Abstract
NMDA receptor antagonists interfere with learning and memory in some tasks, but not others. Some recent accounts have suggested that tasks placing demands on working memory are those most likely to be affected, and the present study tested this hypothesis. The purpose of the study was to adapt a recently developed procedure designed to test working memory capacity, the olfactory memory span task, for use in behavioral pharmacology and to then determine the effects of the NMDA receptor antagonist, dizocilpine (MK-801) on performance in this task. Rats were trained in a non-match-to-sample procedure under conditions in which they had to remember an increasing number of olfactory stimuli as the session progressed. Simple olfactory discrimination trials were interspersed to provide a performance control. Effects of dizocilpine (.03, .10, .17, .3 mg/kg) were determined after stable performances were obtained. Rats were able to sustain stable performances on both the span and simple discrimination tasks with average spans of about 10 items. Accuracy declined as the number of stimuli to remember increased, and dizocilpine impaired accuracy in a dose-dependent and memory-load dependent fashion. The finding that the effects of dizocilpine interacted with the number of stimuli to remember is generally consistent with hypotheses linking NMDA receptors and working memory processes.
Keywords: Working Memory, Olfactory Span, NMDA antagonist, MK-801, dizocilpine
1.0 Introduction
The findings of Morris and his colleagues (Morris, 1989; Morris, Anderson, Lynch, and Baudry, 1986) that NMDA antagonists impaired spatial learning in the Morris Swim Task (MST) at doses that blocked long-term potentiation (LTP) in the hippocampus provided the first pharmacological support for the now widely-accepted hypothesis that some forms of learning are mediated by LTP-like activity. However, questions were quickly raised about Morris’ interpretations because NMDA receptor antagonists produce a host of behavioral impairments that are not specific to learning and memory. Thus, impairment in the MST may reflect processes other than spatial learning, e.g., sensorimotor or motivational effects (Cain, Saucier, Hall, Hargreaves, and Boon, 1996; Keith and Rudy, 1990). In support of a non-mnemonic account of the Morris findings, pre-training experience in the MST abolishes the ability of NMDA antagonists to interfere with new spatial learning except at very high doses that also produce motor impairments (Bannerman, Good, Butcher, Ramsay, and Morris, 1995; Saucier and Cain, 1995). Further, in a repeated acquisition task in the MST, learning of a new spatial location is impaired only at relatively high doses of NMDA antagonists that also interfere with the ability to swim to a previously learned location (Galizio, Keith, Mansfield, and Pitts, 2003; Keith and Galizio, 1997). However, Steele and Morris (1999) observed NMDA antagonist impairments using a procedure in which rats learned to swim to a new platform location each session, but only when the delay between the first and second trials was relatively long (20 minutes and 2 hours); no impairment was observed at shorter delays (15 seconds) comparable to those used in the other repeated acquisition studies. Bannerman, Rawlins & Good (2006) reviewed the literature on NMDA antagonists and learning and concluded that NMDA effects are primarily manifest in tasks that place demands on working memory, for example, Steele & Morris (1999) in the case of spatial learning, but also in studies of non-spatial learning (e.g., Schmitt, Sprengel, Mack, Draft, Seeburg, Deacon, Rawlins, and Bannerman, 2005; Tonkiss and Rawlins, 1991). Bannerman et al. hypothesize that NMDA receptor activity is required for learning that involves certain working memory processes (i.e., those involving single trial learning and rapid selection of conditional information).
Operational definitions of working memory procedures for non-humans typically require that stimuli be presented during only a single learning trial and are only relevant for controlling behavior during a single trial or session (Bannerman et al., 2006; Dudchenko, 2004; Olton, Becker, and Handelmann, 1979). This is in contrast to definitions used in human research in which working memory is described in terms of a short term store of limited capacity requiring controlled attention (Baddeley, 2003; Saults and Cowan, 2007). Although the capacity limits of working memory in humans are still disputed (Cowan, 2001; Miller, 1956), the issue has received very little attention in non-human animals. As a result, a dearth of procedures is available for studying working memory capacity in rodents.
However, the olfactory span task (OST) for rodents (Dudchenko, Wood and Eichenbaum, 2000) incorporates manipulations of memory load into a single-session learning paradigm. The procedure involves the presentation of a single olfactory stimulus (a cup of scented sand) in an arena. Responses to this stimulus (digging) are reinforced through the retrieval of a food reward buried within the scented sand. Following a response to the stimulus, the rat is removed from the arena and the stimulus cup was moved to a random location. A second stimulus cup scented with a different odor is then baited with a food reward and placed in a random position in the arena. The rat is free to respond to either of the scented stimuli present, but only responses to the novel stimulus produce a food reward. On the third trial, the two previously presented olfactory stimuli are moved to new positions and a third odor was introduced. Once again, only responses to the novel odor are reinforced. The procedure is continued in this fashion with the introduction of a novel olfactory stimulus on each trial until up to 24 stimuli are present. Thus the procedure can be viewed as a non-match-to-sample task in which each stimulus serves as a sample during its initial presentation and as a comparison stimulus in each additional trial. Further, it might be best described as an incrementing non-match-to-sample task as the number of comparison stimuli increases on each successive trial.
