Abstract
Isoflurane is known to increase β-amyloid aggregation and neuronal damage. We hypothesized that isoflurane will have similar effects on the polyglutamine huntingtin protein, and will cause alterations in intracellular calcium homeostasis. We tested this hypothesis in striatal cells from the expanded glutamine huntingtin knock-in mouse (STHdhQ111/Q111) and wild type (STHdhQ7/Q7) striatal neurons. The primary cultured neurons were exposed for 24h to equipotent concentrations of isoflurane, sevoflurane and desflurane in the presence or absence of extracellular calcium and with or without xestospongin C, a potent endoplasmic reticulum inositol 1,4,5-trisphosphate (InsP3) receptor antagonist. Aggregation of huntingtin protein, cell viability, and calcium concentrations were measured. Isoflurane, sevoflurane and desflurane all increased the aggregation of huntingtin in STHdhQ111/Q111 cells, with isoflurane having the largest effect. Isoflurane induced greater calcium release from the ER and relatively more cell damage in the STHdhQ111/Q111 Huntington cells than in the wild type STHdhQ7/Q7 striatal cells. However, sevoflurane and desflurane caused less calcium release from the ER and less cell damage. Xestospongin C inhibited the isoflurane-induced calcium release from the ER, aggregation of huntingtin, and cell damage in the STHdhQ111/Q111 cells. In summary, the Q111 form of huntingtin increases the vulnerability of striatal neurons to isoflurane neurotoxicity through combined actions on the ER IP3 receptors. Calcium release from the ER contributes to the anesthetic induced huntingtin aggregation in STHdhQ111/Q111 striatal cells.
Keywords: Anesthesia, Huntington’s disease, Neuronal Death, Calcium, InsP3 receptor, Polyglutamine Aggregation
Introduction
Increasing evidence suggests that isoflurane, a commonly used inhaled anesthetic, induces cell damage by apoptosis in different types of cells, including neurons (Eckenhoff et al., 2004;Liang et al., 2008;Liang et al., 2010;Wei et al., 2005;Wei et al., 2008;Wise-Faberowski et al., 2005;Xie et al., 2006;Zhao et al., 2010). In addition, isoflurane, at clinically relevant concentrations, causes widespread neuronal apoptosis in the developing rat brain with subsequent learning deficits (Jevtovic-Todorovic et al., 2003;Zhao et al., 2010). The mechanism of isoflurane cytotoxicity is still not clear, but it may be associated with an increase of pathologic proteins and/or their aggregation (Bianchi et al., 2008;Eckenhoff et al., 2004;Zhao et al., 2010), disruption of intracellular calcium homeostasis (Liang et al., 2008;Wei et al., 2005;Wei et al., 2008;Zhao et al., 2010), or excitotoxicity (Ikonomidou et al., 2001). Because both the aggregation of pathological proteins and the disruption of intracellular calcium homeostasis may play important roles in neuronal cell death in neurodegenerative diseases, such as Alzheimer’s or Huntington’s disease (Arrasate et al., 2004;Bates, 2003;Lindholm et al., 2006;Orrenius et al., 2003), it is possible that vulnerable neurons are unusually susceptible to isoflurane cytotoxicity. The relationship between anesthesia and the onset and progression of neurodegenerative disorders is of current interest (Baranov et al., 2009). Although there has been no direct evidence that inhalational anesthetics worsen Huntington’s disease in humans, a previous history of general anesthesia has been associated with an early onset of Alzheimer’s disease (Bohnen et al., 1994;Lee et al., 2005a) and with unmasking the symptoms of Parkinson’s disease (Kuehn, 2007;Lee et al., 2005b;Muravchick and Smith, 1995), two other common neurodegenerative diseases with pathological protein aggregation.
Huntington’s disease is an autosomal dominant disorder that is characterized by motor dysfunction, cognitive decline and psychiatric disturbance. It is caused by an expansion in the number of CAG repeats in a gene called huntingtin (Hickey and Chesselet, 2003). CAG codes for the amino acid glutamine, and these polyglutamine regions increase the oligomerization of the huntingtin protein into presumably toxic aggregates (Hickey and Chesselet, 2003). In addition, disruption of calcium homeostasis, especially abnormal calcium release from the endoplasmic reticulum (ER) via inositol 1,4,5-trisphosphate receptors (InsP3R), is thought to play an important role in the neurodegeneration found in Huntington’s disease (Tang et al., 2003;Tang et al., 2004;Tang et al., 2005). Mitochondrial calcium defects also occur early in the pathogenesis of Huntington’s disease (Panov et al., 2002). It is still controversial whether or not huntingtin protein aggregation leads to neuronal degeneration (Ding et al., 2002;Hackam et al., 1998;Shastry, 2003;Wellington et al., 2000). In addition, a relationship between huntingtin aggregation and calcium dysregulation has not been shown.
Because isoflurane increases β-amyloid aggregation (Bianchi et al., 2008;Eckenhoff et al., 2004), disrupts intracellular calcium homeostasis (Liang et al., 2008;Wei et al., 2005;Wei et al., 2008;Zhao et al., 2010), and is more neurotoxic than either sevoflurane or desflurane (Liang et al., 2008;Liang et al., 2010;Yang et al., 2008), we hypothesize that neurons from huntingtin Q111 knock-in mice will be more vulnerable to isoflurane cytotoxicity.
Materials and Methods
Cell Culture
Immortalized knock-in mouse striatal cells carrying the polyglutamine enriched huntingtin (STHdhQ111/Q111) (HD cells) and their wild type control cells (STHdhQ7/Q7) (WT cells) were a generous gift from Dr. Marcy MacDonald, Harvard University Medical School, and were generated and cultured as previously described (Trettel et al., 2000). Briefly, conditionally immortalized WT STHdhQ7/Q7 striatal neuronal progenitor cells expressing endogenous normal huntingtin and homozygous mutant STHdhQ111/Q111 striatal neuronal progenitor cell lines expressing endogenous mutant huntingtin with 111-glutamines were generated from STHdh7/Q7 and STHdhQ111/Q111 littermate embryos. The striatal cell lines were grown in DMEM medium supplemented with 10% fetal calf serum, 400 μg/ml G418 and antibiotics. Monolayer cultures at a density of 0.3×105 cells/cm2 were incubated in plastic flasks in a 95% air, 5% CO2 humidified atmosphere at 33°C. The culture medium was changed every 48 hr. Striatal cells were grown on 25 mm glass coverslips or 24-well plates at a cell density of 0.8–1×105/cm2.
