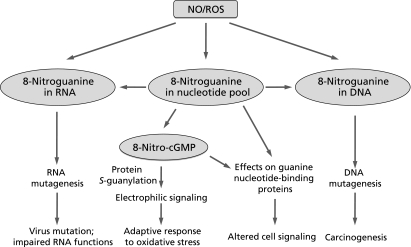
Evidence of this mutagenesis includes the finding that human leukocytes producing O2•−, but not leukocytes from patients with chronic granulomatous disease, were shown to be mutagenic for S. typhimurium TA100.(49) Our earlier study also confirmed that oxidative and nitrative stress induced by a high output of NO and ROS accelerated mutation of the RNA virus.(50) Related to this in vivo RNA virus mutation, our investigations also found that 8-nitroguanine formed by RNOS in the viral genome led to an increased frequency of mutations in an RNA virus (Fig. 1).(32) In addition, authentic 8-nitroguanosine added exogenously to an RNA virus-infected cells caused a dose-dependent increase in the frequency of viral mutations, especially C to U transitions.
Fig. 1.
Biological significance of nitration of guanine nucleotides. NO, nitric oxide; ROS, reactive oxygen species.
An earlier study by Wogan’s group documented that a high NO output induced mutations in an endogenous hypoxanthine-guanine phosphoribosyltransferase gene (hprt) in murine macrophages expressing iNOS.(51) Genetic analysis of the NO-induced mutated gene indicated that the NO-associated mutational spectrum was similar to that arising spontaneously, but small deletions and insertions were found in the NO-induced mutants. The same group showed that mutagenicity was enhanced by NO overproduction in vivo, as assessed by mutation of an exogenously expressed lacZ gene in lacZ-containing pUR288 plasmid-transgenic mice.(52) Excess production of NO by iNOS induced by inflammatory cytokines, possibly through RNOS (particularly peroxynitrite), caused DNA damage and impaired DNA repair in human cholangiocarcinoma cells, as assessed by the comet assay, which suggests an NO-dependent development and progression of cholangiocarcinoma.(53)
It is important to note that guanine nitration appears to cause DNA mutagenesis as well, with a mutation spectrum similar to that induced by peroxynitrite.(54) Thus, 8-nitroguanine in DNA may be rapidly depurinated from DNA in vitro, within 1–4 h under physiological conditions, the result being the formation of mutagenic abasic sites and release of free 8-nitroguanine.(27) Formation of 8-nitroguanine in DNA may therefore facilitate G to T transversion via abasic site formation.(42) In addition, Suzuki et al.(55) used photochemical synthesis to obtain an oligodeoxynucleotide containing a single 8-nitrodeoxyguanosine at a specific position(56) and utilized the oligodeoxynucleotide as a template in primer extension reactions catalyzed by mammalian DNA polymerases. This finding suggests that 8-nitrodeoxyguanosine in DNA can mispair with adenine, thereby directly inducing a G to T transversion in mammalian cells. Consistent with those in vitro findings, 8-nitroguanosine exhibited mutagenic activity in mammalian cells.(57) Treatment of Chinese hamster ovary AS52 cells with 8-nitroguanosine significantly increased the mutation frequency of the xanthine-guanine phosphoribosyltranserase gene, with G to T transversion at the gene locus. Concomitant increase of abasic sites in DNA further supports the notion that depurination-dependent mutagenesis may be operative for 8-nitroguanosine-induced mutation.
A similar mutation spectrum is observed for oxidative damaged nucleic acids caused by peroxynitrite and ROS. Specifically, 8-oxoguanine can pair with both cytosine and adenine during DNA synthesis, and this base mismatching could contribute significantly to spontaneous mutations in genomic DNA.(58) Direct oxidation in G:C pair can result in G to T transversion during replication, whereas 8-oxo-dGTP can be incorporated in DNA opposite to the adenine, leading to the A to C transversion.(59) Mammalian cells are equipped multiple enzyme systems to suppress 8-oxoguanine accumulation in DNA.(60) For example, 8-oxoguanine DNA glycosylase1 (OGG1) excises 8-oxoguanine from 8-oxo-G:C pairs in DNA, thus initiating base excision repair. MutT homolog-1 (MTH1) is an enzyme that hydrolyzes 8-oxo-dGTP to the monophosphate form, thus preventing incorporation of 8-oxo-dGTP into DNA during replication.
