Abstract
The essentiality of polyunsaturated lipids makes membranes susceptible to peroxidative modifications. One of the most contemporary examples includes selective peroxidation of cardiolipin in mitochondria of cells undergoing apoptosis. Cardiolipin peroxidation products are required for the mitochondrial membrane permeabilization, release of pro-apoptotic factors and completion of the cell death program. Therefore, search for effective inhibitors of cardiolipin peroxidation is critical to discovery and development of anti-apoptotic antioxidants. Mitochondria contain significant amounts of α-tocopherol, a well known scavenger of reactive free radicals. In the present study, we used an oxidative lipidomics approach to evaluate the effect of α-tocopherol and its homologues with different lengths of the side-chain such as 2,5,7,8,-tetramethyl-2(4-methylpentyl)-6-chromanol and 2,2,5,7,8-pentamethyl-6-chromanol, on oxidation of tetralinoleoyl cardiolipin induced by cytochrome c in the presence of hydrogen peroxide. Our data indicate that vitamin E homologues inhibit not only accumulation of tetralinoleoyl cardiolipin hydroperoxides but also hydroxy-derivatives of tetralinoleoyl cardiolipin formed in the enzymatic peroxidase half-reaction catalyzed by cytochrome c. This suggests that protective effects of vitamin E homologues against tetralinoleoyl cardiolipin peroxidation catalyzed by cytochrome c/hydrogen peroxide are realized largely due to their effects on the peroxidase activity of cytochrome c towards tetralinoleoyl cardiolipin rather than via their scavenging activity.
Keywords: cytochrome c, cardiolipin peroxidation, peroxidase activity, antioxidants, α-tocopherol, lipidomics
Introduction
Polyunsaturated lipids are essential for life: they represent the structural core of membranes both as uninterrupted bilayer and as microenvironment of transmembrane proteins, they act as precursors of physiological regulators and as a fuel and energy resource.(1) Complex functions of membranes necessitate the asymmetry of lipid distribution of polyunsaturated lipids both across the bilayer and within the two monolayers. This requires specialized intracellular machinery and its collapse is associated with cell death pathways.(2) Recent MS-based analysis demonstrated remarkable diversity of polyunsaturated lipids and identified thousands of their molecular species in each cell.(3)
The essentiality of polyunsaturated lipids makes membranes vulnerable to oxidative damage due to their susceptibility to peroxidation. For decades, the prevailing dogma was that the major factor driving the peroxidation process is the number of double bonds in their molecules.(4) Given that most of membranes contain sufficient amounts of polyunsaturated lipids—far exceeding those utilized during the peroxidation process—the abundance of most classes of phospholipids with four-, five- and six double bonds has been viewed as the major factor defining the meaning and kinetics of free radical-driven peroxidation process propagated randomly in membranes.(5) Not surprisingly, the process of lipid peroxidation has been long associated almost exclusively with cell and tissue injury.(6–8) Recent advancements and developments in lipidomics and oxidative lipidomics uncovered new roles of peroxidized polyunsaturated lipids in cell physiology and signaling and established that the peroxidation products accumulate selectively in particular classes of phospholipids with asymmetric topography.(9)
One of the most contemporary examples includes selective peroxidation of a mitochondria-specific phospholipid, cardiolipin (CL) that is normally confined almost exclusively to the inner mitochondrial membrane and is lacking from the outer mitochondrial membrane.(10) This asymmetric topography of CL is characteristic of normal mitochondria and is mainly due to its synthesis on the matrix side of the inner membrane.(11) The diversity of CL is tissue-specific and ranges from only few kinds of molecular species in the liver and heart, over a dozen of molecular species in the lung and small intestine, and hundreds of different species of CL in the brain.(12,13) While the importance of CL for mitochondrial functions and its association with many mitochondrial membrane proteins has been firmly established,(14–16) the significance and mechanisms controlling tissue-specific molecular diversification are far from being clear; it is possible that a large number of CL molecular species in the brain is utilized for the production of intra- and extracellular regulators and mediators—eicosanoids as well as docosahexanoids.(17)
Universally, early in apoptosis CL transmigrates from the inner to the outer mitochondrial membrane.(18) This migration is facilitated by at least four different mitochondrial proteins: i) scramblase-3 activatable via protein kinase C-delta phosphorylation at Thr21,(19) ii) mitochondrial phosphocreatine kinase that forms an octamer spanning the distance between the two membranes at the contact sites of mitochondria,(20) iii) dinucleotide phosphokinase D acting in a similar manner in its hexameric form, and iv) pro-apoptotic protein tBid.(21) While molecular details and contribution of each of these mechanisms awaits further studies, the fact of the equilibration of CL between the inner and outer membranes, i.e., collapse of CL asymmetry has been demonstrated in apoptosis.(22) The appearance of CL on the membrane surfaces of the intermembrane space facilitates its interactions with an abundant intermembrane space hemoprotein, cytochrome c (cyt c).