To assess performance on the OST, Dudchenko et al. recorded span for each session as well as overall accuracy. Span was defined as the number of consecutive correct choices minus one (because there are no stimuli to remember on the first trial). The spans averaged about eight stimuli and there was an inverse relationship between performance and the number of stimuli to be remembered. Thus, accuracy decreased as the memory load increased. These findings provide some validation of the OST and the task has shown promise for investigating the neurobiological determinants of working memory capacity. For example, OST performance is transiently disrupted by lesions of the basal forebrain cholinergic system (Turchi and Sarter, 2000). The procedure has also been successfully adapted for the testing of mice (Young, Kerr, Kelly, Marston, Spratt, Finlayson, and Sharkey, 2007b); performance decrements have been observed in human amyloid over-expressing (Young, Sharkey, and Finlayson, 2009) and α7-nicotinic cholinergic receptor knockout mice (Young, Crawford, Kelly, Kerr, Marston, Spratt, Finlayson, and Sharkey, 2007a); increases in span are produced by nicotinic agonists (Rushforth, Allison, Wonnacott, and Shoaib, 2010). These effects were generally interpreted in terms of working memory capacity, however, alternative interpretations of some outcomes in the OST are possible. First, control procedures that allow separation of an effect on processes specific to remembering from the disruption of more general processes (e.g. motivation, perception, psychomotor ability) have not always been present. Second, as the number of stimuli to remember is incremented in the OST task, the number of comparison stimuli in the arena also increases. Thus, on any given trial the number of stimuli to remember (memory load) is inherently confounded with the number of comparisons the animal must choose among. Finally, stringent controls are needed to assure that stimulus control is based on the stimulus odors and not the scent of the food reward or odor trails left in the arena. In the present study, we first developed an adaptation of the OST procedure for use in behavioral pharmacology by including within-session controls for the above issues. In view of the hypothesis that NMDA receptors contribute to mechanisms supporting working memory (c.f., Bannerman et al., 2006), we investigated the effects of the NMDA antagonist dizocilpine (MK-801) on OST performance.
2.0 Methods
2.1.1 Subjects
Subjects were five male Holtzman Sprague-Dawley albino rats between 90 and 150 days old at the start of testing. All rats were housed individually in a temperature and humidity regulated vivarium operating on a 12 hour light-dark cycle. All subjects were given continuous access to water in their home cage and food access was restricted such that animals were kept at approximately 85% of their free-feeding weight.
2.1.2 Apparatus
All testing was conducted in an open-field apparatus constructed from a circular table 29.2 cm tall and 94 cm in diameter surrounded by a 32 cm high wall of sheet metal baffling. The Formica surface of the table contained 18 holes, 5.5 cm in diameter that were positioned in two concentric circles. Twelve holes were evenly spaced in an outer ring, 2.5 cm from the wall surrounding the surface of the table. Six holes were evenly spaced in an inner ring, 21.5 cm from the apparatus wall (Figure 1). Plastic cups (2 oz.) were placed in each hole during a trial. Sessions were recorded on a web cam (Logitech, Inc.).
Figure 1.
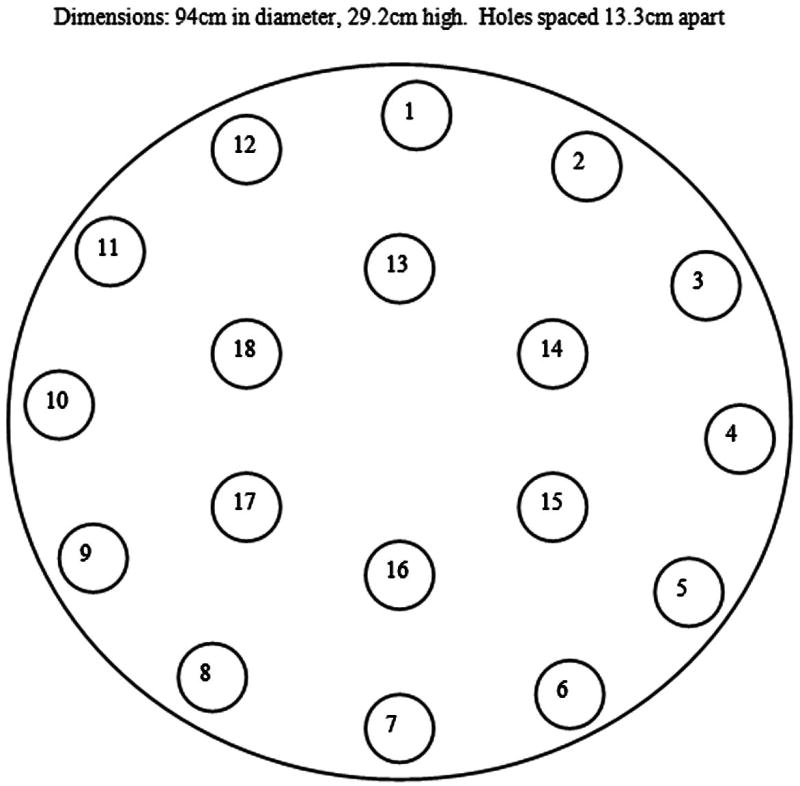
Diagram of the OST arena from above.
2.1.3 Stimuli
Plastic cups were half filled with white, fine grained, play sand and covered with scented lids to present olfactory stimuli. Sand served to weight the cups so that they were not displaced from the holes in the surface of the odor arena. The plastic lids used to present the odors were scented by storing them in airtight plastic containers containing the following household spices and flavorings: allspice, bay, beet, caraway, clove, cinnamon, coriander, cumin, celery, dill, fennel, garlic, ginger, lime, marjoram, mustard, nutmeg, paprika, onion, orange juice, oregano, savory, rosemary, sage, spinach, sumac, thyme, and turmeric. Spices in each storage container were refreshed weekly.
2.2 Procedure
2.2.1 Pretraining
Subjects were introduced to the apparatus and permitted to obtain 45 mg. sucrose pellets from the stimulus cups until subjects consumed all pellets promptly. Subsequently, a shaping procedure was used to train each rat to remove the lids from stimulus cups. On each shaping trial the animal was placed in the arena with a single, baited stimulus cup that was partially covered with a scented lid in one of the hole locations. On each successive trial, the baited cup was moved to random hole locations and the opening of the stimulus cup was more fully obscured until the subject was consistently removing the lids off of fully-obscured stimulus cups on every trial. In all subsequent training and testing the lids used to present odors were placed on top of the stimulus cup such that they fully-obscured the cup opening but were not firmly snapped onto the cup.
