Abstract
Persistent oxidative stress has been associated with carcinogenesis. Iron overload is considered one such condition that causes oxidative stress. Epidemiological studies support a close link between iron overload and carcinogenesis. Reportedly, regular semiannual phlebotomies reduced cancer risk in an otherwise normal population. More specifically, genetic hemochromatosis, chronic viral hepatitis, ovarian endometriosis and asbestosis induce iron overload, which can lead to hepatocellular carcinoma, ovarian carcinoma or mesothelioma in humans. Through a combination of animal experiments and microarray analyses, homozygous deletion of CDKN2A/2B has been recognized as one of the major target genes involved in iron overload-induced carcinogenesis. CDKN2A/2B are the second most frequently inactivated tumor suppressing genes in human cancers. Currently, when infection is becoming sufficiently controlled worldwide, iron regulation may be the next target for human longevity.
Keywords: iron overload, CDKN2A/2B, carcinogenesis
Introduction
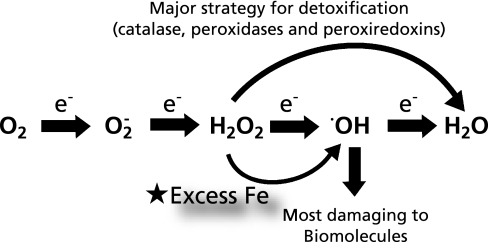
Most living species on earth, including humans, use oxygen. Indeed, we cannot live without oxygen for even 5 min. Why is oxygen so important? This is principally based on the chemical characteristics of oxygen; a series of reductions by one electron each is possible four times, until the oxygen is reduced to a stable species and solvent, H2O (Fig. 1). This characteristic enables efficient electron flow that is essential for any moving material, including motors and robots. During this electron flow, three intermediate species are generated, among which hydroxyl radical (•OH) has been identified as the most damaging to biomolecules. Accordingly, cells strategically prevent the generation of •OH by metabolizing H2O2 directly into H2O via various types of enzymes, including catalase, peroxidases and peroxiredoxins. Even excess O2•− is metabolized to H2O2. The roles of O2•− and H2O2 as signaling molecules have been well established by studies within the past two decades.(1) The adverse effects of oxygen were already recognized in the 1950’s, and oxygen free radicals were hypothesized to be the direct cause of aging.(2)
Fig. 1.
Biological significance of excess iron in the oxygen metabolism.
Cancer has been the leading cause of death in Japan for 30 years, while in most western countries, coronary heart disease is the current leading cause of death. I suspect that cancer would become the predominant cause of death in western countries as well if metabolic syndrome could be overcome by proper preventive exercise and diet. Currently, most of the fatal infections are well controlled worldwide. The longer we live, the more genetic alterations we obtain through persistent oxygen consumption and environmental exposure to genotoxic agents.
Persistent oxidative stress has been associated with carcinogenesis.(3) The causes of oxidative cellular stress stem mainly from direct and indirect exogenous sources. Examples of direct causes are X-radiation and ultraviolet radiation. Thus, exposure to atomic bomb radiation in Hiroshima and Nagasaki was shown to increase leukemia and lymphoma,(4) and ultraviolet radiation has been closely associated with skin cancer.(5) Indirect sources of oxidative stress include inflammation. Chronic Helicobacter pylori infection is associated with gastric cancer and low-grade lymphoma of mucosa-associated lymphoid tissue.(6,7)
Similarly, inflammatory bowel diseases such as Crohn’s disease(8) and ulcerative colitis(9) are associated with colon cancer. In these cases, inflammation causes pain and various other symptoms, and X-radiation is currently under strict legal regulation. However, we should not forget iron overload as a cause of oxidative stress. Iron overload per se does not induce inflammation, nor is it under legal control. Although the pathology of iron overload is really insidious, it can eventually lead to cancer. In this sense, iron overload is really a quiet “time bomb”.
Iron and Carcinogenesis
Iron is ubiquitous, and no living organisms on earth can do without iron. Iron deficiency causes anemia, but excess iron can be a risk for carcinogenesis because iron works as a catalyst for the Fenton reaction, thus promoting the generation of the undesirable molecule, •OH (Fig. 1). Iron stores within the body accumulate insidiously with aging because iron intake exceeds loss and no biologic mechanisms exist for excretion of iron in excess of physiological requirements.(10) It was initially reported in 2008 that iron reduction by phlebotomy not only decreased visceral cancer risk by 35% but also decreased mortality in cancer patients by 60% in a supposedly normal population with peripheral arterial disease.(11) In spite of some criticisms regarding design of this study, this is a highly significant observation supporting other epidemiological studies(12,13) and one should not underestimate the role of iron in carcinogenesis.