Immunohistochemical studies demonstrated that 8-oxoguanine and 8-nitroguanine are also present in the cytosol of various cells and tissues suffering from oxidative/nitrative stress,(26,30,35,61,62) which suggests that not only DNA but also the nucleotide pool, RNA, and other cytosolic compartments are modified chemically via oxidative/nitrative stress. For example, because certain enzymes such as MPO are unlikely to nitrate DNA directly, nitrated nucleic acids may be formed in the nucleotide pool or in RNA by reactive nitrogen species that are generated by such enzyme systems. As shown for certain oxidized and halogenated nucleosides,(63–65) 8-nitroguanine and related nucleosides and nucleotides may be misincorporated into DNA, which can result in mutations. Similarly, 8-nitroguanine and related nucleosides and nucleotides, like oxidized and halogenated nucleosides, may be incorporated into RNA and interfere with RNA function and metabolism.(66–68) The studies summarized here therefore clearly show that oxidative and nitrative DNA damage, as evidenced by increased formation of 8-oxoguanine and 8-nitroguanine, can be induced under various inflammatory conditions. In fact, chronic inflammation induced by various biological, chemical, and physical factors has also been associated with an increased risk of human cancer at many locations.(10,11,69) However, to establish a causal relationship between this type of DNA damage and human cancer, additional studies that utilize a molecular epidemiological approach in a large human population are required.
Redox and Electrophilic Signaling Property of Nitrated Guanine Nucleotides
8-nitro-cGMP has the potential to activate cGMP-dependent protein kinase (PKG) in vascular smooth muscle cells; in addition, 8-nitroguanosine and its derivatives possess a significant redox activity. 8-nitro-cGMP had the highest redox activity among the 8-nitroguanosine derivatives we tested, with redox activity decreasing in the following order: 8-nitro-cGMP > 8-nitroguanosine > 8-nitroguanosine 5'-monophosphate (8-nitro-GMP) ≈ 8-nitro-GTP. 8-nitroguanine had only negligible redox activity.(26,34,35) In the presence of certain oxidoreductases and electron donors such as NADPH, 8-nitroguanosine derivatives were readily reduced to form their anion radicals, after which a single electron was transferred to molecular oxygen to form superoxide anion radical.(26,34)
Electrophilicity is another unique redox property of nitro-nucleotides. Because of their electrophilicity, nitro-nucleotides readily react with nucleophilic thiol compounds of low and high molecular weight to form 8-thioalkoxy-guanosine (8-RS-cGMP) adducts (Fig. 2). We named this unique electrophilic reaction S-guanylation of sulfhydryls.(35,37,70) This reaction seems to occur via a nucleophilic attack by the thiol group of a protein Cys or GSH on C8 of 8-nitro-cGMP, the results being release of the nitro moiety and formation of the 8-RS-cGMP adduct (Fig. 2). The second-order rate constant for the reaction of 8-nitro-cGMP with the sulfhydryl of GSH was determined to be 0.03 M−1 s−1 at pH 7.4 and 37°C.(35) This value is much smaller than values for other electrophiles such as 4-hydroxynonenal, 15-deoxy-Δ(12,14)-prostaglandin J2, and nitrolinoleic and nitrooleic acids. Those compounds have reaction rate constants with GSH of 1.3, 0.7, 355, and 183 M−1 s−1, respectively, at pH 7.4 and 37°C.(37) This comparatively lower second-order rate constant may account for the stable nature of this novel compound in the cellular compartment where GSH is abundant (at ~ mM levels) and may be responsible for the fact that 8-nitro-cGMP causes very selective S-guanylation with sulfhydryls possessing high nucleophilicity, as determined, at least in part, by low pKa values of sulfhydryls of the Cys moiety.
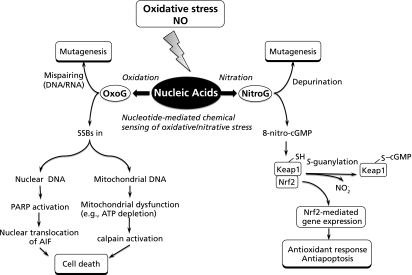
Fig. 2.
Schematic representation for cell stress responses activated via two major nucleotide modifications, such as guanine nitration and oxidation, with subsequent completely different pathways for downstream signaling, leading to cell death and antioxidant cytoprotective effects. NO, nitric oxide; OxoG, 8-oxoguanine; NitroG, 8-nitroguanine; SSBs, single strand breaks; PARP, poly-ADP-ribose polymerase; AIF, apoptosis-inducing factor; cGMP, guanosine 3',5'-cyclic monophosphate; Keap1, Kelch-like ECH-associated protein 1; Nrf2, NF-E2-related factor 2.
