Abstract
Tumor necrosis factor-alpha (TNF-α) is a key proinflammatory cytokine. It is generally believed that TNF-α exerts its effects primarily via TNF receptor subtype-1 (TNFR1). We investigated distinct role of TNFR1 and TNFR2 in spinal cord synaptic transmission and inflammatory pain. Compared to wild-type (WT) mice, TNFR1 and TNFR2 knockout (KO) mice exhibited normal heat sensitivity and unaltered excitatory synaptic transmission in the spinal cord, as revealed by spontaneous excitatory postsynaptic currents (sEPSCs) in lamina II neurons of spinal cord slices. However, heat hyperalgesia after intrathecal TNF-α and the second-phase spontaneous pain in the formalin test were reduced in both TNFR1- and TNFR2-KO mice. In particular, heat hyperalgesia after intraplantar injection of complete Freund's adjuvant (CFA) was decreased in the early phase in TNFR2-KO mice but reduced in both early and later phase in TNFR1-KO mice. Consistently, CFA elicited a transient increase of TNFR2 mRNA levels in the spinal cord on day 1. Notably, TNF-α evoked a drastic increase in sEPSC frequency in lamina II neurons, which was abolished in TNFR1-KO mice and reduced in TNFR2-KO mice. TNF-α also increased NMDA currents in lamina II neurons, and this increase was abolished in TNFR1-KO mice but retained in TNFR2-KO mice. Finally, intrathecal injection of the NMDA receptor antagonist MK-801 prevented heat hyperalgesia elicited by intrathecal TNF-α. Our findings support a central role of TNF-α in regulating synaptic plasticity (central sensitization) and inflammatory pain via both TNFR1 and TNFR2. Our data also uncover a unique role of TNFR2 in mediating early-phase inflammatory pain.
Keywords: proinflammatory cytokine, central sensitization, TNFR1, TNFR2, formalin, complete Freund's adjuvant
1. Introduction
Tumor necrosis factor-α (TNF-α) is a major proinflammatory cytokine produced not only in the immune system but also in the peripheral and central nervous system, especially under the pathological conditions [16;48;51;64;67;68;70]. Increasing evidence suggests a critical role of TNF-α in the pathogenesis of pain [56] including neuropathic pain [55;57;62], inflammatory pain [14;68;72], and cancer pain [13;24]. The peripheral effects of TNF-α on nociceptor sensitization (peripheral sensitization) [37] have been well documented. For example, intraplantar, intradermal, endoneurial, or intramuscular injection of TNF-α elicits heat hyperalgesia and mechanical allodynia [14;54;66;68;76]. TNF-α also modulates the activity of multiple ion channels including TRPV1, Na+ , Ca2+ , and K+ channels [15;36;43] and induces spontaneous activity in primary sensory neurons [52;59].
Several lines of evidence also suggest a central role of TNF-α in producing central sensitization (e.g., enhanced synaptic transmission and hyperexcitability in dorsal horn neurons) [33;39;42;69;75]. First, TNF-α is induced in spinal cord glial cells in several chronic pain conditions [16;27;70]. Second, intrathecal injection of TNF-α produces heat hyperalgesia and mechanical allodynia [23;39;41]. Third, intrathecal injection of TNF-α inhibitor such as etanercept attenuates chronic pain [7;44;55;57]. Fourth, perfusion of spinal cord slices with TNF-α increases the spontaneous excitatory postsynaptic currents (sEPSCs) [39;42;72;75] and enhances NMDA-induced currents in lamina II neurons [13;39;77]. In addition, TNF-α induces the trafficking and surface expression of AMPA receptors (AMPARs), leading to enhanced synaptic transmission in hippocampal neurons [3;60]. After spinal cord injury TNF-α induces rapid trafficking of GluR2-lacking AMPARs to the plasma membrane, leading to cell death of spinal cord motor neurons [19]. Recently, Choi et al. have shown that inflammation also induces a TNF-α-dependent surface trafficking of GluR1 AMPARs in the dorsal horn [11].
TNF-α exerts its effects through two structurally related and functionally distinct receptors, the TNF receptor-1 (TNFR1, p55) and the TNF receptor-2 (TNFR2, p75) [6;9]. Compared to TNFR1, little is known about the function of TNFR2. TNFR1 and TNFR2 may play distinct roles in the nervous system. In culture hippocampus neurons TNFR1 and TNFR2 produce cytotoxic and neuroprotective effects, respectively [9;74]. TNFR1 and TNFR2 also play different roles in regulating neuropathic pain [53;65] and cancer pain [13]. However, it remains unclear whether TNFR1 and TNFR2 differentially regulate synaptic transmission and inflammatory pain.
In this study, we used TNFR1 and TNFR2 knockout (KO) mice, as well as TNFR1/2 double knockout (DKO) mice to determine the distinct role of these two receptors in inflammatory pain conditions following intraplantar injection of formalin or complete freund's adjuvant (CFA) or intrathecal injection of TNF-α. We also used patch clamp recording in isolated spinal cord slices to investigate whether TNF-α could potentiate excitatory synaptic transmission and NMDA receptor activity via different TNF receptors. Our data demonstrated a critical role of TNF-α in regulating central sensitization and inflammatory pain via both TNFR1 and TRNF2. Our data also revealed a predominant role of TNFR1 in mediating all phases of inflammatory pain and a unique role of TNFR2 in mediating early-phase inflammatory pain.
2. Methods
2.1. Animals and pain models
Knockout mice lacking TNFR1 (Tnfrsf1a–/–), TNFR2 (Tnfrsf1b–/–), and TNFR1/R2 (Tnfrsf1a/1b–/–) were purchased from Jackson Laboratories and bred in the Thorn Research Building Animal Facility of Harvard Medical School. Because all these KO mice have the same C57BL/6 background, we used C57BL/6 wild-type mice as controls. TNFR1- and TNFR2-KO mice and TNFR1/R2 DKO mice were viable and showed no developmental defects. Adult mice (8-10 weeks) were used for behavioral studies. Young mice (3-5 weeks) were used for electrophysiological studies, because 1) high quality spinal cord slices and patch clamp recordings could be obtained from young animals, 2) spinal cord circuit is fully developed by postnatal age of two weeks [20], and 3) expression of sodium channels such as Na(v)1.8 and Na(v)1.9 reaches adult levels by postnatal day 7 [4]. All animal procedures performed in this study were approved by the Animal Care Committee of Harvard Medical School. To produce acute inflammatory pain, 20 μl of diluted formalin (5%) was injected into the plantar surface of a hindpaw. To produce persistent inflammatory pain, complete Freund's adjuvant (CFA, 20 μl, 1 mg/ml, Sigma) was injected into the plantar surface of a hindpaw.
2.2. Drugs and drug administration
TNF-α was purchased from R & D. NMDA, AMPA, and MK-801 were obtained from Sigma. For intrathecal injection, spinal cord puncture was made with a 30G needle between the L5 and L6 level to deliver reagents (10 μl) to the cerebral spinal fluid [31]. TNF-α was prepared in saline (2 ng/μl, pH ≈7.0) and intrathecally injected at the dose of 20 ng. This dose was based on our previous study showing dose-dependent heat hyperalgesia following intrathecal TNF-α (0.2-20 ng) [23]. For electrophysiological studies, drugs were applied via bath perfusion.