There are ample human epidemiological data to support the involvement of excessive iron in carcinogenesis. Genetic hemochromatosis is a hereditary disorder of iron sensing and currently 4 genetic types are recognized. Among them, autosomal recessive HFE (OMIM +235200) is a major type.(14) A mutation in the gene encoding SLC40A1 (OMIM #606069) is associated with an autosomal-dominant type of hemochromatosis. It is of note that defects in HAMP (OMIM #602390) or in transferrin receptor 2 (OMIM #604250) also induce hemochromatosis. Major causes of death today in hereditary hemochromatosis are due to either hepatic failure with cirrhosis or hepatocellular carcinoma.(15) In addition, hemochromatosis patients were shown to have 240-fold greater risk developing primary hepatocellular carcinoma than the age-matched control population.(16)
Persistent damage to hepatocytes reduces the production of a peptide hormone, hepcidin, leading to iron absorption and deposition irrespective of existing iron stores.(17) Thus, hepatic iron levels are significantly increased in patients with chronic viral hepatitis B/C. Currently, phlebotomy along with a low iron diet is the second-line therapeutic strategy after α-interferon administration to prevent hepatocellular carcinoma in Japan.(18)
Several epidemiological studies have suggested an association between endometriosis and ovarian cancer by demonstrating a high risk of ovarian cancer in women with a long-standing history (>10 years) of ovarian endometriosis.(19) Here, hemorrhagic retention every month leads to localized iron overload in the ovary. We recently showed that the epithelia of endometriotic cysts are oxidatively stressed and contain high levels of catalytic iron.(20)
Asbestos fibers have been heavily used for industrial applications since World War II because of their durability, heat-resistance and low cost. However, in 1987, IARC designated asbestos fibers as a Group I (definite) carcinogen for humans (http://monographs.iarc.fr/ENG/Classification/crthgr01.php). Epidemiological studies suggest that asbestos fibers that contain iron (a transition metal which catalyzes free radical generation) are more carcinogenic.(21)
Target Gene in Iron-Induced Carcinogenesis
Our research group has been working on iron-induced carcinogenesis for more than 20 years. I came across the ferric nitrilotriacetate (Fe-NTA)-induced renal carcinogenesis model when I was a graduate student. Prof. Shigeru Okada, my mentor, serendipitously discovered this model while he was studying the iron loading effects of Fe-NTA on the liver.(22) Chelation of metals alters their biological effects, presumably because metals are not easily dissolved into solution at neutral pH while chelation makes dissolution possible.(23) This is an excellent model that accurately reproduces both acute renal tubular damage and carcinogenesis.(24) Multiple studies and subsequent papers have been based on the use of this single model, including the development of monoclonal antibodies against 4-hydroxy-2-nonenal-modified proteins(25) and 8-hydroxy-2'-deoxyguanosine.(26)
Fe-NTA is an efficient catalyst for the Fenton reaction to generate •OH. Hydroxyl radical is one of the most reactive chemical species in biology. Thus, it is natural to think that every portion of the genome is randomly damaged by this species and to hypothesize that there would be no specific target genes in this carcinogenesis model. We tried to address this question in the 1990’s and unexpectedly found that target genes do exist. At that time, rat genomic information was largely not available, so we had to literally perform thousands of PCR reactions to find common allelic loss in the genome by using microsatellite markers. Fortunately, we reached a conclusion that CDKN2A/2B, which encode inhibitors for cyclin-dependent kinases, are major target genes in this model. Approximately one third of the cases had homozygous deletion of these tumor suppressing genes.(27) Recently, our results were confirmed with a modern technology called array-based comparative genome hybridization analysis.(28)
Surprisingly, CDKN2A/2B are target genes in human malignant mesothelioma, a type of cancer which has been strongly associated with asbestos exposure. Excessive iron is thought to be a principal pathology.(29) As many as 74% of cases of human malignant mesothelioma had homozygous deletion of CDKN2A.(30) We observed an even higher frequency of homozygous deletion of CDKN2A/2B in rat peritoneal mesothelioma induced either by chryostile, crocidolite or amosite (Jiang L and Toyokuni S, unpublished observation). Interestingly, iron compounds alone (ferric saccharate with nitrilotriacetate) can generate malignant mesothelioma in rats albeit lower incidence and longer incubation period, and we recently found that sarcomatoid types of these mesotheliomas had a high incidence (80%) of homozygous deletion of CDKN2A.(31)
CDKN2A/2B as an Iron Overload-Associated Gene
Based on these three examples of animal experiments, we can hypothesize that excess iron causes deletion of CDKN2A/2B. The genomic sequence at CDKN2A generates another tumor suppressing gene called ARF by alternative splicing that works as an inhibitor of TP53 specific ubiquitin ligase.(32) CDKN2B is a backup for CDKN2A.(33) Thus, this genomic location acts as a guardian of the genome, and genes corresponding to this location are involved in both the RB and TP53 pathways (Fig. 2). It is also interesting to note that P16 protein (the product of CDKN2A) levels decrease with age,(34) and that established cancer cell lines have a high incidence of CDKN2A homozygous deletion.(35) Taken together, these findings suggest that oxidative stress, either by excess iron or a high concentration of oxygen, induced this genetic alteration. CDKN2A/2B are really mysterious genomic points, and we are currently using oxygenomics(36,37) as a strategy to investigate the loci.
