Abstract
Diets rich in fruits and vegetables have been associated with benefits for human health. Those effects have been partially ascribed to their content in flavonoids, compounds that are present in many edible plants and its derived foods. In humans, a significant number of studies has been developed analyzing the effect of foods and beverages rich in flavonoids on the presence and progression of risk factors associated to cardiovascular diseases, including hypertension. Cocoa derived products, rich in flavanols, have been thoroughly studied and demonstrated to be efficient improving endothelial function and decreasing blood pressure in humans and animals. However, the final chemical species and the mechanism/s responsible for these effects have not been completely defined. In this paper we present data supporting the hypothesis that flavanols could define superoxide anion production and then, establish optimal nitric oxide levels and blood pressure.
Keywords: (−)-epicatechin, nitric oxide, oxidants, antioxidants
Introduction
Lifestyle modifications, including dietary habits, have substantial effects on risk factors for cardiovascular disease such as hypertension.(1) Epidemiological evidence demonstrates that diets rich in fruits and vegetables benefit heart and vascular health.(2–11) Molecularly, these beneficial effects of fruits and vegetables have been largely ascribed to their content in flavonoids. These compounds are synthesized in many edible plants and remain present when plants are processed to foods. Grapes and wine, cocoa and chocolate, black and green tea, and soy and soy-derived products, are among the most important sources of flavonoids in the human diet. A significant number of studies has been developed in humans, analyzing the effect of foods and beverages rich in flavonoids on the presence and progression of risk factors associated to cardiovascular disease. Cocoa derived products have been thoroughly studied and demonstrated to be efficient improving endothelial function and decreasing blood pressure (BP).(12–14)
BP is influenced and regulated by a variety of conditions and chemical entities that interact among them in very complex ways. These entities include from atoms, e.g., sodium, to well-orchestrated systems, e.g., the renin-angiotensin system. In this complex scheme, nitric oxide (NO) appears to play a pivotal role in the regulation of vascular homeostasis, and then BP. We will focus the discussion about the effects of dietary flavonoids, mainly those provided by cocoa and cocoa products, on NO bioavailability as a result of regulating steady-state levels of superoxide anion (O2•−) and oxidants in cells and tissues.
Nitric Oxide and Oxidants in Biological Systems
NO is produced from L-arginine in a reaction catalyzed by the enzyme nitric oxide synthase (NOS). This enzyme is present in mammals in different isoforms: endothelial (eNOS), neuronal (nNOS), inducible (iNOS), which is expressed in response to different stimulus, and mitochondrial (mtNOS). NO reacts with several metal centers, molecular oxygen, thiol groups, and some oxygen radicals. NO fulfills its most important physiological action, i.e. vasodilation, by activating guanylyl cyclase through the reaction with its heme group. The guanylyl cyclase catalyzes the dephosphorylation of GTP to cGMP, which serves as a second messenger for signaling smooth muscle relaxation. Alternatively, NO reacts with superoxide anion, the one-electron reduction product of oxygen in a near diffusion controlled reaction to form peroxynitrite (NO + O2•− → ONOO−). This reaction has two physiological consequences: i) the generation of peroxynitrite which can oxidize cell components;(15) and ii) the elimination of NO. In this way, superoxide anion production is a modulator of NO steady-state levels. In mammalian cells, the principal source of superoxide anion is the mitochondrial electron transport, but other enzymatic sources include NADPH oxidase (NOX), xanthine oxidase, arachidonic acid metabolism by cyclooxygenase/lipoxygenase, and cytochrome P450.(16) In endothelial cells the NOX-dependent superoxide production is particularly relevant.
Flavanols
Cocoa, and foods and beverages made with, contain important amounts of flavan-3-ols or flavanols, a subfamily of flavonoids. Flavonoids include a large number of compounds that share a basic chemical structure and are classified in different subfamilies depending on specific substitution patterns.(17) Flavan-3-ols or flavanols are defined by the presence of a hydroxyl group at position 3 (ring C) and constitute the most complex subfamily of flavonoids, ranging from simple monomers to the oligomeric and polymeric forms, called procyanidins.(17) Monomers could be present in the aglycone form or esterified with gallic acid to form the gallate derivatives. Studies on the composition of cocoa products have shown that non-esterified monomers of flavanols and procyanidins are the quantitatively most important type of flavonoids. Those chemical species have been identified as (−)-epicatechin (EC) (Fig. 1A), (+)-catechin (Cat), and mostly B-type procyanidins, that are oligomers of EC.(18,19) Importantly, regardless the manufacturing process applied and the raw material used to produce chocolate, the main flavanol present in chocolate is EC.(19,20)
Fig. 1.