2.3. Quantitative Real-Time PCR
Mice were sacrificed after terminal anesthesia with isoflurane, and the L4-L5 spinal cord segments were rapidly dissected. Total RNA was extracted using RNeasy Plus Mini kit (Qiagen). Quantity and quality of the eluted RNA samples were verified by NanoDrop spectrophotometer (Thermo Fisher Scientific). A total of 0.5 μg of RNA was reverse transcribed using Omniscript reverse transcriptase according to the protocol of the manufacturer (Qiagen). Specific primers for TNFR1 and TNFR2 as well as GAPDH control were designed using IDT SciTools Real-Time PCR software (Integrated DNA Technologies). The sequences of these primers were described in Table-1. We performed gene-specific mRNA analyses using the MiniOpticon Real-Time PCR system (BioRad). Quantitative PCR amplification reactions contained the same amount of RT product including 7.5 μl of 2 × iQSYBR-green mix (BioRad) and 300 nM of forward and reverse primers in a final volume of 15 μl. The thermal cycling conditions comprised 3 min polymerase activation at 95°C, 45 cycles of 10 s denaturation at 95 °C and 30 s annealing and extension at 60°C, followed by a DNA melting curve for the determination of amplicon specificity. All experiments were performed in duplicate. Primer efficiency was obtained from the standard curve and integrated for calculation of the relative gene expression, which was based on real-time PCR threshold values of different transcripts and groups (Berta et al., 2008) [5].
Table I.
Sequences of DNA primers for Real-time PCR.
| Target Gene | Forward Primers | Reverse Primers | Genbank No. |
|---|---|---|---|
| TNFR1 | TGAGTGCGTCCCTTGCAGCC | AACCAGGGGCAACAGCACCG | NM011609 |
| TNFR2 | GTCATGGCGGAGGCCCAAGG | GCGCTGGCTTGGGAAGAGCA | NM011610 |
| GAPDH | TCCATGACAACTTTGGCATTG | CAGTCTTCTGGGTGGCAGTGA | XM001473623 |
2.4. Spinal cord slice preparation
As we previously reported [1;39], a portion of the lumbar spinal cord (L4-L5) was removed from mice (3-5 week old) under urethane anesthesia (1.5 - 2.0 g/kg, i.p.). The spinal cord segment was kept in pre-oxygenated ice-cold Krebs solution. Spinal segment was placed in a shallow groove formed in an agar block and glued to the bottom of the microslicer stage. Transverse slices (600 μm) were cut on a vibrating microslicer. The slices were perfused with Kreb's solution (8-10 ml/min) that was saturated with 95% O2 and 5% CO2 at 36±1°C for at least 1-3 h prior to experiment. The Krebs solution contains (in mM): NaCl 117, KCl 3.6, CaCl2 2.5, MgCl2 1.2, NaH2PO4 1.2, NaHCO3 25, and glucose 11.
2.5. Patch clamp recordings in spinal slices
The whole cell patch-clamp recordings were made from lamina II neurons in voltage clamp mode [1]. Under a dissecting microscope with transmitted illumination, the substantia gelatinosa (SG, lamina II) is clearly visible as a relatively translucent band across the dorsal horn. Patch pipettes were fabricated from thin-walled, borosilicate, glass-capillary tubing (1.5 mm o.d., World Precision Instruments). After establishing the whole-cell configuration, neurons were held at the potential of -70 mV to record spontaneous excitatory postsynaptic currents (sEPSCs). The stock solution of TNF-α (1000x) was diluted with Kreb's solution (pH ≈7.2) before the use. After the baseline recordings, neurons were perfused with TNF-α (10 ng/ml, i.e. 0.59 nM) for 2 min. Our pilot study showed that TNF-α at the concentration of 10 ng/ml but not 1 ng/ml induced a marked increase in sEPSCs. We did not intend to use high concentrations, in order to avoid possible neurotoxic effects of TNF-α at higher concentrations (>10 ng/ml) [3;60]. Neuron was held at the potential of -50 mV or -70 mV for recording NMDA- or AMPA-induced current following bath application of NMDA (50 μM) or AMPA (10 μM), respectively. The concentration of NMDA and AMAP was chosen according to our previous studies [39;40;72]. The resistance of a typical patch pipette is 5-10 MΩ. The internal solution contains (in mM): potassium gluconate 135, KCl 5, CaCl2 0.5, MgCl2 2, EGTA 5, HEPES 5, ATP-Mg 5. Membrane currents were amplified with an Axopatch 200B amplifier (Axon Instruments) in voltage-clamp mode. Signals were filtered at 2 kHz and digitized at 5 kHz. Data were stored with a personal computer using pCLAMP 10 software and analyzed with Mini Analysis (Synaptosoft Inc.).
2.6. Behavioral analysis
Animals were habituated to the testing environment daily for at least two days before baseline testing. The room temperature and humidity remained stable for all experiments. Animals were put in plastic boxes and allowed 30 min for habituation before examination. Heat sensitivity was tested by radiant heat using Hargreaves [28] apparatus (IITC Life Science Inc.) and expressed as paw withdrawal latency (PWL). The radiant heat intensity was adjusted so that basal PWL is between 9-12 seconds, with a cut-off of 20 seconds to prevent tissue damage. For formalin test, the time spent in licking and lifting the affected paws was recorded every 5 min for 45 min. The experimenters were blinded to genotypes of mice.
2.7. Statistical analysis
All data were expressed as mean ± s.e.m. For electrophysiology, those cells showed >5% changes from the baseline levels during drug perfusion were regarded as responding ones and included for statistical analysis [39]. Differences between groups were compared using student t-test or ANOVA followed by Newman-Keuls test. The criterion for statistical significance was P<0.05.
3. Results
3.1. Intrathecal TNF-α induces heat hyperalgesia via both TNFR1 and TNFR2
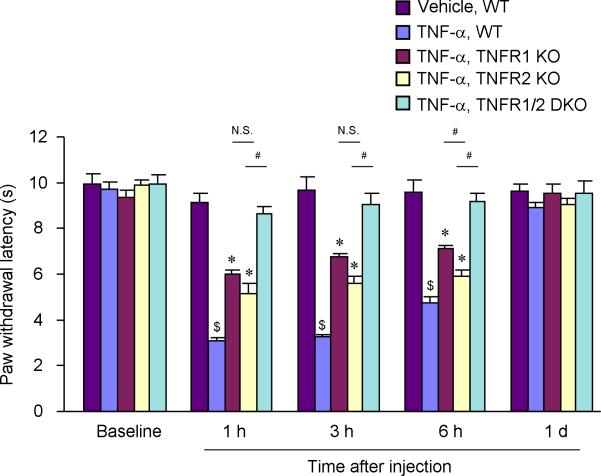
In order to test the central effects of TNF-α, we delivered TNF-α to the spinal cord intrathecally through spinal cord puncture and measured the paw withdrawal latency (PWL) using radiant heat. TNF-α (20 ng) induced a marked reduction in PWL in WT mice, indicating the development of heat hyperalgesia. Compared to vehicle control, this heat hyperalgesia developed within 1 h, maintained at 3 and 6 h, but recovered at 24 h (Fig. 1). Intrathecal injection of the vehicle (saline) did not induce significant heat hyperalgesia at these time points (Fig. 1). Notably, TNFR1-KO, TNFR2-KO, and TNFR1/R2 DKO mice exhibited normal baseline PWLs (P>0.05, compared to WT mice). However, TNF-α-elicited heat hyperalgesia was significantly reduced in both TNFR1- and TNFR2-KO mice (Fig. 1). Although the deficit appeared to be more severe in TNFR1-KO mice, only at one time point (6 h) there was significant difference in PWLs between TNFR1 and TNFR2 KO mice (P<0.05, Fig. 1). While TNFR1- and TNFR2-KO mice showed a partial reduction in TNF-α-induced heat hyperalgesia, TNFR1/R2 DKO mice showed a complete loss of heat hyperalgesia (Fig. 1).
Figure 1.