Fig. 2.
Homozygous deletion of CDKN2A/2B and its biological significance in carcinogenesis.
Conclusion and Strategy for Longevity
During this decade, iron metabolism has been further elucidated and many novel molecules and transporters have been identified.(13) Among the organelles, the mitochondrion is at the center of iron metabolism, producing iron-sulfur cluster and heme. Abnormality of its specific chaperone, frataxin, causes decreased iron storage in mitochondria with concomitant cytoplasmic iron accumulation. This can lead to a neurodegenerative disorder called Friedreich ataxia,(38) thus indicating that iron excess damages neurons as well.
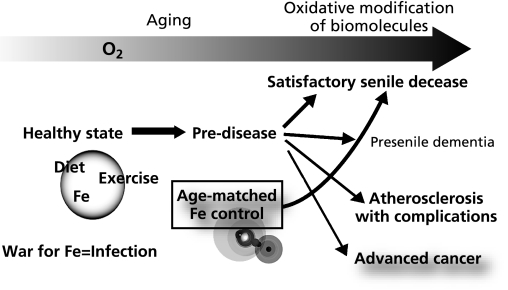
Throughout evolutional and developmental processes, iron has been and will continue to be necessary. However, it is now apparent that iron excess is harmful and even promotes cancer. This has important implications for human longevity. The current mean lifetime for the Japanese was 86.1 years for females and 79.3 years for males in 2009. This is a great victory of the public health service, considering that the mean lifetime expectancy was approximately 50 years immediately following World War II. I believe that it is time to consider the demerits of excess iron. Iron modulation by phlebotomy (blood donation), low iron diet or chelation therapy may be a strategy to prevent cancer and possibly other diseases on the condition that the person is healthy and without anemia (Fig. 3).
Fig. 3.
Strategy for longevity from the standpoint of appropriate iron stores for age.
CDKN2A/2B are intriguing genomic crossroads for cancer and aging, which is involved in both the RB and TP53 pathways. This gene is evolutionally conserved between fish (Xyphophorus maculates and Fugu rubripes) and mammals. Elucidation of the evolution of this gene would help to clarify further grounds for research into longevity.
