Abstract
The striatum is critical for learning and decision making; however, the molecular mechanisms that govern striatum function are not fully understood. The extracellular signal regulated kinase (ERK) cascade is an important signaling pathway that underlies synaptic plasticity, cellular excitability, learning and arousal. This review focuses on the role of ERK signaling in striatum function. ERK is activated in the striatum by coordinated dopamine and glutamate receptor signaling, where it underlies corticostriatal synaptic plasticity and influences striatal cell excitability. ERK activation in the dorsal striatum is necessary for action-outcome learning and performance of goal-directed actions. In the ventral striatum, ERK is necessary for the motivating effects of reward-associated stimuli on instrumental performance. Dysregulation of ERK signaling in the striatum by repeated drug exposure contributes to the development of addictive behavior. These results highlight the importance of ERK signaling in the striatum as a critical substrate for learning and as a regulator of ongoing behavior. Furthermore, they suggest that ERK may be a suitable target for therapeutics to treat disorders of learning and decision making that arise from compromised striatum function.
Keywords: Nucleus accumbens, dorsomedial striatum, dorsolateral striatum, extracellular signal regulated kinase, devaluation, pavlovian-instrumental transfer
I. Introduction
The striatum, the largest of the basal ganglia nuclei, is a critical substrate for learning and decision making. Impairments in learning and decision making accompany a range of disorders that affect the striatum, including substance abuse disorder, obsessive-compulsive disorder, and Parkinson’s disease [1–3]. Furthermore, the application of sophisticated procedures derived from instrumental conditioning in animals has identified parallel corticostriatal circuits that mediate distinct learning and action control processes [4–8]. Nevertheless, the molecular mechanisms that underlie learning and decision making in the striatum are not fully understood. One important regulator of neuronal function is the extracellular signal regulated kinase (ERK) pathway. ERK is a member of mitogen activated protein kinase (MAPK) family, and is critical for nervous system development and plasticity in the adult nervous system [9, 10]. ERK signaling in the nervous system has a critical role in memory formation, affect and arousal. Furthermore, the efficacy of a number of psychoactive substances, such as mood stabilizers and addictive drugs, depend, in part, on their ability to activate ERK in the nervous system.
In the following review, we highlight the role of ERK signaling in the striatum in learning and decision making. We begin with a brief overview of ERK signaling in the nervous system and its role in learning and behavior regulation. This is followed by a discussion of the role of ERK signaling in striatal-based learning and decision-making tasks. We conclude with a description of the role of ERK signaling in substance abuse, in which we discuss how alterations in ERK signaling by repeated drug exposure may compromise striatum function and produce features of addictive behavior.
II. The ERK signaling pathway
Like other MAPK signaling pathways, the ERK cascade consists of three kinases: ERK, of which there are two isoforms, ERK1 and ERK2, and their upstream kinases MEK and raf (see [11, 12] for review). ERK activation occurs in response to a variety of extracellular stimuli and forms an essential pathway for cells to generate adaptive responses to changing environments. These cellular responses include regulation of gene expression and synthesis of new proteins, alteration in cellular structure or metabolism, cellular growth, differentiation and apoptosis. ERK is activated when dual-phosphorylated by MEK at its serine and threonine residues. Raf, which phosphorylates MEK, consists of a family of kinases, and is activated when bound by the GTP-binding protein ras. Ras-ERK activity increases in response to a range of stimuli, including activation of G protein coupled receptors, tyrosine kinase receptors, and ca++ influx [9, 13]. Scaffolding proteins and phosphatases regulate ERK activity, thus providing an additional level of control over ERK function [11]. Upon activation, ERK has many cellular targets and influences a wide range of cellular functions [12]. ERK influences gene expression through its interaction with transcriptional regulators, such as ribosomal s6 kinase (RSK), mitogen- and stress-activated protein kinase-1 (MSK1) as well as the transcription factor elk-1. ERK also influences translation and new protein synthesis [14]. These and many other cellular operations under ERK’s regulation enable the cell to produce a coordinated response to extracellular stimuli.
In the adult nervous system, ERK is intimately involved in synaptic plasticity (see [15] for review). ERK inhibition prevents the induction of various forms of long-term potentiation (LTP) and long-term depression (LTD) in the hippocampus and amygdala [16–20]. ERK activation is necessary for a number of physiological processes that underlie synaptic plasticity, including AMPA receptor trafficking and spine restructuring [21–24]. Among the ways ERK influences synaptic plasticity is through its regulation of transcription and translation [14, 25, 26]. Finally, the effects of various neuromodulators on LTP and LTD induction and maintenance depend on ERK signaling [26–30]. Consistent with its role in synaptic plasticity, ERK has been shown to be necessary for long-term memory formation across a variety of tasks. An increase in ERK activation, measured as the ratio of phosphorylated to total (phosphorylated and non-phospohrylated) ERK, occurs in a number of brain regions during learning. Furthermore, treatments that interfere with ERK signaling such as the MEK/ERK inhibitors SL327, U0126 or PD98059, impair long-term memory retention. ERK inhibition prevents the formation of lasting memories of an event or association, including spatial memory, fear memory, and object recognition memory [31–39]. More recently, genetic approaches have been used to examine ERK function and have yielded similar effects on behavior. ERK2 conditional knockouts show impaired long-term retention in spatial memory and fear conditioning tasks [14, 40]. Based on these data, ERK activation is necessary for the establishment of long-lasting changes in behavior, likely by mediating changes in synaptic strength.
In addition to its role in synaptic plasticity, ERK activation in the nervous system is important for plasticity of cellular intrinsic excitability. This form of plasticity involves changes in the electrical properties of the cell membrane that render neurons more or less responsive to synaptic inputs [41]. In particular, A-type K+ channels located in distal dendrites are an important determinant of cellular excitability by limiting dendritic action potential back-propagation and raising action potential thresholds (see [41, 42] for review). Recently it has been shown that the inwardly rectifying K+ channel Kv4.2 is regulated by ERK (see [43] for review). ERK phosphorylates the pore-forming unit of the Kv4.2 channel, which results in down regulation of this channel’s activity [44–46]. The net effect of this modulation is to increase cellular excitability and facilitate LTP induction in neurons with elevated ERK phosphorylation [46, 47]. Thus, Kv4.2 channel phosphorylation and its effect on membrane electrical properties enable ERK activation to increase cellular excitability and potentially alter cellular information processing and ongoing behavior.
Alterations in cellular excitability have been proposed to have a role in behavior regulation [48, 49]. In this context, ERK’s regulation of cellular excitability may underlie its increasingly recognized role in regulating emotional arousal and affective states. For example, inhibition of ERK activation with the systemic ERK inhibitor SL327 reduces the amount of time rats swim in the forced swim test, which suggests that ERK inhibition has a depressant effect on behavior [50, 51]. Likewise, knockout of ERK1 increases basal ERK2 activation and increases measures of behavioral arousal, an effect that is reversed by ERK inhibition [52, 53]. These studies suggest a link between altered levels of basal ERK activation and mood disorders, such as mania and depression. This is supported by findings that chronic exposure to the stress hormone corticosterone produces a depressive phenotype in rats, reduces basal levels of ERK activation in the hippocampus and reduces performance on measures of motivation and arousal. Exposure to anti-depressants reverses the behavioral effects of corticosterone exposure and restores ERK phosphorylation levels in the hippocampus [54, 55]. One explanation for these behavioral effects is that ERK activation modulates affect and arousal through its effects on cellular excitability. This would explain why ERK inhibition acutely disrupts behavior on these tasks, since it is unlikely that such rapid effects of ERK inhibition could be mediated by alterations in gene expression. Recent evidence showing that olfactory discrimination learning relies on increased basal ERK phosphorylation and changes in cellular intrinsic excitability in the piriform cortex supports this conclusion [56]. Of course, it is unlikely that such a general behavioral measure as arousal is mediated solely by the excitability of neurons in a single brain region. Additionally, changes in basal ERK activation not only influence intrinsic excitability, but also cause structural changes in neurons that are responsible for phenotypes characteristic of mood disorders such as mania and depression [57]. Nevertheless, these results highlight the importance of basal ERK signaling in regulation of arousal and affective states and its impact on ongoing behavior.