Because of its electrophilicity, 8-nitro-cGMP may mediate electrophilic signaling by means of induction of S-guanylation of redox sensor proteins. Among this class of proteins, Kelch-like ECH-associated protein 1 (Keap1) was identified as a highly sensitive S-guanylation target.(35) Keap1 is a negative regulator of NF-E2-related factor 2 (Nrf2), which is a transcription factor that regulates phase-2 detoxifying and antioxidant enzymes for electrophiles and ROS.(71,72) Our chemical analyses revealed that Keap1 expressed by various cultured cells was highly susceptible to S-guanylation induced by NO-dependent 8-nitro-cGMP.(35) In fact, we found that NO and RNOS could activate the Keap1-Nrf2 pathway in macrophages during bacterial infections and in rat C6 glial cells in culture after treatment with proinflammatory stimuli. That 8-nitro-cGMP may act as an endogenous electrophilic ligand and affect Keap1 sulfhydryls via S-guanylation, which would lead to antioxidant signaling, is therefore highly plausible. Cytoprotection and host defense conferred by 8-nitro-cGMP were clearly associated with increased expression of heme oxygenase 1 (HO-1) in cultured macrophages and in vivo during Salmonella infection.(11,35,44) HO-1 is an enzyme with various physiological roles including vasoregulation,(73) cytoprotection,(74) and anti-inflammatory effects.(75) We also reported earlier that HO-1 expression induced by NO contributed to cell survival in certain solid tumor models.(76,77) We recently found, in rat C6 glial cells, that Keap1 is the major target that is S-guanylated by NO exposure and that its S-guanylated structure derives primarily from 8-RS-cGMP adducts.(47) S-Guanylated Keap1 led to Nrf2 activation and subsequent induction of antioxidant enzymes including HO-1, so 8-nitro-cGMP protected cells against the cytotoxic effects of hydrogen peroxide. Proteomic analysis for endogenously modified Keap1 with matrix-assisted laser desorption/ionization time-of-flight-MS/MS revealed that 8-nitro-cGMP S-guanylated the Cys434 of Keap1 (Fig. 2). This finding is therefore the first compelling corroboration of the potential roles of 8-nitro-cGMP in the Nrf2-dependent antioxidant response.
Nucleotide Oxidation Caused by Oxidative Stress Triggering Cell Death Signaling
Recent studies have suggested that 8-oxoguanine formation not only can contribute to mutagenesis, but also may play an important role in the regulation of cell death.(78) Excessive formation of 8-oxoguanine was found to induce cell death via nuclear DNA-dependent and mitochondrial DNA-dependent mechanisms.(78) As mentioned above, 8-oxoguanine in DNA pairing with cytosine is removed by OGG1. In addition to OGG1, MutY homolog (MUTYH) excises adenine opposite 8-oxoguanine in template DNA, with concomitant formation of single strand breaks (SSBs) during the base excision repair. The accumulation of SSBs in nuclear DNA by the action of MUTYH leads to poly-ADP-ribose polymerase-dependent nuclear translocation of apoptosis-inducing factor, and triggers cell death. On the other hand, the accumulation of SSBs in mitochondrial DNA, which is also dependent on MUTYH, caused mitochondrial dysfunction and calcium ion release, thereby activating calpain, and finally triggers cell death. Recent study also demonstrated that excessive production of 8-oxo-dGTP in the nucleotide pool is a major source of oxidized bases, which may in turn cause accumulation of 8-oxoguanine in DNA,(38,79) leading to activate the cell death pathway as mentioned above. Taken together, 8-oxoguanine may function as a signaling molecule triggering the cell death, which appears to cause a completely opposite effect compared with the downstream cytoprotective effect of 8-nitro-cGMP signaling as described above (Fig. 2).
Conclusion
In this article, we overviewed available data on the physiology and pathophysiology of oxidized and nitrated guanine nucleotides, with emphasis on information on mutagenesis and cell signaling related to these nucleotides reported in the last decades. Different RNOS produced under various pathophysiological conditions may oxidize and nitrate guanine and its related nucleosides and nucleotides, which exist as part of DNA or RNA or in free form as an abundant component of the intracellular nucleotide pool. Not only do 8-oxoguaine and 8-nitroguanine function biologically as an endogenous mutagen but it may also serve as a biomarker for ROS- and RNOS-induced nucleic acid damage. More important, the major nitrated guanine nucleotide product, 8-nitro-cGMP, may play a critical role in signal transduction during cellular responses to oxidative stress that are primarily mediated by NO and ROS. This concept was unambiguously confirmed by our recent observation that NO-dependent formation of 8-nitro-cGMP is a potent contributor to activation of the antioxidant signaling pathway controlled by the Keap1-Nrf2 system via unique site-specific S-guanylation of Keap1 in cells. Also, structural evidence of Keap1 S-guanylation at Cys434 in vivo indicated that 8-nitro-cGMP, formed from the major nucleotide sensor GTP that exists in an intracellular pool, appears to act as a critical signaling molecule in the initial defense against nitrative and oxidative stress. Of importance is that, while guanine oxidation to form 8-oxoguanine may activate the cell death pathway, either dependent or independent of the p53 regulation, guanine nitration involving 8-nitro-cGMP-mediated antioxidant responses exhibits a completely opposite cellular protective effect (Fig. 2). Therefore, the nucleotides in the intracellular pool seem to be sensing the oxidative and nitrative stress occurring in cells, as being differentially recognized based on the altered chemical structures of nucleotides (e.g., oxo- and nitro-moieties) modified by ROS and RNOS. In other words, oxidative and nitrative nucleotide modifications may not be simple chemical damages, which mostly lose their biological functions because of the altered nucleotide structures, but may be physiologically relevant phenomena, which allow the cells to evoke the versatile cell signaling for adaptive responses to the various chemical stress. Further clarification of the signaling functions via oxidized and nitrated nucleotides may shed light on the chemical biology and mutation research and may support an emerging paradigm for oxidative stress-related chemical sensing and signaling of NO and ROS via cellular nucleotides functioning potentially as nitrative/oxidative stress sensors.
