Abstract
Stroke is the major cause of acquired epilepsy in the adult population. The mechanisms of ischemia-induced epileptogenesis are not completely understood, but glutamate is associated with both ischemia-induced injury and epileptogenesis. The objective of this study was to develop an in vitro model of epileptogenesis induced by glutamate injury in organotypic hippocampal slice cultures (OHSCs), as observed in stroke-induced acquired epilepsy. OHSCs were prepared from 1-week old Sprague-Dawley rat pups. They were exposed to 3.5 mM glutamate for 35 minutes at 21 days in vitro. Field potential recordings and whole-cell current clamp electrophysiology were used to monitor the development of in vitro seizure events up to 19 days after injury. Propidium iodide uptake assays were used to examine acute cell death following injury. Glutamate exposure produced a subset of hippocampal neurons that died acutely and a larger population of injured but surviving neurons. These surviving neurons manifested spontaneous, recurrent epileptiform discharges in neural networks, characterized by paroxysmal depolarizing shifts and high frequency spiking in both field potential and intracellular recordings. This model also exhibited anticonvulsant sensitivity similar to in vivo models. Our study is the first demonstration of a chronic model of acquired epilepsy in OHSCs following a glutamate injury. This in vitro model of glutamate injury–induced epileptogenesis may help develop therapeutic strategies to prevent epileptogenesis after stroke and elucidate some of the mechanisms that underlie stroke-induced epilepsy in a more anatomically in-tact system.
Keywords: excitotoxicity, stroke, glutamate, patch clamp electrophysiology, rat
1. Introduction
The association between stroke and epilepsy is well recognized, but it is not well understood. Stroke is one of the most common neurological conditions, affecting over half a million Americans per year (Taylor et al., 1996) and is a leading cause of acquired epilepsy (AE), most commonly in the elderly (Annegers et al., 1996; Armstrong et al., 2009; Herman 2002). The development of AE after a stroke has been shown to increase both the morbidity and mortality of the stroke patient (Menon and Shorvon 2009). Thus it is important to prevent the development of AE after stroke. Despite this, the mechanisms underlying the development of epilepsy (epileptogenesis) following stroke-like injury are poorly understood.
Stroke refers to the brain injury that occurs following cerebral ischemia (Sharp et al., 1998). It has been suggested that acute ischemia increases extracellular concentrations of glutamate (Buchkremer-Ratzmann et al., 1998), reduces GABAergic function, and causes functional or structural impairment of GABAergic interneurons (Menon and Shorvon 2009). In addition, injury from stroke also causes selective cell death and apoptosis, changes in membrane potential properties, mitochondrial changes, receptor changes, deafferentation or collateral sprouting (Menon and Shorvon 2009). Together these impaired functional and structural relationships may lead to epileptogenesis- a process that causes a permanent neuronal plasticity change in previously normal brain tissue, leading to the onset of spontaneous recurrent epileptiform discharges (SREDs) (DeLorenzo et al., 2007). Certainly, this period of epileptogenesis represents an important clinical time-frame wherein intervention may be able to inhibit the plasticity changes that lead to the development of seizures.
Development of an in vivo model of stroke-induced AE has met limited success (Karhunen et al., 2005). Our lab has utilized the in vitro hippocampal neuronal culture model of stroke-induced AE (Sun et al., 2001; Sun et al., 2004). The in vitro model utilizes glutamate exposure to produce an injury similar to that seen secondary to ischemic stroke. Subsequent to injury, surviving neurons manifest SREDs analogous to epileptic seizures (Sun et al., 2001; Sun et al., 2004). While this model provides insight into some of the molecular mechanisms involved in epileptogenesis following stroke, it is limited by lack of normal anatomical morphology and circuitry that is important in excitatory feedback in the brain. In contrast, animal models provide proper morphology and neuronal feedback, but they are often time consuming and cost restrictive for use in rapid screening of novel therapeutic compounds. Some of these limitations could be overcome while still using an in vitro system by utilizing organotypic hippocampal slice cultures (OHSCs). OHSCs have been shown to manifest intact neuronal morphology, cellular and anatomical relations and network connections (Noraberg et al., 2005; Sundstrom et al., 2005; Zimmer and Gahwiler 1984). OHSCs have been used to study the acute physiological effects (Albus et al., 2008; Wahab et al., 2010) and some of the morphological changes that occur following excitotoxic injury (Routbort et al., 1999; Thomas et al., 2005). Glutamate toxicity is an important aspect of the ischemic cascade (Buchkremer-Ratzmann et al., 1998). OHSC models have also established similarities in cell death patterns in oxygen glucose deprivation and glutamate injury, suggesting NMDA mediated cell death in both injuries (Lipski et al., 2007, Noraberg et al., 2005). However, a thorough characterization of the physiological changes that occur after excitotoxicity particularly its effect on seizure genesis has not been explored.
In this paper, we describe development of an OHSC model of glutamate injury induced AE. Our novel model utilizes a glutamate injury paradigm to induce a stroke-like injury in OHSCs (Lipski et al., 2007). After a period of epileptogenesis, field potential and intracellular recordings revealed expression of SREDs, the in vitro correlate of “seizures”, in glutamate treated slices as compared to untreated sham control slices. Pharmacological studies using standard anticonvulsant drugs have also been described.
2. Results
2.1 Glutamate exposure produced hippocampal neuronal injury
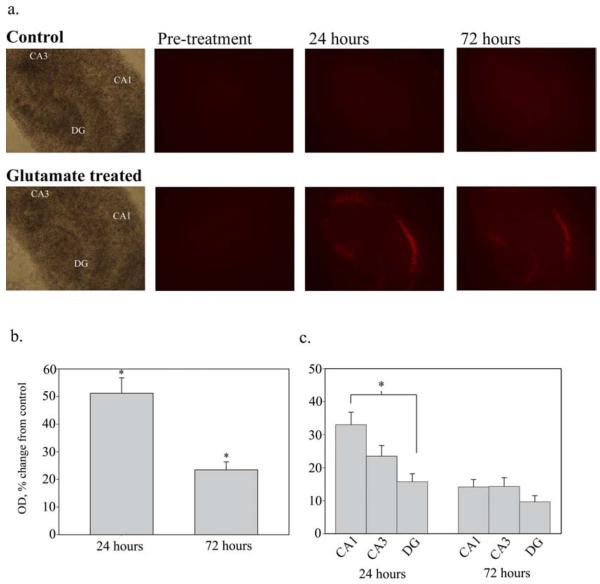
Figure 1a shows PI staining in OHSCs after injury with 3.5 mM glutamate. PI uptake was measured in optical density units of the whole slice. At 24 hours, glutamate injured OHSCs showed an increase in PI uptake of 51.17 ± 5.364% (n= 174) of age-matched controls (0.0 ± 3.09%, n= 121, p<0.001, Mann-Whitney Rank sum test). The increase in PI staining in glutamate treated slices was still significant at 72 hours, with optical density measurements of 23.5 ± 2.89% (n=154) over controls (0.0 ± 2.535%, n=108, p<0.001, Mann-Whitney Rank sum test) (figure 1b). PI staining displayed that the glutamate treatment killed some, but not all neurons, producing a mixed population of live and dead neurons. The CA1 region showed significantly higher cell death than the dentate gyrus at 24 hours (33.0 ± 3.8% vs. 15.8 ± 2.4%, n=154 for both, p<0.05, one way ANOVA with a Fisher’s post-hoc analysis), though this effect was not significant at 72 hours (figure 1c). The CA3 region did not show a significant difference in cell death when compared to either CA1 or DG at either time point.