Summary
ERK is one of the primary cellular signal transduction pathways in the nervous system, and confers adult neurons with plasticity that is crucial for learning and adaptive behavior. ERK enables synaptic plasticity through its regulation of receptor trafficking, transcription and translation, operations that are vital for the formation of lasting memories. ERK enables plasticity of cellular excitability through its phosphorylation of Kv4.2 channels, which, unlike its effects on gene expression, allows ERK activity to rapidly modulate neural activity and behavior. These two components of ERK signaling likely work in parallel to simultaneously confer long-lasting experience-dependent changes in behavior as well as adaptability to changing environmental demands. In the following section, these themes will reappear when considering the role of ERK signaling in the striatum.
III. ERK signaling in the striatum
ERK signaling influences striatal synaptic plasticity and excitability
The striatum is the largest of the basal ganglia nuclei and serves as the primary input structure for basal ganglia-thalamo-cortical circuitry. The striatum receives excitatory glutamatergic inputs from cortical, limbic and thalamic regions, as well as dopaminergic input from the midbrain [58, 59]. Dopaminergic input to the striatum accounts for the majority of dopaminergic signaling in the brain. Glutamatergic and dopaminergic synapses form in close proximity on striatal medium spiny neurons (MSN’s) [59]. Dopamine and glutamate signaling interact in important ways to influence synaptic plasticity at corticostriatal synapses (see [60–62] for review). In particular, the induction of corticostriatal LTP depends on the combined activation of post-synaptic D1 and NMDA receptors [63, 64].
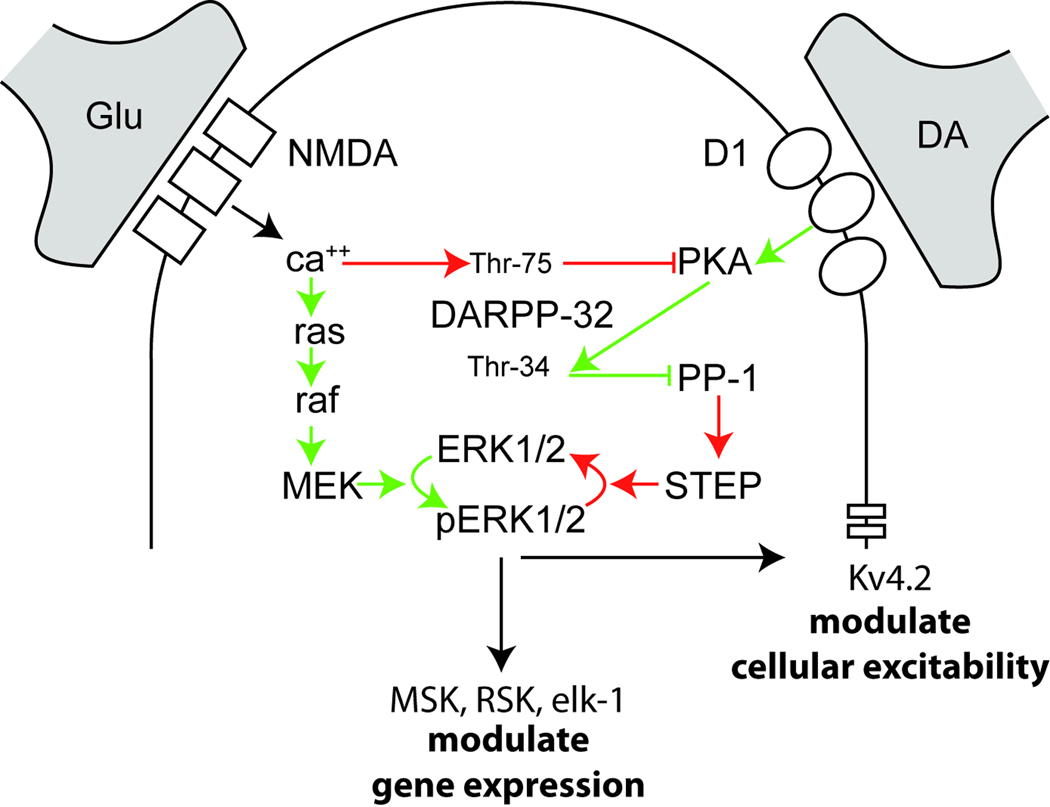
One consequence of combined D1 and NMDA receptor activation in striatal MSN’s is activation of ERK, and indeed ERK activation is necessary for corticostriatal synaptic plasticity [65–67]. This pattern of activation is the result of ERK’s complex regulation by phosphatase cascades and the phosphoprotein DARPP-32 [65, 68, 69] (Figure 1). In striatal neurons, DA D1 receptor activation increases cAMP-PKA activity and phosphorylation by PKA of DARPP-32 at its Thr-34 residue. Thr-34 DARPP-32 becomes a potent inhibitor of a phosphatase cascade that targets ERK, thus freeing ERK from inhibition. NMDA signaling also activates ERK in striatal neurons via calcium-dependent activation of ras [62, 70–72]. However, glutamate negatively regulates ERK signaling through calcium-mediated phosphorylation of DARPP-32 at Thr-75 and dephosphorylation of DARPP-32 at Thr-34, which disinhibits phosphatases that dephosphorylate ERK [73]. Thus, ERK appears to be optimally activated in the healthy striatum under conditions of combined NMDA and DA receptor activation. ERK likely enables corticostriatal plasticity, in part, through regulation of transcription factors such as cAMP response element binding (CREB) protein, as disruption of CREB signaling in the striatum prevents striatal LTP and LTD induction [74].
Figure 1. Regulation of ERK activation in striatal neurons.
In striatal MSN’s, the coordinated activation of DA D1 receptors and NMDA receptors produces maximal ERK activation. D1 receptor stimulation activates cAMP-PKA, which phosphorylates the phosphoprotein DARPP-32 at its Thr-34 residue. Thr-34 DARPP-32 becomes a potent inhibitor of the phosphatase PP-1, which dephosphorylates ERK through its regulation of another phosphatase, striatal enriched phosphatase (STEP). NMDA receptor stimulation results in ca++ influx, activation of ca++ dependent kinases (not shown), which stimulates ras activity. Ras, in turn, activates raf and MEK, the kinases upstream of ERK, resulting in ERK phosphorylation. Ca++ influx also activates a signaling cascade that results in phosphorylation of DARPP-32 at Thr-75. Thr-75 DARPP-32 becomes an inhibitor of PKA activity. Green lines indicate pathways that promote ERK phosphorylation. Red lines indicate pathways that result in ERK dephosphorylation.
In addition to its role in corticostriatal synaptic plasticity, ERK appears to be involved in regulating striatal cell excitability. MSN’s exhibit a hyperpolarized resting membrane potential known as the down state, which is characterized by high input resistance (see [62, 75] for review). Coordinated glutamatergic input shifts MSNs to a less hyperpolarized “up-state”. During the up-state, D1 receptor activation increases cell excitability and facilitates glutamatergic signaling [76–79]. Striatal neurons contain Kv4.2 channels, which play an important role during striatal up-states to limit MSN depolarization [80, 81]. ERK phosphorylates Kv4.2 in striatal neurons, which down-regulates these channels, and ultimately increases cell excitability during up-states and promotes corticostriatal synaptic plasticity [82]. ERK’s ability to modulate MSN excitability during up-states through Kv4.2 phosphorylation may enable it to rapidly modulate striatal activity and behavior.
Striatal ERK signaling and instrumental learning
One of the clearest examples of striatal involvement in learning and control of actions is found in instrumental learning. During instrumental learning animals learn, in part, to associate their actions with specific outcomes or consequences. If outcomes are rewarding (or involve the removal of an aversive stimulus) they serve as positive reinforcers, whereas aversive outcomes (or those that involve removing an appetitive stimulus) act as are negative reinforcers. During instrumental learning, animals form associations between their actions (such as pressing a lever in an operant chamber) and the specific consequences of those actions (such as delivery of food pellets or termination of an aversive footshock). The striatum is critical for this form of learning, and models of reinforcement learning have emphasized the importance of glutamate-DA interactions in the striatum during instrumental action-outcome learning [83–85]. Specifically, DA release in the dorsal striatum is hypothesized to underlie the encoding of action-outcome associations by enabling potentiation of corticostriatal synapses during learning [86]. In support of this hypothesis, inhibition of glutamate and DA receptors in the dorsal striatum interferes with action-outcome encoding [87, 88].