Acknowledgments
This manuscript is dedicated to the first retirement of Prof. Toshikazu Yoshikawa, who has been encouraging me for more than 20 years to study in the field of free radical biology. This work was supported in part by a MEXT grant (Special Coordination Funds for Promoting Science and Technology), a Grant from Takeda Science Foundation, a Grant-in-Aid for Cancer Research from the Ministry of Health, Labor and Welfare of Japan, and a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1.Woo HA, Yim SH, Shin DH, Kang D, Yu DY, Rhee SG. Inactivation of peroxiredoxin I by phosphorylation allows localized H2O2 accumulation for cell signaling. Cell. 2010;140:517–528. doi: 10.1016/j.cell.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 3.Toyokuni S. Molecular mechanisms of oxidative stress-induced carcinogenesis: from epidemiology to oxygenomics. IUBMB Life. 2008;60:441–447. doi: 10.1002/iub.61. [DOI] [PubMed] [Google Scholar]
- 4.Preston D, Kusumi S, Tomonaga M, et al. Cancer incidence in atomic bomb survivors. Part III. Leukemia, lymphoma and multiple myeloma, 1950–1987. Radiat. Res. 1994;137:S68–S97. [PubMed] [Google Scholar]
- 5.Nishigori C, Hattori Y, Toyokuni S. Role of reactive oxygen species in skin carcinogenesis. Antioxiol Redox Singnal. 2004;6:561–570. doi: 10.1089/152308604773934314. [DOI] [PubMed] [Google Scholar]
- 6.Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 7.Naito Y, Yoshikawa T. Carcinogenesis and chemoprevention in gastric cancer associated with Helicobacter pylori infection: role of oxidants and antioxidants. J Clin Biochem Nutr. 2005;36:37–49. [Google Scholar]
- 8.Ekbom A, Helmick C, Zack M, Adami H. Increased risk of large-bowel cancer in Crohn’s disease with colonic involvement. Lancet. 1990;336:357–359. doi: 10.1016/0140-6736(90)91889-i. [DOI] [PubMed] [Google Scholar]
- 9.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zacharski LR, Ornstein DL, Woloshin S, Schwartz LM. Association of age, sex, and race with body iron stores in adults: analysis of NHANES III data. Am Heart J. 2000;140:98–104. doi: 10.1067/mhj.2000.106646. [DOI] [PubMed] [Google Scholar]
- 11.Zacharski LR, Chow BK, Howes PS, et al. Decreased cancer risk after iron reduction in patients with peripheral arterial disease: results from a randomized trial. J Natl Cancer Inst. 2008;100:996–1002. doi: 10.1093/jnci/djn209. [DOI] [PubMed] [Google Scholar]
- 12.Toyokuni S. Iron-induced carcinogenesis: the role of redox regulation. Free Radic Biol Med. 1996;20:553–566. doi: 10.1016/0891-5849(95)02111-6. [DOI] [PubMed] [Google Scholar]
- 13.Toyokuni S. Role of iron in carcinogenesis: cancer as a ferrotoxic disease. Cancer Sci. 2009;100:9–16. doi: 10.1111/j.1349-7006.2008.01001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lyon E, Frank EL. Hereditary hemochromatosis since discovery of the HFE gene. Clin Chem. 2001;47:1147–1156. [PubMed] [Google Scholar]
- 15.Milman N, Pedersen P, á Steig T, Byg KE, Graudal N, Fenger K. Clinically overt hereditary hemochromatosis in Denmark 1948–1985: epidemiology, factors of significance for long-term survival, and causes of death in 179 patients. Ann Hematol. 2001;80:737–744. doi: 10.1007/s002770100371. [DOI] [PubMed] [Google Scholar]
- 16.Bradbear RA, Bain C, Siskind V, et al. Cohort study of internal malignancy in genetic hemochromatosis and other chronic nonalcoholic liver diseases. J Natl Cancer Inst. 1985;75:81–84. [PubMed] [Google Scholar]
- 17.Drakesmith H, Prentice A. Viral infection and iron metabolism. Nat Rev Microbiol. 2008;6:541–552. doi: 10.1038/nrmicro1930. [DOI] [PubMed] [Google Scholar]
- 18.Kato J, Miyanishi K, Kobune M, et al. Long-term phlebotomy with low-iron diet therapy lowers risk of development of hepatocellular carcinoma from chronic hepatitis. Clin J Gastroenterol. 2007;42:830–836. doi: 10.1007/s00535-007-2095-z. [DOI] [PubMed] [Google Scholar]
- 19.Brinton LA, Gridley G, Persson I, Baron J, Bergqvist A. Cancer risk after a hospital discharge diagnosis of endometriosis. Am J Obstet Gynecol. 1997;176:572–579. doi: 10.1016/s0002-9378(97)70550-7. [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi K, Mandai M, Toyokuni S, et al. Contents of endometriotic cysts, especially the high concentration of free iron, are a possible cause of carcinogenesis in the cysts through the iron-induced persistent oxidative stress. Clin Cancer Res. 2008;14:32–40. doi: 10.1158/1078-0432.CCR-07-1614. [DOI] [PubMed] [Google Scholar]