Figure 1.
A. Propidium Iodide uptake - Propidium Iodide (PI) uptake in OHSCs following 35 minute treatment with 3.5mM glutamate. PI staining indicates increased cell death in glutamate treated slices as compared to controls at both 24 and 72 hours following treatment. Note that significant cell death is observed in the CA1 cell region. (4X magnification). B. Comparison of PI uptake - Quantification of PI uptake in OHSCs at 24 and 72 hours after injury, expressed as a percent change from control. PI uptake was measured as mean optical density of the whole slice and normalized to age-matched controls. PI staining indicated a significant increase in cell death at both 24 (n=121, 174 for control and glutamate treated, respectively) and 72 hours (n=108, 154 for control and glutamate treated, respectively) after glutamate treatment (*p<0.001, Mann-Whitney Rank Sum test). C. PI uptake by cell region - At 24 hours, cell death is significantly higher in the CA1 cell region than it is in the dentate gyrus (DG) (*p<0.05, Kruskall-Wallis One Way ANOVA On Ranks). The CA3 region did not have a significant difference in PI uptake from either the CA1 or DG. At 72 hours, there is no significant difference in PI uptake between cell regions. (n=154)
2.2 Glutamate injury induced alterations in OHSC excitability
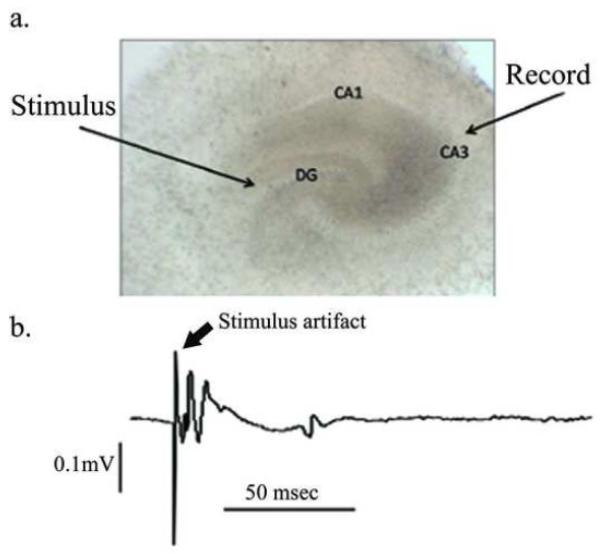
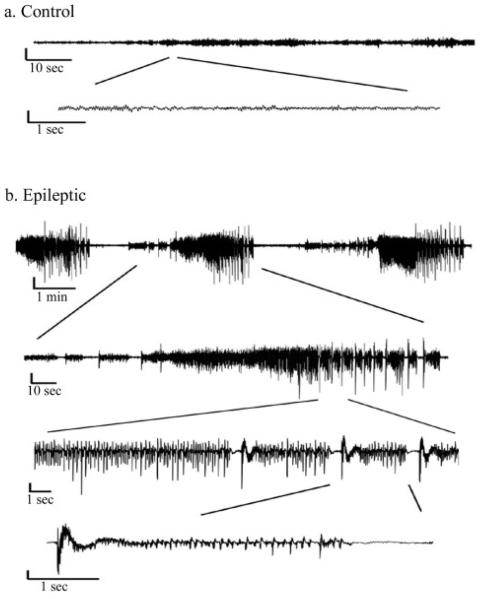
To investigate changes in OHSC excitability, we obtained field potential recordings from the CA3 region of OHSCs 9-12 days after glutamate injury. OHSCs were first tested for viability by stimulating in the dentate gyrus while recording in the CA3 cell layer (figure 2). Representative field potential recordings of control and glutamate treated OHSCs are shown in figure 3. Seizure events were defined as the abrupt onset of a high amplitude burst of rhythmic activity superimposed on a field depolarization shift that lasted ≥3 s, during which the waveforms evolved over time and terminated abruptly (Bausch et al., 2006; Routbort et al., 1999). Field potential recordings showed seizure events in 46.25% of glutamate treated OHSCs (n= 80) compared to 7.14% of control slices (n= 28, p<0.001, Chi-square analysis). OHSCs displaying seizures had an average of 3.73 ± 0.57 seizure events per 2 minute recording, lasting an average of 14.26 ± 3.11 sec. The average maximal amplitude shift was 0.78 ± 0.14 mV. While some control and injured slices displayed individual action potentials and brief depolarizing shifts, these slices were not considered to have seizure events unless the burst of activity occurred for greater than 3 seconds.
Figure 2.
A. Phase contrast photo (4X) of an OHSC at DIV 21 - Cell layers of the dentate gyrus (DG), CA3 and CA1 are clearly visible. B. Field potential recording of OHSC- To test viability of OHSCs during field potential recording, the stimulating electrode is placed in the DG while the recording electrode is placed over the CA3 cell region. The trace shows an example of an evoked field potential recording from a viable OHSC. The stimulus artifact is followed by the field potential.
Figure 3.
Extracellular recordings - Representative extracellular field potential recordings of CA3 cell layer of OHSCs at DIV 30. A. Control - Control OHSCs do not display seizure activity in aCSF. B. Glutamate treated - Representative field potential recording from an OHSC that was subjected to glutamate injury at DIV 21. Seizure activity is characterized by repetitive bursts of activity that last for longer than 3 seconds, changes over time and terminates. All vertical bars = 0.5mV
2.3 Spontaneous recurrent epileptiform discharges in glutamate-injured OHSCs
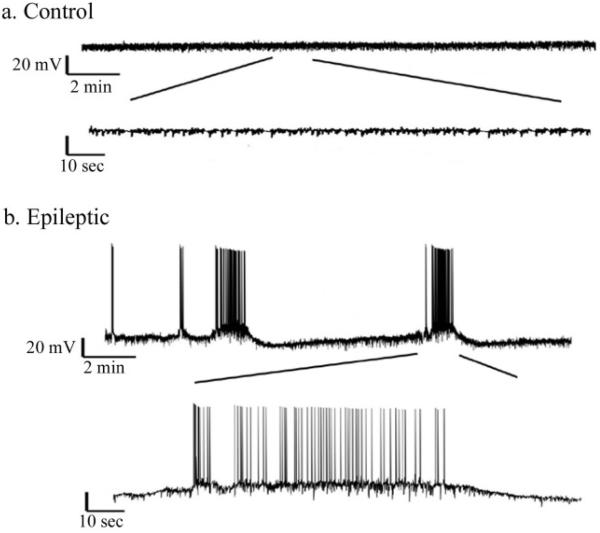
To further confirm seizure activity in glutamate treated OHSCs, whole-cell current clamp methods were employed on CA3 cells in glutamate treated and control slices. Cells in glutamate treated slices displayed SREDs while cells in control slices did not (figure 4). SREDs were defined as a burst of activity with a spike frequency ≥ 3Hz for durations of ≥ 20 sec (Sun et al., 2001). This electrographic recording is analogous to electrographic seizures observed with EEG recordings (McNamara 1994). Cells in control slices showed spontaneous firing as well, but it did not meet the criteria for a SRED. SREDs were observed in 43.7% of glutamate treated slices (n=16), compared to 0% of control slices (n=9, P= 0.027, Fisher exact test). Cells in control slices had an average spike frequency of 0.0637 ± 0.0495 Hz over 257 minutes of recording. Cells from all glutamate treated slices had an average spike frequency of 0.8406 ± 0.545154 Hz over 211 minutes. Of those glutamate treated slices that displayed SREDs, average spike frequency was 1.54384 ± 0.9357 Hz over 105 minutes (P=0.014 compared to control, Mann-Whitney Rank Sum Test), with frequencies over 3 Hz during SRED events. The resting membrane potential of control cells was −63.9 ± 2.8 mV (n=9) and this was not significantly different from all glutamate treated cells (−59.45 ± 2.5 mV, n=16, p=0.25, Student’s t-test) or those cells displaying SREDs (−55.1 ± 3.8 mV, n=7, p=0.08, Student’s t-test). Input resistance was also not significantly different between the two groups (226.87 ± 21.3 MΩ for control, 258.5 ± 53.3 MΩ for glutamate treated cells, p=0.6, Student’s t-test).