Normally cyt c functions as a shuttle between mitochondrial complexes III and IV.(23) Binding with CL and formation of cyt c/CL complex confers peroxidase activity on the hemoprotein by causing its partial unfolding, loosening Met80 bond with the heme-iron and creating a new structure whereby small molecules—such as H2O2—get access to the heme catalytic site.(24) Most importantly, the cyt c/CL complex can catalyze peroxidation of polyunsaturated bound CL. This peroxidation reaction proceeds as a typical enzymatic reaction and generates CL hydroperoxides (CL-OOH) which can be reduced by cyt c to CL hydroxides (CL-OH). In the context of this review, the most important feature of this peroxidation reaction is that it is non-random and in mitochondria includes selective peroxidation of CL. Thus the rule of abundant substrates—polyunsaturated phospholipids—is not obeyed in this process and highly polyunsaturated phosphatidylcholine (PC), phosphatidylethanolamine (PE) (with 4–6 double bonds) do not undergo peroxidation while less polyunsaturated tetra-linoleoyl cardiolipin (TLCL) gets exclusively peroxidized. The selective CL peroxidation has been documented not only in vitro but also during apoptosis induced in the lung and small intestinal tissue, after total body irradiation of mice.(25) Notably, CL peroxidation products are required for the mitochondrial membrane permeabilization, release of pro-apoptotic factors and completion of the cell death program. Therefore, search for effective inhibitors of CL peroxidation is critical to discovery and development of new anti-apoptotic “antioxidants”. This brings the review to the point where mechanisms of antioxidant action of the major lipid-soluble antioxidants of membranes and lipoproteins(26) should be viewed not only as sacrificial chain-breaking radical scavengers but also from the angle of their ability to regulate enzymatic CL peroxidation catalyzed by cyt c/CL complexes.
Vitamin E (α-tocopherol, α-Toc) is the major lipid-soluble antioxidant of biological membranes and lipoproteins. In line with its antioxidant function, clusters of α-Toc have been associated with membrane microdomains enriched in oxidizable highly unsaturated phospholipids.(27) Numerous in vitro experiments have demonstrated its effectiveness and utility in protection against random phospholipid peroxidation.(28–30) It has been also shown that vitamin E homologues with the different length of the side-chain display different effectiveness in inhibiting lipid peroxidation in model biomembranes and liver organelles.(31) The smallest homologue (α-C1-chromanol, PMC) was most effective in spite of the fact that the reaction rate constants of PMC and α-Toc in scavenging peroxyl radicals are very similar (3.8 × 106 M−1 and 3.2 × 106 M−1 respectively).(32) It has been suggested that the lateral mobility of PMC and other short-chain tocopherol homologues in the membrane is mostly responsible for their high radical scavenging activity in membranes.(31–33) Another important redox feature of vitamin E is its ability to be recycled from its phenoxyl (tocopheroxyl) radical, thus enhancing its overall radical scavenging efficiency. The major small-molecule redox partners for recycling of α-Toc are ubiquinol and ascorbate.(34–36) In addition, electron-transport chains of mitochondria and endoplasmic reticulum can act as donors of electrons for the tocopheroxyl radicals, hence contribute to vitamin E recycling.(37,38)
In the present study, we used an oxidative lipidomics approach to evaluate the effect of α-Toc and its homologues with the different length of the side-chain such as α-C6-chromanol (C6) and PMC, on oxidation of TLCL induced by cyt c in the presence of H2O2. Our results show that all three compounds were able to protect TLCL against cyt c/H2O2 induced oxidation as evidenced by inhibition of accumulation of both hydroxy- and hydroperoxy-molecular species of TLCL. We suggest that protective effect of vitamin E homologues is realized not only due to their scavenging activity but also through their effects on the peroxidase activity cyt c/CL complex.
Materials and Methods
Chemicals
1,1,2,2-Tetralinoleoyl cardiolipin (TLCL); TMCL, 1,1,2,2-tetramyristoyl cardiolipin (TMCL); 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC); were obtained from Avanti Polar Lipids Inc. (Albaster, AL). 4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES), diethylenetriaminepentaacetic acid (DTPA), cytochrome c, α-Tocopherol: DL-2,5,7,8,-tetramethyl-2-(4,8,12-trimethyltridecyl)-6-chromanol and HPLC solvents were purchased from Sigma-Aldrich (St. Louis, MO). α-C1-chromanol: 2,2,5,7,8,-pentamethyl-6-chromanol (PMC) was a generous gift from Eisai Co. (Tokyo, Japan) and C6: 2,5,7,8,-Tetramethyl-2(4-methylpentyl)-6-chromanol (C6) was a gift from Prof. Evstigneeva, Institute of Fine Chemical Technology, Moscow, Russia).