2.2.2 Initial Span Training
In this phase of training subjects began non-match to sample training with stimulus cups covered by scented lids. On the first trial of a session, a single baited cup covered with a scented lid was placed in a random location and the other 17 holes were filled with empty cups. The subject was then placed in the apparatus facing North, until a response occurred (operationally defined as any displacement of the lid from a stimulus cup). The rat was then removed from the apparatus to a holding cage for an inter-trial interval (ITI) of approximately 1 min. During the ITI, two stimulus cups were placed in random locations: one with a lid scented with the same odor as on Trial 1 (unbaited) and the other cup was baited and covered with a lid scented with a new odor. If the subject responded to the novel odor on trial 2, it was permitted to consume the food pellet and was then removed from the apparatus. The next trial then began with three stimuli present in the arena (the first two odors without pellets—S-; one novel odor with a pellet—S+). After each trial on which a correct response was scored, another new stimulus (S+) was presented with all odor stimuli used on the preceding trial (S-).
Following errors (when the rat responded to a cup that had been presented on a previous trial), the trial was terminated and the experimenter recorded the subject’s span. Span was defined as the number of consecutive correct choices minus one because the memory load was zero on the first trial of each span. Following each error, the session continued by repeating the entire procedure with new stimuli. That is, the next trial began with a single, baited, stimulus cup with an odor not yet presented during the session, and stimuli continued to increment after each correct response as described above. Sessions were terminated after 24 trials or after 30 minutes had elapsed, whichever came first. Animals were trained in this phase until relatively long spans were regularly produced (at least two consecutive sessions with spans greater than 7).
2.2.3 18-Comparison Span
Experimental sessions were conducted as in the previous phase with the exception that the span task continued to increment for 24 trials regardless of performance. A correction procedure was implemented in this phase such that trials were only terminated after a response to the novel stimulus. Thus, following an incorrect response, the trial continued until the subject responded to the novel stimulus. As the apparatus contained only 18 cup positions, randomly chosen stimuli were omitted from the comparison array on each of the last six trials in a session. In this phase, span and percent correct were recorded for each session and subjects were trained to a performance criterion of at least two sessions with spans of 10 or higher and accuracy of at least 90% correct.
2.2.4 5-Comparison Span
In the 18-Comparison Span procedure there is an inherent confound between the number of stimuli to remember and the number of comparison stimuli in the array. The 5-Comparison Span phase corrected for this by presenting no more than five comparison stimuli in the arena on any trial (although the number of novel samples presented continued to increment as in the previous phase). In this phase, each trial beyond the fourth included one correct comparison stimulus (an odor novel to the session; S+) along with four additional S- comparisons randomly chosen from odors presented as samples in the previous trials of the session. As such, chance performance was 20% for all trials beyond the fourth. The presentation order of stimuli, the comparisons used on any given trial and the placement location for each S+ was randomly determined.
2.2.5 5-Comparison Span with Added Simple Discrimination
After subjects showed stable performances on the 5-Comparison Span task, a simple discrimination was introduced to serve as a within-session control for drug effects not specific to memory span. First, subjects learned a simple discrimination between two odor stimuli not previously used in the span task. Only one of the two stimuli was baited during each trial and the same stimulus was consistently baited across all trials and sessions. Once rats mastered the simple discrimination, trials of the simple discrimination task were interspersed with the 5-comparison span task. The number of trials within a session increased from 24 to 30 trials with 24 span trials and six simple discrimination trials (one simple discrimination trial after every fourth span trial). Thus, the simple discrimination provides a measure of performance on a task which presumably involves reference, rather than working memory, but is otherwise comparable in task demands to the span procedure.
Three additional control conditions were also introduced during this phase. First, the procedure was modified to control for scent marking of the lids. To achieve this, all lids were replaced with fresh lids (of the same scent) after each trial. Thus, each scented lid was used only once per session, though the odor of each stimulus remained unchanged. This procedure was adopted to ensure that accurate performance could not be achieved by rejecting comparison stimuli based on detection of a scent mark left during a previous trial and was implemented during all baseline and drug testing sessions. Second, control sessions with all stimulus cups unbaited were introduced to verify that responding was not influenced by the presence of sucrose pellet odor in the S+ stimulus. In each control session, six span trials interspersed throughout the session were conducted with no pellet in the S+ cup. On these trials sucrose pellets were dropped into the stimulus cup only after the subject had made a correct response. At least five of these control sessions were conducted for each animal, and accuracy on pellet detection trials was then compared to accuracy on normally baited trials to determine if the odor of the sucrose was influencing outcomes. Finally, an experimenter who was blind to the condition rated video-recorded sessions (nine sessions selected arbitrarily across rats) and trial-by-trial scoring was compared with those recorded during the live session to determine inter-rater reliability. Ratings were highly consistent with an overall agreement of 99.3%.
2.2.6 Drug Phase
Rats were trained under these baseline conditions until a stability criterion was met such that the difference between percent correct on the last five sessions and the preceding five sessions was less than 15% of the mean of the ten sessions combined (Perone, 1991). Drug administration began once criterion was met on both span and simple discrimination accuracy. All subjects were tested five days a week (Monday through Friday). Drugs were administered on Tuesdays and Fridays. Mondays and Wednesdays served as recovery days and Thursday sessions were defined as baseline sessions. Dizocilpine (MK-801) maleate (Tocris) was dissolved in 0.9% saline prepared daily and delivered via intraperitoneal injection (I.P.) 30 minutes prior to testing in a volume of 1ml/kg at doses of .03, .10, .17 and .30 mg/kg (expressed as total salt). Each subject received 2 administrations of each dose (including saline) in a random order with the constraint that a complete cycle of dose determinations had to be complete before the next cycle began. Additional dose determinations up to four were performed in cases where there was considerable variability between determinations. High doses of DZP often resulted in gross motor impairment. In such cases, if the rat failed to respond within 2 min, the trial was terminated and scored as an error.