Given the importance of glutamate and DA signaling in the striatum during instrumental learning, ERK signaling may be particularly relevant for this process, given that its activation in striatum depends on combined DA and NMDA receptor stimulation, whereas when these receptors are stimulated separately they weakly activate ERK [65]. Indeed, the requirement for dual-activation of NMDA and D1 receptors in order to activate ERK has lead to the proposal that ERK functions to selectively induce plasticity at corticostriatal neurons during highly relevant learning situations when coordinated DA and NMDA receptor activation is likely to occur, such as during reward learning [65, 89]. Additionally, this selectivity would prevent arbitrary associations from being formed between actions and irrelevant outcomes.
Recent evidence supports the conclusion that ERK is important for instrumental action-outcome learning in the striatum. In order to demonstrate action-outcome (or goal-directed) learning in instrumental conditioning, an animal must be shown to possess some knowledge of the outcome resulting from its actions and uses this information to select among potential actions (see [4, 90] for review). One method of demonstrating this knowledge is through an outcome devaluation paradigm. In this paradigm the goal or outcome of an action is manipulated, with the expectation that the animal’s behavior will adjust to changes in goal value. Using these procedures in rodents, action-outcome learning has been shown to rely on a posterior portion of the dorsomedial striatum (pDMS) [91]. Although still capable of performing instrumental responses, inactivation of the pDMS prior to instrumental learning produces outcome-insensitive responding following devaluation in a subsequent test conducted in extinction, thereby demonstrating that the pDMS has a critical role in action-outcome encoding.
We recently demonstrated that ERK signaling in the pDMS plays an important role in action-outcome learning [92]. We found that when rats are trained to press a lever for a food outcome, this experience will increase ERK phosphorylation in the pDMS compared to yoked controls that experience the same pattern of outcomes, but whose actions have no casual relation to outcome delivery. This suggests that ERK activation in the pDMS is necessary for action-outcome learning. To test this suggestion, we trained rats in several sessions to perform actions (left and right lever press) for a common outcome (20% polycose solution). Subsequently, in a single session, rats learned that left and right lever responses delivered unique outcomes (sucrose pellets and 20% sucrose solution). Prior to learning these new action-outcome associations, rats received intra-pDMS U0126 infusions to interfere with ERK activation. We then tested the rats’ knowledge of action-outcome associations, by devaluing one of the two outcomes prior to a test in which rats could choose between the lever that produced the now devalued outcome or the still valued outcome. Control animals showed a significant devaluation effect, making fewer responses on the action that previously produced the now devalued outcome. In contrast, rats treated with U0126 showed no devaluation effect, responding equally on the valued and devalued lever, indicating that intra-pDMS U0126 prevented new action-outcome learning.
In addition to learning about the consequences of their actions, animals performing an instrumental task may also develop habitual responding, and indeed, this form of learning likely occurs in parallel and largely independent from action-outcome learning. Habitual behavior typically develops more slowly than goal-directed learning and is the product of extensive training. Once established, habitual responses are automatically elicited and performed without consideration of the outcome or consequence of their execution. Habitual responding is thought to be supported by stimulus-response associations. It has shown that habit formation in rodents depends on the dorsolateral striatum (DLS), an area that is richly innervated by sensorimotor areas of the frontal cortex. Lesions of this structure prevent the development of habitual responding, and inactivation after training returns behavior to an outcome-sensitive state [93, 94].
Acquisition of habitual behavior depends on DA innervation of the DLS, suggesting that ERK may be involved in this form of learning [95]. However, it is not known whether the acquisition of habitual responding depends on ERK activation. It has been shown that ERK signaling in the dorsal striatum is necessary for the consolidation of a complex motor skill [96]. Inhibition of ERK activation in the dorsal striatum prevented improvement on the accelerating rotarod task following initial acquisition, suggesting that ERK signaling in the striatum has a role in the consolidation of this form of motor learning. Whether a similar process occurs in the development of habitual responding for rewards is not known.
Based on the findings reviewed above, ERK signaling in the striatum is necessary for instrumental learning: in the DMS, ERK is necessary for action-outcome encoding, whereas in the DLS it may be necessary for the development of habitual behavior (Table 1). ERK is likely involved in these forms of learning through its role in corticostriatal plasticity. We propose that, during learning, ERK is “transiently” activated in the striatum (i.e., ERK activation increases during learning and returns to baseline following a learning episode) and engages transcriptional and translational mechanisms to enable corticostriatal plasticity and memory formation. In the DMS, action-outcome learning likely involves LTP, which is the dominant form of plasticity in this region, and where ERK has a well-described role in LTP induction. The role of synaptic plasticity in the DLS during habit formation is less clear, but may involve corticostriatal LTD. Although a requirement for ERK in corticostriatal LTD has not yet been demonstrated, ERK is necessary for LTD in other brain areas [20].
Table 1.
Role of ERK activation in striatal-based behavio
| Process | Dorsomedial striatum |
Dorsolateral striatum |
Nucleus accumbens |
|---|---|---|---|
| Learning | A-O learning | Habit formation? | S-O learning? |
| Performance | Goal-directed action | Habit performance? | PIT |
Striatal ERK signaling and instrumental performance
In addition to a role in instrumental learning, the striatum is necessary for the performance of instrumental actions. As is the case with learning, instrumental control relies on parallel circuits involving the pDMS and DLS, which mediate performance of goal-directed and habitual action, respectively. Inactivation of the pDMS produces outcome-insensitive (i.e., habitual) instrumental behavior in rats that ordinarily would display outcome-sensitive responding, whereas inactivation of the DLS produces outcome-sensitive (i.e., goal-directed) instrumental behavior in rats that ordinarily would display outcome-insensitive responding [91, 94]. These findings support the notion that parallel corticostriatal circuits mediate goal-directed and habitual action.
We recently demonstrated that ERK activation in the pDMS is necessary for the performance of goal-directed actions. We trained rats over several sessions to perform instrumental actions for a rewarding sucrose solution. We found an increase in ERK activation in the pDMS and DLS following the final training session [92]. To test the involvement of ERK in goal-directed behavior performance, we trained a separate set of rats to associate left and right lever presses with unique outcomes, after which one outcome was devalued and performance on each lever was assessed in an extinction test. Prior to the extinction test, rats received U0126 infusions into the pDMS or DLS. Rats that received U0126 infusions into the pDMS showed no preference for the valued action compared to the devalued action in a choice test. ERK inhibition had no effect on rats’ ability to make instrumental responses generally, nor did it interfere with the palatability of the food outcomes. Rather, it appears that inhibition of ERK activation in the DMS prevents the use of outcome value to guide action selection. Interestingly, ERK inactivation in the DLS also interfered with goal-directed responding, suggesting that the DLS may also play a role in the performance of goal-directed actions.
In addition to outcome value, instrumental actions are also sensitive to cues associated with rewarding outcomes. Animals learn through Pavlovian conditioning to associate stimuli with outcomes or events, and these associations can affect instrumental responding by influencing the choice and vigor of instrumental actions. This process is known as Pavlovian-instrumental transfer (PIT). During a typical PIT paradigm, animals are first trained under Pavlovian and instrumental procedures to separately associate a CS and an action with outcome delivery. During the PIT test, the CS is presented while animals have access to the instrumental levers, and typically CS presentation will cause animals to increase their instrumental performance compared to a neutral stimulus or baseline. This effect is thought to reflect the motivating or arousing state that CS presentations elicit.