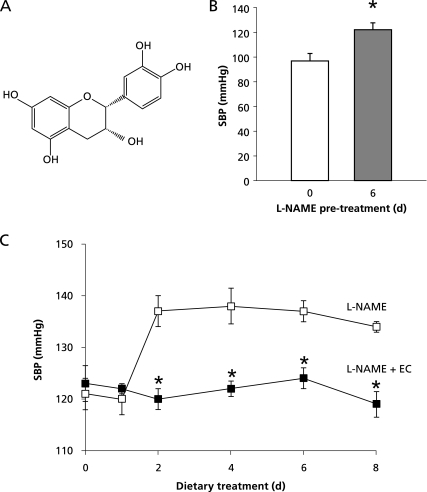
Dietary (−)-epicatechin (EC) and blood pressure. A: chemical structure of EC; B: systolic blood pressure (SBP) of rats subjected to L-NAME pre-treatment (40 mg/kg/d in drinking water) for 6 days; and C: SBP of rats administered with L-NAME (open square), or L-NAME + EC 0.4 g/100 g diet (closed square) during the subsequent 8 days. Sprague–Dawley rats (130–140 g) were maintained under controlled housing conditions. SBP was measured in preconditioned, conscious and restrained rats by tail-cuff plethysmography. Data are presented as means ± SEM. Groups were compared using ANOVA. *: p<0.05 respect to day zero of treatment (B), or to L-NAME treated rats (C).
In terms of the metabolization of flavanols by humans and animals, it has been demonstrated that the gastric environment does not affect flavanols and procyanidins stability, and they transit with minimal modification to the small intestine where can be absorbed.(21) EC reached detectable values in human plasma after dietary administration of chocolate or cocoa derived products.(22–25) These results demonstrate that the EC present in the raw material, i.e. cocoa beans, even after the alterations suffered by manufacturing and metabolization processes, is present in tissues after its ingestion.
Flavanols and Human Blood Pressure
Pioneer observations by McCullough et al.(26) indicated that the Kuna Indians of Panama have a very low incidence of hypertension and cardiovascular disease, but when members of this tribe moved to Panama urban places, their BP increased. The migration leads to cultural changes including an important decrease in cocoa consumption, making cocoa the potential responsible for the observed changes in BP. Epidemiologically, a sub-study of the Zutphen population showed that cocoa (chocolate) consumption was associated with a decrease in BP and cardiovascular mortality.(5) Intervention trials with cocoa and cocoa products have included different groups of subjects: normotensive (young, old, overweight, hypercholesterolemic), pre-hypertensive, hypertensive stage 1, and hypertensive with impaired glucose tolerance. Most of the studies showed that cocoa consumption was associated with a decrease in BP.(27)
Hooper et al.(13) reviewed the effectiveness of different flavonoid subclasses and flavonoid-rich food sources on cardiovascular disease, and one of the risk factors measured was BP. Through the analysis of 133 trials, their three significant conclusions were: i) chocolate decreased diastolic BP and systolic BP (SBP); ii) soy protein isolate (and no other soy derived products) decreased diastolic BP; and iii) acute ingestion of black tea increased SBP and diastolic BP. In the same direction, another meta-analysis about the effects of cocoa and tea on BP, showed that foods rich in cocoa may reduce, while tea intake appears to have no effect on BP.(12) A new and recent meta-analysis evaluating the effect of cocoa rich-foods on BP of hypertensive and normotensive individuals concludes that dark chocolate is superior to placebo in reducing systolic hypertension or diastolic prehypertension.(14) In contrast to studies using chocolate or cocoa preparations, only few studies have been developed on human subjects by using purified flavanols. One study was carried out by dietary supplementation with (+)-catechin in obese and near-obese Japanese children.(28) Results, stratified by the median of the initial BP values, showed that consumption of 75 mg (+)-catechin/d for 24 w was effective in reducing SBP in the above-median category. The studies with isolated flavanols are crucial to assess these compounds as responsible for the antihypertensive effects of flavanol containing foods.