Oxidation of tetra-linoleyl cardiolipin (TLCL) by cyt c/H2O2
Chloroform solutions of TLCL and dioleoyl phosphatidylcholine (DOPC) were mixed and the solvent was evaporated under N2. After evaporation, 20 mM HEPES, pH 7.4, containing 100 µM DTPA was added and the suspension was vortexed and sonicated using a water-bath sonicator (Fisher Scientific F63). The concentration of TLCL and DOPC were 50 µM and 200 µM, respectively. Liposomes were incubated with cyt c (5 µM) in the presence of H2O2 (100 µM) in 20 mM HEPES, pH 7.4, containing 100 µM DTPA for 10 min at 37°C. Reaction was stopped by addition of catalase (2 U/ml). After that, 0.75% of KCL was added, lipids were extracted using Folch procedure(39) and dried under N2. Then lipids were resuspended in chloroform:methanol (2:1) and used for MS analysis. α-Toc (50 µM) or C6 (50 µM) or PMC (50 µM) were introduced to liposomes prior addition of cyt c and H2O2.
Electrospray ionization mass spectrometry
To quantitatively assess molecular species of oxidized TLCL, LC/ESI-MS was performed using a Dionex UltimateTM 3000 HPLC coupled on-line to ESI and a linear ion trap mass spectrometer with the Xcalibur operating system (Thermo Fisher Scientific, San Jose, CA) as previously described.(40,41) The lipids were separated on a normal phase column (Luna 3 µm Silica 100A, 150 × 2 mm, (Phenomenex, Torrance CA)) with flow rate 0.2 ml/min using gradient solvents containing NH4OH (A-chloroform : methanol : 30% NH4OH—80:19.5:0.5 (v/v/v) and B-chloroform : methanol : water : 30% NH4OH—60:34:5.5:0.5 (v/v/v)).(42) Analysis of phospholipid oxidized molecular species (hydroperoxy- and hydroxy-) was performed as previously described.(25) The ESI probe was operated at a voltage differential of 3.5–5.0 kV in the negative ion mode. Capillary temperature was maintained at 150°C. Using full range zoom (200–2000 m/z) in negative ion mode, the spectra were acquired in centroid mode. Doubly-charged ions were used for quantitative assessment of CL and its oxidation products.
Statistics
The results are presented as mean ± SEM values from at least three experiments, and statistical analyses were performed by one-way ANOVA. The statistical significance of differences was set at p<0.05.
Results and Discussion
Oxidation of TLCL by cyt c/H2O2
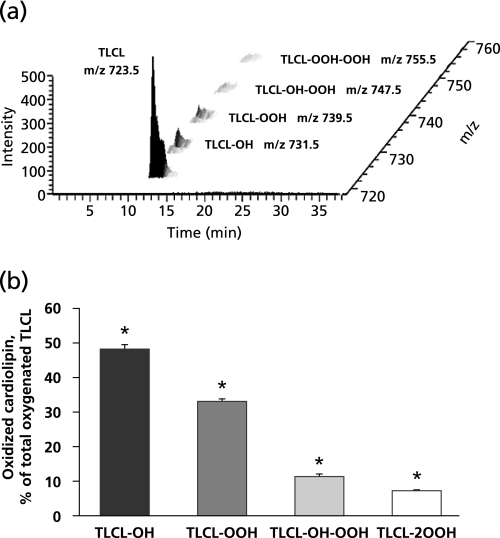
First we quantitatively assessed the oxidation of TLCL in the reaction driven by cyt c in the presence of H2O2. To this end, liposomes (250 µM) containing 20% of TLCL and 80% of DOPC were incubated with cyt c (50 µM) and H2O2 (100 µM) for 10 min at 37°C. At the end of incubation, lipids were extracted and resolved by LC/ESI-MS. Typical 3D-MS map of TLCL oxidized by cyt c/H2O2 is present on Fig. 1a. LC/ESI-MS of oxidized TLCL revealed molecular ions of TLCL with m/z 731.5, 739.5, 747.5, 755.5 corresponding to TLCL species containing one, two, three, and four oxygens, respectively. The characterization of these oxygenated TLCL species was performed using MSn analysis as previously described.(43) We identified the TLCL oxidation products as monohydroxy- (m/z 731.5), monohydroperoxy- (m/z 739.5), monohydroxy-monohydroperoxy- (m/z 747.5), and dihydroperoxy-molecular species (m/z 755.5). Excess of H2O2 can feed the peroxidase cycle of cyt c/CL complexes to produce CL-OOH. Depletion of H2O2 switches the peroxidase reaction of cyt c/CL complexes to utilization of CL-OOH as a source of oxidizing equivalents. This yields a mixture of CL-OOH and CL-OH as the reaction products.(25) Recently we demonstrated that the reaction of cyt c with hydroperoxides may proceed via both homo- and heterolytic pathways.(44)
Fig. 1.