Intrathecal injection of TNF-α induces heat hyperalgesia via both TFNR1 and TNFR2. Spinal infusion of TNF-α (20 ng, i.t.) elicits robust heat hyperalgesia in wide-type (WT) mice, which is reduced in both TNFR1 and TNFR2 knockout (KO) and abolished in TNFR1/2 double knockout (DKO) mice. $P<0.05, compared with WT control with vehicle injection; *P<0.05, compared to WT control with TNF-α injection; #P<0.05, n = 5 mice.
3.2. Both TNFR1 and TNFR2 are required for the second-phase spontaneous pain in the formalin test
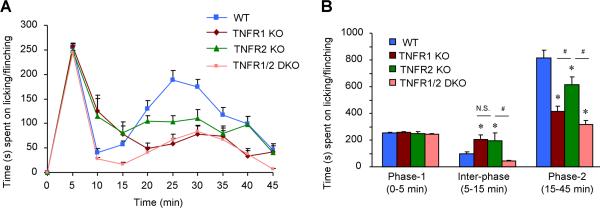
Intraplantar injection of diluted formalin (5%, 20 μl) induced characteristic two-phase spontaneous pain behaviors, as indicated by licking and lifting the affected paws in WT mice (Fig. 2). Formalin-induced spontaneous pain in the first-phase (0-5 min) did not alter in TNFR1-KO, TNFR2-KO, and TNFR1/R2 DKO mice (Fig. 2A,B). By contrast, spontaneous pain in the second phase (15-45 min) was reduced in all 3 lines of KO mice (Fig. 2A,B). However, the decrease in the second-phase pain was more robust in TNFR1-KO and TNFR1/R2 DKO-mice than that in TNFR2-KO mice (Fig. 2B). Surprisingly, spontaneous pain in the quiescent inter-phase (5-15 min) was increased in TNFR1- or TNFR2-KO mice but not in TNFR1/R2 DKO mice (Fig. 2).
Figure 2.
Formalin-induced spontaneous pain in the second-phase requires both TNFR1 and TNFR2. (A) Time course of formalin-induced spontaneous measured every 5 min for 45 min. (B) Spontaneous pain plotted in the first phase (0-5 min), inter-phase (5-15 min), and the second phase (15-45 min). Intraplantar formalin (5%) induces biphasic spontaneous behaviors (licking/lifting the affect paws) in WT mice. The second-phase but not the first-phase pain behaviors are attenuated in both TNFR1-KO, TNFR2-KO and TNFR1/2 DKO mice. Notably, the inter-phase response is increased in TNFR1-KO and TNFR2-KO mice. *P<0.05, compared to WT control; #P<0.05, n = 6 mice. N.S., no significance.
3.3. Distinct role of TNFR1 and TNFR2 in the development and maintenance CFA-induced heat hyperalgesia
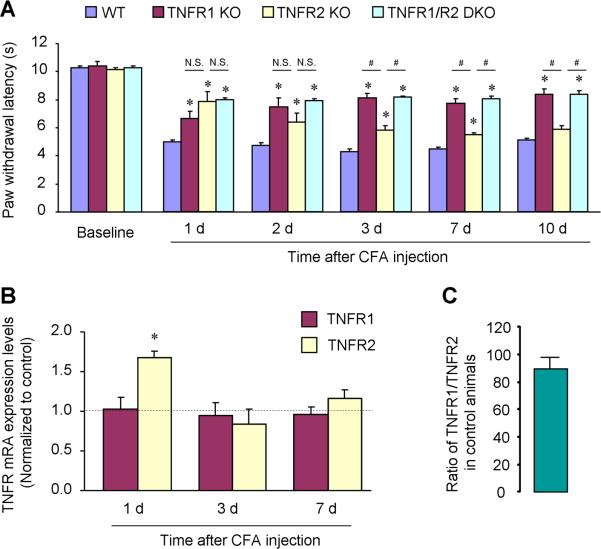
Intraplantar injection of CFA (1 mg/ml, 20 μl) resulted in a persistent heat hyperalgesia in WT mice, starting on day 1 and maintaining on day 10 (Fig. 3A). CFA-evoked heat hyperalgesia was attenuated in all 3 lines of KO mice lacking TNFR1, TNFR2, and TNFR1/R2 (Fig. 3A). However, the contribution of TNFR1 and TNFR2 to the induction and maintenance phase of heat hyperalgesia was different. TNFR1-KO mice displayed a significant reduction in heat hyperalgesia at all the time points examined from day 1 to day 10, and there was no difference in heat hyperalgesia between TNFR1-KO mice and TNFR1/2-DKO mice at all the time points (Fig. 2A). By contrast, TNFR2 mice exhibited a marked reduction in heat hyperalgesia on day 1 and a mild reduction on day 2, 3, and 7 but no reduction on day 10 (Fig. 2A). Statistically, TNFR1-KO mice showed more reduction in heat hyperalgesia than TNFR2-KO mice on day 3, 7, and 10 (P<0.05, Fig. 2A).
Figure 3.
CFA-induced heat hyperalgesia differentially requires TNFR1 and TNFR2. (A) Intraplantar CFA induces persistent heat hyperalgesia for > 10 days in WT mice, which is reduced in TNFR1-KO and TNFR1/2-DKO mice at all the times examined. Of note TNFR2-KO mice only show deficit in the first several days, especially on day 1. *P<0.05, compared to WT control; #P<0.05; N.S., no significance. n = 5 mice. (B) Real-time RT-PCR reveals transient increases in TNFR2 mRNA levels in the spinal cord on CFA day 1. There is no change in TNFR1 mRNA levels after CFA inflammation. *P<0.05, compared to naive control, n = 4 mice. (C) Ratio of spinal mRNA copies of TNFR1/TNFR2 in the naïve control. n = 4 mice.
Real-Time PCR revealed distinct regulation of TNFR1 and TNFR2 mRNA expression in the spinal cord following inflammation (Fig. 2B). CFA induced a transient increase in TNFR2 mRNA levels on day 1 but not on day 3 and 7. However, CFA did not change TNFR1 mRNA levels at all these times (Fig. 2B). Notably, the copy number of TNFR1 mRNA was more than 80 times higher than that of TNFR2 mRNA in naïve animals (Fig. 2C, P<0.05, n=4).
3.4. TNF-α enhances excitatory synaptic transmission (sEPSC) in spinal lamina II neurons via both TNFR1 and TNFR2
To address how TNF-α mediates central sensitization and inflammatory pain, we prepared spinal cord slice and performed whole-cell patch clamp recordings in lamina II neurons where many nociceptive neurons are localized [1;38]. We examined excitatory synaptic transmission by recording spontaneous EPSCs (sEPSCs) in WT and TNFR1- and TNFR2-KO mice. We did not find any difference in basal synaptic transmission between WT and TNFR1- or TNFR2-KO mice: both the frequency and amplitude of sEPSCs were comparable (Table II).
Table II.
