Abstract
The use of narcotics by adolescent females is a growing problem, yet very little is known about the long-term consequences for either the user or her future offspring. In the current study, we utilized an animal model to examine the transgenerational consequences of opiate exposure occurring during this sensitive period. Female rats were exposed to increasing doses of morphine or its saline vehicle twice daily during adolescent development (postnatal days 30–40), after which they remained drug free. At 60 days of age, all females were mated and their adult offspring were tested for anxiety-like behavior and sensitivity to morphine. Specifically, offspring of adolescent morphine (MOR-F1)- or saline (SAL-F1)-exposed mothers were tested for acute locomotor responses in an open field, followed by testing of acute or chronic morphine analgesia on the hot plate. Open field testing indicated alterations in anxiety-like behavior in MOR-F1 female offspring, with effects dependent upon the stage of the estrus cycle. Hot plate testing revealed sex differences in baseline pain threshold and morphine sensitivity in all offspring, regardless of maternal exposure. However, when compared to their SAL-F1 counterparts, MOR-F1 male offspring demonstrated significantly increased sensitivity to the analgesic effects of acute morphine, and developed analgesic tolerance more rapidly following chronic morphine treatment. The findings indicate that prior opiate exposure during early adolescence in females produces sex-specific alterations of both emotionality and morphine sensitivity in their progeny.
Keywords: morphine, adolescence, puberty, transgenerational, offspring, tolerance, analgesia, anxiety
1. INTRODUCTION
During the past decade, there has been a significant increase in the number of adolescents using powerful narcotics [1], with the most commonly used substances being codeine, hydrocodone, and oxycodone [2]. Prescription opioids are considered readily accessible by 40% of adolescent’s surveyed [3] and many adolescents obtain the drugs from their home medicine cabinets. This easy availability has led to opiate use in populations that in years past were unlikely to be exposed to this class of drugs. Indeed, recent epidemiological data indicate that adolescent females (i.e. between the ages of 12 and 17) are one sub-group at increased risk for narcotic misuse [1]. Despite these statistics, few studies have examined the long-term impact of adolescent exposure to opiates. This is unfortunate given that adolescence represents a critical period of neurodevelopment. Moreover, given the role of endogenous opioids in the onset of puberty and the regulation of reproductive function [4], females may be particularly vulnerable to the long-term effects of opiate exposure during this period.
We have previously utilized a rodent model to better understand the long-term effects of adolescent opioid exposure in both the female as well as in her offspring. These studies have demonstrated significant effects on suckling-induced prolactin secretion [5] and neural gene expression [6]. Moreover, both male and female offspring of morphine-exposed females (MOR-F1) developed more rapid and robust behavioral sensitization to morphine when compared to the offspring saline-exposed females (SAL-F1) [7]. Finally, MOR-F1 females demonstrated increased anxiety-like behavior on the elevated plus maze when compared to SAL-F1 females [7]. Thus, exposure to opiates during adolescence appears to have lasting neuroendocrine effects in the female. In addition, the offspring of exposed females, even in the absence of in utero exposure, demonstrate significant neurobehavioral alterations.
In these previous studies we utilized a prolonged morphine exposure (20 days) that essentially persisted throughout adolescent development. Adolescence is transitional period between childhood and adulthood. In the rodent, this developmental stage has been both narrowly and broadly defined [8]. As this period progresses along a continuum and is not distinguished by any one specific event, we prefer the more comprehensive definition of this developmental period, which in rats ranges from postnatal day 28 (PN28) to PN50 [9, 10]. In this context, early-mid adolescence is marked by significant neuroendocrine changes in response to both endogenous and exogenous opioids [4, 11]. These include sexual maturation [12] and the regulation of the hypothalamic-pituitary-gonadal axis [11]. The present study was designed to model female opioid use limited to early-mid adolescence, and to further characterize possible transgenerational effects related to emotionality and opioid sensitivity. Female rats were exposed to increasing doses of morphine or its saline vehicle twice daily during early-mid adolescent development (PN30-PN40). As adults, females were mated and their adult offspring were tested for anxiety-like behavior and sensitivity to morphine analgesia (both acute and chronic). Overall, the findings support and expand upon our previous work, indicating that maternal drug experience in early-mid adolescence produces significant neurobehavioral changes in future offspring.
2. EXPERIMENTAL METHODS AND PROCEDURE
2.1 Animals
Female Sprague-Dawley rats (22 days of age) were purchased from Charles River Breeding Laboratories (Crl:CD(SD)BR; Kingston, NY). All animals the in the study were group-housed in light-(1200-1200 h) and temperature-(21–24°C) controlled rooms and were provided with food and water ad libitum. All animals were maintained in accordance with the National Research Council (NRC) Guide for the Care and Use of Laboratory Animals, and all animal procedures were approved by the Institutional Animals Care and Use Committee of Tufts University.
2.2 Adolescent Morphine Exposure
Beginning at 30 days of age, females were treated with morphine (morphine sulfate; Butler-Schein, Dublin, OH) for a total of 10 days using an increasing dose regimen. The doses used in the current study were based on allometric scaling to approximate human use. Moreover, the use of increasing doses is more compatible with human use patterns, allowing for rising and falling levels of opiates. On Day 1 of exposure, animals received 2.5 mg/kg morphine sulfate (s.c.) twice daily (0800h and 1600h). On each subsequent day, the dose of morphine was increased by 2.5 mg/kg such that by the final day of treatment subjects received two 25 mg/kg injections. Age-matched control animals received the saline vehicle (0.9% NaCl, s.c.) twice daily with volumes adjusted to match those of drug-treated females. Bodyweights were recorded daily. Following this regimen, animals remained undisturbed in the colony until mating.