Figure 4.
Intracellular recording - Representative intracellular whole cell current clamp recordings of CA3 pyramidal cells at DIV 30. A. Control - Cells from control slices did not display repetitive bursting (n=9) B. Glutmate treated - Cells from glutamate treated slices displayed spontaneous recurrent epileptiform discharges (SREDs), similar to activity seen in extracellular field potential recordings. SREDs were observed in 43% of slice cultures following glutamate injury at DIV 21 (n=16).
2.4 SREDs lasted for the life of the OHSCs
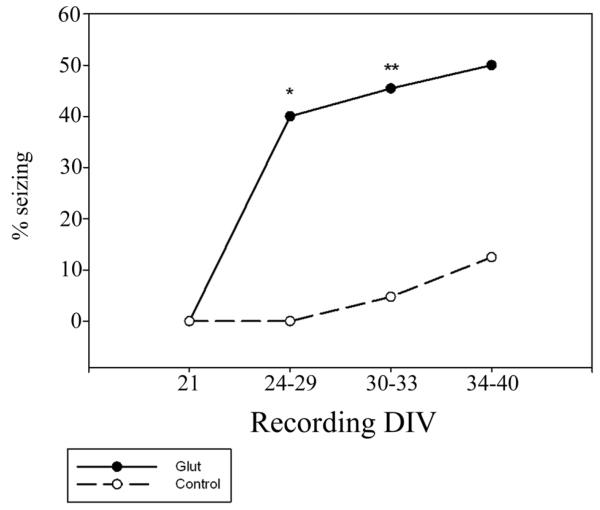
Field potential recordings were performed at different time points after glutamate injury to confirm the optimal time of recording. In glutamate treated slices seizure activity was not observed immediately after the injury, at DIV 21. However, seizure activity was present at DIV 24-29, 3-8 days after injury, in 40% of slices. Seizure activity continued in approximately 50% of treated slices until DIV 40 (figure 5). Consistent with literature findings (Bausch and McNamara 2000; McBain et al., 1989), control OHSCs began showing more spontaneous seizure activity as time in culture increased, with 12.5% showing seizure activity between 34 and 40 days in vitro. At this point in culture, the expression of spontaneous epileptiform activity is thought to arise from an alteration in the balance of excitatory and inhibitory synaptic activity, resulting increased synaptic activity (McBain et al., 1989). Therefore, we established DIV 30-33 as an acceptable window for field potential recording as there is a significant difference in the frequency of seizure events in injured and control slices.
Figure 5.
Development of seizures after injury - After glutamate injury (solid line), a significant percentage of OHSCs display seizure activity in field potential recordings as compared to age matched controls (dashed line). This change is long lasting and was observed up to 40 days in vitro. Within hours of injury (DIV 21), OHSCs did not display seizure events in either the control or injured groups (n=6 each). Between 3 and 8 days after injury (DIV 24-29), 40% of injured OHSCs and 0% of controls displayed seizure events (n=10 each, *p<0.05, Fisher’s exact test). In our established experimental time frame, DIV 30-33, we observed seizure events in 46.25% of glutamate injured OHSCs and 7.14% of controls (n=80, 28, **p<0.001, Chi-square analysis). By DIV 34-40, seizure events were observed in 50% of injured slices and 12.5% of controls (n=8, 9).
2.5 Degree of cell death did not determine development of SREDS
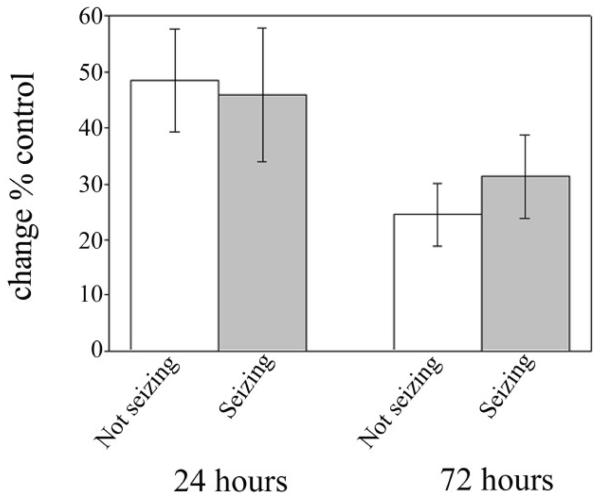
To determine if the varying degree of cell death played a role in the development of seizure events, we analyzed PI staining in the glutamate treated slices that had been used in field potential recordings. As shown in figure 6, slices displaying SREDs showed a 45.89 ± 11.956% (n=34) increase in PI uptake 24 hours after injury when compared to matched controls, while slices that did not show seizure activity showed a 48.5 ± 9.231% (n=39) increase. Similarly, at 72 hours, seizing slices maintained a 45.5 ± 9.23% (n=34) increase while non-seizing slices showed a 31.3 ± 7.445% (n=39) increase over controls. These values are not significantly different (p=0.862 and 0.175, respectively, Student’s t-test) from one another, suggesting that magnitude of cell death following initial injury does not play a significant role in the development of seizure activity in this model. In addition, there was no significant difference in cell death in individual cell areas among OHSCs that displayed seizure activity and those that did not (all P>0.198, data not shown).
Figure 6.
Degree of cell death does not determine seizure activity - After glutamate injury, OHSCs displayed slightly varying degrees of cell death. However, the degree of cell death observed does not appear to play a role in the development of seizure activity. OHSCs were evaluated for cell death at 24 and 72 hours after glutamate injury on DIV 21. They were returned to the incubator until field potential recording at DIV 30-33. OHSCs that did not display seizure activity (white bars, n=39) did not significantly differ in PI uptake from OHSCs displaying seizures (gray bars, n=34).