PIT depends on the NAc: lesions of this structure do not prevent the acquisition or performance of instrumental responding; however, the motivating effect of CS presentation on instrumental performance is abolished in animals with NAc lesions [97, 98]. We found that ERK signaling in the NAc has an important role in reward-seeking behavior, where it appears to be critically involved in PIT [99]. We trained rats over several sessions to associate a 2-min tone stimulus with delivery of food outcomes. In a subsequent test in which the tone was presented in extinction, we found that the tone stimulus increased ERK phosphorylation in the NAc when compared to control animals that were presented with a neutral stimulus. CS presentation also increased CREB phosphorylation in the NAc, suggesting that Pavlovian conditioning produces synaptic plasticity in this structure [100]. To examine the role of CS-evoked ERK activation in the NAc, we trained rats under Pavlovian and instrumental conditioning and assessed their performance in a PIT task. We found that intra-NAc U0126 infusion prior to the PIT task prevented the CS from elevating instrumental responding. ERK inhibition has no effect on instrumental responding generally, which suggests that ERK inhibition has a specific role in enabling cues to motivate instrumental behavior.
Based on these studies, ERK signaling in the striatum appears to be necessary for goal-directed learning as well as the modulation of acquired instrumental actions by outcome value or by cues associated with a rewarding outcome. We propose that ERK activation in the striatum influences performance of instrumental actions by modulating the intrinsic excitability of striatal neurons. As described above, ERK phosphorylates Kv4.2 channels, which increases cellular excitability in striatal neurons. The net effect of this modulation may be to allow synaptic inputs to the striatum representing appetitive stimuli (such as from the BLA [101]) to increase their control of striatal cellular activity, and as a consequence engage reward-seeking behavior. It is not known whether ERK acts as a permissive or instructive signal for modulating instrumental performance. Some evidence suggests that the level of ERK activation evoked during task performance may determine the magnitude of the behavioral effects mediated by ERK [102]. The level of ERK activation in the striatum may therefore act as a modulator of decision processes, with greater ERK activation evoked by a stimulus or event facilitating action initiation [103].
IV. ERK and addiction
Exposure to a variety of substances with abuse potential, including psychostimulants, opioids and nicotine, acutely activate ERK in the striatum and other brain areas [104, 105]. Many enduring behavioral effects of acute drug exposure depend on ERK signaling. For example, pairing a context with drug exposure produces a conditioned place preference, and ERK inhibition prior to drug-context pairing prevents the formation of this preference [105, 106]. Since ERK does not prevent the hyperlocomotion induced by drug exposure, it is likely that the effect of ERK inhibition on CPP is the result of impaired memory formation [107, 108]. ERK is also required for the development of locomotor sensitization, a process that occurs when repeated drug exposure heightens locomotor responses to subsequent drug presentations. ERK inhibition prior to drug exposure prevents the development of locomotor sensitization [109]. Similarly, over-expression of ERK2 increases locomotor sensitization and CPP [67, 110]. These findings suggest that ERK is required for the neuroadaptive changes resulting from repeated drug exposure, and which are responsible for heightened sensitivity to the drug’s acute locomotor effects. Interestingly, ERK is also necessary for the expression of CPP [108, 109]. Furthermore, the ability of stimuli to elicit ERK activation may itself become sensitized by repeated drug exposure, and this may also contribute to heightened relapse to drug-associated cues. Supporting this notion are findings that ERK is involved in the incubation of craving, in which responses to drug-associated stimuli increases with the withdrawal period [111, 112]. Recent studies have shown that drug-associated cues elicit ERK activation in the central nucleus of the amygdala, and after 30 d of withdrawal this cue-driven ERK response is greater than after 1 d of withdrawal, mirroring the effects of these cues on relapse. Similarly, chronic food restriction increases sensitivity to drugs of abuse, and this is associated with enhanced striatal activation of ERK by D1 and NMDA receptor stimulation [113]. Taken together, these results parallel findings from instrumental tasks that ERK is necessary for learning and may also directly influence the expression of reward-seeking behaviors.
Although a single drug exposure acutely affects behavior, repeated drug administration produces a variety of changes in behavior that characterize addiction. Among the many physiological changes produced by repeated drug exposure is a sustained increase in baseline ERK activation in the brain, including in the striatum [114–116]. This change in baseline ERK activation has been observed in the NAc and accompanies changes in NAc excitability that results from repeated drug exposure, a process that depends on AMPA receptor insertion [116, 117]. These findings suggest that ERK is dynamically regulated following repeated drug exposure and withdrawal, that changes in ERK activation directly influence striatal cell excitability, and that these effects may be responsible for the expression of addictive behavior. Based on the role that we have described for ERK signaling in striatum function, sustained ERK activation may promote excessive action initiation, consequently giving rise to impairments in action control. Excessive excitability in the pDMS may give rise to compulsive behavior as actions are repeatedly performed, whereas excessive excitability in the DLS may give rise to impulsive behavior as stimuli repeatedly elicit actions (Figure 2). Such phenotypes have been observed in rodents following repeated drug exposure [118–120]. Within the NAc, excessive excitability may enhance the arousing effects of drug-associated cues on reward-seeking behavior, an effect that has been documented following repeated amphetamine exposure in rodents [121]. It is not known to what extent these behavioral patterns are the result of drug-induced alterations in striatal ERK signaling.
Figure 2. Drugs of abuse potentially alter learning and reward-seeking through ERK dysregulation.
A) During initial drug-taking behavior, acute exposure to abused substances transiently enhances ERK activation and facilitates corticostriatal plasticity and learning. This may establish strong action-outcome associations, which may dominate subsequent goal-directed reward seeking behavior. B) Repeated drug exposure increases basal ERK activation and increases cellular excitability in the NAc. These neuroadaptations may render behavior more impulsive, as synaptic activity representing reward-associated stimuli more easily cause NAc neural activity and elicit reward-seeking behavior.
Conclusions
The role of ERK in addictive behavior in the striatum is consistent with its role in action-outcome learning and control of behavior by reward-associated stimuli more generally. This is not surprising given that drug-taking is a form of appetitive behavior. What is different about abused substances is they activate ERK more strongly than natural rewards and that with repeated exposure they increase basal levels of ERK activation. These properties may be responsible for addictive behavior. Indeed, the effects of abused substances, like the effects of mood regulators on ERK, show how up or down-regulation of baseline ERK activation influences behavior. In the context of ERK’s role in striatum function, we propose that changes in baseline activity give rise to impulsive or cue-driven behavior. By understanding ERK function in the striatum during processing of natural rewards, we may begin to decipher how dysregulation of ERK signaling gives rise to disorders such as addiction.
It is clear that striatal ERK mediates learning and decision processes; however, ERK is not necessary for the generation of motor action or for the processing of hedonics. This specialization in learning and decision making may be the consequence of ERK’s unique regulation in striatum: ERK is maximally activated under conditions of coordinated D1 and NMDA receptor signaling. This functional specialization suggests that ERK could serve as a target of therapeutics to treat disorders of learning and decision making (e.g., to prevent relapse) and spare other striatal functions. There is still much to be known about ERK’s regulation in the nervous system and the mechanisms through which it influences the structure and physiology of striatal neurons. Nevertheless, these data suggest that ERK is a promising target for treating disorders of learning and decision making that arise from compromised striatal function.
Research Highlights
ERK signaling regulates learning, memory, synaptic plasticity and arousal
Dorsal striatum ERK mediates goal-directed learning and performance
Ventral striatum ERK mediates Pavlovian-instrumental transfer
ERK dysregulation may underlie addiction
Acknowledgements
This research was supporteby NIH grants HD059527 to BWB and DA026559 to MWS.