3.0 Results
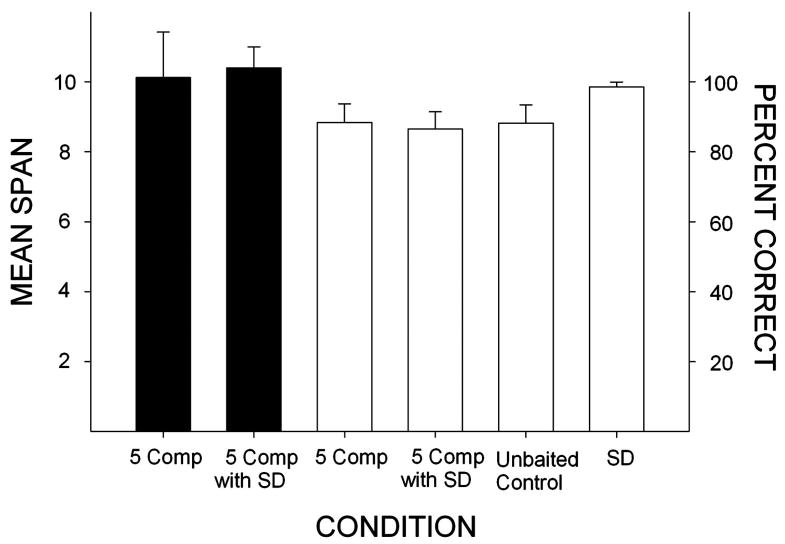
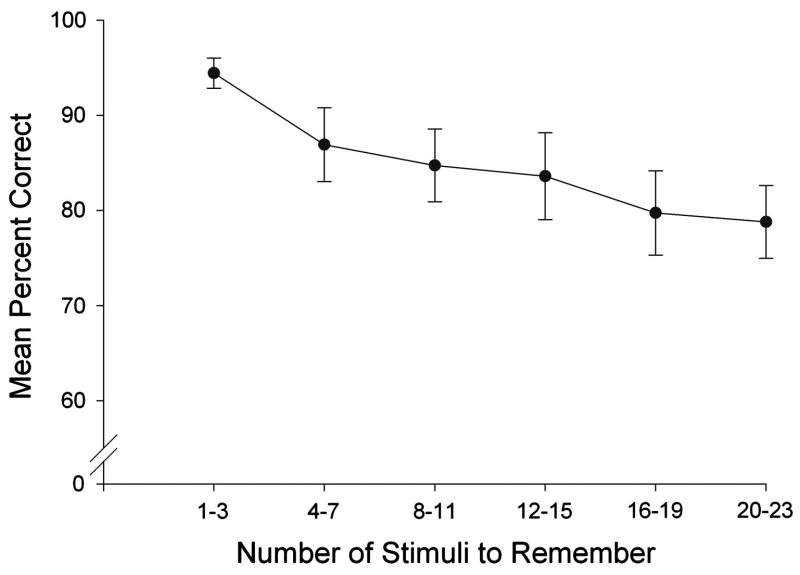
Subjects required an average of 61 sessions to meet criteria on all training phases of the experiment required prior to drug administration. Figure 2 shows mean span (black bars) and overall accuracy (white bars) obtained during the baseline training conditions. Mean spans were slightly above 10 items in the 5-Comparison Phase of the study and were unchanged when simple discrimination trials were interspersed within the session (5-Comparison with SD Phase). Accuracy on the span task was high throughout with over 80% correct in both the 5-Comparison and 5-Comparison with SD Phases of the study. Importantly, accuracy was comparable on trials on which none of the stimulus cups contained a sucrose pellet, and a statistical comparison of percent correct on unbaited control trials with regularly baited trials in the conditions shown in Figure 2 was not significant, (F2, 8 <1). Thus, accurate performances on the span task were not based on tracking the scent of the pellet in the correct stimulus cup. Finally, subjects seldom made errors on the simple discrimination task with accuracies higher than 95% correct (see Figure 2). Figure 3 shows within-session accuracy on the baseline span task. As the number of stimuli to remember increased during the session, accuracy declined from nearly 95% (with 1-3 stimuli) to just below 80% (with 16-23 stimuli). The reliability of these effects were confirmed by a one-way repeated measures ANOVA which revealed a significant effect of trial block, (F5, 20=10.04, p<.05), and post hoc tests (Tukey) showed that accuracy with only 1-3 stimuli to remember was significantly higher than all other conditions (p < .05); accuracy with 4-7 stimuli was significantly higher than the highest memory load (20-23, p < .05), but other pair-wise comparisons were non-significant.
Figure 2.
Mean span (black bars/left axis) and percent correct on the span task (white bars/right axis) obtained during the last five sessions of the 5-Comparison Phase (5 Comp) and the five sessions before drug administration began for the 5-Comparison Phase with Added Simple Discrimination (5 Comp with SD). Also shown are the percent correct on unbaited control trials and on simple discrimination trials during the 5 Comp with SD phase. Error bars represent the standard error.
Figure 3.
Mean percent correct on the span task as a function of the number of stimuli to remember for the final five baseline sessions before drug testing began. Data are presented in blocks of four consecutive trials (the first block includes only three trials (2-4) because there is nothing to remember on Trial 1). Error bars represent the standard error of the mean.