Acknowledgments
We thank Judith B. Gandy for her excellent editing of the manuscript. We also express our gratitude to Drs. Hozumi Motohashi and Masayuki Yamamoto for their stimulating discussion on the signaling functions of 8-nitro-cGMP as related to Keap1 S-guanylation. This work was supported in part by Grants-in-Aid for Scientific Research (S, B, C) and Grants-in-Aid for Scientific Research on Innovative Areas (Research in a Proposed Area) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) and by Grants-in-Aid from the Ministry of Health, Labor and Welfare of Japan.
References
- 1.Griffith OW, Stuehr DJ. Nitric oxide synthases: properties and catalytic mechanism. Annu Rev Physiol. 1995;57:707–736. doi: 10.1146/annurev.ph.57.030195.003423. [DOI] [PubMed] [Google Scholar]
- 2.Murad F. Cyclic guanosine monophosphate as a mediator of vasodilation. J Clin Invest. 1986;78:1–5. doi: 10.1172/JCI112536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bredt DS, Hwang PM, Snyder SH. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990;347:768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- 4.Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992;6:3051–3064. [PubMed] [Google Scholar]
- 5.Patel RP, Moellering D, Murphy-Ullrich J, Jo H, Beckman JS, Darley-Usmar VM. Cell signaling by reactive nitrogen and oxygen species in atherosclerosis. Free Radic Biol Med. 2000;28:1780–1794. doi: 10.1016/s0891-5849(00)00235-5. [DOI] [PubMed] [Google Scholar]
- 6.Madhusoodanan KS, Murad F. NO-cGMP signaling and regenerative medicine involving stem cells. Neurochem Res. 2007;32:681–694. doi: 10.1007/s11064-006-9167-y. [DOI] [PubMed] [Google Scholar]
- 7.Hare JM, Stamler JS. NO/redox disequilibrium in the failing heart and cardiovascular system. J Clin Invest. 2005;115:509–517. doi: 10.1172/JCI200524459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ischiropoulos H, Beckman JS. Oxidative stress and nitration in neurodegeneration: cause, effect, or association? J Clin Invest. 2003;111:163–169. doi: 10.1172/JCI17638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nathan C, Shiloh MU. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc Natl Acad Sci USA. 2000;97:8841–8848. doi: 10.1073/pnas.97.16.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohshima H, Bartsch H. Chronic infections and inflammatory processes as cancer risk factors: possible role of nitric oxide in carcinogenesis. Mutat Res. 1994;305:253–264. doi: 10.1016/0027-5107(94)90245-3. [DOI] [PubMed] [Google Scholar]
- 11.Sawa T, Ohshima H. Nitrative DNA damage in inflammation and its possible role in carcinogenesis. Nitric Oxide. 2006;14:91–100. doi: 10.1016/j.niox.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Zaki MH, Akuta T, Akaike T. Nitric oxide-induced nitrative stress involved in microbial pathogenesis. J Pharmacol Sci. 2005;98:117–129. doi: 10.1254/jphs.crj05004x. [DOI] [PubMed] [Google Scholar]
- 13.Lewis RS, Deen WM. Kinetics of the reaction of nitric oxide with oxygen in aqueous solutions. Chem Res Toxicol. 1994;7:568–574. doi: 10.1021/tx00040a013. [DOI] [PubMed] [Google Scholar]
- 14.Kissner R, Nauser T, Bugnon P, Lye PG, Koppenol WH. Formation and properties of peroxynitrite as studied by laser flash photolysis, high-pressure stopped-flow technique, and pulse radiolysis. Chem Res Toxicol. 1997;10:1285–1292. doi: 10.1021/tx970160x. [DOI] [PubMed] [Google Scholar]
- 15.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 16.Ducrocq C, Blanchard B, Pignatelli B, Ohshima H. Peroxynitrite: an endogenous oxidizing and nitrating agent. Cell Mol Life Sci. 1999;55:1068–1077. doi: 10.1007/s000180050357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Squadrito GL, Pryor WA. Oxidative chemistry of nitric oxide: the roles of superoxide, peroxynitrite, and carbon dioxide. Free Radic Biol Med. 1998;25:392–403. doi: 10.1016/s0891-5849(98)00095-1. [DOI] [PubMed] [Google Scholar]