Abbreviations
- ERK
extracellular signal regulated kinase
- LTP
long-term potentiation
- LTD
long-term depression
- PIT
Pavlovian-instrumental transfer
- pDMS
posterior dorsomedial striatum
- DLS
dorsolateral striatum
- NAc
nucleus accumbens
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Frank MJ, Seeberger LC, O'Reilly RC. By Carrot or by Stick: Cognitive Reinforcement Learning in Parkinsonism. Science. 2004;306:1940–1943. doi: 10.1126/science.1102941. [DOI] [PubMed] [Google Scholar]
- 2.Kalivas PW, Volkow ND. The Neural Basis of Addiction: A Pathology of Motivation and Choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 3.Canales JJ, Graybiel AM. Patterns of gene expression and behavior induced by chronic dopamine treatments. Annals of Neurology. 2000;47:S53–S59. [PubMed] [Google Scholar]
- 4.Balleine BW, Liljeholm M, Ostlund SB. The integrative function of the basal ganglia in instrumental conditioning. Behav Brain Res. 2009;199:43–52. doi: 10.1016/j.bbr.2008.10.034. [DOI] [PubMed] [Google Scholar]
- 5.Yin HH, Ostlund SB, Balleine BW. Reward-guided learning beyond dopamine in the nucleus accumbens: the integrative functions of cortico-basal ganglia networks. Eur J Neurosci. 2008;28:1437–1448. doi: 10.1111/j.1460-9568.2008.06422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J Neurosci. 2007;27:8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wickens JR, Budd CS, Hyland BI, Arbuthnott GW. Striatal contributions to reward and decision making - Making sense of regional variations in a reiterated processing matrix. Reward and Decision Making in Corticobasal Ganglia Networks. 2007;1104:192–212. doi: 10.1196/annals.1390.016. [DOI] [PubMed] [Google Scholar]
- 8.Cohen MX, Frank MJ. Neurocomputational models of basal ganglia function in learning, memory and choice. Behav Brain Res. 2009;199:141–156. doi: 10.1016/j.bbr.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sweatt J. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Samuels IS, Saitta SC, Landreth GE. MAP'ing CNS Development and Cognition: An ERKsome Process. 2009;61:160–167. doi: 10.1016/j.neuron.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene. 2010;26:3100–3112. doi: 10.1038/sj.onc.1210392. [DOI] [PubMed] [Google Scholar]
- 12.Roux PP, Blenis J. ERK and p38 MAPK-Activated Protein Kinases: a Family of Protein Kinases with Diverse Biological Functions. Microbiol Mol Biol Rev. 2004;68:320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dolmetsch RE, Pajvani U, Fife K, Spotts JM, Greenberg ME. Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway. Science. 2001;294:333–339. doi: 10.1126/science.1063395. [DOI] [PubMed] [Google Scholar]
- 14.Kelleher RJ, Govindarajan A, Jung HY, Kang HJ, Tonegawa S. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell. 2004;116:467–479. doi: 10.1016/s0092-8674(04)00115-1. [DOI] [PubMed] [Google Scholar]
- 15.Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci. 2004;5:173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- 16.English JD, Sweatt JD. A requirement for the mitogen-activated protein kinase cascade in hippocampal long term potentiation. J Biol Chem. 1997;272:19103–19106. doi: 10.1074/jbc.272.31.19103. [DOI] [PubMed] [Google Scholar]
- 17.English JD, Sweatt JD. Activation of p42 mitogen-activated protein kinase in hippocampal long term potentiation. J Biol Chem. 1996;271:24329–24332. doi: 10.1074/jbc.271.40.24329. [DOI] [PubMed] [Google Scholar]
- 18.Kanterewicz BI, Urban NN, McMahon DB, Norman ED, Giffen LJ, Favata MF, et al. The extracellular signal-regulated kinase cascade is required for NMDA receptor-independent LTP in area CA1 but not area CA3 of the hippocampus. J Neurosci. 2000;20:3057–3066. doi: 10.1523/JNEUROSCI.20-09-03057.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang YY, Martin KC, Kandel ER. Both protein kinase A and mitogen-activated protein kinase are required in the amygdala for the macromolecular synthesis-dependent late phase of long-term potentiation. J Neurosci. 2000;20:6317–6325. doi: 10.1523/JNEUROSCI.20-17-06317.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thiels E, Kanterewicz BI, Norman ED, Trzaskos JM, Klann E. Long-term depression in the adult hippocampus in vivo involves activation of extracellular signal-regulated kinase and phosphorylation of Elk-1. J Neurosci. 2002;22:2054–2062. doi: 10.1523/JNEUROSCI.22-06-02054.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu JJ, Qin Y, Zhao M, Van Aelst L, Malinow R. Ras and Rap Control AMPA Receptor Trafficking during Synaptic Plasticity. Cell. 2002;110:443–455. doi: 10.1016/s0092-8674(02)00897-8. [DOI] [PubMed] [Google Scholar]
- 22.Kim MJ, Dunah AW, Wang YT, Sheng M. Differential roles of NR2A- and NR2B-containing NMDA receptors in Ras-ERK signaling and AMPA receptor trafficking. Neuron. 2005;46:745–760. doi: 10.1016/j.neuron.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 23.Goldin M, Segal M. Protein kinase C and ERK involvement in dendritic spine plasticity in cultured rodent hippocampal neurons. Eur J Neurosci. 2003;17:2529–2539. doi: 10.1046/j.1460-9568.2003.02694.x. [DOI] [PubMed] [Google Scholar]
- 24.Wu G-Y, Deisseroth K, Tsien RW. Spaced stimuli stabilize MAPK pathway activation and its effects on dendritic morphology. Nat Neurosci. 2001;4:151–158. doi: 10.1038/83976. [DOI] [PubMed] [Google Scholar]
- 25.Davis S, Vanhoutte P, Pages C, Caboche J, Laroche S. The MAPK/ERK cascade targets both Elk-1 and cAMP response element-binding protein to control long-term potentiation-dependent gene expression in the dentate gyrus in vivo. J Neurosci. 2000;20:4563–4572. doi: 10.1523/JNEUROSCI.20-12-04563.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberson ED, English JD, Adams JP, Selcher JC, Kondratick C, Sweatt JD. The mitogen-activated protein kinase cascade couples PKA and PKC to cAMP response element binding protein phosphorylation in area CA1 of hippocampus. J Neurosci. 1999;19:4337–4348. doi: 10.1523/JNEUROSCI.19-11-04337.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gottschalk WA, Jiang H, Tartaglia N, Feng L, Figurov A, Lu B. Signaling mechanisms mediating BDNF modulation of synaptic plasticity in the hippocampus. Learn Mem. 1999;6:243–256. [PMC free article] [PubMed] [Google Scholar]
- 28.Watabe AM, Zaki PA, O'Dell TJ. Coactivation of beta-adrenergic and cholinergic receptors enhances the induction of long-term potentiation and synergistically activates mitogen-activated protein kinase in the hippocampal CA1 region. J Neurosci. 2000;20:5924–5931. doi: 10.1523/JNEUROSCI.20-16-05924.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang CH, Huang CC, Hsu KS. Behavioral stress modifies hippocampal synaptic plasticity through corticosterone-induced sustained extracellular signal-regulated kinase/mitogen-activated protein kinase activation. J Neurosci. 2004;24:11029–11034. doi: 10.1523/JNEUROSCI.3968-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Welsby PJ, Rowan MJ, Anwyl R. Intracellular mechanisms underlying the nicotinic enhancement of LTP in the rat dentate gyrus. Eur J Neurosci. 2009;29:65–75. doi: 10.1111/j.1460-9568.2008.06562.x. [DOI] [PubMed] [Google Scholar]