Oxidation of tetra-linoleyl CL (TLCL) induced by cyt c in the presence of H2O2. (a) 3D-MS map of TLCL oxidized by cyt c/H2O2. Monohydroxy-(TLCL-OH, m/z 731.5), monohydroperoxy-(TLCL-OOH, m/z 739.5), monohydroxy-monohydroperoxy-(TLCL-OH-OOH, m/z 747.5), and dihydroperoxy-molecular species (TLCL-2OOH, m/z 755.5) were detected on MS spectrum of oxidized TLCL. (b) Accumulation of TLCL oxygenated products generated by cyt c in the presence of H2O2. Oxygenated TLCL products were enriched with molecular species containing monohydroxy- and monohydroperoxy groups. Data are means ± SEM, n = 12, *: p<0.05 vs cyt c/H2O2.
Next we performed quantitative assessment of CL oxidation products. Under the incubation conditions employed, total accumulation of oxidized TLCL molecular species was 206 ± 33 pmol/nmol of TLCL. Oxygenated TLCL products were enriched with molecular species containing monohydroxy- and monohydroperoxy groups. Their content was 48.1 ± 5.6% and 32.9 ± 3.7% of total oxygenated TLCL species, respectively (Fig. 1b). The accumulation of oxygenated species of TLCL with three and four oxygens was less pronounced and constituted 11.5 ± 2.7% and 7.4 ± 1.3% of total oxidized TLCL, respectively.
Vitamin E homologues protect TLCL against oxidation induced by cyt c/H2O2
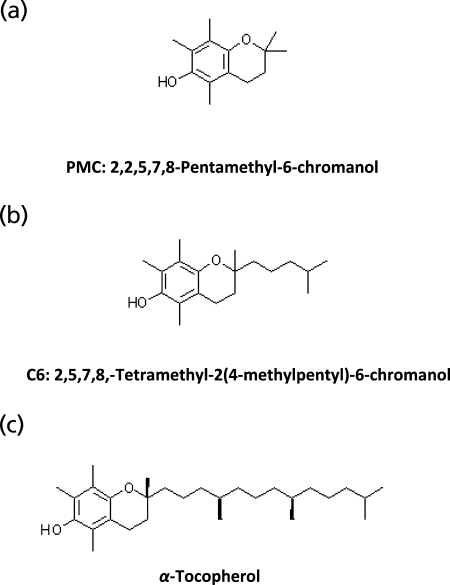
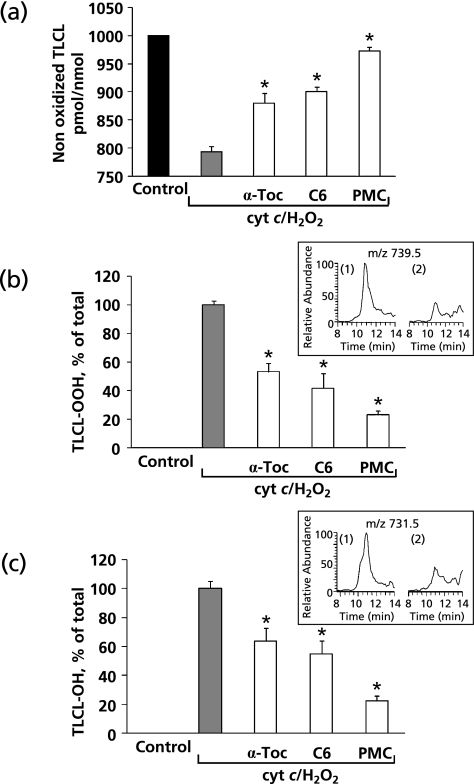
Mitochondria contain significant amounts of α-Toc that has been reported to be an effective scavenger of reactive free radicals.(45) We reasoned that α-Toc and its homologues may be effective in suppressing cyt c-induced TLCL peroxidation. Therefore, we studied the effects of α-Toc and its homologues with different lengths of the side-chain such as C6 and PMC (Fig. 2) on oxidation of TLCL induced by cyt c in the presence of H2O2. We found that all three compounds were able to significantly protect TLCL against oxidation induced by cyt c/H2O2 (Fig. 3). Notably, the protective effects of vitamin E homologues were dependent on the length of the side-chain of the compounds. PMC was most active, while α-Toc was least effective in inhibiting TLCL oxidation. In the presence of PMC, C6 and α-Toc the amounts of non-oxidized TLCL (m/z 723.5) were 972.8 ± 7.4, 900.8 ± 8.1 and 880.3 ± 17.3 pmol/nmol of TLCL as compared to 793.3 ± 9.6 pmol/nmol after incubation with cyt c/H2O2 in the absence of vitamin E or its homologues. Next, we quantitatively assessed the cyt c catalyzed formation of two predominant oxidized molecular species of TLCL, TLCL-OOH (m/z 731.5) and TLCL-OH (m/z 739.5). In the absence of vitamin E homologues, the rate of TLCL-OOH and TLCL-OH accumulation was 7.2 ± 0.2 and 10.3 ± 0.5 pmol/nmol of TLCL per min, respectively. When α-Toc, C6 or PMC were integrated in the liposomes the accumulation of both TLCL-OOH and TLCL-OH was significantly inhibited (Fig. 3b–c). PMC was most effective in the suppression of TLCL