2.3 Mating and Postpartum Care
Twenty days after the final drug or vehicle administration (60 days of age), females were mated with colony males and subsequently monitored for pregnancy. The birth of each litter was designated postnatal day 0 (PND0). On PND1 pups were weighed and genders determined. All litters were culled to 4 males and 4 females. On PND21 litters were weighed prior to weaning. Littermates of each sex were housed together throughout the experiment.
2.4 Open Field Testing
For open field testing of the F1 generation, animals were transported to a behavioral testing room maintained under standard vivarium temperature and lighting conditions. After a 5 minute acclimation to the room, animals were placed individually into a Plexiglas open field arena (length=41 cm, width=41 cm, height=38 cm), and motor activity was monitored for 10 minutes using the SmartFrame® Open Field Activity System (Hamilton-Kinder; Poway, CA). This system consists of 32 photocells (16 × 16, spaced 2.5 cm apart) which continuously monitor the animal’s movement. Data were collected as photobeam breaks in both the center and periphery of the arena using MotorMonitor® software (Hamilton-Kinder). Data were analyzed and expressed as total photobeam breaks in the center and periphery (locomotor activity), and as the percent of total testing time spent in the center of the open field.
2.5 Pain Threshold Testing
Pain threshold was measured in F1 males and females between 0900 and 1100 h. For this test, animals were individually placed onto the hot plate (Ugo Basile Model 7280, Collegeville, PA) with the surface temperature maintained at 54°C. The latency (s) to hindpaw licking (hindpaw latency: HPL) was recorded, with a cut-off period of 30 s used to prevent tissue damage. Immediately following testing, animals were placed into the home cage. Both baseline (untreated) pain threshold and morphine analgesia were determined for each animal (see Experimental Procedure). For data analysis, baseline pain threshold was calculated and expressed as HPL (s). Morphine analgesia was calculated using the standard formula for percent maximum possible effect (% MPE): [HPL(s) − mean baseline HPL(s)]/[30 s − mean baseline (HPL(s)]*100. Data were expressed as either mean % MPE, Median % MPE, or the percent of animals in each group reaching 100% MPE.
2.5 Experimental Procedure
Behavioral testing of the F1 generation commenced once animals reached 60 days of age (adulthood). All animals were tested for both open field behavior and pain threshold. Open field testing was conducted at least one week prior to pain threshold testing.
2.5.1 Open Field Behavior
Rodent locomotor activity in a novel open field is a commonly used measure of “anxiety-like” behavior. Accordingly, a tendency for the animal to remain close to the walls of an enclosure (thigmotaxis) is interpreted as anxiety-like, while center crossings are believed to reflect a state of decreased anxiety (for an excellent review of this method see Prut and Bulzung, 2003) [13]. In the current study, locomotor response to novelty was studied using a single 5 minute exposure to an open field. F1 males were tested between either 0900–1000 h (AM) or 1600–1700 h (PM). Each male was tested only once, with separate groups of animals (one per litter) tested at each time point. Given the known variation in anxiety-like behavior across the estrous cycle in female rats [14, 15], separate groups of F1 females were tested at each estrous cycle stage (one animal per litter per stage). Testing of these animals followed demonstration of two full estrous cycles (based on vaginal cytology).
2.5.2 Baseline Pain Threshold and Acute Morphine Analgesia
To study the acute effects of morphine on pain threshold, HPL was first recorded in untreated animals for 10 consecutive days to establish baseline responding. Baseline responses obtained on days 1 and 10 were compared to determine the extent of habituation. After 24 hr, animals were tested again for baseline HPL at 30 and 15 min prior to treatment with one of three doses of morphine sulfate (1.0, 1.75, or 2.5 mg/kg). All doses were administered subcutaneously in a volume of 1 ml/kg, and were distributed such that only one subject from each litter received a given dose. HPL on the hot plate were measured 30 min later to assess sensitivity to the acute analgesic effects of morphine.
2.5.3 Repeated Morphine Analgesia
The development of morphine tolerance was examined in a separate group of animals. Animals received 10 mg/kg morphine sulfate (1 ml/kg, s.c.) twice-daily (0830 and 1630 h) for a total of 3 days, with a final dose administered at 0830 on day 4. HPL on the hot plate was determined 30 min following each 0830 dose. HPL was then compared across days to determine the effect of repeated morphine treatment on hot plate analgesia and analgesic tolerance.
2.6 Statistical Analyses
Open field activity for male and female offspring was analyzed separately using a two-way ANOVA, with Maternal Adolescent Exposure and Time of Day (males) or Estrous Cycle Stage (females) as factors. For baseline pain threshold, HPL data were analyzed using a three-way Repeated Measures ANOVA, with Maternal Adolescent Exposure, Sex, and Test Day as factors. For acute morphine studies, percent MPE data were analyzed using three-way ANOVA with Maternal Adolescent Exposure, Sex, and morphine Dose as factors. All significant main effects were followed by Tukey’s posthoc tests. For tolerance data, the percent of subjects demonstrating 100% MPE following chronic morphine was analyzed using the Fisher’s Exact Test. The median % MPE on the final day of repeated morphine treatment was analyzed using the Mann-Whitney Rank Sum Test. For all analyses, significance was defined as p < 0.05.