- 31.Selcher JC, Atkins CM, Trzaskos JM, Paylor R, Sweatt JD. A Necessity for MAP Kinase Activation in Mammalian Spatial Learning. Learning & Memory. 1999;6:478–490. doi: 10.1101/lm.6.5.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Villarreal JS, Barea-Rodriguez EJ. ERK phosphorylation is required for retention of trace fear memory. Neurobiology of Learning and Memory. 2006;85:44–57. doi: 10.1016/j.nlm.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 33.Kelly A, Laroche S, Davis S. Activation of mitogen-activated protein kinase/extracellular signal-regulated kinase in hippocampal circuitry is required for consolidation and reconsolidation of recognition memory. J Neurosci. 2003;23:5354–5360. doi: 10.1523/JNEUROSCI.23-12-05354.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atkins C, Selcher J, Petraitis J, Trazaskos J, Sweatt J. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1 doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- 35.Blum S, Moore AN, Adams F, Dash PK. A mitogen-activated protein kinase cascade in the CA1/CA2 subfield of the dorsal hippocampus is essential for long-term spatial memory. J Neurosci. 1999;19:3535–3544. doi: 10.1523/JNEUROSCI.19-09-03535.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu K-T, Walker DL, Davis M. Mitogen-Activated Protein Kinase Cascade in the Basolateral Nucleus of Amygdala Is Involved in Extinction of Fear-Potentiated Startle. J Neurosci. 2001;21:162RC. doi: 10.1523/JNEUROSCI.21-16-j0005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schafe GE, Atkins CM, Swank MW, Bauer EP, Sweatt JD, LeDoux JE. Activation of ERK/MAP Kinase in the Amygdala Is Required for Memory Consolidation of Pavlovian Fear Conditioning. J Neurosci. 2000;20:8177–8187. doi: 10.1523/JNEUROSCI.20-21-08177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Runyan JD, Dash PK. Intra-medial prefrontal administration of SCH-23390 attenuates ERK phosphorylation and long-term memory for trace fear conditioning in rats. Neurobiol Learn Mem. 2004;82:65–70. doi: 10.1016/j.nlm.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 39.Giovannini MG, Pazzagli M, Malmberg-Aiello P, Della Corte L, Rakovska AD, Cerbai F, et al. Inhibition of acetylcholine-induced activation of extracellular regulated protein kinase prevents the encoding of an inhibitory avoidance response in the rat. Neuroscience. 2005;136:15–32. doi: 10.1016/j.neuroscience.2005.07.046. [DOI] [PubMed] [Google Scholar]
- 40.Satoh Y, Endo S, Ikeda T, Yamada K, Ito M, Kuroki M, et al. Extracellular Signal-Regulated Kinase 2 (ERK2) Knockdown Mice Show Deficits in Long-Term Memory; ERK2 Has a Specific Function in Learning and Memory. J Neurosci. 2007;27:10765–10776. doi: 10.1523/JNEUROSCI.0117-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnston D, Narayanan R. Active dendrites: colorful wings of the mysterious butterflies. Trends in Neurosciences. 2008;31:309–316. doi: 10.1016/j.tins.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 42.Shah MM, Hammond RS, Hoffman DA. Dendritic ion channel trafficking and plasticity. Trends in Neurosciences. 2010;33:307–316. doi: 10.1016/j.tins.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Birnbaum SG, Varga AW, Yuan L-L, Anderson AE, Sweatt JD, Schrader LA. Structure and Function of Kv4-Family Transient Potassium Channels. Physiol Rev. 2004;84:803–833. doi: 10.1152/physrev.00039.2003. [DOI] [PubMed] [Google Scholar]
- 44.Adams JP, Anderson AE, Varga AW, Dineley KT, Cook RG, Pfaffinger PJ, et al. The A-type potassium channel Kv4.2 is a substrate for the mitogen-activated protein kinase ERK. J Neurochem. 2000;75:2277–2287. doi: 10.1046/j.1471-4159.2000.0752277.x. [DOI] [PubMed] [Google Scholar]
- 45.Schrader LA, Birnbaum SG, Nadin BM, Ren Y, Bui D, Anderson AE, et al. ERK/MAPK regulates the Kv4.2 potassium channel by direct phosphorylation of the pore-forming subunit. Am J Physiol Cell Physiol. 2006;290:C852–C861. doi: 10.1152/ajpcell.00358.2005. [DOI] [PubMed] [Google Scholar]
- 46.Yuan LL, Adams JP, Swank M, Sweatt JD, Johnston D. Protein kinase modulation of dendritic K+ channels in hippocampus involves a mitogen-activated protein kinase pathway. J Neurosci. 2002;22:4860–4868. doi: 10.1523/JNEUROSCI.22-12-04860.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenkranz JA, Frick A, Johnston D. Kinase-dependent modification of dendritic excitability after long-term potentiation. J Physiol. 2009;587:115–125. doi: 10.1113/jphysiol.2008.158816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang D, Schreurs BG. Characteristics of IA currents in adult rabbit cerebellar Purkinje cells. Brain Research. 2006;1096:85–96. doi: 10.1016/j.brainres.2006.04.048. [DOI] [PubMed] [Google Scholar]
- 49.Zhang W, Linden DJ. The other side of the engram: experience-driven changes in neuronal intrinsic excitability. Nat Rev Neurosci. 2003;4:885–900. doi: 10.1038/nrn1248. [DOI] [PubMed] [Google Scholar]
- 50.Duman CH, Schlesinger L, Kodama M, Russell DS, Duman RS. A Role for MAP Kinase Signaling in Behavioral Models of Depression and Antidepressant Treatment. Biological Psychiatry. 2007;61:661–670. doi: 10.1016/j.biopsych.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 51.Qi H, Mailliet F, Spedding M, Rocher C, Zhang X, Delagrange P, et al. Antidepressants reverse the attenuation of the neurotrophic MEK/MAPK cascade in frontal cortex by elevated platform stress; reversal of effects on LTP is associated with GluA1 phosphorylation. Neuropharmacology. 2009;56:37–46. doi: 10.1016/j.neuropharm.2008.06.068. [DOI] [PubMed] [Google Scholar]
- 52.Engel SR, Creson TK, Hao Y, Shen Y, Maeng S, Nekrasova T, et al. The extracellular signal-regulated kinase pathway contributes to the control of behavioral excitement. Mol Psychiatry. 2009;14:448–461. doi: 10.1038/sj.mp.4002135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tronson NC, Schrick C, Fischer A, Sananbenesi F, Pages G, Pouyssegur J, et al. Regulatory Mechanisms of Fear Extinction and Depression-Like Behavior. Neuropsychopharmacology. 2007;33:1570–1583. doi: 10.1038/sj.npp.1301550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gourley SL, Wu FJ, Kiraly DD, Ploski JE, Kedves AT, Duman RS, et al. Regionally Specific Regulation of ERK MAP Kinase in a Model of Antidepressant-Sensitive Chronic Depression. Biological Psychiatry. 2008;63:353–359. doi: 10.1016/j.biopsych.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gourley SL, Wu FJ, Taylor JR. Corticosterone regulates pERK1/2 map kinase in a chronic depression model. Ann N Y Acad Sci. 2008;1148:509–514. doi: 10.1196/annals.1410.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cohen-Matsliah SI, Brosh I, Rosenblum K, Barkai E. A novel role for extracellular signal-regulated kinase in maintaining long-term memory-relevant excitability changes. J Neurosci. 2007;27:12584–12589. doi: 10.1523/JNEUROSCI.3728-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Einat H, Manji HK. Cellular Plasticity Cascades: Genes-To-Behavior Pathways in Animal Models of Bipolar Disorder. Biological Psychiatry. 2006;59:1160–1171. doi: 10.1016/j.biopsych.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 58.Smith Y, Bevan MD, Shink E, Bolam JP. Microcircuitry of the direct and indirect pathways of the basal ganglia. Neuroscience. 1998;86:353–387. doi: 10.1016/s0306-4522(98)00004-9. [DOI] [PubMed] [Google Scholar]
- 59.Sesack SR, Carr DB, Omelchenko N, Pinto A. Anatomical substrates for glutamate-dopamine interactions: evidence for specificity of connections and extrasynaptic actions. Ann N Y Acad Sci. 2003;1003:36–52. doi: 10.1196/annals.1300.066. [DOI] [PubMed] [Google Scholar]
- 60.Lovinger DM. Neurotransmitter roles in synaptic modulation, plasticity and learning in the dorsal striatum. Neuropharmacology. 2010;58:951–961. doi: 10.1016/j.neuropharm.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Di Filippo M, Picconi B, Tantucci M, Ghiglieri V, Bagetta V, Sgobio C, et al. Short-term and long-term plasticity at corticostriatal synapses: Implications for learning and memory. Behavioural Brain Research. 2009;199:108–118. doi: 10.1016/j.bbr.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 62.Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends in Neurosciences. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 63.Calabresi P, Pisani A, Mercuri NB, Bernardi G. Long-term Potentiation in the Striatum is Unmasked by Removing the Voltage-dependent Magnesium Block of NMDA Receptor Channels. Eur J Neurosci. 1992;4:929–935. doi: 10.1111/j.1460-9568.1992.tb00119.x. [DOI] [PubMed] [Google Scholar]