Frequency and amplitude of spontaneous EPSC (sEPSC) recorded in lamina II neurons in spinal cord slices of wild-type (WT), TNFR1 and TNFR2 knockout (KO) mice. P>0.05, compared to TNFR1-KO and TNFR2-KO mice. n = 14, 16, 13 neurons in WT, TNFR1-KO, and TNFR2-KO group, respectively.
| Frequency (Hz) | Amplitude (pA) | |
|---|---|---|
| WT | 6.07 ± 1.56 | 12.37 ± 1.08 |
| TNFR1 KO | 6..32 ± 1.63 | 12.50 ± 1.52 |
| TNFR2 KO | 6..13 ± 1.58 | 12.10 ±0.99 |
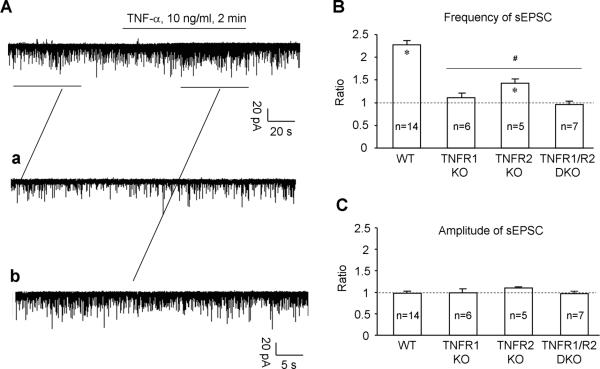
We further assessed the role of TNFR1 and TNFR2 in TNF-α-induced enhancement of excitatory synaptic transmission. Superfusion of spinal cord slices with TNF-α (10 ng/ml, i.e. 0.59 nM) caused a robust (>100%) increase in sEPSC frequency (Fig. 4A), as a result of enhanced glutamate release from presynaptic sites [39;45;73]. This frequency increase by TNF-α was almost abolished in TNFR1-KO and TNFR1/2-DKO mice but only partially reduced in TNFR2-KO mice (Fig. 4A,B). In contrast to frequency changes, TNF-α did not induce amplitude changes in sEPSCs either in WT mice or in TNFR1- and TNFR2-KO mice (Fig. 4A, C).
Figure 4.
TNF-α enhances excitatory synaptic transmission, i.e. spontaneous EPSC frequency (sEPSC) in spinal lamina II neurons via both TNFR1 and TNFR2. (A) Patch-clamp recording in spinal cord slices shows an increase in the frequency of sEPSC after bath application of TNF-α (10 ng/ml, 2 min). Aa and Ab are enlarged recordings before and after TNF-α treatment, respectively. (B) TNF-α increases the frequency of sEPSC in WT mice. This frequency increase is abolished in TNFR1-KO and TNFR1/2 -DKO mice and partially reduced in TNFR2-KO mice. *P<0.05, compared to pretreatment baseline; #P<0.05, compared to WT. The number of recorded neurons is indicated inside each column. (C) TNF-α fails to increases the amplitude of sEPSC in WT mice, in TNFR1-KO and TNFR2-KO mice, as well as in TNFR1/2-DKO mice. All the data are expressed as the ratio of corresponding baseline. The number of recorded neurons is indicated inside each column.
3.5. TNF-α enhances NMDA-induced currents in spinal lamina II neurons via TNFR1 but not TNFR2
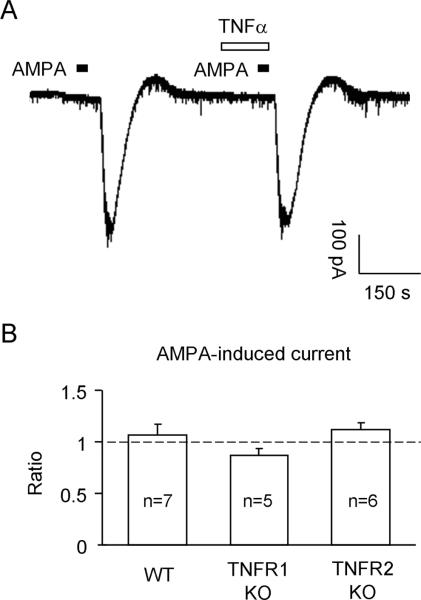
Excitatory synaptic transmission is mediated by AMPA and NMDA receptors of glutamate [72]. Thus, we also examined whether the activity of AMPA and NMDA receptors could be modulated by TNF-α via TNFR1 or TNFR2. We bath applied AMPA and NMDA to spinal cord slices to stimulate AMPA and NMDA receptors. AMPA (10 μM), at the holding potential of -70 mV, induced a robust inward current in WT mice (Fig. 5A). However, TNF-α (10 ng/ml, 2 min) failed to potentiate this current either in WT mice or in TNFR1- or TNFR2-KO mice (Fig. 5B).
Figure 5.
(A) Patch clamp recording in lamina II neurons of spinal cord slices shows no potentiation of AMPA (10 μM)-induced current by TNF-α (10 ng/ml, 2 min). (B) Quantification of AMPA-induced currents in WT mice, TNFR1-KO, and TNFR2-KO mice following TNF-α treatment. The data are expressed as the ratio of the amplitude of AMPA current (after vs. before TNF-α stimulation). The number of recorded neurons is indicated inside each column.
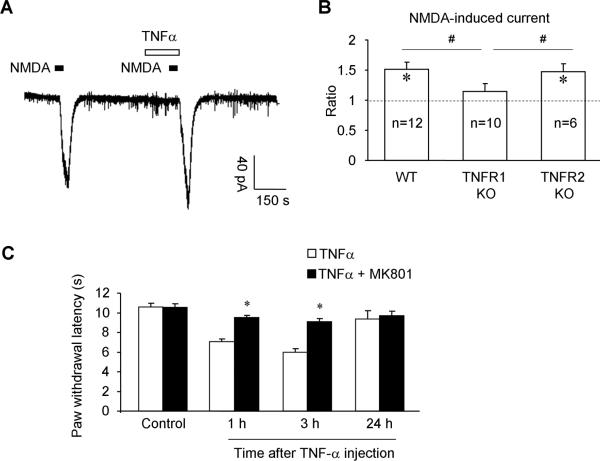
Perfusion of NMDA (50 μM), at the holding potential of -50 mV, also induced a robust inward current in WT mice. Unlike AMPA-induced current, NMDA-induced current was greatly potentiated by TNF-α (10 ng/ml, 2 min) in WT mice (Fig. 6A,B). This potentiation of NMDA current by TNF-α was abrogated in TNFR1-KO mice but not in TNFR2-KO mice (Fig. 6B), indicating a role of TNFR1 but not TNFR2 in NMDA receptor sensitization.
Figure 6.
TNF-α enhances the NMDA-induced current in lamina II neurons of spinal cord slices via TNFR1. (A) Patch clamp recording shows potentiation of NMDA-induced current after perfusion of TNF-α (10 ng/ml, 2min) in spinal cord slices of WT mice. (B) Quantification of NMDA-induced currents in lamina II neurons of WT mice and TNFR1-KO and TNFR2-KO mice, following TNF-α treatment. The data are expressed as the ratio of the amplitude of NMDA current. * P <0.05, compared to the first NMDA-induced current; #P<0.05. Note that TNF-α enhances NMDA currents in WT and TNFR2-KO mice but not in TNFR1-KO mice. The number of recorded neurons is indicated inside each column. (C) Intrathecal injection of TNF-α (10 ng) induces heat hyperalgesia in naïve mice, which is prevented by intrathecal pretreatment of the NMDA receptor antagonist MK-801 (10 nmol), given 30 min before TNF-α injection. *P <0.05, compared to TNF-α, n = 6 mice.
Finally, to determine a behavioral consequence of NMDA receptor sensitization by TNF-α, we examined whether administration of the NMDA receptor antagonist MK-801 could attenuate TNF-α-induced pain. MK-801 (10 nmol) intrathecally administrated prior to TNF-α injection (20 ng, intrathecal), prevented the development of TNF-α-induced heat hyperalgesia (Fig. 6C).