- 18.Akaike T, Noguchi Y, Ijiri S, et al. Pathogenesis of influenza virus-induced pneumonia: involvement of both nitric oxide and oxygen radicals. Proc Natl Acad Sci USA. 1996;93:2448–2453. doi: 10.1073/pnas.93.6.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sawa T, Akaike T, Maeda H. Tyrosine nitration by peroxynitrite formed from nitric oxide and superoxide generated by xanthine oxidase. J Biol Chem. 2000;275:32467–32474. doi: 10.1074/jbc.M910169199. [DOI] [PubMed] [Google Scholar]
- 20.Okamoto T, Akaike T, Sawa T, Miyamoto Y, van der Vliet A, Maeda H. Activation of matrix metalloproteinases by peroxynitrite-induced protein S-glutathiolation via disulfide S-oxide formation. J Biol Chem. 2001;276:29596–29602. doi: 10.1074/jbc.M102417200. [DOI] [PubMed] [Google Scholar]
- 21.Eiserich JP, Cross CE, Jones AD, Halliwell B, van der Vliet A. Formation of nitrating and chlorinating species by reaction of nitrite with hypochlorous acid. A novel mechanism for nitric oxide-mediated protein modification. J Biol Chem. 1996;271:19199–19208. doi: 10.1074/jbc.271.32.19199. [DOI] [PubMed] [Google Scholar]
- 22.Panasenko OM, Briviba K, Klotz LO, Sies H. Oxidative modification and nitration of human low-density lipoproteins by the reaction of hypochlorous acid with nitrite. Arch Biochem Biophys. 1997;343:254–259. doi: 10.1006/abbi.1997.0171. [DOI] [PubMed] [Google Scholar]
- 23.Wang C, Deen WM. Nitric oxide delivery system for cell culture studies. Ann Biomed Eng. 2003;31:65–79. doi: 10.1114/1.1533072. [DOI] [PubMed] [Google Scholar]
- 24.Brennan ML, Wu W, Fu X, et al. A tale of two controversies: defining both the role of peroxidases in nitrotyrosine formation in vivo using eosinophil peroxidase and myeloperoxidase-deficient mice, and the nature of peroxidase-generated reactive nitrogen species. J Biol Chem. 2002;277:17415–17427. doi: 10.1074/jbc.M112400200. [DOI] [PubMed] [Google Scholar]
- 25.Eiserich JP, Hristova M, Cross CE, et al. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature. 1998;391:393–397. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- 26.Akaike T, Okamoto S, Sawa T, et al. 8-nitroguanosine formation in viral pneumonia and its implication for pathogenesis. Proc Natl Acad Sci USA. 2003;100:685–690. doi: 10.1073/pnas.0235623100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohshima H, Sawa T, Akaike T. 8-nitroguanine, a product of nitrative DNA damage caused by reactive nitrogen species: formation, occurrence, and implications in inflammation and carcinogenesis. Antioxid Redox Signal. 2006;8:1033–1045. doi: 10.1089/ars.2006.8.1033. [DOI] [PubMed] [Google Scholar]
- 28.Sawa T, Tatemichi M, Akaike T, Barbin A, Ohshima H. Analysis of urinary 8-nitroguanine, a marker of nitrative nucleic acid damage, by high-performance liquid chromatography-electrochemical detection coupled with immunoaffinity purification: association with cigarette smoking. Free Radic Biol Med. 2006;40:711–720. doi: 10.1016/j.freeradbiomed.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 29.Tazawa H, Tatemichi M, Sawa T, et al. Oxidative and nitrative stress caused by subcutaneous implantation of a foreign body accelerates sarcoma development in Trp53+/− mice. Carcinogenesis. 2007;28:191–198. doi: 10.1093/carcin/bgl128. [DOI] [PubMed] [Google Scholar]
- 30.Terasaki Y, Akuta T, Terasaki M, et al. Guanine nitration in idiopathic pulmonary fibrosis and its implication for carcinogenesis. Am J Respir Crit Care Med. 2006;174:665–673. doi: 10.1164/rccm.200510-1580OC. [DOI] [PubMed] [Google Scholar]