peroxidation. The rate of TLCL-OOH and TL-OH formation was decreased to 1.7 ± 0.2 and 2.3 ± 0.3 pmol/nmol of TLCL per min, respectively. Consequently, in the presence of α-Toc and C6, 3.8 ± 0.4 and 3.0 ± 0.7 pmol of TLCL-OOH/nmol of TLCL/min and 6.5 ± 0.1 and 5.6 ± 0.9 pmol of TLCL-OH/nmol of TLCL/min were generated in cyt c driven reaction. Vitamin E homologues, particularly PMC, can effectively compete with TLCL as substrates of cyt c/CL peroxidase reaction to prevent TLCL oxidation. It is also possible that radical scavenging activity may contribute to the suppression of TLCL peroxidation initiated by cyt c in the presence of H2O2. This “scavenging effects” can be realized during accumulation of TLCL hydroperoxides (TLCL-OOH). However, the peroxidase half-reaction, during which molecules of TLCL-OOH are reduced to TLCL-OH could be only affected via the enzymatic cyt c-catalyzed mechanism. This suggests that these vitamin E homologues exert a non-random protection against CL oxidation by affecting the peroxidase activity of cyt c/TLCL complexes.
Fig. 2.
Structural formulas of the vitamin E homologues. (a) 2,2,5,7,8-Pentamethyl-6-chromanol (PMC), (b) 2,5,7,8,-Tetramethyl-2(4-methylpentyl)-6-chromanol (C6), (c) α-Tocopherol (α-Toc).
Fig. 3.
Vitamin E homologues protect TLCL against oxidation induced by cyt c in the presence of H2O2. (a) Protective effect of vitamin E homologues on oxidation of TLCL. Data are means ± SEM, n = 12, *p<0.05 vs cyt c/H2O2. (b) Accumulation of monohydroperoxy molecular species of TLCL (TLCL-OOH) induced by cyt c/H2O2 in the absence and in the presence of vitamin E homologues. Insert: Typical base peak of molecular ion with m/z 739.5 corresponding to monohydroperoxy molecular species of TLCL (1) cyt c/H2O2; (2) cyt c/H2O2 + PMC. Data are means ± SEM, n = 6, *p<0.05 vs cyt c/H2O2. (c) Accumulation of monohydroxy molecular species of TLCL (TLCL-OH) induced by cyt c/H2O2 in the absence and in the presence of vitamin E homologues. Insert: Typical base peak of molecular ion with m/z 731.5 corresponding to monohydroxy molecular species of TLCL: (1) cyt c/H2O2; (2) cyt c/H2O2 + PMC. Data are means ± SEM, n = 6, *: p<0.05 vs cyt c/H2O2.
This conclusion is also supported by the previous work that has demonstrated that vitamin E homologues can serve as substrates(46,47) and inhibitors for different peroxidases.(48) It has been shown that cyt c is able to interact with vitamin E homologues and generate tocopheroxyl radicals(49) in model system as well as in mitochondria.(49) Moreover, the latter can be readily reduced back by cyt c.(49) Thus inhibition of peroxidase activity of cyt c can significantly contribute to the protective effect of vitamin E and its homologues, against oxidative damage to mitochondria.
Acknowledgments
Supported by NIH HL70755, HL094488; U19 AIO68021; by NIOSH OH008282; La Junta de Extremadura,Orden 2008050288 (A.K.S.A.).