2.6 Phenobarbital and phenytoin but not ethosuximide inhibited epileptiform activity
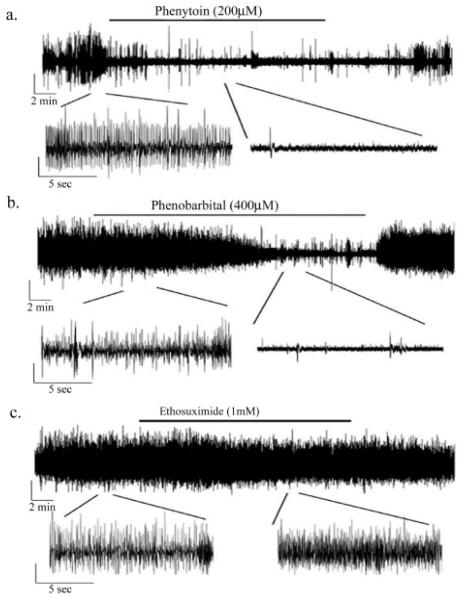
To better understand the characteristics of the in vitro seizure events, we employed several standard AEDs to see their effect on seizure activity in this model. Given the refractory nature of other seizure models in OHSCs, chosen AED concentrations were slightly higher than equivalent anticonvulsant plasma concentrations in rats (Albus et al., 2008). Perfusion of phenytoin (200 μM) during extracellular recording reversibly reduced the amplitude and frequency of the field potential seizure activity (figure 7a). After 18 minutes of drug perfusion, phenytoin decreased the field potential amplitude by 23.4 ± 9.3 % (P=0.045) and frequency by 83.8 ± 4.6% (p<0.001, n=4). Phenobarbital (400 μM) also acted as a reversible anti-seizure agent in this model, though the onset of its action was longer (figure 7b). Field potential amplitude decreased by 23.3 ± 8.5% (p=0.035) and frequency decreased by 77.3 ± 12.7% (p<0.001) following 18 minutes of drug perfusion (n=4). On the other hand, ethosuximide (1 mM), a T-type, voltage gated calcium channel blocker effective in treating generalized absence seizures (Rogawski and Porter 1990), had no effect on seizure activity in this model (figure 7c). Amplitude (3.7 ± 3.6% decrease) and frequency (27.6 ± 29.6% increase) remained unchanged (p>0.05, n=4). Thus, the seizure activity induced by glutamate injury in our model responded to therapeutically relevant concentrations of anticonvulsants analogous to the setting of generalized tonic clonic and partial complex seizures (Macdonald and Kelly 1995).
Figure 7.
Standard AEDs are effective at blocking seizure activity - Representative field potential recordings of the effect of standard anti-epileptic drugs on seizure activity in glutamate treated slices at DIV 30. A. Phenytoin (200μM) is effective at blocking seizure activity during a 20 minute perfusion and can be washed out. On average, phenytoin decreased field potential amplitude by 23.4 ± 9.3 % (P=0.045) and frequency by 83.8 ± 4.6% (p<0.001, n=4) B. Phenobarbital (400μM) is also effective at blocking seizure activity during the 20 minute period of perfusion, amplitude decreased by 23.3 ± 8.5% (p=0.035) and frequency decreased by 77.3 ± 12.7% (p<0.001) following 15 minutes of drug perfusion (n=4). C. Ethosuximide (1 mM), is not effective at blocking seizure activity in glutamate injured OHSCs (p>0.05, n=4). All vertical bars = 0.1mV
3. Discussion
The experiments in this study document a novel model of epileptogenesis in OHSCs following glutamate injury. Similar to the excitotoxic injuries associated with both ischemic and anoxic stroke events, glutamate injury in this model produced a mixed population of neurons characterized by both cell survival and cell death. As suggested by our initial hypothesis, neurons that survived the glutamate exposure become the substrate for the development of epileptogenesis as indicated by increased excitability of the CA3 cell layer in a significant number of OHSCs. The OHSC model of glutamate injury induced spontaneous seizure events may provide insights into the development of stroke induced AE. It also offers a powerful tool to screen potential pharmacological agents to treat seizures and develop therapeutic interventions to prevent the development of AE after stroke.
The seizure events seen in extracellular recordings expressed many characteristics of overt electrographic epileptic seizures. Seizure events started and terminated spontaneously and were synchronized in nature, as they represented a population of synchronized neurons (figure 3). The seizure events produced by excitotoxic glutamate injury manifested larger spike amplitude than would be seen if the activity originated from a single neuron, suggesting that the activity occurred in a group of neurons. The typical seizure pattern observed in OHSCs after glutamate injury also included an abrupt onset of activity superimposed on a large field potential shift, consistent with the classic paroxysmal depolarizing shift associated with electrographic seizure discharges in both in vitro and in vivo models of epilepsy (Bausch et al., 2006; Dichter and Ayala 1987). Finally, the seizures produced by glutamate injury responded to the anticonvulsant drugs phenobarbital and phenytoin, but not to ethosuximide (figure 7). These results demonstrated that OHSCs subjected to injury by glutamate exposure could be transformed into neuronal networks manifesting seizures for the life of the culture, producing an in vitro model of epilepsy.
Various studies of OHSCs have confirmed that cells develop and mature similar to age-matched cells in vivo (Bahr et al., 1995, Stoppini et al. 1991). In this respect, OHSCs are often used at times comparable to the appropriate in vivo age. Our OHSCs are cultured at P8 and injured at DIV 21, thereby showing the equivalent cellular maturity of young adult rats at P29. OHSCs from P8 pups have been shown to develop mature synaptic properties within a few days in culture (Muller et al., 1993). In addition, OHSCs have been shown to respond to seizure inducing stimuli over a wide range of time points anywhere from 7-56 days in culture (Albus et al., 2008). We therefore believe our slice cultures are at an appropriate age to respond to a glutamate injury at DIV 21.
Organotypic hippocampal slice cultures have been utilized in many models of excitotoxicity, including kainic acid (Routbort et al., 1999), NMDA (Ring et al., 2010), and oxygen glucose deprivation injuries (Lipski et al., 2007). However, few of these studies have examined the long-term physiological changes that occur in OHSCs after excitotoxic injury, thereby exploring their potential as a model of acquired epilepsy. Recently, Bausch and McNamara (Bausch and McNamara 2004) used a kainic acid injury paradigm in OHSCs and found no significant difference in seizure rates from controls when recording from granule cells in physiological buffer 30 days after injury. They were however, able to show that the granule cells were more hyperexcitable and there was considerable mossy fiber sprouting in the injured slices. Various other studies have used excitotoxic injuries to study neuroprotective agents and morphological changes (Boscia et al., 2006; Cho et al., 2007; Lipski et al., 2007; Ring et al., 2010; Routbort et al., 1999; Thomas et al., 2005). Our model takes this a step further by characterizing the physiological changes after an excitotoxic injury with glutamate. A study by Lahtinen et al. (Lahtinen et al., 2001) utilized a higher concentration of glutamate (10 mM) to examine acute electrophysiological consequences of glutamate injury in OHSCs. Within 2 hours of the injury, they found increased hyperexcitability in the CA3 cell region. In addition, they reported attenuation of cell death with TTX following glutamate injury, indicating that some cell death during the initial 24 hour period may be caused by neuronal excitability following the initial injury. Indeed, clinical and in vivo studies have shown that seizures often occur acutely following an ischemic injury (Menon and Shorvon, 2009, Karhunen et al., 2006), but this does not necessarily lead to epileptogenesis and acquired epilepsy. This would suggest that the post-insult activity observed by Lahtinen et al. is not necessarily indicative of chronic epileptogenesis. In contrast, our study employs a more moderate glutamate injury (3.5mM glutamate for 35 minutes) and we examine cell death at both 24 hours and 72 hours. We establish that cell death subsides following the 24 hour period, as the PI uptake is significantly decreased at 72 hours. In addition, we examine epileptogenesis in OHSCs 9-12 days following glutamate injury indicating long lasting changes in neuronal excitability following glutamate injury. It is important to stress this issue, since our study is the first demonstration of the development of spontaneous recurrent epileptiform discharges (epilepsy) in the OHSCs and represents the first model of post injury acquired “epilepsy” in this model.