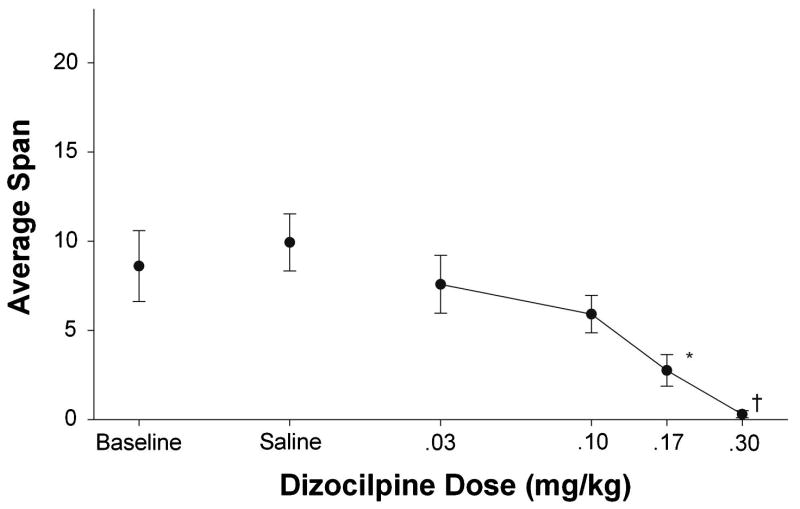
Figure 4 shows the effect of DZP on one index of working memory, olfactory span. DZP produced dose-dependent reductions in span. Spans observed at the .17 and .30 mg/kg doses were reduced relative to control sessions (baseline and saline). The .30 mg/kg dose suppressed overall responding and so this condition was omitted from the statistical analysis. The analysis of the remaining five conditions confirmed the conclusion that DZP decreased span (F4, 16=3.99, p<.05). Post hoc analyses confirmed that the .17 mg/kg dose was significantly different from saline. However, reductions in span could still have been due to global performance impairment produced by the .17 mg/kg DZP dose.
Figure 4.
Mean span as a function of DZP dose Error bars represent the standard error. Asterisks indicate values that differed significantly from saline (p<.05). † denotes doses omitted from statistical analyses.
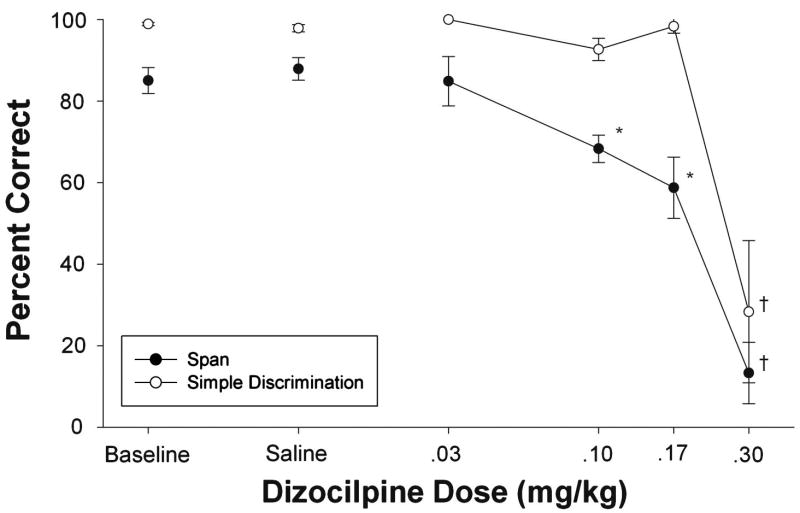
Figure 5 shows effects of DZP on another measure: percent correct on all trials of the span task (black circles) and on the simple discrimination trials (white circles). This latter measure can be viewed as an index of performance on a reference memory task requiring sensorimotor capacities and motivation comparable to those demanded by the span task. DZP caused dose-dependent impairments in percent correct on both tasks. Accuracy levels on both tasks were unaffected at the .03 mg/kg dose. However, both the .10 and .17 mg/kg doses caused decreases in accuracy on the span task without affecting performance of the simple discrimination. The .30 mg/kg dose severely disrupted responding in both tasks. Note that an error was scored when a subject did not produce a response within 2 minutes on any given trial. At the .30 mg/kg dose, many trials were ended without a response, accounting for the below chance levels of performance. For this reason, the .30 mg/kg dose was omitted from the statistical analysis of percent correct. A Two-Way DZP Dose by Task (Span/Simple Discrimination) repeated-measures ANOVA was conducted for the remaining conditions and revealed significant main effects of Dose (F4, 16=9.76, p<.05) and Task (F1, 4=32.68, p<.05) as well as a significant Dose by Task interaction (F4, 16=10.38, p<.05). Post-hoc analysis of each component revealed that significant impairments were observed only in the span task at the .10 and .17 mg/kg doses (p<.05). These doses can be described as producing selective impairments because performance on the span task was affected at doses that spared simple discrimination accuracy.
Figure 5.
Mean percent correct as a function of dizocilpine dose. Closed circles represent mean percent correct on span task trials while open circles represent percent correct performance on simple discrimination trials. Error bars represent the standard error. Asterisks indicate values that differed significantly from saline (P<.05). † denotes doses omitted from statistical analyses.