Anesthetic exposures
In this study, unless stated, cells are exposed to three volatile anesthetics in equipotent concentrations. The concentration of isoflurane used is 0.8 mM, which is equal to 2.4% or 2 MAC (minimum alveolar concentration). For sevoflurane, this concentration is 0.92mM, which is equal to 4% or 2 MAC. The concentration of desflurane is 1.32 mM, which is equal to 12.3% or 2 MAC. Hereafter, unless stated, the anesthetic concentrations will be referred to as 2 MAC. There is one instance, where 1 MAC isoflurane is also used, which is equal to 1.2% or 0.4 mM.
Cytosolic calcium measurements
HD and WT striatal cells grown on coverslips were exposed to the three volatile anesthetics in a sealed gas tight chamber (Warner Instrument Inc., Hamden, CT, USA) connected with multiple inflow infusion tubes and one outflow tube, which provided a constant flow of buffer to the chamber, inside the culture incubator (Bellco Glass, Inc., Vineland, NJ, USA). The cells were first washed with Krebs-Ringer buffer through one inflow tube for the baseline [Ca2+]c measurements, and then were exposed to approximately 2 MAC isoflurane, sevoflurane or desflurane in buffer at room temperature via separate inflow infusion tubes driven by a syringe pump (Braintree Scientific Inc., Braintree, MA). In a pilot study, samples of the anesthetics in both the inflow and outflow tubes were also collected and their concentrations measured by high performance liquid chromatography (System Gold, Beckmam Coulter, Fullerton, CA, USA) to confirm constant anesthetic concentrations in the buffered solutions.
Anesthetic cytotoxicity measurements
HD and WT striatal cells grown on 24-well plates were exposed to isoflurane at 1 or 2 MAC, or to sevoflurane or desflurane at 2 MAC, for 24 h in a gas tight chamber inside the culture incubator (Bellco Glass, Inc., Vineland, NJ, USA), with a carrying gas (5%CO2/21%O2/balanced N2, AirGas East, Bellmawr, NJ, USA) going through a calibrated agent-specific vaporizer as we described previously (Wei et al., 2005;Wei et al., 2008). The cells in the control group were only exposed to the carrying gas (5%CO2/21%O2/balanced N2) outside the exposure chamber for 24 h. Gas phase concentrations in the gas chamber were checked with infrared absorbance of the effluent gas, and constantly monitored and maintained at the designed concentration throughout experiments, using an infrared Ohmeda 5330 agent monitor (Coast to Coast Medical, Fall River, MA, USA). Since the cell culture plates were inside the sealed anesthetic exposure chamber, which was continuously perfused with a constant concentration of anesthetic, the anesthetic concentration in the cell culture medium remained stable as previously reported (Wei et al., 2005) and there is no evidence of degradation of the anesthetics in cell culture over a 24 h period.
Immunocytochemistry of Huntingtin aggregation
We determined huntingtin protein aggregation in cells by methods described previously with some modifications (Waelter et al., 2001). HD and WT striatal cells grown on glass coverslips were exposed to three inhaled anesthetics at 2 MAC for 24 h, with or without pretreatment with xestospongin C, in the sealed anesthetic chamber within an incubator, and then were placed in a regular incubator for a 24 h recovery period., The striatal cells were washed 3× with PBS, fixed in 4% paraformaldehyde for 15 min at room temperature, and permeabilized with 0.1%Triton X-100 for 5 min. Cells were incubated in 5% normal donkey serum for 1 h to block nonspecific binding, and then incubated in 5% BSA/PBS containing anti-huntingtin antibody which is an affinity purified goat polyclonal antibody raised against a peptide mapping near the C-terminus of huntingtin of human origin (Santa Cruz Biotechnology, Inc, sc-8768, 1:50 dilution) at 4°C overnight. Cells were washed 3× with PBS and incubated at room temperature with Alexa Fluor 594 Donkey anti-goat antibody (Molecular Probes, 1:1000 dilution) for 1 h, and then incubated with Hoechst 33342 (1:1000) in PBS for 2 min. For the positive controls of huntingtin aggregates, we treated both types of cells with a protease inhibitor, ALLN (N-acetyl-Leu-Leu-norleucinal), as previously described (Rajan et al., 2001). Huntingtin aggregates were viewed with an Olympus IX70 fluorescent microscope (excitation =594, emission=617). The percentage of cells that contained aggregates within each group was determined by eye by two persons blinded to the treatment. For each experimental condition, the percentage of cells with aggregates on each coverslip were observed and averaged from 10 fields using a 40× magnification lens. The number of coverslips examined represented the n for each experimental condition. A minimum of 817 cells from at least three separate experiments were used to determine the average percentage of aggregates for each group.