3. RESULTS
3.1 Body Weight in Exposed Females and Offspring
As shown in Table 1, there was a significant effect of morphine on bodyweight gain over the ten days of exposure in the adolescent females (T[15] = 2.51, p = 0.02). No differences in maternal bodyweight on PN1 (p = 0.3), litter BW on PN1 (p = 0.5) or litter BW on PN21 (p = 0.8). Thus, adolescent exposure does not significantly influence growth parameters during the postnatal period.
Table 1.
Body weight changes (grams) of females exposed to either saline or morphine during early-mid adolescence and their F1 offspring during postnatal development.
| Adolescent Exposure | BW Gain PN30-PN40 | Maternal BW PN1 | Litter BW PN1 | Litter BW PN21 |
|---|---|---|---|---|
| Saline | 52.7±2.8 | 291.1±9.1 | 49.7±1.1 | 463.7±11.7 |
| Morphine | 44.5±1.4* | 308.8±13.7 | 51.3±1.7 | 458.9±15.5 |
p < 0.05 versus saline-treated controls
3.2 Open Field Behavior
Analysis of overall locomotor activity revealed no significant main effects of Maternal Adolescent Exposure in F1 males and females. Likewise, no main effects of Time of Day (F1 males) or Estrous Cycle Stage (F1 females) on overall locomotor activity were observed. There was, however, an interaction between Maternal Adolescent Exposure and Estrous Cycle Stage (F[1,65] = 3.2, p < 0.05) in F1 females. As shown in Figure 1 (upper panel), post hoc analysis revealed a significant reduction in locomotor activity during metestrus (p < 0.05) in MOR-F1 females, as well as a trend toward increased on estrus (p = 0.07).
Figure 1.
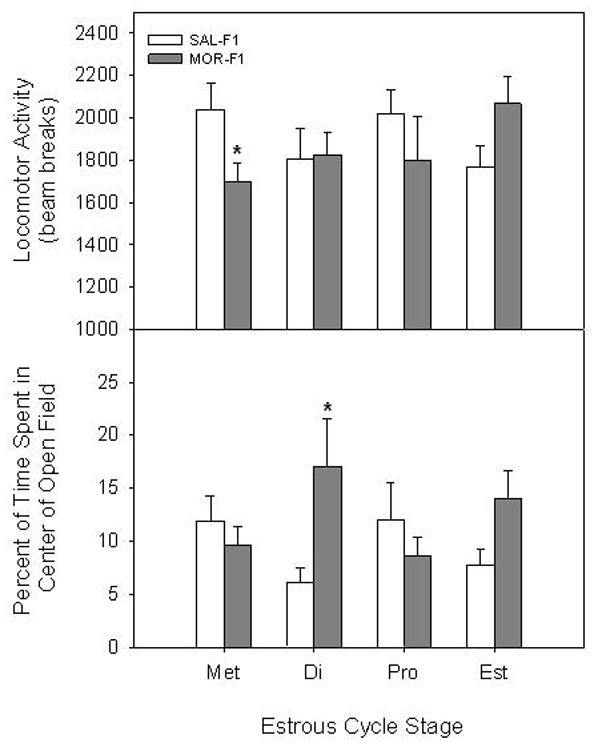
Effects of early adolescent morphine treatment on anxiety-like behavior in adult offspring. Behavioral responses in a novel open field were examined for 10 min in F1 females across the estrous cycle. Data are locomotor activity (mean ± SEM; upper panel) and percent time spent in the center of the open field (mean ± SEM; lower panel) for separate groups of 6–10 animals. Met=metestrus, Di=diestrus, Pro=proestrus, Est=estrus.
*p < 0.05 vs SAL-F1 within Estrous Cycle Stage.
Analysis of anxiety-like behavior revealed sex-dependent effects of Maternal Adolescent Exposure. Specifically, a two-way ANOVA conducted on percent time spent in the center of the open field indicated a significant interaction between Maternal Adolescent Exposure and Estrous Cycle Stage in F1 females (F[1,65] = 2.8, p < 0.05). As shown in Figure 1 (lower panel), post hoc analyses indicated that MOR-F1 females spent significantly more time in the center on diestrus (p < 0.05), with a trend toward increased time during estrus (p=0.09). In contrast, no effects of Maternal Adolescent Exposure or Time of Day on anxiety-like behavior were observed for F1 males (Data not shown).
3.3 Baseline Pain Threshold and Acute Morphine Analgesia
There was no main effect of Maternal Adolescent Exposure on baseline HPL responses in F1 animals. However, significant main effects of Sex (F[1,131] = 14.9, p < 0.001), Test Day, (F[1,131] = 54.6, p < 0.001), and their interaction (F[1,131] = 6.45, p < 0.02) were observed. As shown in Figure 2, both F1 males and F1 females demonstrated a significant decline in HPL from Day 1 to Day 10 (both p’s < 0.001). Moreover, females had significantly lower HPL when compared to males. This effect, however, was significant only on the first day of testing (p < 0.001).