- 64.Kerr JN, Wickens JR. Dopamine D-1/D-5 receptor activation is required for long-term potentiation in the rat neostriatum in vitro. J Neurophysiol. 2001;85:117–124. doi: 10.1152/jn.2001.85.1.117. [DOI] [PubMed] [Google Scholar]
- 65.Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, Corvol JC, et al. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci U S A. 2005;102:491–496. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Calabresi P, Gubellini P, Centonze D, Picconi B, Bernardi G, Chergui K, et al. Dopamine and cAMP-Regulated Phosphoprotein 32 kDa Controls Both Striatal Long-Term Depression and Long-Term Potentiation, Opposing Forms of Synaptic Plasticity. J Neurosci. 2000;20:8443–8451. doi: 10.1523/JNEUROSCI.20-22-08443.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mazzucchelli C, Vantaggiato C, Ciamei A, Fasano S, Pakhotin P, Krezel W, et al. Knockout of ERK1 MAP kinase enhances synaptic plasticity in the striatum and facilitates striatal-mediated learning and memory. Neuron. 2002;34:807–820. doi: 10.1016/s0896-6273(02)00716-x. [DOI] [PubMed] [Google Scholar]
- 68.Svenningsson P, Nishi A, Fisone G, Girault JA, Nairn AC, Greengard P. DARPP-32: an integrator of neurotransmission. Annu Rev Pharmacol Toxicol. 2004;44:269–296. doi: 10.1146/annurev.pharmtox.44.101802.121415. [DOI] [PubMed] [Google Scholar]
- 69.Greengard P, Allen PB, Nairn AC. Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron. 1999;23:435–447. doi: 10.1016/s0896-6273(00)80798-9. [DOI] [PubMed] [Google Scholar]
- 70.Perkinton MS, Sihra TS, Williams RJ. Ca2+-Permeable AMPA Receptors Induce Phosphorylation of cAMP Response Element-Binding Protein through a Phosphatidylinositol 3-Kinase-Dependent Stimulation of the Mitogen-Activated Protein Kinase Signaling Cascade in Neurons. J Neurosci. 1999;19:5861–5874. doi: 10.1523/JNEUROSCI.19-14-05861.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fasano S, D'Antoni A, Orban PC, Valjent E, Putignano E, Vara H, et al. Ras-guanine nucleotide-releasing factor 1 (Ras-GRF1) controls activation of extracellular signal-regulated kinase (ERK) signaling in the striatum and long-term behavioral responses to cocaine. Biol Psychiatry. 2009;66:758–768. doi: 10.1016/j.biopsych.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mao L, Tang Q, Samdani S, Liu Z, Wang JQ. Regulation of MAPK/ERK phosphorylation via ionotropic glutamate receptors in cultured rat striatal neurons. Eur J Neurosci. 2004;19:1207–1216. doi: 10.1111/j.1460-9568.2004.03223.x. [DOI] [PubMed] [Google Scholar]
- 73.Paul S, Nairn AC, Wang P, Lombroso PJ. NMDA-mediated activation of the tyrosine phosphatase STEP regulates the duration of ERK signaling. Nat Neurosci. 2003;6:34–42. doi: 10.1038/nn989. [DOI] [PubMed] [Google Scholar]
- 74.Pittenger C, Fasano S, Mazzocchi-Jones D, Dunnett SB, Kandel ER, Brambilla R. Impaired Bidirectional Synaptic Plasticity and Procedural Memory Formation in Striatum-Specific cAMP Response Element-Binding Protein-Deficient Mice. J Neurosci. 2006;26:2808–2813. doi: 10.1523/JNEUROSCI.5406-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kreitzer AC. Physiology and Pharmacology of Striatal Neurons. Annual Review of Neuroscience. 2009;32:127–147. doi: 10.1146/annurev.neuro.051508.135422. [DOI] [PubMed] [Google Scholar]
- 76.Carter AG, Sabatini BL. State-dependent calcium signaling in dendritic spines of striatal medium spiny neurons. Neuron. 2004;44:483–493. doi: 10.1016/j.neuron.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 77.Surmeier DJ, Bargas J, Hemmings HC, Jr, Nairn AC, Greengard P. Modulation of calcium currents by a D1 dopaminergic protein kinase/phosphatase cascade in rat neostriatal neurons. Neuron. 1995;14:385–397. doi: 10.1016/0896-6273(95)90294-5. [DOI] [PubMed] [Google Scholar]
- 78.Flores-Hernandez J, Cepeda C, Hernandez-Echeagaray E, Calvert CR, Jokel ES, Fienberg AA, et al. Dopamine Enhancement of NMDA Currents in Dissociated Medium-Sized Striatal Neurons: Role of D1 Receptors and DARPP-32. J Neurophysiol. 2002;88:3010–3020. doi: 10.1152/jn.00361.2002. [DOI] [PubMed] [Google Scholar]
- 79.Hallett PJ, Spoelgen R, Hyman BT, Standaert DG, Dunah AW. Dopamine D1 Activation Potentiates Striatal NMDA Receptors by Tyrosine Phosphorylation-Dependent Subunit Trafficking. J Neurosci. 2006;26:4690–4700. doi: 10.1523/JNEUROSCI.0792-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tkatch T, Baranauskas G, Surmeier DJ. Kv4.2 mRNA abundance and A-type K(+) current amplitude are linearly related in basal ganglia and basal forebrain neurons. J Neurosci. 2000;20:579–588. doi: 10.1523/JNEUROSCI.20-02-00579.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Song WJ, Tkatch T, Baranauskas G, Ichinohe N, Kitai ST, Surmeier DJ. Somatodendritic depolarization-activated potassium currents in rat neostriatal cholinergic interneurons are predominantly of the A type and attributable to coexpression of Kv4.2 and Kv4.1 subunits. J Neurosci. 1998;18:3124–3137. doi: 10.1523/JNEUROSCI.18-09-03124.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Varga AW, Anderson AE, Adams JP, Vogel H, Sweatt JD. Input-Specific Immunolocalization of Differentially Phosphorylated Kv4.2 in the Mouse Brain. Learning & Memory. 2000;7:321–332. doi: 10.1101/lm.35300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Horvitz JC. Dopamine gating of glutamatergic sensorimotor and incentive motivational input signals to the striatum. Behavioural Brain Research. 2002;137:65–74. doi: 10.1016/s0166-4328(02)00285-1. [DOI] [PubMed] [Google Scholar]
- 84.Horvitz JC. Stimulus-response and response-outcome learning mechanisms in the striatum. Behav Brain Res. 2009;199:129–140. doi: 10.1016/j.bbr.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wickens JR, Horvitz JC, Costa RM, Killcross S. Dopaminergic mechanisms in actions and habits. J Neurosci. 2007;27:8181–8183. doi: 10.1523/JNEUROSCI.1671-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reynolds JNJ, Hyland BI, Wickens JR. A cellular mechanism of reward-related learning. Nature. 2001;413:67–70. doi: 10.1038/35092560. [DOI] [PubMed] [Google Scholar]
- 87.Lex B, Hauber W. The role of dopamine in the prelimbic cortex and the dorsomedial striatum in instrumental conditioning. Cereb Cortex. 2010;20:873–883. doi: 10.1093/cercor/bhp151. [DOI] [PubMed] [Google Scholar]
- 88.Yin HH, Knowlton BJ, Balleine BW. Blockade of NMDA receptors in the dorsomedial striatum prevents action-outcome learning in instrumental conditioning. Eur J Neurosci. 2005;22:505–512. doi: 10.1111/j.1460-9568.2005.04219.x. [DOI] [PubMed] [Google Scholar]
- 89.Girault JA, Valjent E, Caboche J, Herve D. ERK2: a logical AND gate critical for drug-induced plasticity? Curr Opin Pharmacol. 2007;7:77–85. doi: 10.1016/j.coph.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 90.Balleine BW, Dickinson A. Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology. 1998;37:407–419. doi: 10.1016/s0028-3908(98)00033-1. [DOI] [PubMed] [Google Scholar]
- 91.Yin HH, Ostlund SB, Knowlton BJ, Balleine BW. The role of the dorsomedial striatum in instrumental conditioning. Eur J Neurosci. 2005;22:513–523. doi: 10.1111/j.1460-9568.2005.04218.x. [DOI] [PubMed] [Google Scholar]