Flavanols, Nitric Oxide and Oxidative Stress in Human Studies
The BP-lowering effect of cocoa-rich products have been correlated with: i) increases in plasma or urine NO-derived species; ii) improvement in NO-mediated flow-mediated dilation (FMD) as an indicative of vascular function; and iii) reduced oxidative stress. Plasma NO metabolites (measured as S-nitrosothiols) were increased in prehypertensive and hypertensive patients after 18 weeks consuming daily 6.3 g of chocolate (30 mg of polyphenols).(29) The oral ingestion of a high-flavanol cocoa drink (917 mg of flavonols) resulted in a transient increase in NO-derived species concentration in plasma and urine of healthy subjects.(30) This increase was associated with the improvement of FMD, and with plasma flavanol levels. More recently, the oral ingestion of 200 mg of quercetin or EC was also associated with increased plasma and urine NO metabolites.(31) FMD was improved in hypertensive individuals (with and without impaired glucose tolerance) in association with decreased BP after 2 weeks consuming 100 g of chocolate.(32,33) Engler et al.(34) treated normotensive individuals for 2 weeks with chocolate and did not find any difference in BP and oxidative stress parameters, but a significant increase in FMD. However, Fraga et al.(35) also working with a normotensive group and chocolate supplementation for 2 weeks, found positive results: decrease in BP associated to improvement in oxidative stress markers (plasma malondialdehyde, vitamin E/low density lipoprotein (LDL), vitamin E/cholesterol). More recently, an intervention study administering cocoa beverage to a group of subjects with essential hypertension for 2 weeks was developed.(36) There were no changes in BP, but the insulin-mediated changes in brachial diameter were higher in cocoa treated patients than in placebo group. In another study, it was observed that two different doses of polyphenols (500 mg or 1000 mg during 2 weeks) were equally effective in reducing BP.(37)
One important factor to consider when an integrative conclusion wants to be drawn from all these investigations is the composition of the chocolate used. Unfortunately, there is not a standardized chocolate or a consistent standardization (qualitative and/or quantitative) of the chocolate components through the different studies. Animal studies using purified compounds are a valid alternative to advance on the comprehension of the mechanisms underlying the BP lowering effect of cocoa derived products.
Flavanols, Nitric Oxide and Oxidative Stress in Animal Studies
Mechanistic aspects defining the beneficial effect of flavonoid-rich diets on endothelial function and BP have been studied in a variety of animal experimental models.(27,38) However, only a few studies have been carried out by using cocoa derived products. A commercially available natural flavonoid-enriched cocoa powder was tested in spontaneously hypertensive rats (SHR). A single oral administration of different doses of the product (50–600 mg/kg) produced an antihypertensive effect in SHR without modifications in the BP of normotensive Wistar-Kyoto rats. The maximum effect on BP, caused by 300 mg/kg of powder, produced a BP decrease similar to the one obtained with 50 mg/kg captopril (an angiotensin converting enzyme inhibitor).(39) More recently, the same flavonoid-enriched cocoa powder was studied as antihypertensive in SHR in a long-term treatment. Animals received the flavonoid-enriched cocoa powder in the drinking water at doses 100, 200 and 400 mg/kg/d during 20 weeks. All the doses attenuated the development of hypertension and improved the endothelial function in SHR.(40) However, the higher antihypertensive effect was observed in the group treated with the lowest dose.(40)
A more mechanistic approach has been studied by using a model of type 2 diabetes, the Otsuka Long-Evans Tokushima Fatty (OLETF) rats, compared with nondiabetic Long-Evans Tokushima Otsuka (LETO) rats. All rats were daily treated with (+)-catechin (30 mg/kg/day) or saline for 12 weeks. OLETF rats showed an increase in BP, associated with higher NOX activity and expression of two NOX subunits (p22phox and p47phox) in aortic wall during the studied period. Treatment of the OLETF rats with Cat resulted in the maintenance of BP, arterial NOX activity and expression, and the oxidative stress conditions in values similar to that observed in saline-treated LETO rats.(41)
Being EC (Fig. 1A) the most abundant flavanol in cocoa, the potential of this compound as responsible for the cocoa and chocolate BP-lowering effect is of singular relevance. Sprague-Dawley rats were pre-treated with the NO-synthase inhibitor Nω-nitro-L-arginine methyl ester (L-NAME) in the drinking water during 6 days. After that period, an increase of 25 mm Hg to 122 ± 6 mm Hg was observed (Fig. 1B). From that day, the rats were maintained during 8 days under the same L-NAME treatment, but receiving different diets: one group was fed with a control diet (L-NAME group) and the second group with an EC-enriched diet (0.4 g EC/100 g diet) (L-NAME + EC group). SBP was measured during the following 8 days in both dietary groups. At the end of that period, the group receiving control diet showed an additional increase in BP. Meanwhile, the group receiving the diet supplemented with EC maintained the initial BP values (Fig. 1C). These results suggest that the presence of EC in the diet can prevent the sustained BP increase induced by a deficiency in NO production associated to the L-NAME treatment.