Measurements of cytosolic calcium concentration ([Ca2+]c)
[Ca2+]c were made using fura-2 fluorescence (Molecular probe, Eugene, OR) with a photometer coupled to an Olympus IX70 inverted microscope and IPLab v3.7 imaging processing and analysis software (Biovision Technologies, Exton, PA). The protocol to determine [Ca2+]c was similar to that which we previously described with some modifications (Hiroi et al., 2005;Liang et al., 2008). Briefly, HD and WT striatal cells were grown on 25 mm round glass cover slips in DMEM medium for four days. On day 5, the cells were washed 3× with Krebs-Ringer buffer (HEPES 10 mM, NaCl 145 mM, KCl 5 mM, NaHPO4 0.5 mM, Glucose 10 mM, MgSO4 1 mM, CaCl2 1 mM, pH 7.4), loaded with 2.5 μM fura-2/AM (Molecular Probes, Eugene, OR, USA) in Krebs-Ringer buffer for 30 min at room temperature, and washed 3× with Krebs-Ringer buffer. For experiments without extracellular calcium, calcium was not added to the regular Krebs-Ringer buffer and 0.2 mM EGTA was added to the buffer. The cells were then placed in a sealed chamber (Warner Instrument Inc., Hamden, CT, USA) connected with multiple inflow infusion tubes and one outflow tube, which provided constant flow to the chamber. The cells were washed with Krebs-Ringer buffer through one inflow tube for the baseline measurements of [Ca2+]c, and then were exposed to the three different volatile anesthetics in buffer at 2 MAC via separate inflow infusion tubes, for 18 min. The fluorescence signals were measured with excitation at 340 and 380 alternatively and emission at 510 nM. The F340/F380 ratios, which correlate with the cytosolic calcium concentrations, were constantly determined during the 18 min anesthetic exposure. The fluorescence measurements were calibrated by bathing cells in the buffer (HEPES 10 mM, KCl 130 mM, NaCl 17.5 mM, Glucose 1 mM) containing ionomycin 20 mM for maximum or 20 mM EGTA for minimum calcium. The maximum and minimum calcium measurements were corrected for auto fluorescence (determined before the cells were loaded with fura-2/AM). The [Ca2+]I was calculated by the ratio method described previously (Grynkiewicz et al., 1985), using 224 nM as the Kd of fura-2. The final results for the [Ca2+]c were averaged from a minimum of 30 cells from at least three separate experiments for each anesthetic.
Cytotoxicity assays
HD and WT striatal cells grown on 24-well plates were exposed to isoflurane, sevoflurane and desflurane at 2 MAC, for 24 h in the incubation chamber as we described previously (Yang et al., 2008), and then relocated to the cell culture incubator for another 24 h before measuring MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt; MTS] reduction with a protocol similar to our previous cytotoxicity assay using MTT (3-(4,5-dimethyithiazol-2-yl)-2,5-diphenyl-tetrazolium bromide) reduction (Wei et al., 2005). The MTS assay (Promega Corporation, Madison, WI) is a colorimetric method for determining the number of viable cells. Twenty μl of MTS reagent was added to each well of the 24-well assay plate containing the samples in 500 μl culture medium and the plate was then incubated for 2 h at 33°C in a humidified, 5% CO2 atmosphere. The absorbance at 490 nm was read using a plate reader (OPSYS MR™ Absorbance Reader, Dynex Technologies, Chantilly, VA). The results of the MTS reduction assays were expressed as the percentage of control without anesthetic treatment.
Statistical Analysis
Results were expressed as mean ± SEM. Results were analyzed by unpaired t-tests for comparison of the isoflurane-induced peak elevation of [Ca2+]c with or without pretreatment with xestospongin C in HD striatal cells. All other results were analyzed by either one-way ANOVA followed by Newman-Keuls multiple comparison tests or two-way ANOVA followed by Bonferroni post-tests. P<0.05 was considered as statistical significance.
Results
Inhaled anesthetics increased aggregation of Q111 Huntingtin protein
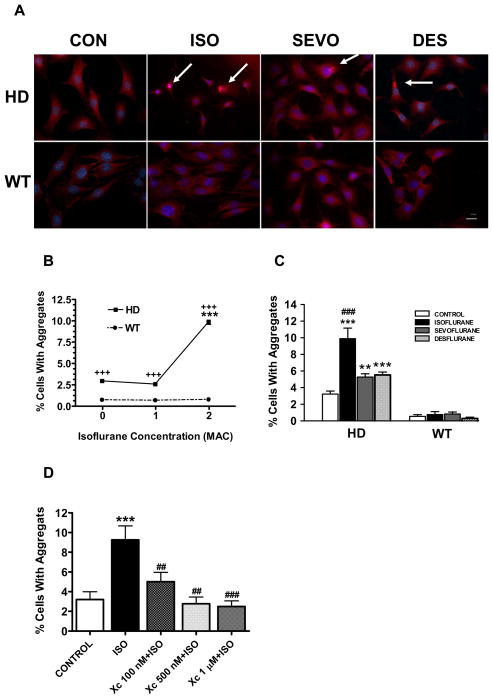
Huntingtin protein aggregation was rarely seen in wild type cells containing a normal length polyglutamine segment (Q7) whether or not they were exposed to isoflurane (Figure 1A). Huntingtin aggregation was observed primarily in the cytosol of the HD cells and rarely in the nuclear space. The aggregate appearance was similar to those produced by the over expression of polyglutamine proteins (56- and 80- glutamine) (Ding et al., 2002) and to those produced by the protease inhibitor ALLN (N-acetyl-Leu-Leu-norleucinal) (Rajan et al., 2001), which we used here as a positive control. Isoflurane increased aggregation of huntingtin only in HD striatal cells (Figure 1B). The three commonly used inhalational anesthetics (isoflurane, sevoflurane and desflurane), at clinically relevant concentrations, all significantly increased the aggregation of huntingtin protein in HD but not in WT striatal cells, with isoflurane having greater potency for this effect than sevoflurane or desflurane (Figure 1C). Xestospongin C, a potent IP3 receptor antagonist (Gafni et al., 1997;Oka et al., 2002), significantly inhibited isoflurane mediated Huntingtin aggregation (Figure 1D).
Figure 1. Isoflurane, sevoflurane and desflurane increased huntingtin protein aggregation in STHdhQ111/Q111 (HD) but not STHdhQ7/Q7 (WT) striatal cells.