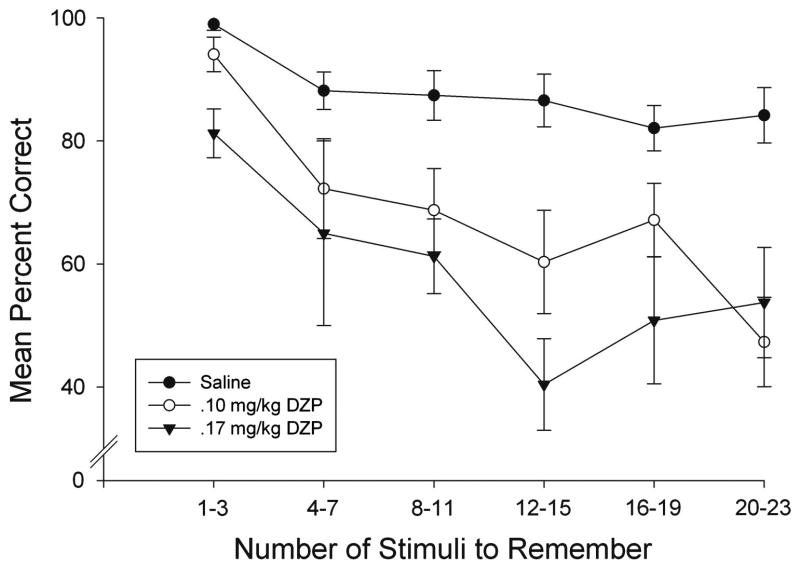
The effects of doses that selectively impaired percent correct performance on the span task (.10 and .17 mg/kg) were further analyzed within session to determine the extent to which impairments were dependent on the memory load (the number of stimuli to be remembered on a given trial). Figure 6 shows the mean percent correct performance after saline and DZP administration as a function of the number of stimuli to remember. As observed earlier in training (Figure 3), there was a shallow decline in accuracy as the number of stimuli to remember increased in baseline and after saline. The .17 mg/kg DZP dose reduced accuracy independently of memory load with equivalent impairment observed at the beginning and at the end of the session. This conclusion was supported statistically by a Dose (.17 mg/kg DZP vs. saline) X Trial Block (memory load) ANOVA which yielded significant effects of both Dose (F1, 4=31.73, p<.05) and Trial Block (F5, 20=7.43, p<.05), but no significant interaction (F5, 20=2.55, p>.05). In contrast, accuracy at the .1 mg/kg DZP dose was equivalent to saline at the outset, but declined much more sharply as the number of stimuli to remember increased. There was a main effect for Dose (F1, 4=31.74, p<.05), Trial Block (F5, 20=6.58, p<.05), and a significant interaction (F5, 20=3.62, p<.05). Simple Main Effects tests revealed significant decreases in accuracy as a function of the number of stimuli to remember under both saline and .1 DZP conditions (p < .05). Under saline conditions, the function was quite shallow and the condition with 1-3 stimuli to remember differed only from the conditions with 16-19 and 20-23 stimuli (p < .05). Other pair-wise comparisons under saline were non-significant. However, at the .1 dose of DZP subjects performed better at the lowest memory load condition (1-3 stimuli to remember) than all other memory load conditions. In addition, subjects performed significantly worse on the highest memory load condition (20-23 stimuli to remember) when compared with all other load conditions Importantly, there was no significant difference between saline and .1 DZP with 1-3 stimuli to remember (p > .05), but significant differences were obtained at each of the higher memory loads (p < .05). Thus, the effects of .1 DZP appeared to depend on the increased number of stimuli to remember as the session continued, that is, on the memory load.
Figure 6.
Mean percent correct on the span task as a function of the number of stimuli to remember after saline (closed circles), .10 mg/kg DZP (open circles) and .17 DZP (triangles). Data are presented in blocks of four consecutive trials (the first block includes only trials 2-4 because there is nothing to remember on Trial 1). Error bars represent the standard error of the mean.
4.0 Discussion
The present study replicates and extends the findings of Dudchenko et al. (2000) and others (Rushforth et al., 2010; Young et al., 2009) that the OST can provide a sensitive, within-session measure of the effects of increasing the number of stimuli to remember on delayed-matching to sample performance. Average olfactory spans and within-session declines in accuracy as the memory load increased in the present study were comparable to those observed in previous studies with this procedure. Importantly, these effects were demonstrated under control conditions not always present in the previous OST research. For example, the use of scented lids that were displaced by rats enhanced response definition (relative to sand digging measures) and led to virtually perfect inter-rater reliability. To ensure that responding was not under the control of scent markings by subjects, the scented lids used in the task were replaced with fresh lids (presenting the same odor as the lid they replaced) in between each trial. In addition, non-baited control sessions were implemented to verify that responding was not influenced by the presence of the sucrose pellet reinforcer in the S+ stimulus cup. No decreases in accuracy were observed under these non-baited conditions. The use of the scent marking control procedure and the results of non-baited probe sessions provide convincing evidence that behavior was indeed under the control of the olfactory cues presented by the experimenter.
In previous studies the number of comparison stimuli incremented along with the number of stimuli to remember creating a confound between the number of comparison choices in the array and memory load. In our adaptation of the OST, five comparison stimuli were presented on each trial (beyond the 4th trial) and thus, effects of distraction by an increasing number of comparison stimuli were separated from the number of stimuli that the rat needed to remember for accurate performance. Performance was most accurate when there were only 1-3 stimuli to remember (Figures 3 and 6), however, there were fewer comparison stimuli during these trials. There were continued decreases in accuracy as the memory load increased during baseline (Figure 3). Although these decreases occurred at a shallower slope the further declines cannot be attributed to the number of comparison choices. Interestingly, the function obtained under saline conditions during the drug phase was even flatter, and rats showed less decline in accuracy as the number of stimuli to remember increased, perhaps due to increased experience with the task.
Several other controls implemented in this version OST procedure strengthen the inferences that can be drawn from performance of the task. The addition of a simple discrimination task interspersed with the OST trials provided a further within-session control for the effects of drugs on aspects of performance required for olfactory discrimination, but not specific to within-session memory processes (e.g., sensory-motor effects, motivation, etc.). Although many sessions of training were required to develop stable performances on this complex task, this drawback is offset by the result that the use of within-subject controls permitted detection of treatment effects with relatively few subjects. Indeed, the finding of stable baseline performances with these controls appears to make the OST well suited for the within-subject analysis of drug effects, and the analysis of DZP effects confirmed the value of the technique.
The NMDA receptor antagonist DZP caused dose-dependent reductions in overall accuracy on the span and simple discrimination tasks. Rats showed marked impairment on all measures, including the simple discrimination, at the .30 mg/kg dose, but because overall responding was suppressed, all outcomes at this dose must be regarded as reflecting a general behavioral impairment. However, at the .10 and .17 mg/kg doses, DZP produced impairments in accuracy on the OST, but not on simple discrimination performance. As with the OST, accurate performance of the simple discrimination component required that subjects navigate the arena, discriminate olfactory cues and produce the lid displacement response. As such, it can be concluded that the selective impairments seen at the .10 and .17 mg/kg doses of DZP were not indicative of sensorimotor or motivational disturbances. Within-session analysis permitted further elaboration of the nature of DZP effects. The .17 mg/kg dose of DZP reduced accuracy throughout the session, i.e., the impairment was independent of memory load. Thus, although the .17 mg/kg dose of DZP reduced overall session accuracy on the span task (but not simple discrimination accuracy), the fact that effects were present at even the smallest memory loads does not fully support an interpretation in terms of memory capacity. However, at the .1 mg/kg dose, accuracy was not affected at the outset, but declined much more sharply than in control conditions as the number of stimuli to remember increased. Accuracy on the simple discrimination was maintained at a high level throughout the session, so this decline did not appear to be due to overall within-session performance decrements. Thus, the effects of .1 mg/kg DZP appeared to specifically depend on processes related to the number of stimuli to remember, that is, on the memory load.