The Hypothesis of Nitric Oxide Bioavailability
The concept of NO as a modulator of BP is centered in the fact that sufficient NO bioavailability is associated with normal vasodilation and normal BP. On the other hand, decreases in NO steady-state concentration can lead to a failure in smooth muscle relaxation and the consequent hypertension. As indicated, under physiological conditions, most NO is produced by endothelial cells due to eNOS activity. In response to inflammatory stimuli NO can also be produced by iNOS in adventitia cells.(42) The small size and lipophilic nature of NO allow the rapid diffusion of NO through cell membranes to reach its target cells, the smooth muscle cells.(15)
The principal sources of superoxide anion in the vasculature are NOX activities, a family of oxidases that catalyze superoxide anion production using NADPH as the electron donor. Three, out of five NOX identified in animal tissues, have been reported in the rat vasculature: NOX1 in smooth muscle cells; NOX2 in endothelial and adventitial cells; and NOX4 in endothelial cells.(43) Superoxide anion produced in the vascular wall will “consume” the NO diffusing from the endothelial cells being determinant of NO availability in the smooth muscle cells.
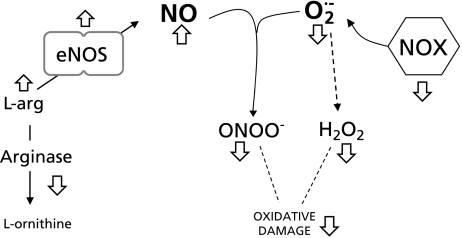
The processes involved in the maintenance of NO steady-state level have multiple steps susceptible to be regulated by flavanols (Fig. 2): i) decrease in superoxide anion and related oxidants concentration in the cells by direct reaction (free radical scavenging action of EC);(44) ii) decrease in superoxide anion concentration in the cell by diminishing NOX expression and/or activity; iii) increase in NO production by augmentation of eNOS expression and/or activity; iv) modulation of enzymes and receptors associated to ROS production, e.g., angiotensin II-mediated pathways,(42,45) and v) protection of oxidative loss of tetrahydrobiopterin to avoid eNOS uncoupling (a condition in which eNOS produces large quantities of superoxide anion rather than NO).(46) The final condition of optimal NO steady-state concentration can be reached by either, increasing its synthesis (without modification in its degradation), or by diminishing superoxide anion-dependent NO loss. In the latter situation, an undesirable high peroxynitrite formation is avoided and then, cells are protected from oxidative and nitrosative damage.
Fig. 2.
NO and superoxide anion in the vasculature. Normal BP should be the resultant of sufficient steady-state concentration of NO. White arrows indicate the steps in which (−)-epicatechin has been shown to exert biochemical actions resulting in its antihypertensive actions.
The basic chemical features of EC, shared with most of the flavonoids, allow them to act as classic antioxidants, i.e. free radical scavengers and/or redox-active metal chelators. Even though those antioxidant reactions are thermodynamically favored, the actual concentration of flavonoids reached in the vasculature makes quantitatively unfeasible these direct antioxidant mechanisms.(47) Then, other biochemical mechanisms, probably related to more specific interactions with proteins or lipids, should be responsible for the in vivo effects of flavanols decreasing oxidative stress. These mechanisms should be attained at the low flavanol concentration observed (or estimated) to occur in most human and animal tissues.
EC has been reported as responsible for eNOS activation in human coronary artery endothelial cells culture.(48) EC treatment activates eNOS via serine 633 and serine 1177 phosphorylation and threonine 495 dephosphorylation, inducing eNOS dissociation from the inhibitory protein caveolin-1. The authors propose that EC effects are calcium-dependent and related to actions exerted at the cell membrane level. In this line, we have postulated the lipid rafts as targets for EC action.(49)
Other possible mechanism to increase NO production is to preserve the intracellular arginine pool. Arginine, which is the eNOS substrate, is also metabolized by the enzyme arginase. Inhibition of arginase activity will limit NO production. Experiments in human umbilical vein endothelial cells (HUVEC) suggest the action of EC diminishing mRNA expression and activity of arginase.(50) Moreover, dietary intervention with flavanol-rich cocoa caused decreases in arginase activity in rat kidney and human erythrocytes.(50)
In an interesting mechanistic study, EC was associated with increased NO levels in HUVEC.(51) The authors observed a decrease in superoxide anion concentration as a result of a direct inhibition of NOX by EC metabolites. In addition, it was showed that while EC is an efficient superoxide anion scavenger, its O-methylated metabolites directly inhibit NOX. These metabolites have been reported as predominant species after EC ingestion by human and rats, and are structurally similar to apocynin, a well-known NOX inhibitor.(51) Supporting this hypothesis, inhibition of the enzyme catechol-O-methyltransferase in HUVEC preceding the EC treatments, prevented the NO increase. This study indicates that methylation of EC could be essential to inhibit NOX, and as a consequence, superoxide anion production.