Figure 2.
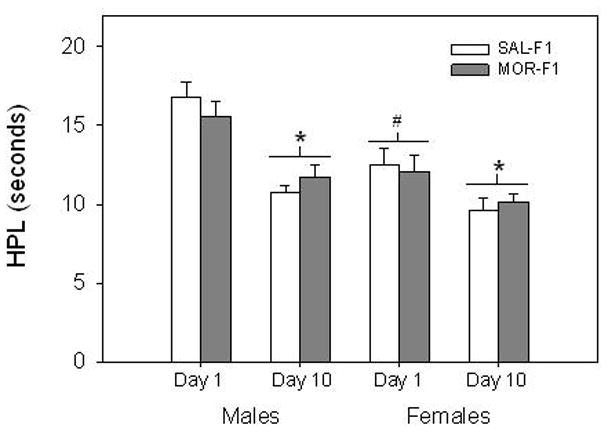
Effects of early adolescent morphine treatment on baseline pain threshold in adult offspring. Untreated F1 males and females were tested daily for baseline pain threshold using the hot plate. Data are mean (± SEM) HPL (s) for the first and last day of testing for groups of 30–36 animals. *p < 0.001 vs Day 1 for within each sex condition. #p<0.001 vs F1 males on Day 1.
Analysis of analgesic responses of the F1 generation to morphine yielded several findings. Results of a three-way ANOVA indicated main effects of both Sex (F[1,96] = 11.24, p < 0.001) and morphine Dose (F[2,96] = 13.6, p < 0.001) on % MPE, though only a trend toward an effect of Maternal Adolescent Exposure was found (F[1,96] = 3.5, p = 0.06). However, there were significant interactions between Maternal Adolescent Exposure and Sex (F[1,96] = 3.9, p < 0.05), as well as between Sex and morphine Dose (F[2,96] = 3.7, p < 0.05). Based on these interactions indicating Sex as a significant factor, data obtained from males and females were analyzed separately via two-way ANOVA (Maternal Adolescent Exposure X morphine Dose). In F1 males (Figure 3, left panel), there was a significant main effect of Maternal Adolescent Exposure (F[1,46] = 4.85, p < 0.05), with MOR-F1 males displaying an augmented analgesic response across all three doses when compared to SAL-F1 males. In addition, there was a significant main effect of morphine Dose (F[2,46] = 8.35, p < 0.001), with % MPE significantly increased in the 2.5 mg/kg dose when compared to 1.0 mg/kg. For F1 females (Figure 3, right panel), a significant main effect of morphine Dose (F[2,46] = 8.35, p < 0.001) on % MPE was also observed (2.5 mg/kg vs 1.0 mg/kg, p < 0.05). However, there was no significant effect of Maternal Adolescent Exposure on morphine analgesia in F1 females.
Figure 3.
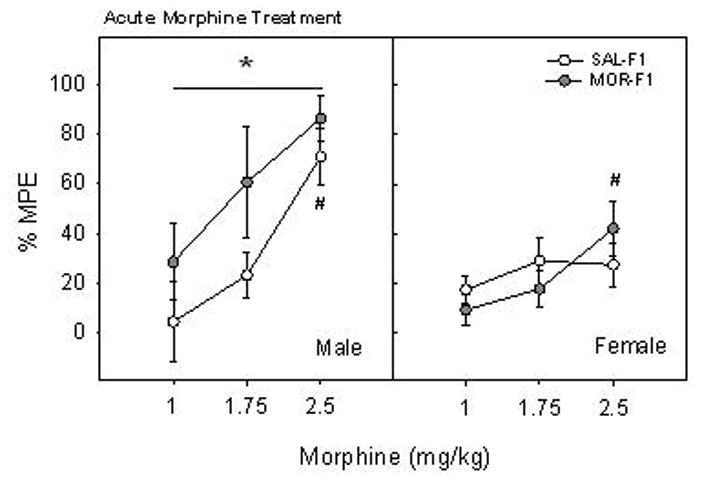
Effects of early adolescent morphine treatment on analgesic sensitivity to acute morphine in adult offspring. F1 males and females were treated with one of three doses of morphine and were tested for thermal pain threshold after 30 min. Data are % MPE on the hot plate (mean ± SEM) for groups of 7–9 animals. *p < 0.05 vs SAL-F1 collapsed across doses. #p<0.001 vs 1 mg/kg collapsed across maternal adolescent exposure condition.
3.4 Repeated Morphine Analgesia
Analysis of analgesic responses of F1 animals to morphine following 4 days of treatment indicated an effect of Maternal Adolescent Exposure and its interaction with offspring Sex. All F1 males administered 10 mg/kg morphine on Treatment Day 1 failed to demonstrate a HPL within the 30 s cutoff, and therefore were assigned 100% MPE (Figure 4, left panel). While the percent of animals responding at 100% MPE declined across Treatment Day for all F1 males, more rapid and robust tolerance was observed in the MOR-F1 group. Indeed, only 20% of animals in the MOR-F1 group responded at 100% MPE by Treatment Day 4 as compared to 80% of those in the SAL-F1 group (Fishers Exact Test, p < 0.05). Moreover, by Treatment Day 4, MOR-F1 males had significantly lower median % MPE scores when compared to SAL-F1 males (SAL-F1=100, MOR-F1=68.9: Mann–Whitney U = 56, p < 0.042). In contrast, no significant differences in analgesic response to morphine as a function of Maternal Adolescent Exposure were observed in F1 females (Figure 4, right panel).