These findings may provide some insight to the controversy regarding NMDA antagonist effects on learning and memory. The present study provides an example of specific impairment by an NMDA antagonist on performance in a non-spatial learning/memory task. Although there other such examples (e.g. Baron and Moerschbaecher, 1996; Pitts, Buda, Keith, Cerutti, and Galizio, 2006; Schmitt et al., 2005), the point remains important as it has frequently been argued that NMDA antagonist-induced impairments are specific to spatial processes (Caramanos and Shapiro, 1994; Uekita and Okaichi, 2005). The present findings may also help clarify the conditions that are critical to evidence impairments induced by NMDA antagonists. For example, NMDA antagonists generally do not impair spatial learning in animals with spatial pretraining when appropriate performance controls are provided except at high doses that impair general performance measures (Keith and Galizio, 1997; Saucier and Cain, 1995). An exception is when a long delay between trials places a more considerable memory demand on the animal (Steele and Morris, 1999). The present findings that DZP specifically impaired performance on the OST with its incrementing memory demands would appear to be consistent with the Steele and Morris findings and with the theoretical synthesis of Bannerman et al. (2006), that NMDA antagonist impairments are expected in situations that place demands on working memory in cases when trial specific information is necessary to choose from response options (a “one-trial what/where, what/when memory mechanism”). In general, the present results can be viewed as supporting the Bannerman et al. hypothesis; however, there is an important inconsistency. The processes proposed by Bannerman et al. are postulated to be mediated by hippocampal LTP, but Dudchenko et al. (2000) found that olfactory span performance was not hippocampally-dependent (in contrast to performance on a spatial span task). It would appear that NMDA-gated activity in other brain regions may be necessary to explain the present findings.
Theoretical integration must remain tentative at present given our limited understanding of variables affecting performance on the OST. For example, it seems important to assess the effects of additional drugs and perhaps other neurobiological manipulations to determine the specificity of the NMDA antagonist effect observed here and to provide further characterization of the processes assessed by the procedure. Although it may be tempting to view the OST as a direct measure of working memory capacity (c.f., Rushforth et al., 2010; Young et al., 2007b; Young et al., 2009), there are features of the present study that suggest such an interpretation is premature. Consider that the average span obtained under baseline conditions in the present study was about 10 items. In the typical working memory capacity task (e.g., digit span), the span provides the operational definition of memory capacity. In the present study, this was clearly not the case. Inspection of Figures 3 and 6 revealed that accuracy remained well above chance levels throughout the session with rats averaging 80% correct or better even at the end of the session with 20 or more stimuli to remember. Performances did decline somewhat as the memory load increased (at least under baseline conditions), showing that rats were sensitive to the memory load, but it would appear that the span measure did not identify the capacity limit, nor was that capacity reached within the 23-item memory load of the present study. Clearly more research with procedures such as the OST is needed to better characterize the pharmacological and neurobiological variables that determine performance on tasks with varying memory loads.
The olfactory span task was validated as a measure of working memory in rodents.
Accuracy in the olfactory task decreased as the number of stimuli to remember increased
NMDA antagonist, dizocilpine (MK801) decreased accuracy on the span task at doses that did not interfere with other aspects of performance (.1 and .17 mg/kg).
Dizocilpine effects interacted with the number of stimuli to remember in the span showing the its disruptive effects were more pronounced as the memory load increased.
Acknowledgments
The research was supported in part by DA029252 to Mark Galizio. This study formed part of a thesis submitted by Dave A MacQueen, who is now a doctoral student at the University of South Florida, to fulfill requirements for the Masters degree at the University of North Carolina Wilmington. The authors thank Chris Greenwell, Thea Guze, and L. Brooke Poerstal for assistance in the development of the procedures and data collection and Patrick McKinney and Nicholas Hahn, who designed and built the apparatus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baddeley A. Working memory: looking back and looking forward. Nat Rev Neurosci. 2003;4:829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Good MA, Butcher SP, Ramsay M, Morris RG. Distinct components of spatial learning revealed by prior training and NMDA receptor blockade. Nature. 1995;378:182–186. doi: 10.1038/378182a0. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JN, Good MA. The drugs don’t work-or do they? Pharmacological and transgenic studies of the contribution of NMDA and GluR-A-containing AMPA receptors to hippocampal-dependent memory. Psychopharmacology (Berl) 2006;188:552–566. doi: 10.1007/s00213-006-0403-6. [DOI] [PubMed] [Google Scholar]
- Baron SP, Moerschbaecher JM. Disruption of learning by excitatory amino acid receptor antagonists. Behav Pharmacol. 1996;7:573–584. [PubMed] [Google Scholar]