- 31.Yasuhara R, Miyamoto Y, Akaike T, et al. Interleukin-1β induces death in chondrocyte-like ATDC5 cells through mitochondrial dysfunction and energy depletion in a reactive nitrogen and oxygen species-dependent manner. Biochem J. 2005;389:315–323. doi: 10.1042/BJ20041996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshitake J, Akaike T, Akuta T, et al. Nitric oxide as an endogenous mutagen for Sendai virus without antiviral activity. J Virol. 2004;78:8709–8719. doi: 10.1128/JVI.78.16.8709-8719.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshitake J, Kato K, Yoshioka D, et al. Suppression of NO production and 8-nitroguanosine formation by phenol-containing endocrine-disrupting chemicals in LPS-stimulated macrophages: involvement of estrogen receptor-dependent or -independent pathways. Nitric Oxide. 2008;18:223–228. doi: 10.1016/j.niox.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Sawa T, Akaike T, Ichimori K, et al. Superoxide generation mediated by 8-nitroguanosine, a highly redox-active nucleic acid derivative. Biochem Biophys Res Commun. 2003;311:300–306. doi: 10.1016/j.bbrc.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 35.Sawa T, Zaki MH, Okamoto T, et al. Protein S-guanylation by the biological signal 8-nitroguanosine 3',5'-cyclic monophosphate. Nat Chem Biol. 2007;3:727–735. doi: 10.1038/nchembio.2007.33. [DOI] [PubMed] [Google Scholar]
- 36.Kasai H. Analysis of a form of oxidative DNA damage, 8-hydroxy-2'-deoxyguanosine, as a marker of cellular oxidative stress during carcinogenesis. Mutat Res. 1997;387:147–163. doi: 10.1016/s1383-5742(97)00035-5. [DOI] [PubMed] [Google Scholar]
- 37.Sawa T, Arimoto H, Akaike T. Regulation of redox signaling involving chemical conjugation of protein thiols by nitric oxide and electrophiles. Bioconjug Chem. 2010;21:1121–1129. doi: 10.1021/bc900396u. [DOI] [PubMed] [Google Scholar]
- 38.Nakabeppu Y, Oka S, Sheng Z, Tsuchimoto D, Sakumi K. Programmed cell death triggered by nucleotide pool damage and its prevention by MutT homolog-1 (MTH1) with oxidized purine nucleoside triphosphatase. Mutat Res. 2010 doi: 10.1016/j.mrgentox.2010.06.006. DOI:10.1016/j.mrgentox.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 39.Loft S, Vistisen K, Ewertz M, Tjønneland A, Overvad K, Poulsen HE. Oxidative DNA damage estimated by 8-hydroxydeoxyguanosine excretion in humans: influence of smoking, gender and body mass index. Carcinogenesis. 1992;13:2241–2247. doi: 10.1093/carcin/13.12.2241. [DOI] [PubMed] [Google Scholar]
- 40.Wu LL, Chiou CC, Chang PY, Wu JT. Urinary 8-OHdG: a marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clin Chim Acta. 2004;339:1–9. doi: 10.1016/j.cccn.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 41.Seet RC, Lee CY, Lim EC, et al. Oxidative damage in Parkinson disease: Measurement using accurate biomarkers. Free Radic Biol Med. 2010;48:560–566. doi: 10.1016/j.freeradbiomed.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 42.Yermilov V, Rubio J, Ohshima H. Formation of 8-nitroguanine in DNA treated with peroxynitrite in vitro and its rapid removal from DNA by depurination. FEBS Lett. 1995;376:207–210. doi: 10.1016/0014-5793(95)01281-6. [DOI] [PubMed] [Google Scholar]
- 43.Masuda M, Nishino H, Ohshima H. Formation of 8-nitroguanosine in cellular RNA as a biomarker of exposure to reactive nitrogen species. Chem Biol Interact. 2002;139:187–197. doi: 10.1016/s0009-2797(01)00299-x. [DOI] [PubMed] [Google Scholar]
- 44.Zaki MH, Fujii S, Okamoto T, et al. Cytoprotective function of heme oxygenase 1 induced by a nitrated cyclic nucleotide formed during murine salmonellosis. J Immunol. 2009;182:3746–3756. doi: 10.4049/jimmunol.0803363. [DOI] [PubMed] [Google Scholar]
- 45.Hoki Y, Murata M, Hiraku Y, et al. 8-Nitroguanine as a potential biomarker for progression of malignant fibrous histiocytoma, a model of inflammation-related cancer. Oncol Rep. 2007;18:1165–1169. [PubMed] [Google Scholar]
- 46.Yuasa I, Ma N, Matsubara H, Fukui Y, Uji Y. Inducible nitric oxide synthase mediates retinal DNA damage in Goto-Kakizaki rat retina. Jpn J Ophthalmol. 2008;52:314–322. doi: 10.1007/s10384-008-0542-x. [DOI] [PubMed] [Google Scholar]