Figure 4.
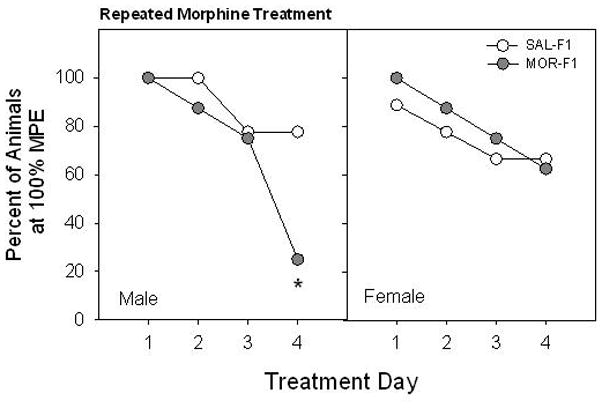
Effects of early adolescent morphine treatment on the development of morphine tolerance in adult offspring. F1 males and females were tested for thermal pain threshold following each daily treatment with morphine (10 mg/kg). Data are percent of animals attaining 100 % MPE for groups of 8–9 animals. *p < 0.05 vs SAL-F1 on Treatment Day 4.
4. DISCUSSION
The present study indicates that exposure of female rats to morphine in early adolescence has transgenerational consequences, the nature of which are dependent upon the sex of the offspring. It was found that the effects of maternal adolescent morphine treatment on female offspring were associated with decreased anxiety-like behavior. These effects were estrous cycle-dependent, with MOR-F1 females exhibiting increased time in the center of the open field during diestrus and estrus. No effects of maternal adolescent morphine treatment on open field behavior were found in male offspring. In contrast, hot plate testing revealed increased sensitivity to acute morphine analgesia and more rapid development of morphine tolerance in MOR-F1 males, while no changes in morphine sensitivity in MOR-F1 females was observed. These findings indicate that exposure to morphine, even when confined to early adolescence, can significantly impact the phenotype of future progeny in a sex-specific manner.
Our previous work on the transgenerational effects of adolescent morphine exposure utilized a prolonged injection regimen with higher doses of morphine [7]. The current findings indicate that transgenerational effects continue to be observed with a shorter duration of exposure, lower doses of morphine, and with an increased period of abstinence between the cessation of morphine administration and mating. It is unclear whether the age at the time of exposure (i.e. early-mid adolescence) is a key factor in modulating these offspring effects. It should be noted, however, that while the current results suggest that similar systems are affected by both exposure regimens, some differences in the nature of these offspring effects are evident, particularly with regard to anxiety-like behavior. For example, we previously reported a significant increase in anxiety-like behavior on the elevated plus maze in MOR-F1 females [7]. In that study, all females were examined during estrus. Similar effects were not observed during estrus in the current study. Indeed, MOR-F1 females tended to demonstrate reduced anxiety-like behavior and increased locomotor activity when tested in the open field during estrus. Whether this represents a fundamental difference based on the duration of treatment or a difference based on the precise nature of the task (e.g. level of stress associated with the task) is unknown. Regardless, the results of the current study indicate that significant alterations in anxiety-like behavior as well as exploratory behavior are modulated by maternal adolescent treatment and that these effects are estrous cycle-dependent. Moreover, they support our previous findings that the transgenerational effects of anxiety-like behavior are observed in F1 female but not F1 males.
In MOR-F1 females, significantly reduced anxiety-like behavior was observed on diestrus but not on proestrus. Similar results have been reported in the adult female offspring of mice exposed to brief maternal separation and these effects were specific to the open field as similar results were not observed on the elevated plus maze [16]. No significant differences in measures of maternal behavior were observed in that study. While we did not directly examine alterations in maternal care in the current study, our previous findings using a more prolonged morphine exposure during adolescence did not reveal any significant effects on gross measures of maternal behavior (retrieval and crouching latencies; [17]). In addition, we observed no significant effects on bodyweight growth as a function of maternal adolescent exposure. Nonetheless, it is possible that subtle alterations in the maternal behavior of females exposed to morphine during early-mid adolescence may play a role in the effects observed in adult female offspring. Certainly, there are many studies that indicate a significant role of maternal behavior in adult emotionality [16, 18–20]. Moreover, studies have also revealed significant alterations in the endogenous opioid system following maternal separation paradigms [21, 22]. We are currently conducting studies examining the potential role of maternal behaviors in transgenerational effects of adolescent morphine exposure. These studies will utilize both home cage observation and cross-fostering designs to begin to dissociate maternal influences in this phenomenon.
In the current study, acute administration of low, threshold-doses of morphine significantly prolonged HPL in MOR-F1 males. In addition, MOR-F1 males also became tolerant to the analgesic effects of morphine more quickly. Acute analgesic responses to morphine and the development of tolerance are believed to reflect the sensitivity of brain opioid systems. Accordingly, the current study suggests enhanced opioid sensitivity in the offspring of females exposed to morphine during adolescence. There is a significant association with analgesic sensitivity and abuse liability [23, 24], with enhanced morphine sensitivity associated with increased risk of abuse. Together with our previous findings on morphine sensitization, these data strongly suggest that maternal adolescent morphine exposure may increase the risk of drug abuse vulnerability in her male offspring. The question of whether such transgenerational effects could be triggered by maternal drug exposure in early life is intriguing. Currently, the role of prior maternal drug use in the familial transmission of substance abuse liability is a relatively unexplored area of study.