Finally, changes in eNOS and/or NOX expression in aorta have been reported in animals treated with purified compounds, such as Cat,(41) the flavonol quercetin,(38) the polyphenol resveratrol(52) and dietary extracts rich in flavonoids.(53,54) However, there is no data about EC modifying the expression of those enzymes in the aortic wall.
Conclusions
Flavanol containing foods, specially cocoa and cocoa-derived products have demonstrated to have BP-lowering effects in both, humans and animals. These effects could be related to the maintenance of optimal NO levels, and could be associated with lowering superoxide anion production in the vasculature.
Acknowledgments
This work was supported by grants of the University of Buenos Aires, UBACyT (2010-2013), ANPCyT (PICT 00994). MCL is ANPCyT fellowship. CGF and MG are members of the Scientific Investigator Career, CONICET, Argentina.
Abbreviations
- BP
blood pressure
- NO
nitric oxide
- O2•−
superoxide anion
- NOS
nitric oxide synthase
- NOX
NADPH oxidase
- EC
(−)-epicatechin
- Cat
(+)-catechin
- SBP
systolic blood pressure
- FMD
flow-mediated dilation
- LDL
low density lipoprotein
- SHR
spontaneously hypertensive rats
- OLETF rat
Otsuka Long-Evans Tokushima Fatty rats
- LETO rat
nondiabetic Long-Evans Tokushima Otsuka rats
- L-NAME
Nω-nitro-L-arginine methyl ester
- HUVEC
human umbilical vein endothelial cells
References
- 1.Maruthur NM, Wang NY, Appel LJ. Lifestyle interventions reduce coronary heart disease risk: results from the PREMIER Trial. Circulation. 2009;119:2026–2031. doi: 10.1161/CIRCULATIONAHA.108.809491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 3.Liu S, Manson JE, Lee IM, et al. Fruit and vegetable intake and risk of cardiovascular disease: the Women’s Health Study. Am J Clin Nutr. 2000;72:922–928. doi: 10.1093/ajcn/72.4.922. [DOI] [PubMed] [Google Scholar]
- 4.Joshipura KJ, Hu FB, Manson JE, et al. The effect of fruit and vegetable intake on risk for coronary heart disease. Ann Intern Med. 2001;134:1106–1114. doi: 10.7326/0003-4819-134-12-200106190-00010. [DOI] [PubMed] [Google Scholar]
- 5.Buijsse B, Feskens EJ, Kok FJ, Kromhout D. Cocoa intake, blood pressure, and cardiovascular mortality: the Zutphen Elderly Study. Arch Intern Med. 2006;166:411–417. doi: 10.1001/archinte.166.4.411. [DOI] [PubMed] [Google Scholar]
- 6.Holt EM, Steffen LM, Moran A, et al. Fruit and vegetable consumption and its relation to markers of inflammation and oxidative stress in adolescents. J Am Diet Assoc. 2009;109:414–421. doi: 10.1016/j.jada.2008.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iqbal R, Anand S, Ounpuu S, et al. Dietary patterns and the risk of acute myocardial infarction in 52 countries: results of the INTERHEART study. Circulation. 2008;118:1929–1937. doi: 10.1161/CIRCULATIONAHA.107.738716. [DOI] [PubMed] [Google Scholar]
- 8.Lichtenstein AH, Appel LJ, Brands M, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114:82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 9.Hung HC, Joshipura KJ, Jiang R, et al. Fruit and vegetable intake and risk of major chronic disease. J Natl Cancer Inst. 2004;96:1577–1584. doi: 10.1093/jnci/djh296. [DOI] [PubMed] [Google Scholar]
- 10.Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993;342:1007–1011. doi: 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- 11.Mursu J, Voutilainen S, Nurmi T, Tuomainen TP, Kurl S, Salonen JT. Flavonoid intake and the risk of ischaemic stroke and CVD mortality in middle-aged Finnish men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Br J Nutr. 2008;100:890–895. doi: 10.1017/S0007114508945694. [DOI] [PubMed] [Google Scholar]