A. Huntingtin aggregates (arrows) determined with immunohistochemistry using anti-huntingtin antibody 24 h after the completion of the anesthetic treatment were found primarily in the cytosol of HD but rarely in the WT striatal cells treated with 2 MAC (minimum alveolar concentration) isoflurane (ISO), sevoflurane (SEVO) and desflurane (DES) for 24 h. The nuclei were stained with Hoecsht 33342 (blue). Scale bar = 20 μm. B. Isoflurane increased aggregation of mutated huntingtin protein in HD striatal cells in a dose-dependent manner. Data represents mean±SEM of percentage of cells with aggregates from at least three separate experiments (Total number of cells counted: Control, 1025; 1 MAC isoflurane, 1064; 2 MAC isoflurane, 817). For each experimental condition, N≥9. ***P<0.001 compared to 0 and 1 MAC isoflurane in HD cells, +++ P<0.001 compared to isoflurane treatment in WT cells. C. Isoflurane, sevoflurane and desflurane all significantly increased the percentage of cells with huntingtin aggregates in HD striatal cells. Data represents mean±SEM of percentage of cells with aggregates from at least three separate experiments in controls or after 24 h treatment of 2 MAC isoflurane, sevoflurane or desflurane (Total cells counted: Control, 1500; Isoflurane, 872; Sevoflurane, 817; Desflurane, 892). N≥10 for each experimental condition. ** P<0.01, *** P<0.001 compared to control. ###P<0.001 compared to either sevoflurane or desflurane treatment. D. IP3 receptor antagonist, xestospongin C (Xc), dose-dependently inhibited isoflurane mediated huntingtin aggregation. Data represents mean±SEM of percentage of cells with aggregates from at least three separate experiments in controls or after 24 h treatment of 2 MAC isoflurane from at least three separate experiments (Total cells counted, 781). N≥8 for each experimental condition. ***P<0.001 compared to control; ##P<0.01, ###P<0.001 compared to ISO treatment alone. All data were analyzed by either one-way ANOVA followed by Newman-Keuls multiple comparison tests (D) or two-way ANOVA followed by Bonferroni post-tests (B, C).
Isoflurane induced greater calcium release from the ER in Huntington’s disease knock-in striatal cells than in wild type control
Isoflurane at 0.8 mM induced a significantly greater and faster elevation of the [Ca2+]c in HD as compared to WT cells, which occurred in both the presence and absence of extracellular calcium (Figure 2A and B). Our pilot study confirmed that the [Ca2+]c baseline did not change significantly even after continuously exposing these cells for 20 min to the excitation UV light, nor did the cell viability change significantly, as determined by a trypan blue assay (data not shown).
Figure 2. Isoflurane induced higher cytosolic calcium levels in STHdhQ111/Q111 (HD) than in STHdhQ7/Q7 (WT) striatal cells.
A. Averaged typical response of the cytosolic calcium concentration ([Ca2+]c) induced by isoflurane at a clinically relevant concentration (0.8 mM) in WT and HD cells in the presence or absence of extracellular calcium. B. Comparison of the peak elevation of [Ca2+]c induced by 0.8 mM isoflurane between WT and HD striatal cells. For each experimental condition, data represents mean+SEM from a minimum of 30 cells (N≥30) from at least three separate experiments. *** P<0.01 compared to those in the presence of 1 mM extracellular calcium. ###P<0.001 compared to their corresponding response in WT striatal cells. All data were analyzed by one-way ANOVA followed by Newman-Keuls multiple comparison tests.
Xestospongin C inhibited isoflurane-mediated calcium release from the ER
We further tested whether or not calcium release from the ER via InsP3R contributed to the isoflurane-induced elevation in the [Ca2+]c in HD striatal cells. Pretreatment with the potent InsP3R antagonist, xestospongin C, for 30 min nearly abolished the isoflurane-induced calcium release from the ER, even in the absence of extracellular calcium (Figure 3A and B).
Figure 3. IP3 receptor antagonist xestospongin C significantly inhibited isoflurane mediated calcium release from the ER.
A. Averaged typical response of the [Ca2+]c induced by 0.8 mM isoflurane (ISO) in the HD STHdhQ111/Q111 striatal cells in the absence of extracellular calcium (representing calcium release from the ER) with or without co-treatment of Xestospongin C (Xc), a potent IP3 receptor antagonist. B. Xc at 1 μM nearly abolished isoflurane-induced elevation of the [Ca2+]c in HD cells in absence of extracellular calcium. For each experimental condition, data represents mean+SEM from a minimum of 25 neurons (N≥25 for all experimental groups) from at least three separate experiments. For each experimental condition, data represents mean+SEM from a minimum of 12 neurons (N≥12) from at least three separate experiments. *** P<0.001 compared to isoflurane treatment alone. Data were analyzed using unpaired student t test.
Isoflurane induced greater calcium release from the ER than sevoflurane or desflurane
We compared the effects of three inhaled anesthetics at equipotent concentrations on the cytosolic calcium concentration in HD and WT striatal cells. Isoflurane induced a significantly greater peak elevation of [Ca2+]c than either sevoflurane or desflurane in both striatal cell types (Figure 4), regardless of the presence or absence of extracellular calcium. There were no significant differences between sevoflurane and desflurane in their effectiveness to raise the [Ca2+]c (Figure 4, E, F).
Figure 4. Isoflurane induced greater calcium release from the ER than sevoflurane or desflurane in both STHdhQ111/Q111 (HD) and STHdhQ7/Q7 (WT) striatal cells.
Averaged typical response of the [Ca2+]c to 2 MAC isoflurane, sevoflurane and desflurane in striatal cells, in the presence (A, B) or absence (C, D) of 1 mM extracellular (EC) calcium. Comparison of the peak elevation of [Ca2+]c induced by equipotent exposures to isoflurane, sevoflurane and desflurane between WT and HD cells in the presence (E) and absence (F) of extracellular calcium. For each experimental condition, data represents mean+SEM from a minimum of 33 neurons (N≥33) from at least three separate experiments. MAC, Minimum Alveolar Concentration. ***P<0.001 compared to the response induced by sevoflurane or desflurane respectively. #P<0.05, ###P<0.001 compared to WT cells treated with isoflurane. All data were analyzed by two-way ANOVA followed by Bonferroni post-tests (E. F).