With regard to differences in morphine sensitivity, no significant effects of maternal morphine exposure were observed in MOR-F1 females. Sex differences in morphine analgesia have been widely reported [25, 26] with males consistently demonstrating increased sensitivity to the analgesic properties of opiates [27–29]. Similar effects were observed in the current study, with few analgesic effects observed in females with lower doses. A number of mechanisms may underlie these sex differences, including differential distribution of μ-opioid receptors, estrogenic effects on endogenous opioid function, and sex differences in brain dopamine and/or glutamate systems [25, 26, 30, 31]. Previous studies have also observed sex differences in the development of tolerance [32–34] although the data are somewhat mixed with some demonstrating enhanced morphine tolerance in males [30, 32], others more rapid tolerance in females [35], and still others indicating no sex differences [36, 37]. There is a rich body of literature detailing the impact of a variety of parameters on sex differences in morphine potency and tolerance, including the effects of the dosing regimen, the route of administration and the test used to measure analgesic response as well as the strain of the animal. Thus, it is possible that transgenerational effects of adolescent morphine exposure on morphine-induced analgesia may yet be revealed in MOR-F1 females under different test conditions.
The mechanism underlying the transmission of these effects remains unknown. It is possible that a modification in the postnatal environment induces the observed effects on both anxiety-like behavior and morphine analgesia in MOR-F1 animals. Indeed, maternal separation has been shown to induce significant alterations in both emotionality and morphine sensitization [38]. On the other hand, the endogenous opioid system is a critical regulator of the prenatal environment [39–42], and studies have shown that blocking endogenous opioids during pregnancy has significant consequences for offspring [43]. Exposure of adolescent female rats to a similar increasing dose regimen of morphine results in significant long-term alterations in their endogenous opioid system [6]. Such persistent changes could result in adaptations within the prenatal environment, including differences in the levels of circulating hormones. Interestingly, there is increasing evidence that prenatal stress can impact anxiety-like behavior and drug abuse liability. These effects are likely mediated via alterations in the HPA axis. Thus, one possible mechanism underlying the observed effects could be a shift in the HPA axis resulting in in utero conditions that are similar to those observed in prenatally stressed mothers. Indeed, it is possible that both pre- and postnatal factors mediated by alterations in endogenous opioid regulation of the HPA and/or HPG axis may mediate these transgenerational effects. Finally, recent studies in the field of epigenetics suggest that changes in DNA methylation, via effects on gene transcription, may play a role in intergenerational transfer of behavioral phenotypes. However, to date there is no evidence to support direct germ line transmission of specific DNA methylation from mother to offspring.
5. Conclusions
The current findings demonstrate significant transgenerational effects of adolescent morphine exposure in female rats. That persistent effects of prior drug use can be observed in the next generation, even in the complete absences of in utero or postnatal exposure, represents a considerable modification in our understanding of the long-term impact of female drug use. To what extent the timing of the exposure to the adolescent period is a significant factor in offspring effects remains to be determined. Moreover, whether these effects are due to epigenetic modifications in the germline, alterations within the in utero environment, or differences in postnatal maternal-infant interactions is also unknown. Overall, what the current findings indicate is that exposure to morphine prior to mating, can significantly modify the adult phenotype of future offspring, including alterations in opioid sensitivity. As such, these transgenerational effects represent an example of an epigenetic modification induced by maternal drug history which could play a role in enhancing her offspring’s vulnerability to opiate-related disorders.
Research Highlights.
Female adolescent opiate exposure induces transgenerational alterations in morphine sensitivity in adult male offspring.
Female adolescent opiate exposure induces transgenerational alterations in morphine tolerance in adult male offspring.
Female adolescent opiate exposure induces transgenerational alterations in open field behavior, which is estrous cycle dependent, in adult female offspring.