- 12.Taubert D, Roesen R, Schömig E. Effect of cocoa and tea intake on blood pressure: a meta-analysis. Arch Intern Med. 2007;167:626–634. doi: 10.1001/archinte.167.7.626. [DOI] [PubMed] [Google Scholar]
- 13.Hooper L, Kroon PA, Rimm EB, et al. Flavonoids, flavonoid-rich foods, and cardiovascular risk: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2008;88:38–50. doi: 10.1093/ajcn/88.1.38. [DOI] [PubMed] [Google Scholar]
- 14.Ried K, Sullivan T, Fakler P, Frank OR, Stocks NP. Does chocolate reduce blood pressure? A meta-analysis. BMC Med. 2010;8:39. doi: 10.1186/1741-7015-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ignarro LJ. Nitric oxide as a unique signaling molecule in the vascular system: a historical overview. J Physiol Pharmacol. 2002;53:503–514. [PubMed] [Google Scholar]
- 16.Pendyala S, Usatyuk PV, Gorshkova IA, Garcia JG, Natarajan V. Regulation of NADPH oxidase in vascular endothelium: the role of phospholipases, protein kinases, and cytoskeletal proteins. Antioxid Redox Signal. 2009;11:841–860. doi: 10.1089/ars.2008.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaganath I, Crozier A. Dietary flavonoids and phenolic compounds. In: Fraga CG, editor. Plant Phenolics and Human Health: Biochemistry, Nutrition and Pharmacology Hoboken. New Jersey: John Wiley & Sons; 2010. pp. 1–49. [Google Scholar]
- 18.Cooper KA, Campos-Giménez E, Jiménez Alvarez, Nagy K, Donovan JL, Williamson G. Rapid reversed phase ultra-performance liquid chromatography analysis of the major cocoa polyphenols and inter-relationships of their concentrations in chocolate. J Agric Food Chem. 2007;55:2841–2847. doi: 10.1021/jf063277c. [DOI] [PubMed] [Google Scholar]
- 19.Andres-Lacueva C, Monagas M, Khan N, et al. Flavanol and flavonol contents of cocoa powder products: influence of the manufacturing process. J Agric Food Chem. 2008;56:3111–3117. doi: 10.1021/jf0728754. [DOI] [PubMed] [Google Scholar]
- 20.Miller KB, Hurst WJ, Flannigan N, et al. Survey of commercially available chocolate- and cocoa-containing products in the United States. 2. Comparison of flavan-3-ol content with nonfat cocoa solids, total polyphenols, and percent cacao. J Agric Food Chem. 2009;57:9169–9180. doi: 10.1021/jf901821x. [DOI] [PubMed] [Google Scholar]
- 21.Rios LY, Bennett RN, Lazarus SA, Rémésy C, Scalbert A, Williamson G. Cocoa procyanidins are stable during gastric transit in humans. Am J Clin Nutr. 2002;76:1106–1110. doi: 10.1093/ajcn/76.5.1106. [DOI] [PubMed] [Google Scholar]
- 22.Rein D, Lotito S, Holt RR, Keen CL, Schmitz HH, Fraga CG. Epicatechin in human plasma: in vivo determination and effect of chocolate consumption on plasma oxidation status. J Nutr. 2000;130:2109S–2114S. doi: 10.1093/jn/130.8.2109S. [DOI] [PubMed] [Google Scholar]
- 23.Wang JF, Schramm DD, Holt RR, et al. A dose-response effect from chocolate consumption on plasma epicatechin and oxidative damage. J Nutr. 2000;130:2115S–2119S. doi: 10.1093/jn/130.8.2115S. [DOI] [PubMed] [Google Scholar]
- 24.Holt RR, Schramm DD, Keen CL, Lazarus SA, Schmitz HH. Chocolate consumption and platelet function. JAMA. 2002;287:2212–2213. doi: 10.1001/jama.287.17.2212. [DOI] [PubMed] [Google Scholar]
- 25.Serafini M, Bugianesi R, Maiani G, Valtuena S, De Santis, Crozier A. Plasma antioxidants from chocolate. Nature. 2003;424:1013. doi: 10.1038/4241013a. [DOI] [PubMed] [Google Scholar]
- 26.McCullough ML, Chevaux K, Jackson L, et al. Hypertension, the Kuna, and the epidemiology of flavanols. J Cardiovasc Pharmacol. 2006;47(Suppl 2):S103–109. doi: 10.1097/00005344-200606001-00003. discussion 19–21. [DOI] [PubMed] [Google Scholar]