4. Discussion
We have demonstrated an essential role of TNF-α in regulating acute and persistent inflammatory pain, as well as central sensitization, indicated by TNF-α-evoked increases in both sEPSC frequencies and NMDA-induced currents in lamina II neurons in spinal cord slices. We have also revealed similar and distinct roles of TNFR1 and TNFR2 in mediating inflammatory pain and TNF-α-evoked synaptic and neuronal changes in lamina II neurons. First, neither TNFR1 nor TNFR2 was required for producing baseline heat pain in naïve animals and mediating excitatory synaptic transmission (sEPSC) in the normal (non-injured) conditions. Second, both TNFR1 and TNFR2 were necessary for inducing the second-phase spontaneous pain in the formalin test and heat hyperalgesia following intrathecal TNF-α injection and CFA inflammation. Third, both TNFR1 and TNFR2 were required for TNF-α-induced sEPSC frequency increase, whereas only TNFR1 was required for TNF-α-induced NMDA current increase. Fourth, CFA induced a transient increase (day 1) in TNFR2 mRNA expression in the spinal cord without changing TNFR1 mRNA expression. Finally, in the CFA model TNFR1 was required for inducing heat hyperalgesia at all the time points (1-10 d) examined, but TNFR2 was mainly required for the early-phase development of heat hyperalgesia.
4.1. TNF-α induces central sensitization
Central sensitization plays an essential role in the development and maintenance of persistent pain [33;69]. Central sensitization could be enhanced and maintained by activation of spinal cord glial cells such as microglia and astrocytes and release of proinflammatory cytokines (e.g., TNF-α and IL-1β), chemokines (e.g., CCL2), or growth factor (e.g., BDNF) from these glial cells [17;22;29;34;46;63]. In the spinal cord, TNF-α is mainly produced by microglia, although other cells types such as astroctyes also produce TNF-α [27;34;71]. Activation of p38 MAP kinase in spinal microglia was implicated in the synthesis and release of TNF-α [30;35;61;78]. We provided both behavioral and electrophysiological evidence to support a critical role of TNF-α in regulating central sensitization. In support of our previous studies [39;72], intrathecal TNF-α induced marked heat hyperalgesia (Fig. 1). In an antigen-induced arthritis model, spinally administered etanercept acutely reduces pain-related behavior and attenuates both the development and the maintenance of inflammation. Of interest intrathecal etanercept is more effective in reducing pain and inflammation than systemic etanercept [7].
Formalin-induced spontaneous pain has two phases, separated by an inter-phase. While the first-phase pain is a result of direct activation of nociceptors and peripheral sensitization, the second-phase pain could be a result of central sensitization [12;18;32;49]. A relatively quiescent inter-phase is thought to be mediated by a descending antinociceptive mechanism from the brainstem [21]. Our data showed that both TNFR1 and TNFR2 were required for the second-phase spontaneous pain (Fig. 2), supporting a role of TNF-α in central sensitization. By contrast, the first-phase pain was completely intact in TNFR1-KO, TNFR2-KO, and TNFR1/2-KO mice. A possible explanation is that after formalin injection TNF-α may not be immediately released in the first phase. Surprisingly, the inter-phase response was enhanced in both TNFR1 and TNFR2-KO mice (Fig. 2B), suggesting a possible involvement of TNF-α in brainstem descending inhibition [21].
How can TNF-α modulate central sensitization? TNF-α might promote central sensitization by enhancing glutamate release from presynaptic terminals and increasing the activity of NMDA receptor, a key play in central sensitization [69]. The frequency of sEPSCs in lamina II neurons drastically increased following TNF-α treatment (Fig. 4), as a result of enhanced glutamate release from presynaptic terminals. Several lines of evidence suggest that activation of TRPV1 contributes to the sEPSC frequency increase by TNF-α. First, TNF-α activates TRPV1 [36;47], and TRPV1 activation by capsaicin substantially increases the sEPSC frequency [73]. Second, the TRP antagonist capsazepine suppresses the TNF-α-induced sEPSC frequency increase [72]. Third, heat hyperalgesia elicited by intrathecal TNF-α is abolished in TRPV1 KO mice [72]. We also found a marked increase in NMDA-induced currents in lamina II neurons following TNF-α perfusion, which was supported by our behavioral result that MK-801 prevented heat hyperalgesia induced by intrathecal TNF-α. Thus TNF-α can also induce heat hyperalgesia via the activation of spinal NMDA receptors. Consistently, formalin-induced second-phase pain was reduced by both TNFR deletion and NMDA receptor blockade [26]. However, we should not exclude the involvement of other ion channels, since our recording conditions for sEPSC (-70 mV) and NMDA current (-50 mV) may not be optimal for the activation of some Na+ channels (e. g., Nav1.9).
Inflammation has also been shown to induce TNF-α-dependent surface trafficking of AMPA receptor (GluR1) in the dorsal horn [11]. However, TNF-α failed to increase the amplitude of sEPSC (Fig. 4C), which is mediated by postsynaptic AMPA receptors [73]. Neither did TNF-α enhance AMPA-induced currents in dorsal horn neurons (Fig. 5), in agreement with previous recordings in dorsal horn neurons [39;75]. This discrepancy in potentiation of AMPA receptors by TNF-α may result from different types of spinal neurons examined (lamina II neurons vs. other lamina neurons). Also, we should not rule out that TNF-α may enhance AMPA currents under injury conditions.
One limitation of blinded patch clamp recordings is we cannot be certain about the chemical properties of the neurons we recorded, since there are at least 4 types of neurons in the lamina II (substantia gelatinosa): islet (inhibitory), radial, central, and vertical neurons [25]. However, excitatory interneurons dominate the lamina II [50]. A recent study by Zhang et al. (2010) reported that TNF-α could also inhibit spontaneous inhibitory postsynaptic currents (sIPSCs) in GAD67-positive inhibitory neurons in the lamina II [77]. In contrast, we did not observe any effect of TNF-α on IPSCs lamina II neurons in rats [39] and mice (data not shown). One possible explanation for this discrepancy is different types of neurons (excitatory vs inhibitory) were recorded in the present study and the recent study by Zhang et al. (2010) [77].
4.2. Distinct role of TNFR1 and TNFR2 in central sensitization and inflammatory pain
Although TNFR1 and TNFR2 have different signaling mechanisms, they have cross-talk through their intralcellular parts [8]. TNFR1 has been regarded as a predominant receptor, whereas the role of TNFR2 was underestimated. TNFR1 is constructively expressed in all tissues, whereas TNFR2 expression is tightly regulated [8;9]. TNFR1 has high affinity for soluble TNF, which is commonly used as TNF-α receptors agonist [2;8;9]. TNFR1 contains a death domain and mediates TNF-α-induced cell death in hippocampal neurons. Conversely, TNFR2 does not contain death domain and instead mediates TNF-α-induced neuronal survival [9;74]. Interestingly, in the Alzheimer's disease brain TNFR1 levels and binding affinity are increased, but TNFR2 levels and binding affinity are decreased [10].
TNFR2 also plays a unique role in neuropathic pain [58;65] and cancer pain [13;24]. Epineural injection of TNFR1 but not TNFR2 neutralizing antibody reduces heat hyperalgesia and mechanical allodynia in the chronic construction injury (CCI) model [58]. CCI-induced thermal hyperalgesia is absent in TNFR1-KO mice, whereas CCI-induced mechanical and cold alldynia are reduced in both TNFR1- and TNFR2-KO mice [65]. Of interest intrathecally injected TNFR1 agonist but not TNFR2 agonist slightly reduces mechanical and thermal withdrawal thresholds in rats, but co-injection results in an additive pain behavior [53]. Further, TNFR1 agonist induces discharge of sensory neurons in normal conditions, whereas TNFR2 agonist increases the discharge after nerve injury, suggesting a unique role of TNFR2 in injury-induced excitation of sensory neurons [53]. In a mouse skin cancer model, TNFR2 gene deletion results in attenuated heat hyperalgesia and reduced TRPV1 expression in tumor-bearing mice, whereas TNFR1 gene deletion has only a minor effect [13]. However, evoked pain and spinal gliosis in a bone cancer pain model require both TNFR1 and TNFR2 [24].