Isoflurane, but not sevoflurane or desflurane at equipotent concentrations, induced more cell damage in HD than in WT striatal cells
We examined the correlation between the potency of volatile anesthetics to induce cytotoxicity. Exposure to 2 MAC isoflurane for 24 h induced relatively more MTS reduction (early stage of cell damage) in the HD than in WT striatal cells (Figure 5), although both cell types were not overly vulnerable to isoflurane-induced cell damage. Interestingly, sevoflurane and desflurane, at equipotent concentrations to isoflurane, did not induce similar cell damage (MTS reduction) in either HD (Figure 5A) or WT striatal neurons (Figure 5B). In addition, exposure to lower concentrations of isoflurane (1 MAC) for 24 h did not cause significant MTS reduction even in HD striatal neurons (data not shown).
Figure 5. Q111 Huntingtin renders striatal cells relatively more vulnerable to isoflurane neurotoxicity.
STHdhQ111/Q111 (HD) and STHdhQ7/Q7 (WT) striatal cells grown in 24 well plates were exposed to 2 MAC of isoflurane (ISO), sevoflurane (SEVO) and desflurane (DES) for 24 hour. After an additional 24 hours of recovery, MTS reductions were measured and normalized to the corresponding control (without inhaled anesthetic treatment). Comparison of MTS reduction in HD (A) and WT (B) striatal cells after exposure to three different inhaled anesthetics. For each experimental condition, data represents means±SEM from a minimum of 17 repeats (N≥17) from at least three separate experiments. ***P<0.001 compared to corresponding control without isoflurane treatments. +++P<0.001 compared to sevoflurane or desflurane treatment. All data were analyzed by two-way ANOVA followed by Bonferroni post-tests.
Discussion
We have demonstrated that commonly used inhalational anesthetics (isoflurane, sevoflurane and desflurane) significantly increased aggregation of Q111 huntingtin protein in HD cells as compared to non-exposed HD cells. As expected, aggregates were rarely observed in the wild type striatal cells, even in the presence of isoflurane, confirming that the aggregation is directly related to the expanded polyglutamine region (Ding et al., 2002). It is not clear if the increase in aggregation induced by isoflurane was a direct result of the interaction between the anesthetic and the Q111 huntingtin protein, as in the case of amyloid beta (Bianchi et al., 2008;Eckenhoff et al., 2004). The inhibition of isoflurane mediated huntingtin aggregation by the InsP3R antagonist xestospongin C suggests that calcium release from intracellular stores may play a role in huntingtin protein aggregation. These results are consistent with previous reports that demonstrated mutated huntingtin protein increased the activity of the InsP3R (Tang et al., 2003;Tang et al., 2005), which in turn could increase calcium release from the ER and lead to further huntingtin protein aggregation, forming a vicious cycle.
It is still controversial whether the aggregation of the expanded polyglutamine huntingtin plays an important role in the neuronal damage observed in Huntington’s disease. While studies have suggested that huntingtin protein aggregates may be the cause of neuronal degeneration in different models of Huntington’s disease (Hackam et al., 1998;Wellington et al., 2000), others could not establish a direct link between huntingtin aggregation and neuronal damage (Ding et al., 2002;Shastry, 2003). Since isoflurane can cause cytotoxicity and cognitive decline in the absence of protein aggregation (Bianchi et al., 2008;Wei et al., 2005), we cannot directly link neurodegeneration to the isoflurane-induced huntingtin aggregation. Furthermore, the results from this study indicate that all three inhalational anesthetics at equipotent exposures significantly increased huntingtin aggregation in HD cells, but only isoflurane induced significant cell damage. These results suggest that isoflurane cytotoxicity is probably not directly linked to the increase in huntingtin aggregation.
Isoflurane increased the [Ca2+]c more in HD cells than in WT cells in both the presence and absence of extracellular calcium. Consistent with our previous study (Wei et al., 2008), the data here clearly indicate that the ER calcium stores contributed significantly to the elevation of the [Ca2+]c induced by isoflurane. Because Q111 huntingtin has been reported to increase the activity of the InsP3R on the ER membrane (Tang et al., 2003;Tang et al., 2004;Tang et al., 2005), and because isoflurane is thought to activate the InsP3R (Peng et al., 2009;Wei et al., 2008;Yang et al., 2008;Zhao et al., 2010), it is possible that isoflurane induced the elevation in the [Ca2+]c due to an exaggerated release from the ER via the InsP3R. The even greater increase in cytosolic calcium concentration found after isoflurane exposure in the presence of extracellular calcium suggests that extracellular calcium also contributes to the anesthetic mediated elevation in the cytosolic calcium concentration. In fact, our recent study on the effects of isoflurane on primary cortical neurons(Zhao et al., 2010), indicated that the influx of calcium form the extracellular space was a major contribution to the elevation of the cytosolic calcium concentration. In addition, since the InsP3R antagonist, xestospongin C, nearly abolished the isoflurane mediated elevation in the cytosolic calcium concentration, even in the presence of extracellular calcium, it is possible that isoflurane initially causes calcium release from the ER via activation of the InsP3R, and then triggers calcium influx from the extracellular space via capacitive calcium entry mechanisms (Hewavitharana et al., 2007;Putney, Jr. et al., 2001).
Inhaled anesthetics may have differing potencies in their ability to release calcium from intracellular calcium stores (Liang et al., 2008;Liang et al., 2010;Yang et al., 2008). There are several possibilities why isoflurane evoked a significantly higher [Ca2+]c than either sevoflurane or desflurane in both WT and HD striatal cells in this study. For example, the three different anesthetics may have altered the [Ca2+]c by acting on the same receptor, but with different potencies, or by a distinct mechanism, such as neurolemmal voltage-gated calcium channels. Our results in both WT and HD cells are consistent with previous reports that isoflurane has a higher potency to induce calcium release from intracellular calcium stores than sevoflurane or desflurane (Kindler et al., 1999;Kunst et al., 2000;Liang et al., 2008;Yang et al., 2008).