- 27.Galleano M, Pechanova O, Fraga CG. Hypertension, nitric oxide, oxidants, and dietary plant polyphenol. Curr Pharm Biotechnol. 2010;11:837–848. doi: 10.2174/138920110793262114. [DOI] [PubMed] [Google Scholar]
- 28.Matsuyama T, Tanaka Y, Kamimaki I, Nagao T, Tokimitsu I. Catechin safely improved higher levels of fatness, blood pressure, and cholesterol in children. Obesity (Silver Spring) 2008;16:1338–1348. doi: 10.1038/oby.2008.60. [DOI] [PubMed] [Google Scholar]
- 29.Taubert D, Roesen R, Lehmann C, Jung N, Schömig E. Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide: a randomized controlled trial. JAMA. 2007;298:49–60. doi: 10.1001/jama.298.1.49. [DOI] [PubMed] [Google Scholar]
- 30.Schroeter H, Heiss C, Balzer J, et al. (–)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc Natl Acad Sci USA. 2006;103:1024–1029. doi: 10.1073/pnas.0510168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loke WM, Hodgson JM, Proudfoot JM, McKinley AJ, Puddey IB, Croft KD. Pure dietary flavonoids quercetin and (–)-epicatechin augment nitric oxide products and reduce endothelin-1 acutely in healthy men. Am J Clin Nutr. 2008;88:1018–1025. doi: 10.1093/ajcn/88.4.1018. [DOI] [PubMed] [Google Scholar]
- 32.Grassi D, Desideri G, Necozione S, et al. Blood pressure is reduced and insulin sensitivity increased in glucose-intolerant, hypertensive subjects after 15 days of consuming high-polyphenol dark chocolate. J Nutr. 2008;138:1671–1676. doi: 10.1093/jn/138.9.1671. [DOI] [PubMed] [Google Scholar]
- 33.Grassi D, Necozione S, Lippi C, et al. Cocoa reduces blood pressure and insulin resistance and improves endothelium-dependent vasodilation in hypertensives. Hypertension. 2005;46:398–405. doi: 10.1161/01.HYP.0000174990.46027.70. [DOI] [PubMed] [Google Scholar]
- 34.Engler MB, Engler MM, Chen CY, et al. Flavonoid-rich dark chocolate improves endothelial function and increases plasma epicatechin concentrations in healthy adults. J Am Coll Nutr. 2004;23:197–204. doi: 10.1080/07315724.2004.10719361. [DOI] [PubMed] [Google Scholar]
- 35.Fraga CG, Actis-Goretta L, Ottaviani JI, et al. Regular consumption of a flavanol-rich chocolate can improve oxidant stress in young soccer players. Clin Dev Immunol. 2005;12:11–17. doi: 10.1080/10446670410001722159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muniyappa R, Hall G, Kolodziej TL, Karne RJ, Crandon SK, Quon MJ. Cocoa consumption for 2 wk enhances insulin-mediated vasodilatation without improving blood pressure or insulin resistance in essential hypertension. Am J Clin Nutr. 2008;88:1685–1696. doi: 10.3945/ajcn.2008.26457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Almoosawi S, Fyfe L, Ho C, Al-Dujaili E. The effect of polyphenol-rich dark chocolate on fasting capillary whole blood glucose, total cholesterol, blood pressure and glucocorticoids in healthy overweight and obese subjects. Br J Nutr. 2010;103:842–850. doi: 10.1017/S0007114509992431. [DOI] [PubMed] [Google Scholar]
- 38.Perez-Vizcaino F, Duarte J, Jimenez R, Santos-Buelga C, Osuna A. Antihypertensive effects of the flavonoid quercetin. Pharmacol Rep. 2009;61:67–75. doi: 10.1016/s1734-1140(09)70008-8. [DOI] [PubMed] [Google Scholar]
- 39.Cienfuegos-Jovellanos E, Quiñones Mdel, Muguerza B, Moulay L, Miguel M, Aleixandre A. Antihypertensive effect of a polyphenol-rich cocoa powder industrially processed to preserve the original flavonoids of the cocoa beans. J Agric Food Chem. 2009;57:6156–6162. doi: 10.1021/jf804045b. [DOI] [PubMed] [Google Scholar]
- 40.Quiñones M, Sánchez D, Muguerza B, et al. Long-term intake of CocoanOX attenuates the development of hypertension in spontaneously hypertensive rats. Food Chemistry. 2010;122:1013–1019. [Google Scholar]