- 47.Fujii S, Sawa T, Ihara H, et al. The critical role of nitric oxide signaling, via protein S-guanylation and nitrated cyclic GMP, in the antioxidant adaptive response. J Biol Chem. 2010;285:23970–23984. doi: 10.1074/jbc.M110.145441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akaike T. Role of free radicals in viral pathogenesis and mutation. Rev Med Virol. 2001;11:87–101. doi: 10.1002/rmv.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weitzman SA, Stossel TP. Mutation caused by human phagocytes. Science. 1981;212:546–547. doi: 10.1126/science.6259738. [DOI] [PubMed] [Google Scholar]
- 50.Akaike T, Fujii S, Kato A, et al. Viral mutation accelerated by nitric oxide production during infection in vivo. FASEB J. 2000;14:1447–1454. doi: 10.1096/fj.14.10.1447. [DOI] [PubMed] [Google Scholar]
- 51.Zhuang JC, Lin C, Lin D, Wogan GN. Mutagenesis associated with nitric oxide production in macrophages. Proc Natl Acad Sci USA. 1998;95:8286–8291. doi: 10.1073/pnas.95.14.8286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gal A, Wogan GN. Mutagenesis associated with nitric oxide production in transgenic SJL mice. Proc Natl Acad Sci USA. 1996;93:15102–15107. doi: 10.1073/pnas.93.26.15102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jaiswal M, LaRusso NF, Burgart LJ, Gores GJ. Inflammatory cytokines induce DNA damage and inhibit DNA repair in cholangiocarcinoma cells by a nitric oxide-dependent mechanism. Cancer Res. 2000;60:184–190. [PubMed] [Google Scholar]
- 54.Juedes MJ, Wogan GN. Peroxynitrite-induced mutation spectra of pSP189 following replication in bacteria and in human cells. Mutat Res. 1996;349:51–61. doi: 10.1016/0027-5107(95)00152-2. [DOI] [PubMed] [Google Scholar]
- 55.Suzuki N, Yasui M, Geacintov NE, Shafirovich V, Shibutani S. Miscoding events during DNA synthesis past the nitration-damaged base 8-nitroguanine. Biochemistry. 2005;44:9238–9245. doi: 10.1021/bi050276p. [DOI] [PubMed] [Google Scholar]
- 56.Shafirovich V, Mock S, Kolbanovskiy A, Geacintov NE. Photochemically catalyzed generation of site-specific 8-nitroguanine adducts in DNA by the reaction of long-lived neutral guanine radicals with nitrogen dioxide. Chem Res Toxicol. 2002;15:591–597. doi: 10.1021/tx015593l. [DOI] [PubMed] [Google Scholar]
- 57.Kaneko K, Akuta T, Sawa T, et al. Mutagenicity of 8-nitroguanosine, a product of nitrative nucleoside modification by reactive nitrogen oxides, in mammalian cells. Cancer Lett. 2008;262:239–247. doi: 10.1016/j.canlet.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 58.Shibutani S, Takeshita M, Grollman AP. Insertion of specific bases during DNA synthesis past the oxidation-damaged base 8-oxodG. Nature. 1991;349:431–434. doi: 10.1038/349431a0. [DOI] [PubMed] [Google Scholar]
- 59.Sekiguchi M, Tsuzuki T. Oxidative nucleotide damage: consequences and prevention. Oncogene. 2002;21:8895–8904. doi: 10.1038/sj.onc.1206023. [DOI] [PubMed] [Google Scholar]
- 60.Nakabeppu Y, Sakumi K, Sakamoto K, Tsuchimoto D, Tsuzuki T, Nakatsu Y. Mutagenesis and carcinogenesis caused by the oxidation of nucleic acids. Biol Chem. 2006;387:373–379. doi: 10.1515/BC.2006.050. [DOI] [PubMed] [Google Scholar]
- 61.Toyokuni S, Tanaka T, Hattori Y, et al. Quantitative immunohistochemical determination of 8-hydroxy-2'-deoxyguanosine by a monoclonal antibody N45.1: its application to ferric nitrilotriacetate-induced renal carcinogenesis model. Lab Invest. 1997;76:365–374. [PubMed] [Google Scholar]