Abbreviations
- cyt c
cytochrome c
- CL
cardiolipin
- TLCL
1,1',2,2'-tetralinoleoyl cardiolipin
- TMCL
1,1',2,2'-tetramyristoyl cardiolipin
- DOPC
PC, 1,2-dioleoyl-sn-glycero-3-phosphocholine
- α-Toc
α-Tocopherol, DL-2,5,7,8,-tetramethyl-2-(4,8,12-trimethyltridecyl)-6-chromanol
- C6
α-C6-chromanol, 2,5,7,8,-tetramethyl-2(4-methylpentyl)-6-chromanol
- PMC
α-C1-chromanol, 2,2,5,7,8,-pentamethyl-6-chromanol
- H2O2
hydrogen peroxide
- HEPES
4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid
- DTPA
diethylenetriaminepentaacetic acid
- HPLC
high-pressure liquid chromatography
- LC/ESI-MS
liquid chromatography/electrospray ionisation-mass spectrometry
References
- 1.Eyster KM. The membrane and lipids as integral participants in signal transduction: lipid signal transduction for the non-lipid biochemist. Adv Physiol Educ. 2007;31:5–16. doi: 10.1152/advan.00088.2006. [DOI] [PubMed] [Google Scholar]
- 2.Sorice MA, Circella IM, Cristea T, et al. Cardiolipin and its metabolites move from mitochondria to other cellular membranes during death receptor-mediated apoptosis. Cell Death Differ. 2004;11:1133–1145. doi: 10.1038/sj.cdd.4401457. [DOI] [PubMed] [Google Scholar]
- 3.Blanksby SJ, Mitchell TW. Advances in mass spectrometry for lipidomics. Ann Rev Anal Chem. 2010;3:433–465. doi: 10.1146/annurev.anchem.111808.073705. [DOI] [PubMed] [Google Scholar]
- 4.Sevanian A, Hochstein P. Mechanisms and consequences of lipid peroxidation in biological systems. Annu Rev Nutr. 1985;5:365–390. doi: 10.1146/annurev.nu.05.070185.002053. [DOI] [PubMed] [Google Scholar]
- 5.Girotti AW. Mechanisms of lipid peroxidation. J Free Radic Biol Med. 1985;1:87–95. doi: 10.1016/0748-5514(85)90011-x. [DOI] [PubMed] [Google Scholar]
- 6.Spiteller G. Lipid peroxidation in aging and age-dependent diseases. Exp Gerontol. 2001;36:1425–1457. doi: 10.1016/s0531-5565(01)00131-0. [DOI] [PubMed] [Google Scholar]
- 7.Hennig B, Chow CK. Lipid peroxidation and endothelial cell injury: implications in atherosclerosis. Free Radic Biol Med. 1988;4:99–106. doi: 10.1016/0891-5849(88)90070-6. [DOI] [PubMed] [Google Scholar]
- 8.McCall JM, Braughler JM, Hall ED. Lipid peroxidation and the role of oxygen radicals in CNS injury. Acta Anaesthesiol Belg. 1987;38:373–379. [PubMed] [Google Scholar]
- 9.Fabisiak JP, Tyurina YY, Tyurin VA, Kagan VE. Quantification of selective phosphatidylserine oxidation during apoptosis. Methods Mol Biol. 2005;291:449–456. doi: 10.1385/1-59259-840-4:449. [DOI] [PubMed] [Google Scholar]
- 10.Esposti MD, Cristea IM, Gaskell SJ, Nakao Y, Dive C. Proapoptotic Bid binds to monolysocardiolipin, a new molecular connection between mitochondrial membranes and cell death. Cell Death Differ. 2003;10:1300–1309. doi: 10.1038/sj.cdd.4401306. [DOI] [PubMed] [Google Scholar]
- 11.Schlame M, Haldar D. Cardiolipin is synthesized on the matrix side of the inner membrane in rat liver mitochondria. J Biol Chem. 1993;268:74–79. [PubMed] [Google Scholar]
- 12.Cheng H, Mancuso DJ, Jiang X, et al. Shotgun lipidomics reveals the temporally dependent, highly diversified cardiolipin profile in the mammalian brain: temporally coordinated postnatal diversification of cardiolipin molecular species with neuronal remodeling. Biochemistry. 2008;47:5869–5880. doi: 10.1021/bi7023282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tyurina YY, Tyurin VA, Kapralova VI, et al. Mass-spectrometric characterization of phospholipids and their hydroperoxide derivatives in vivo: effects of total body irradiation. Methods Mol Biol. 2009;580:153–183. doi: 10.1007/978-1-60761-325-1_9. [DOI] [PubMed] [Google Scholar]
- 14.Awasthi YC, Chuang TF, Keenan TW, Crane FL. Association of cardiolipin and cytochrome oxidase. Biochem Biophys Res Commun. 1970;39:822–832. doi: 10.1016/0006-291x(70)90397-9. [DOI] [PubMed] [Google Scholar]
- 15.Schnyder T, Cyrklaff M, Fuchs K, Wallimann T. Crystallization of mitochondrial creatine kinase on negatively charged lipid layers. J Struct Biol. 1994;112:136–147. doi: 10.1006/jsbi.1994.1015. [DOI] [PubMed] [Google Scholar]