Acknowledgments
The authors would like to thank Robert Bridges, Ph.D. for the use of his laboratory facilities. This work was supported by a grant from the National Institute on Drug Abuse, R03 DA14613 and R01 DA25674 awarded to EMB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sung HE, Richter L, Vaughan R, Johnson PB, Thom B. Nonmedical use of prescription opioids among teenagers in the united states: Trends and correlates. J Adolesc Health. 2005;37:44–51. doi: 10.1016/j.jadohealth.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 2.McCabe SE, Cranford JA, Boyd CJ, Teter CJ. Motives, diversion and routes of administration associated with nonmedical use of prescription opioids. Addict Behav. 2007;32:562–575. doi: 10.1016/j.addbeh.2006.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnston LOMPM, Bachman JG, Schulenberg JE. Overview of key findings, 2007. National Institute on Drug Abuse (NIDA); 2008. Monitoring the future: National results on adolescent drug use. [Google Scholar]
- 4.Genazzani AR, Bernardi F, Monteleone P, Luisi S, Luisi M. Neuropeptides, neurotransmitters, neurosteroids, and the onset of puberty. Ann N Y Acad Sci. 2000;900:1–9. doi: 10.1111/j.1749-6632.2000.tb06210.x. [DOI] [PubMed] [Google Scholar]
- 5.Byrnes EM. Chronic morphine exposure during puberty decreases postpartum prolactin secretion in adult female rats. Pharmacol Biochem Behav. 2005a;80:445–451. doi: 10.1016/j.pbb.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 6.Byrnes EM. Chronic morphine exposure during puberty induces long-lasting changes in opioid-related mrna expression in the mediobasal hypothalamus. Brain Res. 2008;1190:186–192. doi: 10.1016/j.brainres.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 7.Byrnes EM. Transgenerational consequences of adolescent morphine exposure in female rats: Effects on anxiety-like behaviors and morphine sensitization in adult offspring. Psychopharmacology (Berl) 2005b;182:537–544. doi: 10.1007/s00213-005-0122-4. [DOI] [PubMed] [Google Scholar]
- 8.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 9.Andersen SL. Trajectories of brain development: Point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27:3–18. doi: 10.1016/s0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 10.Ellgren M, Artmann A, Tkalych O, Gupta A, Hansen HS, Hansen SH, Devi LA, Hurd YL. Dynamic changes of the endogenous cannabinoid and opioid mesocorticolimbic systems during adolescence. Thc effects. Eur Neuropsychopharmacol. 2008;18:826–834. doi: 10.1016/j.euroneuro.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cicero TJ, Schmoeker PF, Meyer ER, Miller BT, Bell RD, Cytron SM, Brown CC. Ontogeny of the opioid-mediated control of reproductive endocrinology in the male and female rat. J Pharmacol Exp Ther. 1986;236:627–633. [PubMed] [Google Scholar]
- 12.Reis FM, Reis AM. Effect of prepuberal chronic morphine administration on the onset of puberty in pituitary-grafted female rats. Braz J Med Biol Res. 1992;25:201–203. [PubMed] [Google Scholar]
- 13.Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- 14.Marcondes FK, Miguel KJ, Melo LL, Spadari-Bratfisch RC. Estrous cycle influences the response of female rats in the elevated plus-maze test. Physiol Behav. 2001;74:435–440. doi: 10.1016/s0031-9384(01)00593-5. [DOI] [PubMed] [Google Scholar]
- 15.Frye CA, Petralia SM, Rhodes ME. Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3alpha,5alpha-thp. Pharmacol Biochem Behav. 2000;67:587–596. doi: 10.1016/s0091-3057(00)00392-0. [DOI] [PubMed] [Google Scholar]
- 16.Romeo RD, Mueller A, Sisti HM, Ogawa S, McEwen BS, Brake WG. Anxiety and fear behaviors in adult male and female c57bl/6 mice are modulated by maternal separation. Horm Behav. 2003;43:561–567. doi: 10.1016/s0018-506x(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 17.Byrnes EM. Chronic morphine exposure during puberty decreases postpartum prolactin secretion in adult female rats. Pharmacol Biochem Behav. 2005;80:445–451. doi: 10.1016/j.pbb.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 18.D’Amato FR, Cabib S, Ventura R, Orsini C. Long-term effects of postnatal manipulation on emotionality are prevented by maternal anxiolytic treatment in mice. Dev Psychobiol. 1998;32:225–234. doi: 10.1002/(sici)1098-2302(199804)32:3<225::aid-dev6>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 19.Hofer MA. Early relationships as regulators of infant physiology and behavior. Acta Paediatr Suppl. 1994;397:9–18. doi: 10.1111/j.1651-2227.1994.tb13260.x. [DOI] [PubMed] [Google Scholar]
- 20.Champagne F, Meaney MJ. Like mother, like daughter. Evidence for non-genomic transmission of parental behavior and stress responsivity. Prog Brain Res. 2001;133:287–302. doi: 10.1016/s0079-6123(01)33022-4. [DOI] [PubMed] [Google Scholar]
- 21.Kalinichev M, Easterling KW, Holtzman SG. Long-lasting changes in morphine-induced locomotor sensitization and tolerance in long-evans mother rats as a result of periodic postpartum separation from the litter: A novel model of increased vulnerability to drug abuse? Neuropsychopharmacology. 2003;28:317–328. doi: 10.1038/sj.npp.1300068. [DOI] [PubMed] [Google Scholar]