Our model utilizes glutamate as a mode of excitotoxicity, as excessive glutamate concentration is an important aspect of the ischemic prenumbra in stroke (Davalos et al., 1997). The involvement of glutamate in epileptogenesis has been implicated in whole animal, (Croucher et al., 1988; Croucher and Bradford 1990; Rice and DeLorenzo 1998) slice, (Anderson et al., 1990; Stasheff et al., 1989) and cell culture (Sombati and Delorenzo 1995; Sun et al., 2001) models of epilepsy. To induce epileptogenesis, these models all used continuous neuronal spiking produced by seizures, (Rice and DeLorenzo 1998) repeated high-frequency excitation (Croucher et al., 1988; Croucher and Bradford 1990; Stasheff et al., 1989), or low extracellular magnesium environments (Anderson et al., 1986; Sombati and Delorenzo 1995) while only one used a glutamate-induced prolonged, reversible depolarization as used in this study (Sun et al., 2001; Sun et al., 2004). Many of these models have implicated activation of the N-methyl-D-aspartate receptor (NMDAR) for epileptogenesis (Croucher et al., 1988; Croucher and Bradford 1990; DeLorenzo et al., 1998; Rice and DeLorenzo 1998; Stasheff et al., 1989). Interestingly, epileptiform discharges have also been produced by growing OHSCs in culture in the presence of tetrodotoxin (TTX) to block activity or D(–)-2-amino-5-phosphonopentanoic acid (D-APV) to block NMDAR activation (Bausch et al., 2006). Removal of these agents resulted in the expression of seizure-like activity (Bausch et al., 2006). This distinctly different culture model uses the inhibition of glutamate receptors to induce hyperexcitability. Control OHSCs displayed a low occurrence of seizures, possibly as a result of collateral synaptic connections that are known to form after long term culture of hippocampal tissue (Bausch and McNamara 2000; McBain et al., 1989). Although the mechanism producing hyperexcitability in this model has not been fully delineated, it has been shown that inhibition of glutamate receptors in neurons in culture produces alterations in NMDAR subunit expression that are regulated by synaptic activity during development (Hoffmann et al., 2000; Yashiro and Philpot 2008). It is possible that alterations in NMDAR subunit expression may underlie the development of hyperexcitability in this model. Though glutamate exposure may induce changes in receptor subunit expression in the glutamate injury–induced epileptogenesis model, these potential changes probably occur through a separate mechanism. It will be interesting to investigate the mechanism of glutamate injury-induced epileptogenesis in future studies.
The potential role of selective neuronal death in glutamate injury–induced epileptogenesis requires further investigation, especially in light of the fact that inhibitory neurons are typically less vulnerable to excitotoxicity than excitatory neurons (Tecoma and Choi 1989). Although differential cell death may affect the balance between the number of inhibitory and excitatory neurons, resulting in a larger number of surviving inhibitory neurons, (Tecoma and Choi 1989) the glutamate-induced injury produced “epilepsy” in the OHSCs despite the potential alterations in neuronal subpopulations. Our experiments suggest that the severity of injury is not a large factor in epileptogenesis, as there was no difference in the degree of cell death in those OHSCs that developed seizures and those that did not (figure 6). Further studies are needed to determine the role of selective cell death in this model. In addition, the possible roles of gap junctions (Dudek et al., 1998), ischemia-induced alterations in second-messenger systems, and gene changes (Morris et al., 2000) in mediating epileptogenesis represent important future directions for research that can be conveniently studied in this system.
The association between stroke and epilepsy has been demonstrated clinically, and stroke is the most common cause of acquired epilepsy in adults (Hauser et al., 1991). However, the mechanisms by which cerebral ischemia initiates epileptogenesis are not understood. The glutamate injury produced in this model of epileptogenesis resembles some of the phenomena associated with stroke. Increases in extracellular glutamate, (Bullock et al., 1995; Davalos et al., 1997) excitotoxic delayed neuronal death (Choi 2000) associated with the ischemic penumbra (Dirnagl et al., 1999), and a delayed period of epileptogenesis are all present in this model. To our knowledge, this study demonstrates, for the first time, spontaneous, recurrent, epileptiform activity in organotypic hippocampal slice cultures induced by glutamate injury. This model of glutamate injury–induced epileptogenesis may offer new insights into the development and maintenance of the epileptic condition after a neurological trauma such as stroke and therefore may provide therapeutic strategies to develop both novel anti-epileptogenic and anticonvulsant agents to prevent stroke-induced epilepsy.
4. Experimental Procedures
4.1 Organotypic hippocampal slice culture preparation
Slice cultures were prepared using the method of Stoppini et al. (Stoppini et al., 1991), as previously reported by (Schanuel et al., 2008). All animal use protocols are in strict accordance with the National Institute of Health guidelines and are approved by the International Animal Care and Use Committee of Virginia Commonwealth University. Postnatal day 8 (P8) Sprague-Dawley rat pups (Harlan, Frederick, MD, USA) were deeply anesthetized with isoflurane and decapitated. The brains were removed, and hippocampi were dissected out and cut into 350-μm transverse sections using a McIlwain tissue chopper (Brinkmann Instruments, Canada) and placed into Hank’s balanced salt solution [HBSS – Gibco BRL (Invitrogen, Carlsbad, CA, USA)] supplemented with 0.5% sucrose and 1% penicillin-streptomycin. The middle four to six slices of each hippocampus, including part of the entorhinal cortex, were placed onto tissue culture membrane inserts (Millipore, Bedford, MA) in a 6-well tissue culture dish containing medium consisting of 50% minimum essential medium, 25% horse serum and 25% HBSS, 1% penicillin-streptomycin [all from Gibco BRL (Invitrogen, Carlsbad, CA)] and supplemented with 36 mM glucose, and 25 mM Hepes (Sigma, St. Louis, MO, USA) (pH 7.2). Cultures were maintained at 37°C under room air +5% CO2. After 1 day in culture, culture medium was replaced with fresh medium containing no antibiotics. Culture medium was replaced two times a week thereafter.
4.2 Glutamate injury
After 21 days in vitro (DIV21), slice culture media was removed and replaced by media containing 3.5mM glutamate and cultures were returned to the incubator for a period of 35 minutes (Lipski et al., 2007). Glutamate containing media was then removed and slice cultures were washed with HBSS at 37°C two times before returning to normal media. Control slices received normal media (without glutamate) during the 35 minute time period, followed by washing with HBSS twice before returning to normal media.