We examined the involvement of TNFR1 and TNFR2 in three different inflammatory pain conditions. Our data supported an important role of TNFR2 in mediating central sensitization and inflammatory pain. TNFR2-KO mice exhibited the following phenotypes: (1) reduced heat hyperalgeisa after intrathecal TNF-α, (2) decreased second-phase pain in the formalin test, (3) reduced early-phase heat hyepralgesia in the CFA model, and (4) abrogated sEPSC frequency increase by TNF-α. Of interest TNFR2 but not TNFR1 was transiently up-regulated on day 1 after CFA injection, suggesting a distinct role of TNFR2 in early phase development of inflammatory pain.
However, our data also showed a predominant role of TNFR1 in mediating TNF-α's effects. First, TNFR1 was required for all the effects involving TNFR2. Second, TNFR1 but not TNFR2 mediated TNF-α-induced potentiation of NMDA currents and CFA-induced heat hyperalgesia in the late phase (day 10). Third, TNFR1-KO mice and TNFR1/2-DKO mice displayed almost identical phenotypes with the exception of intrathecal TNF-α-induced heat hyperalgesia.
In summary, we have demonstrated a critical role of TNF-α in inflammatory pain by regulating central sensitization, specifically illustrated as synaptic plasticity (sEPSC frequency increase as a result of increased glutamate release) and neuronal plasticity (NMDA receptor hyperactivity) in spinal lamina II neurons. TNF-α may initiate inflammatory pain via TNFR1/TNFR2-mediated glutamate release and maintain inflammatory pain via TNFR1-mediated activation of NMDA receptors in the dorsal horn. Although TNFR1 plays a predominant role in mediating all the effects of TNF-α, TNFR2 has a particular role in mediating early development of inflammatory pain. Given an important central role of TNF-α in regulating synaptic and neuronal plasticity, targeting TNF-α signaling in the central nervous system, in addition to the peripheral nervous system and immune system, would increase the analgesic efficacy of the anti-TNF-α treatments.
Acknowledgements
All the authors claim that they have no competing financial interest in study. This work was supported by NIH grants NS54932, NS67686, and DE17794 to RRJ and National Science Fund of China (NSFC) 30600171 to LZ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This study reveals a critical role of TNF-α in regulating spinal cord synaptic plasticity and central sensitization and uncovers distinct role of TNFR1 and TNFR2 in regulating different phases of inflammatory pain.
References
- 1.Baba H, Ji RR, Kohno T, Moore KA, Ataka T, Wakai A, Okamoto M, Woolf CJ. Removal of GABAergic inhibition facilitates polysynaptic A fiber-mediated excitatory transmission to the superficial spinal dorsal horn. Mol Cell Neurosci. 2003;24:818–830. doi: 10.1016/s1044-7431(03)00236-7. [DOI] [PubMed] [Google Scholar]
- 2.Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001;11:372–377. doi: 10.1016/s0962-8924(01)02064-5. [DOI] [PubMed] [Google Scholar]
- 3.Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von ZM, Beattie MS, Malenka RC. Control of synaptic strength by glial TNFalpha. Science. 2002;295:2282–2285. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- 4.Benn SC, Costigan M, Tate S, Fitzgerald M, Woolf CJ. Developmental expression of the TTX-resistant voltage-gated sodium channels Nav1.8 (SNS) and Nav1.9 (SNS2) in primary sensory neurons. J Neurosci. 2001;21:6077–6085. doi: 10.1523/JNEUROSCI.21-16-06077.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berta T, Poirot O, Pertin M, Ji RR, Kellenberger S, Decosterd I. Transcriptional and functional profiles of voltage-gated Na(+) channels in injured and non-injured DRG neurons in the SNI model of neuropathic pain. Mol Cell Neurosci. 2008;37:196–208. doi: 10.1016/j.mcn.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Beutler B, Van HC. Unraveling function in the TNF ligand and receptor families. Science. 1994;264:667–668. doi: 10.1126/science.8171316. [DOI] [PubMed] [Google Scholar]
- 7.Boettger MK, Weber K, Grossmann D, Gajda M, Bauer R, Bar KJ, Schulz S, Voss A, Geis C, Brauer R, Schaible HG. Spinal tumor necrosis factor alpha neutralization reduces peripheral inflammation and hyperalgesia and suppresses autonomic responses in experimental arthritis: a role for spinal tumor necrosis factor alpha during induction and maintenance of peripheral inflammation. Arthritis Rheum. 2010;62:1308–1318. doi: 10.1002/art.27380. [DOI] [PubMed] [Google Scholar]
- 8.Carpentier I, Coornaert B, Beyaert R. Function and regulation of tumor necrosis factor type 2. Curr Med Chem. 2004;11:2205–2212. doi: 10.2174/0929867043364694. [DOI] [PubMed] [Google Scholar]
- 9.Chadwick W, Magnus T, Martin B, Keselman A, Mattson MP, Maudsley S. Targeting TNF-alpha receptors for neurotherapeutics. Trends Neurosci. 2008;31:504–511. doi: 10.1016/j.tins.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng X, Yang L, He P, Li R, Shen Y. Differential Activation of Tumor Necrosis Factor Receptors Distinguishes between Brains from Alzheimer's Disease and Non-Demented Patients. J Alzheimers Dis. 2009 doi: 10.3233/JAD-2010-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi JI, Svensson CI, Koehrn FJ, Bhuskute A, Sorkin LS. Peripheral inflammation induces tumor necrosis factor dependent AMPA receptor trafficking and Akt phosphorylation in spinal cord in addition to pain behavior. Pain. 2010;149:243–253. doi: 10.1016/j.pain.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coderre TJ, Melzack R. The contribution of excitatory amino acids to central sensitization and persistent nociception after formalin-induced tissue injury. J Neurosci. 1992;12:3665–3670. doi: 10.1523/JNEUROSCI.12-09-03665.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Constantin CE, Mair N, Sailer CA, Andratsch M, Xu ZZ, Blumer MJ, Scherbakov N, Davis JB, Bluethmann H, Ji RR, Kress M. Endogenous tumor necrosis factor alpha (TNFalpha) requires TNF receptor type 2 to generate heat hyperalgesia in a mouse cancer model. J Neurosci. 2008;28:5072–5081. doi: 10.1523/JNEUROSCI.4476-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cunha FQ, Poole S, Lorenzetti BB, Ferreira SH. The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia. Br J Pharmacol. 1992;107:660–664. doi: 10.1111/j.1476-5381.1992.tb14503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Czeschik JC, Hagenacker T, Schafers M, Busselberg D. TNF-alpha differentially modulates ion channels of nociceptive neurons. Neurosci Lett. 2008;434:293–298. doi: 10.1016/j.neulet.2008.01.070. [DOI] [PubMed] [Google Scholar]
- 16.DeLeo JA, Colburn RW, Rickman AJ. Cytokine and growth factor immunohistochemical spinal profiles in two animal models of mononeuropathy. Brain Res. 1997;759:50–57. doi: 10.1016/s0006-8993(97)00209-6. [DOI] [PubMed] [Google Scholar]
- 17.DeLeo JA, Yezierski RP. The role of neuroinflammation and neuroimmune activation in persistent pain. Pain. 2001;90:1–6. doi: 10.1016/s0304-3959(00)00490-5. [DOI] [PubMed] [Google Scholar]
- 18.Dickenson AH, Sullivan AF. Peripheral origins and central modulation of subcutaneous formalin-induced activity of rat dorsal horn neurones. Neurosci Lett. 1987;83:207–211. doi: 10.1016/0304-3940(87)90242-4. [DOI] [PubMed] [Google Scholar]