Increasing evidence suggests that the disruption of intracellular calcium homeostasis, especially excessive calcium release from the ER, may contribute to the neurodegeneration in Huntington’s disease (Tang et al., 2003;Tang et al., 2004;Tang et al., 2005). Our previous study also demonstrated that inhibition of InsP3Rs on the ER membrane by its potent antagonist, xestospongin C, significantly ameliorated both the isoflurane induced calcium release from the ER and the neuronal apoptosis observed in Huntington’s and Alzheimer’s disease (Liang et al., 2008;Wei et al., 2008). The results from this study further support the hypothesis that neurodegeneration and calcium release are linked. Compared to the WT cells, isoflurane was more neurotoxic to HD cells than sevoflurane or desflurane. This pattern was mimicked in the calcium measurements.
This study was limited by the overall resistance of the striatal cells to isoflurane-mediated cell damage, although the HD striatal cells were relatively more vulnerable to isoflurane neurotoxicity than the WT striatal cells. It seems that there exists considerable variation in cell sensitivity to isoflurane induced cell damage. For example, the minimal concentration and exposure time to induce apoptosis in a lymphocyte cell line was 1 MAC isoflurane for 6 h (Wei et al., 2008) whereas only 0.75 MAC isoflurane for 12 h was needed to induce apoptosis in normal human peripheral lymphocytes (Matsuoka et al., 2001). However, in rat cerebral cortical neurons or PC12 cells, 2 MAC isoflurane for 24 h was needed to induce cell damage by apoptosis (Wei et al., 2005). In the current study, immortalized striatal neurons also needed a minimal exposure of 2 MAC isoflurane for 24 h to induce relatively modest cell damage. Although the mechanism for this vulnerability variance among different types of cells is elusive, it appears that neurons are relatively less vulnerable to isoflurane-mediated neurotoxicity (Liang et al., 2008;Wei et al., 2005;Wei et al., 2007;Wei et al., 2008). Because a causal relationship between isoflurane-mediated neuronal death and cognitive dysfunction has not been established, it is possible that general anesthetics may affect postoperative cognitive function without causing significant neuronal damage in the brain, possibly through synaptic dysfunction.
In summary, isoflurane enhances ER calcium release and cytotoxicity in HD striatal cells, whereas sevoflurane and desflurane at equipotent exposures do not. Our results further suggest that enhanced activity of the InsP3R may contribute to anesthetic induced aggregation of Q111 huntingtin, calcium release from the ER, and cell damage, and that inhaled anesthetics directly contribute to pathologic protein aggregation.
Acknowledgments
The research work was performed in and should be attributed to the Department of Anesthesiology, University of Pennsylvania, Philadelphia, PA 19104, USA
The authors thank Dr. Marcy MacDonald from the Molecular Neurogenetics Unit, Center for Human Genetic Research, Massachusetts General Hospital, Boston, Massachusetts for providing the STHdhQ111/Q111 and STHdhQ7/Q7 striatal cells. We thank Dr. Hong Lin from the Department of Pathology and Laboratory Medicine, University of Pennsylvania for assistance with experiments on protein aggregation. We thank Dr. Roderic Eckenhoff from the Department of Anesthesiology, Dr. Randal Pittman from the Department of Pharmacology, University of Pennsylvania for their intellectual involvement and support. This research work was supported by National Institute of General Medical Science (NIGMS) K08 grant (1-K08-GM-073224-01, to H.W.), R01 grant (1-R01GM084979-01, 3R01GM084979-02S1 to H.W.) and March of Dimes Birth Defects Foundation Research Grant (#12-FY05-62 and #12-FY08-167, to H.W.) and the Research Fund from the Department of Anesthesiology and Critical Care, University of Pennsylvania (to H.W.).
Abbreviations
- MAC
minimum alveolar concentration, the anesthetic concentration that is required to prevent a motor response to a surgical stimulus in 50% of individuals
Footnotes
Conflicts of interest statement
The authors declare that there are no conflicts of interest related to this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arrasate M, Mitra S, Schweitzer ES, Segal MR, Finkbeiner S. Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature. 2004;431:805–810. doi: 10.1038/nature02998. [DOI] [PubMed] [Google Scholar]
- Baranov D, Bickler PE, Crosby GJ, Culley DJ, Eckenhoff MF, Eckenhoff RG, Hogan KJ, Jevtovic-Todorovic V, Palotas A, Perouansky M, Planel E, Silverstein JH, Wei H, Whittington RA, Xie Z, Zuo Z. Consensus statement: First International Workshop on Anesthetics and Alzheimer’s disease. Anesth Analg. 2009;108:1627–1630. doi: 10.1213/ane.0b013e318199dc72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates G. Huntingtin aggregation and toxicity in Huntington’s disease. Lancet. 2003;361:1642–1644. doi: 10.1016/S0140-6736(03)13304-1. [DOI] [PubMed] [Google Scholar]