- 21.McDonald AD, McDonald JC, Pooley FO. Mineral fibre content of lung in mesothelial tumours in North America. Ann Occup Hyg. 1982;26:417–422. [PubMed] [Google Scholar]
- 22.Okada S, Midorikawa O. Induction of the rat renal adenocarcinoma by Fe-nitrilotriacetate (Fe-NTA) Jpn Arch Intern Med. 1982;29:485–491. [Google Scholar]
- 23.Toyokuni S, Okada S, Hamazaki S, Fujioka M, Li JL, Midorikawa O. Cirrhosis of the liver induced by cupric nitrilotriacetate in Wistar rats. An experimetnal model of copper toxicosis. Am J Pathol. 1989;134:1263–1274. [PMC free article] [PubMed] [Google Scholar]
- 24.Ebina Y, Okada S, Hamazaki S, Ogino F, Li JL, Midorikawa O. Nephrotoxicity and renal cell carcinoma after use of iron- and aluminum-nitrilotriacetate complexes in rats. J Natl Cancer Inst. 1986;76:107–113. [PubMed] [Google Scholar]
- 25.Toyokuni S, Miyake N, Hiai H, et al. The monoclonal antibody specific for the 4-hydroxy-2-nonenal histidine adduct. FEBS Lett. 1995;359:189–191. doi: 10.1016/0014-5793(95)00033-6. [DOI] [PubMed] [Google Scholar]
- 26.Toyokuni S, Tanaka T, Hattori Y, et al. Quantitative immunohistochemical determination of 8-hydroxy-2'-deoxyguanosine by a monoclonal antibody N45.1: its application to ferric nitrilotriacetate-induced renal carcinogenesis model. Lab Invest. 1997;76:365–374. [PubMed] [Google Scholar]
- 27.Tanaka T, Iwasa Y, Kondo S, Hiai H, Toyokuni S. High incidence of allelic loss on chromosome 5 and inactivation of p15INK4B and p16INK4A tumor suppressor genes in oxystress-induced renal cell carcinoma of rats. Oncogene. 1999;18:3793–3797. doi: 10.1038/sj.onc.1202707. [DOI] [PubMed] [Google Scholar]
- 28.Liu YT, Shang DG, Akatsuka S, et al. Chronic oxidative stress causes amplification and overexpresson of ptprz1 protein tyrosine phosphatase to activate β-catenin pathway. Am J Pathol. 2007;171:1978–1988. doi: 10.2353/ajpath.2007.070741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang L, Nagai H, Ohara H, et al. Characteristics and modifying factors of asbestos-induced oxidative DNA damage. Cancer Sci. 2008;99:2142–2151. doi: 10.1111/j.1349-7006.2008.00934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Illei PB, Rusch VW, Zakowski MF, Ladanyi M. Homozygous deletion of CDKN2A and codeletion of the methylthioadenosine phosphorylase gene in the majority of pleural mesotheliomas. Clin Cancer Res. 2003;9:2108–2113. [PubMed] [Google Scholar]
- 31.Hu Q, Akatsuka S, Yamashita Y, et al. Homozygous deletion of CDKN2A/2B is a hallmark of iron-induced high-grade rat mesothelioma. Lab Invest. 2010;90:360–373. doi: 10.1038/labinvest.2009.140. [DOI] [PubMed] [Google Scholar]
- 32.Honda R, Yasuda H. Association of p19(ARF) with Mdm2 inhibits ubiquitin ligase activity of Mdm2 for tumor suppressor p53. EMBO J. 1999;18:22–27. doi: 10.1093/emboj/18.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krimpenfort P, Ijpenberg A, Song JY, et al. p15(Ink4b) is a critical tumour suppressor in the absence of p16(Ink4a) Nature. 2007;448:943–946. doi: 10.1038/nature06084. [DOI] [PubMed] [Google Scholar]
- 34.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 35.Herman JG, Merlo A, Mao L, et al. Inactivation of the CDKN2/p16/MTS1 gene is frequently associated with aberrant DNA methylation in all common human cancers. Cancer Res. 1995;55:4525–4530. [PubMed] [Google Scholar]
- 36.Akatsuka S, Aung TT, Dutta KK, et al. Contrasting genome-wide distribution of 8-hydroxyguanine and acrolein-modified adenine during oxidative stress-induced renal carcinogenesis. Am J Pathol. 2006;169:1328–1342. doi: 10.2353/ajpath.2006.051280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toyokuni S, Akatsuka S. What has been learned from the studies of oxidative stress-induced carcinogenesis: proposal of the concept of oxygenomics. J Clin Biochem Nutr. 2006;39:3–10. [Google Scholar]
- 38.Cavadini P, O’Neill HA, Benada O, Isaya G. Assembly and iron-binding properties of human frataxin, the protein deficient in Friedreich ataxia. Hum Mol Genet. 2002;11:217–227. doi: 10.1093/hmg/11.3.217. [DOI] [PubMed] [Google Scholar]