- 19.Ferguson AR, Christensen RN, Gensel JC, Miller BA, Sun F, Beattie EC, Bresnahan JC, Beattie MS. Cell death after spinal cord injury is exacerbated by rapid TNF alpha-induced trafficking of GluR2-lacking AMPARs to the plasma membrane. J Neurosci. 2008;28:11391–11400. doi: 10.1523/JNEUROSCI.3708-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fitzgerald M. The development of nociceptive circuits. Nat Rev Neurosci. 2005;6:507–520. doi: 10.1038/nrn1701. [DOI] [PubMed] [Google Scholar]
- 21.Franklin KB, Abbott FV. Pentobarbital, diazepam, and ethanol abolish the interphase diminution of pain in the formalin test: evidence for pain modulation by GABAA receptors. Pharmacol Biochem Behav. 1993;46:661–666. doi: 10.1016/0091-3057(93)90558-b. [DOI] [PubMed] [Google Scholar]
- 22.Gao YJ, Ji RR. Chemokines, neuronal-glial interactions, and central processing of neuropathic pain. Pharmacol Ther. 2010;126:56–68. doi: 10.1016/j.pharmthera.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao YJ, Zhang L, Samad OA, Suter MR, Yasuhiko K, Xu ZZ, Park JY, Lind AL, Ma Q, Ji RR. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J Neurosci. 2009;29:4096–4108. doi: 10.1523/JNEUROSCI.3623-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geis C, Graulich M, Wissmann A, Hagenacker T, Thomale J, Sommer C, Schafers M. Evoked pain behavior and spinal glia activation is dependent on tumor necrosis factor receptor 1 and 2 in a mouse model of bone cancer pain. Neuroscience. 2010 doi: 10.1016/j.neuroscience.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 25.Grudt TJ, Perl ER. Correlations between neuronal morphology and electrophysiological features in the rodent superficial dorsal horn. J Physiol. 2002;540:189–207. doi: 10.1113/jphysiol.2001.012890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haley JE, Sullivan AF, Dickenson AH. Evidence for spinal N-methyl-D-aspartate receptor involvement in prolonged chemical nociception in the rat. Brain Res. 1990;518:218–226. doi: 10.1016/0006-8993(90)90975-h. [DOI] [PubMed] [Google Scholar]
- 27.Hao S, Mata M, Glorioso JC, Fink DJ. Gene transfer to interfere with TNFalpha signaling in neuropathic pain. Gene Ther. 2007;14:1010–1016. doi: 10.1038/sj.gt.3302950. [DOI] [PubMed] [Google Scholar]
- 28.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 29.Hathway GJ, Vega-Avelaira D, Moss A, Ingram R, Fitzgerald M. Brief, low frequency stimulation of rat peripheral C-fibres evokes prolonged microglial-induced central sensitization in adults but not in neonates. Pain. 2009;144:110–118. doi: 10.1016/j.pain.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hide I, Tanaka M, Inoue A, Nakajima K, Kohsaka S, Inoue K, Nakata Y. Extracellular ATP triggers tumor necrosis factor-alpha release from rat microglia. J Neurochem. 2000;75:965–972. doi: 10.1046/j.1471-4159.2000.0750965.x. [DOI] [PubMed] [Google Scholar]
- 31.Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- 32.Ji RR, Baba H, Brenner GJ, Woolf CJ. Nociceptive-specific activation of ERK in spinal neurons contributes to pain hypersensitivity. Nat Neurosci. 1999;2:1114–1119. doi: 10.1038/16040. [DOI] [PubMed] [Google Scholar]
- 33.Ji RR, Kohno T, Moore KA, Woolf CJ. Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci. 2003;26:696–705. doi: 10.1016/j.tins.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 34.Ji RR, Suter MR. p38 MAPK, microglial signaling, and neuropathic pain. Mol Pain. 2007;3:33. doi: 10.1186/1744-8069-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jin SX, Zhuang ZY, Woolf CJ, Ji RR. p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J Neurosci. 2003;23:4017–4022. doi: 10.1523/JNEUROSCI.23-10-04017.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jin X, Gereau RW. Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-alpha. J Neurosci. 2006;26:246–255. doi: 10.1523/JNEUROSCI.3858-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 38.Kato G, Kawasaki Y, Koga K, Uta D, Kosugi M, Yasaka T, Yoshimura M, Ji RR, Strassman AM. Organization of intralaminar and translaminar neuronal connectivity in the superficial spinal dorsal horn. J Neurosci. 2009;29:5088–5099. doi: 10.1523/JNEUROSCI.6175-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawasaki Y, Zhang L, Cheng JK, Ji RR. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci. 2008;28:5189–5194. doi: 10.1523/JNEUROSCI.3338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kohno T, Wang H, Amaya F, Brenner GJ, Cheng JK, Ji RR, Woolf CJ. Bradykinin enhances AMPA and NMDA receptor activity in spinal cord dorsal horn neurons by activating multiple kinases to produce pain hypersensitivity. J Neurosci. 2008;28:4533–4540. doi: 10.1523/JNEUROSCI.5349-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kwon MS, Shim EJ, Seo YJ, Choi SS, Lee JY, Lee HK, Suh HW. Differential modulatory effects of cholera toxin and pertussis toxin on pain behavior induced by TNF-alpha, interleukin-1beta and interferon-gamma injected intrathecally. Arch Pharm Res. 2005;28:582–586. doi: 10.1007/BF02977762. [DOI] [PubMed] [Google Scholar]
- 42.Li J, Xie W, Zhang JM, Baccei ML. Peripheral nerve injury sensitizes neonatal dorsal horn neurons to tumor necrosis factor-alpha. Mol Pain. 2009;5:10. doi: 10.1186/1744-8069-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu BG, Dobretsov M, Stimers JR, Zhang JM. Tumor Necrosis Factor-alpha Suppresses Activation of Sustained Potassium Currents in Rat Small Diameter Sensory Neurons. Open Pain J. 2008;1:1. doi: 10.2174/1876386300801010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marchand F, Tsantoulas C, Singh D, Grist J, Clark AK, Bradbury EJ, McMahon SB. Effects of Etanercept and Minocycline in a rat model of spinal cord injury. Eur J Pain. 2009;13:673–681. doi: 10.1016/j.ejpain.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 45.Medvedeva YV, Kim MS, Usachev YM. Mechanisms of prolonged presynaptic Ca2+ signaling and glutamate release induced by TRPV1 activation in rat sensory neurons. J Neurosci. 2008;28:5295–5311. doi: 10.1523/JNEUROSCI.4810-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nicol GD, Lopshire JC, Pafford CM. Tumor necrosis factor enhances the capsaicin sensitivity of rat sensory neurons. J Neurosci. 1997;17:975–982. doi: 10.1523/JNEUROSCI.17-03-00975.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ohtori S, Takahashi K, Moriya H, Myers RR. TNF-alpha and TNF-alpha receptor type 1 upregulation in glia and neurons after peripheral nerve injury: studies in murine DRG and spinal cord. Spine (Phila Pa 1976 ) 2004;29:1082–1088. doi: 10.1097/00007632-200405150-00006. [DOI] [PubMed] [Google Scholar]
- 49.Puig S, Sorkin LS. Formalin-evoked activity in identified primary afferent fibers: systemic lidocaine suppresses phase-2 activity. Pain. 1996;64:345–355. doi: 10.1016/0304-3959(95)00121-2. [DOI] [PubMed] [Google Scholar]