- Bianchi SL, Tran T, Liu C, Lin S, Li Y, Keller JM, Eckenhoff RG, Eckenhoff MF. Brain and behavior changes in 12-month-old Tg2576 and nontransgenic mice exposed to anesthetics. Neurobiol Aging. 2008;29:1002–1010. doi: 10.1016/j.neurobiolaging.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnen N, Warner MA, Kokmen E, Kurland LT. Early and midlife exposure to anesthesia and age of onset of Alzheimer’s disease. Int J Neurosci. 1994;77:181–185. doi: 10.3109/00207459408986029. [DOI] [PubMed] [Google Scholar]
- Ding QX, Lewis JJ, Strum KM, Dimayuga E, Bruce-Keller AJ, Dunn JC, Keller JN. Polyglutamine expansion, protein aggregation, proteasome activity, and neural survival. J Biol Chem. 2002;277:13935–13942. doi: 10.1074/jbc.M107706200. [DOI] [PubMed] [Google Scholar]
- Eckenhoff RG, Johansson JS, Wei H, Carnini A, Kang B, Wei W, Pidikiti R, Keller JM, Eckenhoff MF. Inhaled anesthetic enhancement of amyloid-beta oligomerization and cytotoxicity. Anesthesiology. 2004;101:703–709. doi: 10.1097/00000542-200409000-00019. [DOI] [PubMed] [Google Scholar]
- Gafni J, Munsch JA, Lam TH, Catlin MC, Costa LG, Molinski TF, Pessah IN. Xestospongins: potent membrane permeable blockers of the inositol 1,4,5-trisphosphate receptor. Neuron. 1997;19:723–733. doi: 10.1016/s0896-6273(00)80384-0. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A New Generation of Ca-2+ Indicators with Greatly Improved Fluorescence Properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hackam AS, Singaraja R, Wellington CL, Metzler M, McCutcheon K, Zhang TQ, Kalchman M, Hayden MR. The influence of Huntingtin protein size on nuclear localization and cellular toxicity. J Cell Biol. 1998;141:1097–1105. doi: 10.1083/jcb.141.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewavitharana T, Deng XX, Soboloff J, Gill DL. Role of STIM and orai proteins in the store-operated calcium signaling pathway. Cell Calcium. 2007;42:173–182. doi: 10.1016/j.ceca.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Hickey MA, Chesselet MF. Apoptosis in Huntington’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:255–265. doi: 10.1016/S0278-5846(03)00021-6. [DOI] [PubMed] [Google Scholar]
- Hiroi T, Wei H, Hough C, Leeds P, Chuang DM. Protracted lithium treatment protects against the ER stress elicited by thapsigargin in rat PC12 cells: roles of intracellular calcium, GRP78 and Bcl-2. Pharmacogenomics J. 2005;5:102–111. doi: 10.1038/sj.tpj.6500296. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Koch C, Genz K, Hoerster F, Felderhoff-Mueser U, Tenkova T, Dikranian K, Olney JW. Neurotransmitters and apoptosis in the developing brain. Biochem Pharmacol. 2001;62:401–405. doi: 10.1016/s0006-2952(01)00696-7. [DOI] [PubMed] [Google Scholar]
- Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindler CH, Eilers H, Donohoe P, Ozer S, Bickler PE. Volatile anesthetics increase intracellular calcium in cerebrocortical and hippocampal neurons. Anesthesiology. 1999;90:1137–1145. doi: 10.1097/00000542-199904000-00029. [DOI] [PubMed] [Google Scholar]
- Kuehn BM. Anesthesia-Alzheimer disease link probed. Jama-Journal of the American Medical Association. 2007;297:1760. doi: 10.1001/jama.297.16.1760. [DOI] [PubMed] [Google Scholar]
- Kunst G, Stucke AG, Graf BM, Martin E, Fink RH. Desflurane induces only minor Ca2+ release from the sarcoplasmic reticulum of mammalian skeletal muscle. Anesthesiology. 2000;93:832–836. doi: 10.1097/00000542-200009000-00034. [DOI] [PubMed] [Google Scholar]
- Lee TA, Wolozin B, Weiss KB, Bednar MM. Assessment of the emergence of Alzheimer’s disease following coronary artery bypass graft surgery or percutaneous transluminal coronary angioplasty. J Alzheimers Dis. 2005a;7:319–324. doi: 10.3233/jad-2005-7408. [DOI] [PubMed] [Google Scholar]
- Lee TA, Wolozin B, Weiss KB, Bednar MM. Assessment of the emergence of Alzheimer’s disease following coronary artery bypass graft surgery or percutaneous transluminal coronary angioplasty. J Alzheimers Dis. 2005b;7:319–324. doi: 10.3233/jad-2005-7408. [DOI] [PubMed] [Google Scholar]
- Liang G, Wang QJ, Li Y, Kang B, Eckenhoff MF, Eckenhoff RG, Wei HF. A presenilin-1 mutation renders neurons vulnerable to isoflurane toxicity. Anesth Analg. 2008;106:492–500. doi: 10.1213/ane.0b013e3181605b71. [DOI] [PubMed] [Google Scholar]
- Liang G, Ward C, Peng J, Zhao Y, Huang B, Wei H. Isoflurane causes greater neurodegeneration than an equivalent exposure of sevoflurane in the developing brain of neonatal mice. Anesthesiology. 2010;112:1325–1334. doi: 10.1097/ALN.0b013e3181d94da5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm D, Wootz H, Korhonen L. ER stress and neurodegenerative diseases. Cell Death Differ. 2006;13:385–392. doi: 10.1038/sj.cdd.4401778. [DOI] [PubMed] [Google Scholar]
- Matsuoka H, Kurosawa S, Horinouchi T, Kato M, Hashimoto Y. Inhalation anesthetics induce apoptosis in normal peripheral lymphocytes in vitro. Anesthesiology. 2001;95:1467–1472. doi: 10.1097/00000542-200112000-00028. [DOI] [PubMed] [Google Scholar]
- Muravchick S, Smith DS. Parkinsonian Symptoms During Emergence from General-Anesthesia. Anesthesiology. 1995;82:305–307. doi: 10.1097/00000542-199501000-00039. [DOI] [PubMed] [Google Scholar]