- 16.Heimburg T, Marsh D. Investigation of secondary and tertiary structural changes of cytochrome c in complexes with anionic lipids using amide hydrogen exchange measurements: an FTIR study. Biophys J. 1993;65:2408–2417. doi: 10.1016/S0006-3495(93)81299-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muralikrishna Adibhatla R, Hatcher JF. Phospholipase A2, reactive oxygen species, and lipid peroxidation in cerebral ischemia. Free Radic Biol Med. 2006;40:376–387. doi: 10.1016/j.freeradbiomed.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 18.Van Mau, Kajava AV, Bonfils C, Martinou JC, Harricane MC. Interactions of Bax and tBid with lipid monolayers. J Membr Biol. 2005;207:1–9. doi: 10.1007/s00232-005-0799-7. [DOI] [PubMed] [Google Scholar]
- 19.He YJ, Liu J, Grossman D, et al. Phosphorylation of mitochondrial phospholipid scramblase 3 by protein kinase C-delta induces its activation and facilitates mitochondrial targeting of tBid. J Cell Biochem. 2007;101:1210–1221. doi: 10.1002/jcb.21243. [DOI] [PubMed] [Google Scholar]
- 20.Maniti O, Lecompte MF, Marcillat O, et al. Mitochondrial creatine kinase binding to phospholipid monolayers induces cardiolipin segregation. Biophys J. 2009;96:2428–2438. doi: 10.1016/j.bpj.2008.12.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim TH, Zhao Y, Ding WX, et al. Bid-cardiolipin interaction at mitochondrial contact site contributes to mitochondrial cristae reorganization and cytochrome C release. Mol Biol Cell. 2004;15:3061–3072. doi: 10.1091/mbc.E03-12-0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia FM, Troiano L, Moretti L, et al. Early changes in intramitochondrial cardiolipin distribution during apoptosis. Cell Growth Differ. 2002;13:449–455. [PubMed] [Google Scholar]
- 23.Errede B, Kamen MD. Comparative kinetic studies of cytochromes c in reactions with mitochondrial cytochrome c oxidase and reductase. Biochemistry. 1978;17:1015–1027. doi: 10.1021/bi00599a012. [DOI] [PubMed] [Google Scholar]
- 24.Godoy LC, Munoz-Pinedo C, Castro L, et al. Disruption of the M80-Fe ligation stimulates the translocation of cytochrome c to the cytoplasm and nucleus in nonapoptotic cells. Proc Natl Acad Sci USA. 2009;106:2653–2658. doi: 10.1073/pnas.0809279106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tyurina YY, Tyurin VA, Epperly MW, Greenberger JS, Kagan VE. Oxidative lipidomics of gamma-irradiation-induced intestinal injury. Free Radic Biol Med. 2008;44:299–314. doi: 10.1016/j.freeradbiomed.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 26.Kagan VE, Serbinova EA, Forte T, Scita G, Packer L. Recycling of vitamin E in human low density lipoproteins. J Lipid Res. 1992;33:385–397. [PubMed] [Google Scholar]
- 27.Atkinson J, Harroun T, Wassall SR, Stillwell W, Katsaras J. The location and behavior of α-tocopherol in membranes. Mol Nutr Food Res. 2010;54:641–651. doi: 10.1002/mnfr.200900439. [DOI] [PubMed] [Google Scholar]
- 28.Forsmark P, Aberg F, Norling B, Nordenbrand K, Dallner G, Ernster L. Inhibition of lipid peroxidation by ubiquinol in submitochondrial particles in the absence of vitamin E. FEBS Lett. 1991;285:39–43. doi: 10.1016/0014-5793(91)80720-n. [DOI] [PubMed] [Google Scholar]
- 29.Kagan VE, Tyurin VA, Kitanova SA, Serbinova EA, Quinn PJ, Stoichev Ts. S. Action of a homologous series of ubiquinols on lipid peroxidation in brain mitochondrial and synaptosomal membranes. Bulletin of Experimental Biology and Medicine. 1988;107:468–471. [PubMed] [Google Scholar]
- 30.Niki E, Noguchi N. Dynamics of antioxidant action of vitamin E. Acc Chem Res. 2004;37:45–51. doi: 10.1021/ar030069m. [DOI] [PubMed] [Google Scholar]
- 31.Kagan VE, Serbinova EA, Packer L. Recycling and antioxidant activity of tocopherol homologs of differing hydrocarbon chain lengths in liver microsomes. Arch Biochem Biophys. 1990;282:221–225. doi: 10.1016/0003-9861(90)90108-b. [DOI] [PubMed] [Google Scholar]
- 32.Lucarini M, Pedulli GF. Overview of antioxidant activity of Vitamin E. In: Preedy VR, Watson RR, editors. The Encyclopedia of Vitamin E. CAB international (UK); 2007. pp. 3–10. [Google Scholar]
- 33.Kagan VE, Serbinova EA, Koynova GM, et al. Antioxidant action of ubiquinol homologues with different isoprenoid chain length in biomembranes. Free Radic Biol Med. 1990;9:117–126. doi: 10.1016/0891-5849(90)90114-x. [DOI] [PubMed] [Google Scholar]