4.3 Neuronal death assay
Delayed neuronal death was assessed 24 and 72 hours after glutamate treatment by measuring uptake of the fluorescent dye Propidium Iodide (PI, Sigma), using established procedures (Lipski et al., 2007; Pomper et al., 2001). Greater PI uptake indicates greater cell death. Cultures were exposed to 2 μM PI for 4 hours in normal culture media. Before recording dye uptake under fluorescent light, a phase contrast picture of each slice was taken so that orientation in the field could be maintained in subsequent recordings at 24 and 72 hours. Dye uptake was recorded on an Olympus CK40 inverted microscope coupled to a QColor 3 camera and QCapture software (Olympus, Center Valley, PA). All optical parameters (illumination aperture, neutral density filters etc.) as well as the camera exposure time and electronic gain, were standardized and kept constant. The fluorescent signal was measured densitometrically using the Image-J software (NIH). The perimeter of each slice was outlined (identified in phase contrast image) and the mean pixel value (0–255) was recorded and converted to a scale of 0-100. All measurements were made after subtracting background fluorescence (region positioned immediately outside the culture). Cell death was expressed as a percent increase of mean pixel value of date matched controls. To assess differential cell death by region, cell regions were identified and circled as regions of interest in the phase contrast image. Propidium iodide staining was then measured densitometrically in each of the 3 cell regions (CA1, CA3, DG). As in whole hippocampus recordings, cell death was expressed as a percent increase of mean pixel value of each cell region in age date-matched controls.
4.4 Field potential recording
At DIV 30-33, a portion of the tissue culture insert membrane containing a single cultured slice was placed into an interface slice recording chamber (Harvard Apparatus, Holliston MA) mounted on a vibration table and viewed through a dissecting microscope (Diagnostic Instruments, Sterling Heights, MI). Slice cultures were perfused (2–3 ml/min) with artificial cerebrospinal fluid (aCSF) composed of (in mM) 120 NaCl, 3.5 KCl, 1.3 MgSO4, 2.5 CaCl2, 1.25 NaH2PO4, 25.6 NaHCO3, and 10 glucose, equilibrated with 95% O2-5% CO2. Recording pipettes were pulled on a Flaming Brown P-80 PC Micropipette puller (Sutter Instrument Co, Novato, CA) filled with 3.5 M NaCl for extracellular recordings to achieve a resistance of 2-4 MΩ. Data were collected with an AxoClamp 2B (Molecular Devices, Union City, CA) recording amplifier and FLA-01 Bessel filter unit (Cygnus Technologies, Inc, Delaware Water Gap, PA) and pCLAMP 9.0 software (Molecular Devices). After a 15 minute equilibration period, field potentials were recorded at 34°C in the CA3 pyramidal cell layer. If spontaneous activity was not observed in the first few minutes, slices were tested for viability by hilar stimulation (0.3-ms square pulse, 0.03 Hz, 50–150 μA) using a bipolar concentric electrode (FHC, Inc., Bowdoin, ME) and a stimulator (World Precision Instruments, Sarasota, FL). Slices were considered acceptable if stimulation elicited an action potential spike that immediately followed the stimulus artifact with a response threshold ≤150 μA (Bausch and McNamara 2000; Bausch et al., 2006). Neither the amplitude of the spike nor the shape of the waveform was used as criterion for acceptable recordings. Each slice culture was recorded at 2 minute intervals (2 minutes on, 2 minutes off) over a 20 minute time period, for a total of 10 minutes of recorded activity. Recordings were scored for seizure events by two separate individuals who were blinded to the treatment group.
4.5 Intracellular recording
Whole-cell current-clamp recordings were performed on visually identified pyramidal neurons in the CA3 region of OHSCs (one cell per slice). A portion of the culture membrane containing a single OHSC was excised and transferred to a recording chamber mounted on a Axioskop 2 FS Plus upright microscope 9 (Carl Zeiss, Inc., Thornwood, NY), equipped with an IR/DIC camera. Slices were perfused at 1-2ml/min with aCSF using a Dynamax peristaltic pump (Rainin, Oakland CA). Patch microelectrodes had a resistance of 2 to 4 MΩ resistance when filled with an internal solution of (in mM) 140 K+ gluconate, 1MgCl2, 10 HEPES, 1.1 Ethylene glycol-bis (ß-aminoethyl ether)-N, N,N’,N’-tetraacetic acid (EGTA), 4 Na2 ATP, 15 Tris Phosphocreatine, pH 7.2 with NaoH and osmolarity adjusted to 310 mOsm with sucrose. Recordings were obtained using MultiClamp 700B amplifier in current clamp mode. Data were digitized by a DigiData 1440A, low-pass filtered at 2 kHz, sampled at 10 kHz and recorded using pClamp 10.0 software. Cells were acceptable for recording if they maintained a membrane potential of −40mV or more hyperpolarized and were able to spike when a current pulse was injected. Cells were omitted if series resistance was not stable within 15% during recording.
4.6 Drug perfusion
To determine efficacy of AEDs in this model, phenobarbital, phenytoin, and ethosuximide (all from Sigma) were added to the aCSF during perfusion and field potential recording at DIV 30-33. Drugs were dissolved as stock solutions and working solutions were prepared fresh daily. Phenobarbital and ethosuximide stocks were dissolved in sterile water while phenytoin was dissolved in dimethyl sulfoxide (DMSO). The final concentration of DMSO in the bath solution was less than 0.01%. Drugs were perfused for 20 minutes followed by a 20 minute wash out period with aCSF. The order of drug perfusion was randomized for each slice. Field potential recording was continuous for 40 minutes, rather than intermittent as in previous recordings. Drug effect was analyzed by evaluating average field potential frequency and amplitude for 2 minutes before drug perfusion began and again in the final 2 minutes of drug perfusion. Drug effect is expressed as a percent decrease in average field potential frequency and amplitude during AED perfusion, as compared to the values before drug perfusion.
4.7 Data analyses
Data are expressed as mean ± SEM. To determine significance between treatment groups for neuronal death assays, Mann-Whitney Rank sum or one way ANOVA tests were employed with a Fisher’s post-hoc where appropriate. For extracellular recordings, Chi-square analysis or Fisher’s exact test was used. For intracellular recording, Fisher’s exact test was used to evaluate seizure occurrence, while Mann-Whitney Rank sum and Student’s t-tests were used to evaluate additional electrophysiological properties. For neuronal death assays, glutamate treated slices were normalized to control slices harvested from the same animal. For intracellular recording, 1 neuron was patch-clamped per OHSC. All experiments were performed over the period of several weeks to months so that the results were representative of multiple cultures. A P-value < 0.05 was considered significant. Statistical analysis was performed using SigmaStat 2.0 software and graphs were drawn using SigmaPlot 11 (Systat Software, San Jose, CA, USA).
Research Highlights.
We have developed a novel hippocampal slice culture model for acquired epilepsy
Unprovoked seizures are observed in slice cultures following glutamate injury
Cell death pattern and anticonvulsant sensitivity similar to in vivo models
Novel model to study epileptogenesis
High through-put screening of antiepileptic drugs
Acknowledgements
This work was supported by the CounterACT Program, National Institutes of Health Office of the Director, and the National Institute of Neurological Disorders and Stroke [Grant Number UO1NS058213] to RJD. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the federal government. This study is also supported by National Institute of Neurological Disorders and Stroke [Grant numbers RO1NS051505 and RO1NS052529] to RJD.