- 22.Matthews K, Robbins TW. Early experience as a determinant of adult behavioural responses to reward: The effects of repeated maternal separation in the rat. Neurosci Biobehav Rev. 2003;27:45–55. doi: 10.1016/s0149-7634(03)00008-3. [DOI] [PubMed] [Google Scholar]
- 23.Franklin KB. Analgesia and abuse potential: An accidental association or a common substrate? Pharmacol Biochem Behav. 1998;59:993–1002. doi: 10.1016/s0091-3057(97)00535-2. [DOI] [PubMed] [Google Scholar]
- 24.Franklin KB. Analgesia and the neural substrate of reward. Neurosci Biobehav Rev. 1989;13:149–154. doi: 10.1016/s0149-7634(89)80024-7. [DOI] [PubMed] [Google Scholar]
- 25.Craft RM. Sex differences in analgesic, reinforcing, discriminative, and motoric effects of opioids. Exp Clin Psychopharmacol. 2008;16:376–385. doi: 10.1037/a0012931. [DOI] [PubMed] [Google Scholar]
- 26.Loyd DR, Wang X, Murphy AZ. Sex differences in micro-opioid receptor expression in the rat midbrain periaqueductal gray are essential for eliciting sex differences in morphine analgesia. J Neurosci. 2008;28:14007–14017. doi: 10.1523/JNEUROSCI.4123-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.South SM, Edwards SR, Smith MT. Antinociception versus serum concentration relationships following acute administration of intravenous morphine in male and female sprague-dawley rats: Differences between the tail flick and hot plate nociceptive tests. Clin Exp Pharmacol Physiol. 2009;36:20–28. doi: 10.1111/j.1440-1681.2008.05019.x. [DOI] [PubMed] [Google Scholar]
- 28.Cicero TJ, Nock B, Meyer ER. Sex-related differences in morphine’s antinociceptive activity: Relationship to serum and brain morphine concentrations. J Pharmacol Exp Ther. 1997;282:939–944. [PubMed] [Google Scholar]
- 29.Cicero TJ, Nock B, Meyer ER. Gender-related differences in the antinociceptive properties of morphine. J Pharmacol Exp Ther. 1996;279:767–773. [PubMed] [Google Scholar]
- 30.Mousavi Z, Shafaghi B, Kobarfard F, Jorjani M. Sex differences and role of gonadal hormones on glutamate level in the nucleus accumbens in morphine tolerant rats. A microdialysis study. Eur J Pharmacol. 2007;554:145–149. doi: 10.1016/j.ejphar.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 31.Nemmani KV, Grisel JE, Stowe JR, Smith-Carliss R, Mogil JS. Modulation of morphine analgesia by site-specific n-methyl-d-aspartate receptor antagonists: Dependence on sex, site of antagonism, morphine dose, and time. Pain. 2004;109:274–283. doi: 10.1016/j.pain.2004.01.035. [DOI] [PubMed] [Google Scholar]
- 32.Loyd DR, Morgan MM, Murphy AZ. Sexually dimorphic activation of the periaqueductal gray-rostral ventromedial medullary circuit during the development of tolerance to morphine in the rat. Eur J Neurosci. 2008;27:1517–1524. doi: 10.1111/j.1460-9568.2008.06100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bartok RE, Craft RM. Sex differences in opioid antinociception. J Pharmacol Exp Ther. 1997;282:769–778. [PubMed] [Google Scholar]
- 34.Hoffmann O, Plesan A, Wiesenfeld-Hallin Z. Genetic differences in morphine sensitivity, tolerance and withdrawal in rats. Brain Res. 1998;806:232–237. doi: 10.1016/s0006-8993(98)00768-9. [DOI] [PubMed] [Google Scholar]
- 35.Hopkins E, Rossi G, Kest B. Sex differences in systemic morphine analgesic tolerance following intrathecal morphine injections. Brain Res. 2004;1014:244–246. doi: 10.1016/j.brainres.2004.03.056. [DOI] [PubMed] [Google Scholar]
- 36.Holtman JR, Jr, Sloan JW, Wala EP. Morphine tolerance in male and female rats. Pharmacol Biochem Behav. 2004;77:517–523. doi: 10.1016/j.pbb.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 37.Barrett AC, Cook CD, Terner JM, Craft RM, Picker MJ. Importance of sex and relative efficacy at the mu opioid receptor in the development of tolerance and cross-tolerance to the antinociceptive effects of opioids. Psychopharmacology (Berl) 2001;158:154–164. doi: 10.1007/s002130100821. [DOI] [PubMed] [Google Scholar]
- 38.Kalinichev M, Easterling KW, Holtzman SG. Early neonatal experience of long-evans rats results in long-lasting changes in reactivity to a novel environment and morphine-induced sensitization and tolerance. Neuropsychopharmacology. 2002;27:518–533. doi: 10.1016/S0893-133X(02)00326-3. [DOI] [PubMed] [Google Scholar]
- 39.Douglas AJ, Bicknell RJ, Russell JA. Pathways to parturition. Adv Exp Med Biol. 1995;395:381–394. [PubMed] [Google Scholar]
- 40.Brunton PJ, Russell JA. Attenuated hypothalamo-pituitary-adrenal axis responses to immune challenge during pregnancy: The neurosteroid opioid connection. J Physiol. 2008;586:369–375. doi: 10.1113/jphysiol.2007.146233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Douglas AJ, Russell JA. Endogenous opioid regulation of oxytocin and acth secretion during pregnancy and parturition. Prog Brain Res. 2001;133:67–82. doi: 10.1016/s0079-6123(01)33006-6. [DOI] [PubMed] [Google Scholar]
- 42.Akil H, Watson SJ, Young E, Lewis ME, Khachaturian H, Walker JM. Endogenous opioids: Biology and function. Annu Rev Neurosci. 1984;7:223–255. doi: 10.1146/annurev.ne.07.030184.001255. [DOI] [PubMed] [Google Scholar]
- 43.McLaughlin PJ, Tobias SW, Lang CM, Zagon IS. Opioid receptor blockade during prenatal life modifies postnatal behavioral development. Pharmacol Biochem Behav. 1997;58:1075–1082. doi: 10.1016/s0091-3057(97)00307-9. [DOI] [PubMed] [Google Scholar]