- 50.Santos SF, Rebelo S, Derkach VA, Safronov BV. Excitatory interneurons dominate sensory processing in the spinal substantia gelatinosa of rat. J Physiol. 2007;581:241–254. doi: 10.1113/jphysiol.2006.126912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schafers M, Geis C, Svensson CI, Luo ZD, Sommer C. Selective increase of tumour necrosis factor-alpha in injured and spared myelinated primary afferents after chronic constrictive injury of rat sciatic nerve. Eur J Neurosci. 2003;17:791–804. doi: 10.1046/j.1460-9568.2003.02504.x. [DOI] [PubMed] [Google Scholar]
- 52.Schafers M, Lee DH, Brors D, Yaksh TL, Sorkin LS. Increased sensitivity of injured and adjacent uninjured rat primary sensory neurons to exogenous tumor necrosis factor-alpha after spinal nerve ligation. J Neurosci. 2003;23:3028–3038. doi: 10.1523/JNEUROSCI.23-07-03028.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schafers M, Sommer C, Geis C, Hagenacker T, Vandenabeele P, Sorkin LS. Selective stimulation of either tumor necrosis factor receptor differentially induces pain behavior in vivo and ectopic activity in sensory neurons in vitro. Neuroscience. 2008;157:414–423. doi: 10.1016/j.neuroscience.2008.08.067. [DOI] [PubMed] [Google Scholar]
- 54.Schafers M, Sorkin LS, Sommer C. Intramuscular injection of tumor necrosis factor-alpha induces muscle hyperalgesia in rats. Pain. 2003;104:579–588. doi: 10.1016/S0304-3959(03)00115-5. [DOI] [PubMed] [Google Scholar]
- 55.Schafers M, Svensson CI, Sommer C, Sorkin LS. Tumor necrosis factor-alpha induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J Neurosci. 2003;23:2517–2521. doi: 10.1523/JNEUROSCI.23-07-02517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sommer C, Kress M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci Lett. 2004;361:184–187. doi: 10.1016/j.neulet.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 57.Sommer C, Schafers M, Marziniak M, Toyka KV. Etanercept reduces hyperalgesia in experimental painful neuropathy. J Peripher Nerv Syst. 2001;6:67–72. doi: 10.1046/j.1529-8027.2001.01010.x. [DOI] [PubMed] [Google Scholar]
- 58.Sommer C, Schmidt C, George A. Hyperalgesia in experimental neuropathy is dependent on the TNF receptor 1. Exp Neurol. 1998;151:138–142. doi: 10.1006/exnr.1998.6797. [DOI] [PubMed] [Google Scholar]
- 59.Sorkin LS, Xiao WH, Wagner R, Myers RR. Tumour necrosis factor-alpha induces ectopic activity in nociceptive primary afferent fibres. Neuroscience. 1997;81:255–262. doi: 10.1016/s0306-4522(97)00147-4. [DOI] [PubMed] [Google Scholar]
- 60.Stellwagen D, Beattie EC, Seo JY, Malenka RC. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. J Neurosci. 2005;25:3219–3228. doi: 10.1523/JNEUROSCI.4486-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Svensson CI, Marsala M, Westerlund A, Calcutt NA, Campana WM, Freshwater JD, Catalano R, Feng Y, Protter AA, Scott B, Yaksh TL. Activation of p38 mitogen-activated protein kinase in spinal microglia is a critical link in inflammation-induced spinal pain processing. J Neurochem. 2003;86:1534–1544. doi: 10.1046/j.1471-4159.2003.01969.x. [DOI] [PubMed] [Google Scholar]
- 62.Sweitzer S, Martin D, DeLeo JA. Intrathecal interleukin-1 receptor antagonist in combination with soluble tumor necrosis factor receptor exhibits an anti-allodynic action in a rat model of neuropathic pain. Neuroscience. 2001;103:529–539. doi: 10.1016/s0306-4522(00)00574-1. [DOI] [PubMed] [Google Scholar]
- 63.Tsuda M, Inoue K, Salter MW. Neuropathic pain and spinal microglia: a big problem from molecules in “small” glia. Trends Neurosci. 2005;28:101–107. doi: 10.1016/j.tins.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 64.Uceyler N, Tscharke A, Sommer C. Early cytokine gene expression in mouse CNS after peripheral nerve lesion. Neurosci Lett. 2008;436:259–264. doi: 10.1016/j.neulet.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 65.Vogel C, Stallforth S, Sommer C. Altered pain behavior and regeneration after nerve injury in TNF receptor deficient mice. J Peripher Nerv Syst. 2006;11:294–303. doi: 10.1111/j.1529-8027.2006.00101.x. [DOI] [PubMed] [Google Scholar]
- 66.Wagner R, Myers RR. Endoneurial injection of TNF-alpha produces neuropathic pain behaviors. Neuroreport. 1996;7:2897–2901. doi: 10.1097/00001756-199611250-00018. [DOI] [PubMed] [Google Scholar]
- 67.Wagner R, Myers RR. Schwann cells produce tumor necrosis factor alpha: expression in injured and non-injured nerves. Neuroscience. 1996;73:625–629. doi: 10.1016/0306-4522(96)00127-3. [DOI] [PubMed] [Google Scholar]
- 68.Woolf CJ, Allchorne A, Safieh-Garabedian B, Poole S. Cytokines, nerve growth factor and inflammatory hyperalgesia: the contribution of tumour necrosis factor alpha. Br J Pharmacol. 1997;121:417–424. doi: 10.1038/sj.bjp.0701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 70.Xu JT, Xin WJ, Zang Y, Wu CY, Liu XG. The role of tumor necrosis factor-alpha in the neuropathic pain induced by Lumbar 5 ventral root transection in rat. Pain. 2006;123:306–321. doi: 10.1016/j.pain.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 71.Xu JT, Xin WJ, Zang Y, Wu CY, Liu XG. The role of tumor necrosis factor-alpha in the neuropathic pain induced by Lumbar 5 ventral root transection in rat. Pain. 2006;123:306–321. doi: 10.1016/j.pain.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 72.Xu ZZ, Zhang L, Liu T, Park JY, Berta T, Yang R, Serhan CN, Ji RR. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat Med. 2010;16:592–7, 1p. doi: 10.1038/nm.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang K, Kumamoto E, Furue H, Yoshimura M. Capsaicin facilitates excitatory but not inhibitory synaptic transmission in substantia gelatinosa of the rat spinal cord. Neurosci Lett. 1998;255:135–138. doi: 10.1016/s0304-3940(98)00730-7. [DOI] [PubMed] [Google Scholar]
- 74.Yang L, Lindholm K, Konishi Y, Li R, Shen Y. Target depletion of distinct tumor necrosis factor receptor subtypes reveals hippocampal neuron death and survival through different signal transduction pathways. J Neurosci. 2002;22:3025–3032. doi: 10.1523/JNEUROSCI.22-08-03025.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Youn DH, Wang H, Jeong SJ. Exogenous tumor necrosis factor-alpha rapidly alters synaptic and sensory transmission in the adult rat spinal cord dorsal horn. J Neurosci Res. 2008;86:2867–2875. doi: 10.1002/jnr.21726. [DOI] [PubMed] [Google Scholar]
- 76.Zelenka M, Schafers M, Sommer C. Intraneural injection of interleukin-1beta and tumor necrosis factor-alpha into rat sciatic nerve at physiological doses induces signs of neuropathic pain. Pain. 2005;116:257–263. doi: 10.1016/j.pain.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 77.Zhang H, Nei H, Dougherty PM. A p38 mitogen-activated protein kinase-dependent mechanism of disinhibition in spinal synaptic transmission induced by tumor necrosis factor-alpha. J Neurosci. 2010;30:12844–12855. doi: 10.1523/JNEUROSCI.2437-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhou Z, Peng X, Hao S, Fink DJ, Mata M. HSV-mediated transfer of interleukin-10 reduces inflammatory pain through modulation of membrane tumor necrosis factor alpha in spinal cord microglia. Gene Ther. 2008;15:183–190. doi: 10.1038/sj.gt.3303054. [DOI] [PMC free article] [PubMed] [Google Scholar]