Abbreviations
- AE
acquired epilepsy
- SRED
spontaneous recurrent epileptiform discharges
- OHSC
organotypic hippocampal slice culture
- AED
anti epileptic drug
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Section: Neurophysiology, Neuropharmacology and other forms of Intercellular Communication
References
- Albus K, Wahab A, Heinemann U. Standard antiepileptic drugs fail to block epileptiform activity in rat organotypic hippocampal slice cultures. Br J Pharmacol. 2008;154:709–24. doi: 10.1038/bjp.2008.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson WW, Stasheff SF, Swartzwelder HS, Wilson WA. Regenerative, all-or-none electrographic seizures in the rat hippocampal slice in mg-free and physiological medium. Brain Res. 1990;532:288–298. doi: 10.1016/0006-8993(90)91771-8. [DOI] [PubMed] [Google Scholar]
- Anderson WW, Lewis DV, Swartzwelder HS, Wilson WA. Magnesium-free medium activates seizure-like events in the rat hippocampal slice. Brain Res. 1986;398:215–219. doi: 10.1016/0006-8993(86)91274-6. [DOI] [PubMed] [Google Scholar]
- Annegers JF, Rocca WA, Hauser WA. Causes of epilepsy: Contributions of the rochester epidemiology project. Mayo Clin Proc. 1996;71:570–575. doi: 10.4065/71.6.570. [DOI] [PubMed] [Google Scholar]
- Armstrong C, Morgan RJ, Soltesz I. Pursuing paradoxical proconvulsant prophylaxis for epileptogenesis. Epilepsia. 2009;50:1657–69. doi: 10.1111/j.1528-1167.2009.02173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahr BA, Kessler M, Rivera S, Vanderklish PW, Hall RA, Mutneja MS, Gall C, Hoffman KB. Stable maintenance of glutamate receptors and other synaptic components in long-term hippocampal slices. Hippocampus. 1995;5:425–439. doi: 10.1002/hipo.450050505. [DOI] [PubMed] [Google Scholar]
- Bausch SB, McNamara JO. Contributions of mossy fiber and CA1 pyramidal cell sprouting to dentate granule cell hyperexcitability in kainic acid-treated hippocampal slice cultures. J Neurophysiol. 2004;92:3582–3595. doi: 10.1152/jn.01028.2003. [DOI] [PubMed] [Google Scholar]
- Bausch SB, McNamara JO. Synaptic connections from multiple subfields contribute to granule cell hyperexcitability in hippocampal slice cultures. J Neurophysiol. 2000;84:2918–2932. doi: 10.1152/jn.2000.84.6.2918. [DOI] [PubMed] [Google Scholar]
- Bausch SB, He S, Petrova Y, Wang X, McNamara JO. Plasticity of both excitatory and inhibitory synapses is associated with seizures induced by removal of chronic blockade of activity in cultured hippocampus. J Neurophysiol. 2006;96:2151–2167. doi: 10.1152/jn.00355.2006. [DOI] [PubMed] [Google Scholar]
- Boscia F, Annunziato L, Taglialatela M. Retigabine and flupirtine exert neuroprotective actions in organotypic hippocampal cultures. Neuropharmacology. 2006;51:283–294. doi: 10.1016/j.neuropharm.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Buchkremer-Ratzmann I, August M, Hagemann G, Witte OW. Epileptiform discharges to extracellular stimuli in rat neocortical slices after photothrombotic infarction. J Neurol Sci. 1998;156:133–137. doi: 10.1016/s0022-510x(98)00034-3. [DOI] [PubMed] [Google Scholar]
- Bullock R, Zauner A, Woodward J, Young HF. Massive persistent release of excitatory amino acids following human occlusive stroke. Stroke. 1995;26:2187–2189. doi: 10.1161/01.str.26.11.2187. [DOI] [PubMed] [Google Scholar]
- Cho S, Wood A, Bowlby MR. Brain slices as models for neurodegenerative disease and screening platforms to identify novel therapeutics. Curr Neuropharmacol. 2007;5:19–33. doi: 10.2174/157015907780077105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D. Stroke. Neurobiol Dis. 2000;7:552–558. doi: 10.1006/nbdi.2000.0354. [DOI] [PubMed] [Google Scholar]
- Croucher MJ, Bradford HF. NMDA receptor blockade inhibits glutamate-induced kindling of the rat amygdala. Brain Res. 1990;506:349–352. doi: 10.1016/0006-8993(90)91279-p. [DOI] [PubMed] [Google Scholar]
- Croucher MJ, Bradford HF, Sunter DC, Watkins JC. Inhibition of the development of electrical kindling of the prepyriform cortex by daily focal injections of excitatory amino acid antagonists. Eur J Pharmacol. 1988;152:29–38. doi: 10.1016/0014-2999(88)90832-1. [DOI] [PubMed] [Google Scholar]
- Davalos A, Castillo J, Serena J, Noya M. Duration of glutamate release after acute ischemic stroke. Stroke. 1997;28:708–710. doi: 10.1161/01.str.28.4.708. [DOI] [PubMed] [Google Scholar]
- DeLorenzo RJ, Sun DA, Blair RE, Sombati S. An in vitro model of stroke-induced epilepsy: Elucidation of the roles of glutamate and calcium in the induction and maintenance of stroke-induced epileptogenesis. Int Rev Neurobiol. 2007;81:59–84. doi: 10.1016/S0074-7742(06)81005-6. [DOI] [PubMed] [Google Scholar]
- DeLorenzo RJ, Pal S, Sombati S. Prolonged activation of the N-methyl-D-aspartate receptor-Ca2+ transduction pathway causes spontaneous recurrent epileptiform discharges in hippocampal neurons in culture. Proc Natl Acad Sci U S A. 1998;95:14482–14487. doi: 10.1073/pnas.95.24.14482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter MA, Ayala GF. Cellular mechanisms of epilepsy: A status report. Science. 1987;237:157–164. doi: 10.1126/science.3037700. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: An integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Dudek FE, Yasumura T, Rash JE. ‘Non-synaptic’ mechanisms in seizures and epileptogenesis. Cell Biol Int. 1998;22:793–805. doi: 10.1006/cbir.1999.0397. [DOI] [PubMed] [Google Scholar]
- Hauser WA, Annegers JF, Kurland LT. Prevalence of epilepsy in rochester, minnesota: 1940-1980. Epilepsia. 1991;32:429–445. doi: 10.1111/j.1528-1157.1991.tb04675.x. [DOI] [PubMed] [Google Scholar]
- Herman ST. Epilepsy after brain insult: Targeting epileptogenesis. Neurology. 2002;59:S21–6. doi: 10.1212/wnl.59.9_suppl_5.s21. [DOI] [PubMed] [Google Scholar]
- Hoffmann H, Gremme T, Hatt H, Gottmann K. Synaptic activity-dependent developmental regulation of NMDA receptor subunit expression in cultured neocortical neurons. J Neurochem. 2000;75:1590–1599. doi: 10.1046/j.1471-4159.2000.0751590.x. [DOI] [PubMed] [Google Scholar]
- Karhunen H, Jolkkonen J, Sivenius J, Pitkanen A. Epileptogenesis after experimental focal cerebral ischemia. Neurochem Res. 2005;30:1529–1542. doi: 10.1007/s11064-005-8831-y. [DOI] [PubMed] [Google Scholar]