- Cain DP, Saucier D, Hall J, Hargreaves EL, Boon F. Detailed behavioral analysis of water maze acquisition under APV or CNQX: contribution of sensorimotor disturbances to drug-induced acquisition deficits. Behav Neurosci. 1996;110:86–102. doi: 10.1037//0735-7044.110.1.86. [DOI] [PubMed] [Google Scholar]
- Caramanos Z, Shapiro ML. Spatial memory and N-methyl-D-aspartate receptor antagonists APV and MK-801: memory impairments depend on familiarity with the environment, drug dose, and training duration. Behav Neurosci. 1994;108:30–43. doi: 10.1037//0735-7044.108.1.30. [DOI] [PubMed] [Google Scholar]
- Cowan N. The magical number 4 in short-term memory: a reconsideration of mental storage capacity. Behav Brain Sci. 2001;24:87–114. doi: 10.1017/s0140525x01003922. discussion 114-185. [DOI] [PubMed] [Google Scholar]
- Dudchenko PA. An overview of the tasks used to test working memory in rodents. Neurosci Biobehav Rev. 2004;28:699–709. doi: 10.1016/j.neubiorev.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Dudchenko PA, Wood ER, Eichenbaum H. Neurotoxic hippocampal lesions have no effect on odor span and little effect on odor recognition memory but produce significant impairments on spatial span, recognition, and alternation. J Neurosci. 2000;20:2964–2977. doi: 10.1523/JNEUROSCI.20-08-02964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galizio M, Keith JR, Mansfield WJ, Pitts RC. Repeated spatial acquisition: effects of NMDA antagonists and morphine. Exp Clin Psychopharmacol. 2003;11:79–90. doi: 10.1037//1064-1297.11.1.79. [DOI] [PubMed] [Google Scholar]
- Keith JR, Galizio M. Acquisition in the Morris swim task is impaired by a benzodiazepine but not an NMDA antagonist: A new procedure for distinguishing acquisition and performance effects. Psychobiology. 1997;25:217–228. [Google Scholar]
- Keith JR, Rudy JW. Why NMDA-receptor-dependent long-term potentiation may not be a mechanism of learning and memory: Reappraisal of the NMDA-receptor blockade strategy. Psychobiology. 1990;18:251–257. [Google Scholar]
- Miller GA. The magical number seven plus or minus two: some limits on our capacity for processing information. Psychol Rev. 1956;63:81–97. [PubMed] [Google Scholar]
- Morris RG. Synaptic plasticity and learning: selective impairment of learning rats and blockade of long-term potentiation in vivo by the N-methyl-D-aspartate receptor antagonist AP5. J Neurosci. 1989;9:3040–3057. doi: 10.1523/JNEUROSCI.09-09-03040.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Olton DS, Becker JT, Handelmann E. Hippocampus, space and memory. Behav Brain Sci. 1979;2:313–366. [Google Scholar]
- Perone M. Experimental design in the analysis of free-operant behavior. In: Iverson IH, Lattal KA, editors. Experimental Analysis of Behavior: Part 1. Amsterdam: Elsevier Science; 1991. pp. 135–172. [Google Scholar]
- Pitts RC, Buda DR, Keith JR, Cerutti DT, Galizio M. Chlordiazepoxide and dizocilpine, but not morphine, selectively impair acquisition under a novel repeated-acquisition and performance task in rats. Psychopharmacology (Berl) 2006;189:135–143. doi: 10.1007/s00213-006-0538-5. [DOI] [PubMed] [Google Scholar]
- Rushforth SL, Allison C, Wonnacott S, Shoaib M. Subtype-selective nicotinic agonists enhance olfactory working memory in normal rats: a novel use of the odour span task. Neurosci Lett. 2010;471:114–118. doi: 10.1016/j.neulet.2010.01.022. [DOI] [PubMed] [Google Scholar]
- Saucier D, Cain DP. Spatial learning without NMDA receptor-dependent long-term potentiation. Nature. 1995;378:186–189. doi: 10.1038/378186a0. [DOI] [PubMed] [Google Scholar]
- Saults JS, Cowan N. A central capacity limit to the simultaneous storage of visual and auditory arrays in working memory. J Exp Psychol Gen. 2007;136:663–684. doi: 10.1037/0096-3445.136.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt WB, Sprengel R, Mack V, Draft RW, Seeburg PH, Deacon RM, Rawlins JN, Bannerman DM. Restoration of spatial working memory by genetic rescue of GluR-A-deficient mice. Nat Neurosci. 2005;8:270–272. doi: 10.1038/nn1412. [DOI] [PubMed] [Google Scholar]
- Steele RJ, Morris RG. Delay-dependent impairment of a matching-to-place task with chronic and intrahippocampal infusion of the NMDA-antagonist D-AP5. Hippocampus. 1999;9:118–136. doi: 10.1002/(SICI)1098-1063(1999)9:2<118::AID-HIPO4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Tonkiss J, Rawlins JN. The competitive NMDA antagonist AP5, but not the non-competitive antagonist MK801, induces a delay-related impairment in spatial working memory in rats. Exp Brain Res. 1991;85:349–358. doi: 10.1007/BF00229412. [DOI] [PubMed] [Google Scholar]
- Turchi J, Sarter M. Cortical cholinergic inputs mediate processing capacity: effects of 192 IgG-saporin-induced lesions on olfactory span performance. Eur J Neurosci. 2000;12:4505–4514. [PubMed] [Google Scholar]
- Uekita T, Okaichi H. NMDA antagonist MK-801 does not interfere with the use of spatial representation in a familiar environment. Behav Neurosci. 2005;119:548–556. doi: 10.1037/0735-7044.119.2.548. [DOI] [PubMed] [Google Scholar]
- Young JW, Crawford N, Kelly JS, Kerr LE, Marston HM, Spratt C, Finlayson K, Sharkey J. Impaired attention is central to the cognitive deficits observed in alpha 7 deficient mice. Eur Neuropsychopharmacol. 2007a;17:145–155. doi: 10.1016/j.euroneuro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Young JW, Kerr LE, Kelly JS, Marston HM, Spratt C, Finlayson K, Sharkey J. The odour span task: a novel paradigm for assessing working memory in mice. Neuropharmacology. 2007b;52:634–645. doi: 10.1016/j.neuropharm.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Young JW, Sharkey J, Finlayson K. Progressive impairment in olfactory working memory in a mouse model of Mild Cognitive Impairment. Neurobiol Aging. 2009;30:1430–1443. doi: 10.1016/j.neurobiolaging.2007.11.018. [DOI] [PubMed] [Google Scholar]