- Oka T, Sato K, Hori M, Ozaki H, Karaki H. Xestospongin C, a novel blocker of IP3 receptor, attenuates the increase in cytosolic calcium level and degranulation that is induced by antigen in RBL-2H3 mast cells. Br J Pharmacol. 2002;135:1959–1966. doi: 10.1038/sj.bjp.0704662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: The calcium-apoptosis link. Nature Reviews Molecular Cell Biology. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- Panov AV, Gutekunst CA, Leavitt BR, Hayden MR, Burke JR, Strittmatter WJ, Greenamyre JT. Early mitochondrial calcium defects in Huntington’s disease are a direct effect of polyglutamines. Nat Neurosci. 2002;5:731–736. doi: 10.1038/nn884. [DOI] [PubMed] [Google Scholar]
- Peng Y, Cheung KH, Foskett J, Li J, Wei H. Isoflurane activates inositol 1,4,5-trisphosphate receptors. Program # 818.11/C4; 2009; 2009 Neuroscience Meeting Planner; Chicago, IL: Society for Neuroscience; 2009. Online. [Google Scholar]
- Putney JW, Jr, Broad LM, Braun FJ, Lievremont JP, Bird GS. Mechanisms of capacitative calcium entry. J Cell Sci. 2001;114:2223–9. doi: 10.1242/jcs.114.12.2223. [DOI] [PubMed] [Google Scholar]
- Rajan RS, Illing ME, Bence NF, Kopito RR. Specificity in intracellular protein aggregation and inclusion body formation. Proc Natl Acad Sci U S A. 2001;98:13060–13065. doi: 10.1073/pnas.181479798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shastry BS. Neurodegenerative disorders of protein aggregation. Neurochem Int. 2003;43:1–7. doi: 10.1016/s0197-0186(02)00196-1. [DOI] [PubMed] [Google Scholar]
- Tang TS, Slow E, Lupu V, Stavrovskaya IG, Sugimori M, Llinas R, Kristal BS, Hayden MR, Bezprozvanny I. Disturbed Ca2+ signaling and apoptosis of medium spiny neurons in Huntington’s disease. Proc Natl Acad Sci U S A. 2005;102:2602–2607. doi: 10.1073/pnas.0409402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang TS, Tu H, Chan EY, Maximov A, Wang Z, Wellington CL, Hayden MR, Bezprozvanny I. Huntingtin and huntingtin-associated protein 1 influence neuronal calcium signaling mediated by inositol-(1,4,5) triphosphate receptor type 1. Neuron. 2003;39:227–239. doi: 10.1016/s0896-6273(03)00366-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang TS, Tu HP, Orban PC, Chan EYW, Hayden MR, Bezprozvanny I. HAP1 facilitates effects of mutant huntingtin on inositol 1,4,5-trisphosphate-induced Ca2+ release in primary culture of striatal medium spiny neurons. Eur J Neurosci. 2004;20:1779–1787. doi: 10.1111/j.1460-9568.2004.03633.x. [DOI] [PubMed] [Google Scholar]
- Trettel F, Rigamonti D, Hilditch-Maguire P, Wheeler VC, Sharp AH, Persichetti F, Cattaneo E, MacDonald ME. Dominant phenotypes produced by the HD mutation in STHdh(Q111) striatal cells. Hum Mol Genet. 2000;9:2799–2809. doi: 10.1093/hmg/9.19.2799. [DOI] [PubMed] [Google Scholar]
- Waelter S, Boeddrich A, Lurz R, Scherzinger E, Lueder G, Lehrach H, Wanker EE. Accumulation of mutant huntingtin fragments in aggresome-like inclusion bodies as a result of insufficient protein degradation. Mol Biol Cell. 2001;12:1393–1407. doi: 10.1091/mbc.12.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H, Kang B, Wei W, Liang G, Meng QC, Li Y, Eckenhoff RG. Isoflurane and sevoflurane affect cell survival and BCL-2/BAX ratio differently. Brain Res. 2005;1037:139–147. doi: 10.1016/j.brainres.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Wei H, Liang G, Yang H. Isoflurane preconditioning inhibited isoflurane-induced neurotoxicity. Neurosci Lett. 2007;425:59–62. doi: 10.1016/j.neulet.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei HF, Liang G, Yang H, Wang QJ, Hawkins B, Madesh M, Wang SP, Eckenhoff RG. The common inhalational anesthetic isoflurane induces apoptosis via activation of inositol 1,4,5-trisphosphate receptors. Anesthesiology. 2008;108:251–260. doi: 10.1097/01.anes.0000299435.59242.0e. [DOI] [PubMed] [Google Scholar]
- Wellington CL, Singaraja R, Ellerby L, Savill J, Roy S, Leavitt B, Cattaneo E, Hackam A, Sharp A, Thornberry N, Nicholson DW, Bredesen DE, Hayden MR. Inhibiting caspase cleavage of huntingtin reduces toxicity and aggregate formation in neuronal and nonneuronal cells. J Biol Chem. 2000;275:19831–19838. doi: 10.1074/jbc.M001475200. [DOI] [PubMed] [Google Scholar]
- Wise-Faberowski L, Zhang H, Ing R, Pearlstein RD, Warner DS. Isoflurane-induced neuronal degeneration: an evaluation in organotypic hippocampal slice cultures. Anesth Analg. 2005;101:651–657. doi: 10.1213/01.ane.0000167382.79889.7c. [DOI] [PubMed] [Google Scholar]
- Xie Z, Dong Y, Maeda U, Alfille P, Culley DJ, Crosby G, Tanzi RE. The common inhalation anesthetic isoflurane induces apoptosis and increases amyloid beta protein levels. Anesthesiology. 2006;104:988–994. doi: 10.1097/00000542-200605000-00015. [DOI] [PubMed] [Google Scholar]
- Yang H, Liang G, Hawkins BJ, Madesh M, Pierwola A, Wei HF. Inhalational anesthetics induce cell damage by disruption of intracellular calcium homeostasis with different potencies. Anesthesiology. 2008;109:243–250. doi: 10.1097/ALN.0b013e31817f5c47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Liang G, Chen Q, Joseph DJ, Meng Q, Eckenhoff RG, Eckenhoff MF, Wei H. Anesthetic-induced neurodegeneration mediated via inositol 1,4,5-trisphosphate receptors. J Pharmacol Exp Ther. 2010;333:14–22. doi: 10.1124/jpet.109.161562. [DOI] [PMC free article] [PubMed] [Google Scholar]