- Karhunen H, Nissinen J, Sivenius J, Jolkkonen J, Pitkanen A. A long-term video-EEG and behavioral follow-up after endothelin-1 induced middle cerebral artery occlusion in rats. Epilepsy Res. 2006;72:25–38. doi: 10.1016/j.eplepsyres.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Lahtinen H, Autere AM, Paalasmaa P, Lauri SE, Kaila K. Post-insult activity is a major cause of delayed neuronal death in organotypic hippocampal slices exposed to glutamate. Neuroscience. 2001;105:131–137. doi: 10.1016/s0306-4522(01)00168-3. [DOI] [PubMed] [Google Scholar]
- Lipski J, Wan CK, Bai JZ, Pi R, Li D, Donnelly D. Neuroprotective potential of ceftriaxone in in vitro models of stroke. Neuroscience. 2007;146:617–629. doi: 10.1016/j.neuroscience.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Kelly KM. Antiepileptic drug mechanisms of action. Epilepsia. 1995;36(Suppl 2):S2–12. doi: 10.1111/j.1528-1157.1995.tb05996.x. [DOI] [PubMed] [Google Scholar]
- McBain CJ, Boden P, Hill RG. Rat hippocampal slices ‘in vitro’ display spontaneous epileptiform activity following long-term organotypic culture. J Neurosci Methods. 1989;27:35–49. doi: 10.1016/0165-0270(89)90051-4. [DOI] [PubMed] [Google Scholar]
- McNamara JO. Cellular and molecular basis of epilepsy. J Neurosci. 1994;14:3413–3425. doi: 10.1523/JNEUROSCI.14-06-03413.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon B, Shorvon SD. Ischaemic stroke in adults and epilepsy. Epilepsy Res. 2009;87:1–11. doi: 10.1016/j.eplepsyres.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Morris TA, Jafari N, DeLorenzo RJ. Chronic DeltaFosB expression and increased AP-1 transcription factor binding are associated with the long term plasticity changes in epilepsy. Brain Res Mol Brain Res. 2000;79:138–149. doi: 10.1016/s0169-328x(00)00112-1. [DOI] [PubMed] [Google Scholar]
- Muller D, Buchs PA, Stoppini L. Time course of synaptic development in hippocampal organotypic cultures. Brain Res. Dev. Brain Res. 1993a;71:93–100. doi: 10.1016/0165-3806(93)90109-n. [DOI] [PubMed] [Google Scholar]
- Noraberg J, Poulsen FR, Blaabjerg M, Kristensen BW, Bonde C, Montero M, Meyer M, Gramsbergen JB, Zimmer J. Organotypic hippocampal slice cultures for studies of brain damage, neuroprotection and neurorepair. Curr Drug Targets CNS Neurol Disord. 2005;4:435–452. doi: 10.2174/1568007054546108. [DOI] [PubMed] [Google Scholar]
- Pomper JK, Graulich J, Kovacs R, Hoffmann U, Gabriel S, Heinemann U. High oxygen tension leads to acute cell death in organotypic hippocampal slice cultures. Dev Brain Res. 2001;126:109–116. doi: 10.1016/s0165-3806(00)00132-2. [DOI] [PubMed] [Google Scholar]
- Rice AC, DeLorenzo RJ. NMDA receptor activation during status epilepticus is required for the development of epilepsy. Brain Res. 1998;782:240–247. doi: 10.1016/s0006-8993(97)01285-7. [DOI] [PubMed] [Google Scholar]
- Ring A, Tanso R, Noraberg J. The use of organotypic hippocampal slice cultures to evaluate protection by non-competitive NMDA receptor antagonists against excitotoxicity. Altern Lab Anim. 2010;38:71–82. doi: 10.1177/026119291003800108. [DOI] [PubMed] [Google Scholar]
- Rogawski MA, Porter RJ. Antiepileptic drugs: Pharmacological mechanisms and clinical efficacy with consideration of promising developmental stage compounds. Pharmacol Rev. 1990;42:223–286. [PubMed] [Google Scholar]
- Routbort MJ, Bausch SB, McNamara JO. Seizures, cell death, and mossy fiber sprouting in kainic acid-treated organotypic hippocampal cultures. Neuroscience. 1999;94:755–765. doi: 10.1016/s0306-4522(99)00358-9. [DOI] [PubMed] [Google Scholar]
- Schanuel SM, Bell KA, Henderson SC, McQuiston AR. Heterologous expression of the invertebrate FMRFamide–gated sodium channel as a mechanism to selectively activate mammalian neurons. Neuroscience. 2008;155:374–386. doi: 10.1016/j.neuroscience.2008.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp FR, Swanson RA, Honkaniemi J, Kogure K, Massa SM. Stroke: Pathophysiology, diagnosis, and management. In: Barnett HJM, Mohr JP, Stein BM, Yatsu FM, editors. Neurochemistry and Molecular Biology. Churchill Livingstone; Philadelphia: 1998. pp. 51–83. [Google Scholar]
- Sombati S, Delorenzo RJ. Recurrent spontaneous seizure activity in hippocampal neuronal networks in culture. J Neurophysiol. 1995;73:1706–1711. doi: 10.1152/jn.1995.73.4.1706. [DOI] [PubMed] [Google Scholar]
- Stasheff SF, Anderson WW, Clark S, Wilson WA. NMDA antagonists differentiate epileptogenesis from seizure expression in an in vitro model. Science. 1989;245:648–651. doi: 10.1126/science.2569762. [DOI] [PubMed] [Google Scholar]
- Stoppini L, Buchs P, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Sun DA, Sombati S, DeLorenzo RJ. Glutamate injury-induced epileptogenesis in hippocampal neurons: An in vitro model of stroke-induced “epilepsy”. Stroke. 2001;32:2344–2350. doi: 10.1161/hs1001.097242. [DOI] [PubMed] [Google Scholar]
- Sun DA, Sombati S, Blair RE, DeLorenzo RJ. Long-lasting alterations in neuronal calcium homeostasis in an in vitro model of stroke-induced epilepsy. Cell Calcium. 2004;35:155–163. doi: 10.1016/j.ceca.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Sundstrom L, Morrison B, 3rd, Bradley M, Pringle A. Organotypic cultures as tools for functional screening in the CNS. Drug Discov Today. 2005;10:993–1000. doi: 10.1016/S1359-6446(05)03502-6. [DOI] [PubMed] [Google Scholar]
- Taylor TN, Davis PH, Torner JC, Holmes J, Meyer JW, Jacobson MF. Lifetime cost of stroke in the united states. Stroke. 1996;27:1459–1466. doi: 10.1161/01.str.27.9.1459. [DOI] [PubMed] [Google Scholar]
- Tecoma ES, Choi DW. GABAergic neocortical neurons are resistant to NMDA receptor-mediated injury. Neurology. 1989;39:676–682. doi: 10.1212/wnl.39.5.676. [DOI] [PubMed] [Google Scholar]
- Thomas AM, Corona-Morales AA, Ferraguti F, Capogna M. Sprouting of mossy fibers and presynaptic inhibition by group II metabotropic glutamate receptors in pilocarpine-treated rat hippocampal slice cultures. Neuroscience. 2005;131:303–320. doi: 10.1016/j.neuroscience.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Wahab A, Albus K, Heinemann U. Drug refractoriness of epileptiform activity in organotypic hippocampal slice cultures depends on the mode of provocation. Epilepsy Res. 2010;90:304–308. doi: 10.1016/j.eplepsyres.2010.06.007. [DOI] [PubMed] [Google Scholar]
- Yashiro K, Philpot BD. Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology. 2008;55:1081–1094. doi: 10.1016/j.neuropharm.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer J, Gahwiler BH. Cellular and connective organization of slice cultures of the rat hippocampus and fascia dentata. J Comp Neurol. 1984;228:432–446. doi: 10.1002/cne.902280310. [DOI] [PubMed] [Google Scholar]