- 62.Zhang J, Perry G, Smith MA, et al. Parkinson’s disease is associated with oxidative damage to cytoplasmic DNA and RNA in substantia nigra neurons. Am J Pathol. 1999;154:1423–1429. doi: 10.1016/S0002-9440(10)65396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Henderson JP, Byun J, Williams MV, et al. Bromination of deoxycytidine by eosinophil peroxidase: a mechanism for mutagenesis by oxidative damage of nucleotide precursors. Proc Natl Acad Sci USA. 2001;98:1631–1636. doi: 10.1073/pnas.041146998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morris SM. The genetic toxicology of 5-fluoropyrimidines and 5-chlorouracil. Mutat Res. 1993;297:39–51. doi: 10.1016/0165-1110(93)90006-9. [DOI] [PubMed] [Google Scholar]
- 65.Nakabeppu Y, Tsuchimoto D, Furuichi M, Sakumi K. The defense mechanisms in mammalian cells against oxidative damage in nucleic acids and their involvement in the suppression of mutagenesis and cell death. Free Radic Res. 2004;38:423–429. doi: 10.1080/10715760410001688348. [DOI] [PubMed] [Google Scholar]
- 66.Li X, Patel R, Melamed MR, Darzynkiewicz Z. The cell cycle effects and induction of apoptosis by 5-bromouridine in cultures of human leukaemic MOLT-4 and HL-60 cell lines and mitogen-stimulated normal lymphocytes. Cell Prolif. 1994;27:307–319. doi: 10.1111/j.1365-2184.1994.tb01428.x. [DOI] [PubMed] [Google Scholar]
- 67.Sierakowska H, Shukla RR, Dominski Z, Kole R. Inhibition of pre-mRNA splicing by 5-fluoro-, 5-chloro-, and 5-bromouridine. J Biol Chem. 1989;264:19185–19191. [PubMed] [Google Scholar]
- 68.Taddei F, Hayakawa H, Bouton M, et al. Counteraction by MutT protein of transcriptional errors caused by oxidative damage. Science. 1997;278:128–130. doi: 10.1126/science.278.5335.128. [DOI] [PubMed] [Google Scholar]
- 69.Ohshima H, Tatemichi M, Sawa T. Chemical basis of inflammation-induced carcinogenesis. Arch Biochem Biophys. 2003;417:3–11. doi: 10.1016/s0003-9861(03)00283-2. [DOI] [PubMed] [Google Scholar]
- 70.Saito Y, Taguchi H, Fujii S, et al. 8-Nitroguanosines as chemical probes of the protein S-guanylation. Chem Commun. 2008;7:5984–5986. doi: 10.1039/b810771h. [DOI] [PubMed] [Google Scholar]
- 71.Dinkova-Kostova AT, Holtzclaw WD, Kensler TW. The role of Keap1 in cellular protective responses. Chem Res Toxicol. 2005;18:1779–1791. doi: 10.1021/tx050217c. [DOI] [PubMed] [Google Scholar]
- 72.Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004;10:549–557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 73.Motterlini R, Gonzales A, Foresti R, Clark JE, Green CJ, Winslow RM. Heme oxygenase-1-derived carbon monoxide contributes to the suppression of acute hypertensive responses in vivo. Circ Res. 1998;83:568–577. doi: 10.1161/01.res.83.5.568. [DOI] [PubMed] [Google Scholar]
- 74.Zuckerbraun BS, Billiar TR, Otterbein SL, et al. Carbon monoxide protects against liver failure through nitric oxide-induced heme oxygenase 1. J Exp Med. 2003;198:1707–1716. doi: 10.1084/jem.20031003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Otterbein LE, Bach FH, Alam J, et al. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 76.Doi K, Akaike T, Fujii S, et al. Induction of haem oxygenase-1 nitric oxide and ischaemia in experimental solid tumours and implications for tumour growth. Br J Cancer. 1999;80:1945–1954. doi: 10.1038/sj.bjc.6690624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tanaka S, Akaike T, Fang J, et al. Antiapoptotic effect of haem oxygenase-1 induced by nitric oxide in experimental solid tumour. Br J Cancer. 2003;88:902–909. doi: 10.1038/sj.bjc.6600830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oka S, Ohno M, Tsuchimoto D, Sakumi K, Furuichi M, Nakabeppu Y. Two distinct pathways of cell death triggered by oxidative damage to nuclear and mitochondrial DNAs. EMBO J. 2008;27:421–432. doi: 10.1038/sj.emboj.7601975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ichikawa J, Tsuchimoto D, Oka S, et al. Oxidation of mitochondrial deoxynucleotide pools by exposure to sodium nitroprusside induces cell death. DNA Repair (Amst) 2008;7:418–430. doi: 10.1016/j.dnarep.2007.11.007. [DOI] [PubMed] [Google Scholar]