- 92.Shiflett MW, Brown RA, Balleine BW. Acquisition and performance of goal-directed instrumental actions depends on ERK signaling in distinct regions of dorsal striatum in rats. J Neurosci. 2010;30:2951–2959. doi: 10.1523/JNEUROSCI.1778-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yin HH, Knowlton BJ, Balleine BW. Lesions of dorsolateral striatum preserve outcome expectancy but disrupt habit formation in instrumental learning. Eur J Neurosci. 2004;19:181–189. doi: 10.1111/j.1460-9568.2004.03095.x. [DOI] [PubMed] [Google Scholar]
- 94.Yin HH, Knowlton BJ, Balleine BW. Inactivation of dorsolateral striatum enhances sensitivity to changes in the action-outcome contingency in instrumental conditioning. Behav Brain Res. 2006;166:189–196. doi: 10.1016/j.bbr.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 95.Faure A, Haberland U, Conde F, El Massioui N. Lesion to the nigrostriatal dopamine system disrupts stimulus-response habit formation. J Neurosci. 2005;25:2771–2780. doi: 10.1523/JNEUROSCI.3894-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bureau G, Carrier M, Lebel M, Cyr M. Intrastriatal inhibition of extracellular signal-regulated kinases impaired the consolidation phase of motor skill learning. Neurobiology of Learning and Memory. 2010;94:107–115. doi: 10.1016/j.nlm.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 97.Corbit LH, Muir JL, Balleine BW. The role of the nucleus accumbens in instrumental conditioning: Evidence of a functional dissociation between accumbens core and shell. J Neurosci. 2001;21:3251–3260. doi: 10.1523/JNEUROSCI.21-09-03251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hall J, Parkinson JA, Connor TM, Dickinson A, Everitt BJ. Involvement of the central nucleus of the amygdala and nucleus accumbens core in mediating Pavlovian influences on instrumental behaviour. Eur J Neurosci. 2001;13:1984–1992. doi: 10.1046/j.0953-816x.2001.01577.x. [DOI] [PubMed] [Google Scholar]
- 99.Shiflett MW, Martini RP, Mauna JC, Foster RL, Peet E, Thiels E. Cue-elicited reward-seeking requires extracellular signal-regulated kinase activation in the nucleus accumbens. Journal of Neuroscience. 2008;28:1434–1443. doi: 10.1523/JNEUROSCI.2383-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shiflett MW, Mauna JC, Chipman AM, Peet E, Thiels E. Appetitive Pavlovian conditioned stimuli increase CREB phosphorylation in the nucleus accumbens. Neurobiol Learn Mem. 2009;92:451–454. doi: 10.1016/j.nlm.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shiflett MW, Balleine BW. At the limbic-motor interface: disconnection of basolateral amygdala from nucleus accumbens core and shell reveals dissociable components of incentive motivation. Eur J Neurosci. 2010;32:1735–1743. doi: 10.1111/j.1460-9568.2010.07439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y. Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat Neurosci. 2005;8:212–219. doi: 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
- 103.Doya K. Modulators of decision making. Nat Neurosci. 2008;11:410–416. doi: 10.1038/nn2077. [DOI] [PubMed] [Google Scholar]
- 104.Valjent E, Pages C, Herve D, Girault JA, Caboche J. Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain. Eur J Neurosci. 2004;19:1826–1836. doi: 10.1111/j.1460-9568.2004.03278.x. [DOI] [PubMed] [Google Scholar]
- 105.Valjent E, Corvol J-C, Pages C, Besson M-J, Maldonado R, Caboche J. Involvement of the Extracellular Signal-Regulated Kinase Cascade for Cocaine-Rewarding Properties. J Neurosci. 2000;20:8701–8709. doi: 10.1523/JNEUROSCI.20-23-08701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gerdjikov TV, Ross GM, Beninger RJ. Place preference induced by nucleus accumbens amphetamine is impaired by antagonists of ERK or p38 MAP kinases in rats. Behav Neurosci. 2004;118:740–750. doi: 10.1037/0735-7044.118.4.740. [DOI] [PubMed] [Google Scholar]
- 107.Sarrazin Ng, Di Blasi F, Roullot-Lacarrière Vr, Rougé-Pont Fo, Le Roux A, Costet P, et al. Transcriptional Effects of Glucocorticoid Receptors in the Dentate Gyrus Increase Anxiety-Related Behaviors. PLoS One. 2009;4:e7704. doi: 10.1371/journal.pone.0007704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Miller CA, Marshall JF. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron. 2005;47:873–884. doi: 10.1016/j.neuron.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 109.Valjent E, Corbille AG, Bertran-Gonzalez J, Herve D, Girault JA. Inhibition of ERK pathway or protein synthesis during reexposure to drugs of abuse erases previously learned place preference. Proc Natl Acad Sci U S A. 2006;103:2932–2937. doi: 10.1073/pnas.0511030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ferguson SM, Fasano S, Yang P, Brambilla R, Robinson TE. Knockout of ERK1 enhances cocaine-evoked immediate early gene expression and behavioral plasticity. Neuropsychopharmacology. 2006;31:2660–2668. doi: 10.1038/sj.npp.1301014. [DOI] [PubMed] [Google Scholar]
- 111.Lu L, Koya E, Zhai H, Hope BT, Shaham Y. Role of ERK in cocaine addiction. Trends Neurosci. 2006;29:695–703. doi: 10.1016/j.tins.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 112.Li Y-Q, Li F-Q, Wang X-Y, Wu P, Zhao M, Xu C-M, et al. Central Amygdala Extracellular Signal-Regulated Kinase Signaling Pathway Is Critical to Incubation of Opiate Craving. J Neurosci. 2008;28:13248–13257. doi: 10.1523/JNEUROSCI.3027-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hilario MR, Clouse E, Yin HH, Costa RM. Endocannabinoid signaling is critical for habit formation. Front Integr Neurosci. 2007;1:6. doi: 10.3389/neuro.07.006.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Berhow MT, Hiroi N, Nestler EJ. Regulation of ERK (Extracellular Signal Regulated Kinase), Part of the Neurotrophin Signal Transduction Cascade, in the Rat Mesolimbic Dopamine System by Chronic Exposure to Morphine or Cocaine. J Neurosci. 1996;16:4707–4715. doi: 10.1523/JNEUROSCI.16-15-04707.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ortiz J, Harris HW, Guitart X, Terwilliger RZ, Haycock JW, Nestler EJ. Extracellular signal-regulated protein kinases (ERKs) and ERK kinase (MEK) in brain: regional distribution and regulation by chronic morphine. J Neurosci. 1995;15:1285–1297. doi: 10.1523/JNEUROSCI.15-02-01285.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Schumann J, Yaka R. Prolonged Withdrawal from Repeated Noncontingent Cocaine Exposure Increases NMDA Receptor Expression and ERK Activity in the Nucleus Accumbens. J Neurosci. 2009;29:6955–6963. doi: 10.1523/JNEUROSCI.1329-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Boudreau AC, Reimers JM, Milovanovic M, Wolf ME. Cell Surface AMPA Receptors in the Rat Nucleus Accumbens Increase during Cocaine Withdrawal But Internalize after Cocaine Challenge in Association with Altered Activation of Mitogen-Activated Protein Kinases. J Neurosci. 2007;27:10621–10635. doi: 10.1523/JNEUROSCI.2163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nelson A, Killcross S. Amphetamine Exposure Enhances Habit Formation. J Neurosci. 2006;26:3805–3812. doi: 10.1523/JNEUROSCI.4305-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Taylor JR, Jentsch JD. Repeated intermittent administration of psychomotor stimulant drugs alters the acquisition of pavlovian approach behavior in rats: Differential effects of cocaine, d-amphetamine and 3,4-methylenedioxymethamphetamine ("ecstasy") Biological Psychiatry. 2001;50:137–143. doi: 10.1016/s0006-3223(01)01106-4. [DOI] [PubMed] [Google Scholar]
- 120.Olausson P, Jentsch JD, Taylor JR. Repeated nicotine exposure enhances reward-related learning in the rat. Neuropsychopharmacology. 2003;28:1264–1271. doi: 10.1038/sj.npp.1300173. [DOI] [PubMed] [Google Scholar]
- 121.Wyvell CL, Berridge KC. Incentive sensitization by previous amphetamine exposure: increased cue-triggered "wanting" for sucrose reward. J Neurosci. 2001;21:7831–7840. doi: 10.1523/JNEUROSCI.21-19-07831.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]