- 41.Ihm SH, Lee JO, Kim SJ, et al. Catechin prevents endothelial dysfunction in the prediabetic stage of OLETF rats by reducing vascular NADPH oxidase activity and expression. Atherosclerosis. 2009;206:47–53. doi: 10.1016/j.atherosclerosis.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 42.Zhang H, Du Y, Cohen RA, Chobanian AV, Brecher P. Adventitia as a source of inducible nitric oxide synthase in the rat aorta. Am J Hypertens. 1999;12:467–475. doi: 10.1016/s0895-7061(98)00271-4. [DOI] [PubMed] [Google Scholar]
- 43.Li JM, Shah AM. ROS generation by nonphagocytic NADPH oxidase: potential relevance in diabetic nephropathy. J Am Soc Nephrol. 2003;14:S221–S226. doi: 10.1097/01.asn.0000077406.67663.e7. [DOI] [PubMed] [Google Scholar]
- 44.Steffen Y, Gruber C, Schewe T, Sies H. Mono-O-methylated flavanols and other flavonoids as inhibitors of endothelial NADPH oxidase. Arch Biochem Biophys. 2008;469:209–219. doi: 10.1016/j.abb.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 45.Brewster UC, Setaro JF, Perazella MA. The renin-angiotensin-aldosterone system: cardiorenal effects and implications for renal and cardiovascular disease states. Am J Med Sci. 2003;326:15–24. doi: 10.1097/00000441-200307000-00003. [DOI] [PubMed] [Google Scholar]
- 46.Paravicini TM, Touyz RM. Redox signaling in hypertension. Cardiovasc Res. 2006;71:247–258. doi: 10.1016/j.cardiores.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 47.Fraga CG. Plant polyphenols: how to translate their in vitro antioxidant actions to in vivo conditions. IUBMB Life. 2007;59:308–315. doi: 10.1080/15216540701230529. [DOI] [PubMed] [Google Scholar]
- 48.Ramirez-Sanchez I, Maya L, Ceballos G, Villarreal F. (−)-epicatechin activation of endothelial cell endothelial nitric oxide synthase, nitric oxide, and related signaling pathways. Hypertension. 2010;55:1398–1405. doi: 10.1161/HYPERTENSIONAHA.109.147892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Galleano M, Verstraeten SV, Oteiza PI, Fraga CG. Antioxidant actions of flavonoids: thermodynamic and kinetic analysis. Arch Biochem Biophys. 2010;501:23–30. doi: 10.1016/j.abb.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 50.Schnorr O, Brossette T, Momma TY, et al. Cocoa flavanols lower vascular arginase activity in human endothelial cells in vitro and in erythrocytes in vivo. Arch Biochem Biophys. 2008;476:211–215. doi: 10.1016/j.abb.2008.02.040. [DOI] [PubMed] [Google Scholar]
- 51.Steffen Y, Schewe T, Sies H. (–)-Epicatechin elevates nitric oxide in endothelial cells via inhibition of NADPH oxidase. Biochem Biophys Res Commun. 2007;359:828–833. doi: 10.1016/j.bbrc.2007.05.200. [DOI] [PubMed] [Google Scholar]
- 52.Miatello R, Vázquez M, Renna N, Cruzado M, Zumino AP, Risler N. Chronic administration of resveratrol prevents biochemical cardiovascular changes in fructose-fed rats. Am J Hypertens. 2005;18:864–870. doi: 10.1016/j.amjhyper.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 53.Agouni A, Lagrue-Lak-Hal AH, Mostefai HA, et al. Red wine polyphenols prevent metabolic and cardiovascular alterations associated with obesity in Zucker fatty rats (Fa/Fa) PLoS One. 2009;4:e5557. doi: 10.1371/journal.pone.0005557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mahn K, Borrás C, Knock GA, et al. Dietary soy isoflavone induced increases in antioxidant and eNOS gene expression lead to improved endothelial function and reduced blood pressure in vivo. FASEB J. 2005;19:1755–1757. doi: 10.1096/fj.05-4008fje. [DOI] [PubMed] [Google Scholar]