- 34.Mukai K, Itoh S, Morimoto H. Stopped-flow kinetic study of vitamin E regeneration reaction with biological hydroquinones (reduced forms of ubiquinone, vitamin K, and tocopherolquinone) in solution. J Biol Chem. 1992;267:22277–22281. [PubMed] [Google Scholar]
- 35.Nagaoka SI, Inoue M, Nishioka C, et al. Tunneling effect in antioxidant, prooxidant, and regeneration reactions of vitamin E. J Phys Chem. 2000;104:856–862. [Google Scholar]
- 36.Samhan-Arias AK, Duarte RO, Martin-Romero FJ, Moura JJ, Gutiérrez-Merino C. Reduction of ascorbate free radical by the plasma membrane of synaptic terminals from rat brain. Arch Biochem Biophys. 2008;469:243–254. doi: 10.1016/j.abb.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 37.Maguire JJ, Kagan V, Ackrell BA, Serbinova E, Packer L. Succinate-ubiquinone reductase linked recycling of α-tocopherol in reconstituted systems and mitochondria: requirement for reduced ubiquinone. Arch Biochem Biophys. 1992;292:47–53. doi: 10.1016/0003-9861(92)90049-3. [DOI] [PubMed] [Google Scholar]
- 38.Kagan VE, Tyurina YY. Recycling and redox cycling of phenolic antioxidants. Ann NY Acad Sci. 1998;854:425–234. doi: 10.1111/j.1749-6632.1998.tb09921.x. [DOI] [PubMed] [Google Scholar]
- 39.Folch J, Lees M, Stanley GHS. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 40.Tyurin VA, Tyurina YY, Kochanek PM, et al. Oxidative lipidomics of programmed cell death. Methods Enzymol. 2008;442:375–393. doi: 10.1016/S0076-6879(08)01419-5. [DOI] [PubMed] [Google Scholar]
- 41.Tyurina YY, Tyurin VA, Kapralova VI, et al. Mass-spectrometric characterization of phospholipids and their hydroperoxide derivatives in vivo: effects of total body irradiation. Methods Mol Biol. 2009;580:153–183. doi: 10.1007/978-1-60761-325-1_9. [DOI] [PubMed] [Google Scholar]
- 42.Malavolta M, Bocci F, Boselli E, Frega NG. Normal phase liquid chromatography-electrospray ionization tandem mass spectrometry analysis of phospholipid molecular species in blood mononuclear cells: application to cystic fibrosis. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;810:173–186. doi: 10.1016/j.jchromb.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 43.Tyurina YY, Tyurin VA, Epperly MW, Greenberger JS, Kagan VE. Oxidative lipidomics of gamma-irradiation-induced intestinal injury. Free Radic Biol Med. 2008;44:299–314. doi: 10.1016/j.freeradbiomed.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 44.Belikova NA, Tyurina YY, Borisenko G, et al. Heterolytic reduction of fatty acid hydroperoxides by cytochrome c/cardiolipin complexes: antioxidant function in mitochondria. J Am Chem Soc. 2009;131:11288–11289. doi: 10.1021/ja904343c. [DOI] [PubMed] [Google Scholar]
- 45.Staats DA, Lohr D, Colby HD. Relationship between mitochondrial lipid peroxidation and α-tocopherol levels in the guinea-pig adrenal cortex. Biochim Biophys Acta. 1988;961:279–284. doi: 10.1016/0005-2760(88)90074-4. [DOI] [PubMed] [Google Scholar]
- 46.Nakamura M, Hayashi T. Oxidation mechanism of vitamin E analogue (Trolox C, 6-hydroxy-2,2,5,7,8-pentamethylchroman) and vitamin E by horseradish peroxidase and myoglobin. Arch Biochem Biophys. 1992;299:313–319. doi: 10.1016/0003-9861(92)90280-a. [DOI] [PubMed] [Google Scholar]
- 47.Kagan VE, Yalowich JC, Borisenko GG, et al. Mechanism-based chemopreventive strategies against etoposide-induced acute myeloid leukemia: free radical/antioxidant approach. Mol Pharmacol. 1999;56:494–506. doi: 10.1124/mol.56.3.494. [DOI] [PubMed] [Google Scholar]
- 48.Jiang Q, Yin X, Lill MA, Danielson ML, Freiser H, Huang J. Long-chain carboxychromanols, metabolites of vitamin E, are potent inhibitors of cyclooxygenases. Proc Natl Acad Sci USA. 2008;105:20464–20469. doi: 10.1073/pnas.0810962106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kagan VE, Serbinova EA, Safadi A, Catudioc JD. NADPH-dependent inhibition of lipid peroxidation in rat liver microsomes. Biochem Biophys Res Commun. 1992;186:74–80. doi: 10.1016/s0006-291x(05)80777-6. [DOI] [PubMed] [Google Scholar]