Abstract
Tissue dissociation enzymes are critical reagents that affect the yield and quality of human pancreatic islets required for islet transplantation. The FDA’s oversight of this procedure recommends laboratories set acceptance criteria for enzymes used in the manufacture of islet products for transplantation. Presently, many laboratories base this selection on personal experience since biochemical analysis is not predictive of success of the islet isolation procedure. This review identifies the challenges of correlating results from enzyme biochemical analysis to their effectiveness in human islet isolation and suggests a path forward to address these challenges to improve control of the islet manufacturing process.
Keywords: tissue dissociation, collagenase, islet isolation, protease, biochemical characterization, type 1 diabetes
Improvements in the immunosuppressive therapy used to treat adult type 1 diabetes patients receiving islet transplants have shown insulin independence rates comparable to those obtained after pancreas transplant alone(1). A critical issue that must be addressed to translate these advances to routine therapeutic use is to comply with the United States Food and Drug Administration (FDA) Guidelines for manufacturing human islets for allo-transplantation. The initial set of guidelines for islet allo-transplantation were published for comment in May, 2008 and finalized in September, 2009(2). These guidelines state that critical manufacturing steps used to generate a current good manufacturing practice (cGMP) human islet product should be controlled by establishing specifications to set limits for process variation. Specifically, the guidelines state the manufacturing controls should evaluate the weight or units of tissue dissociation enzymes (TDEs) and assess varying digestion conditions such as time, temperature, and shaking to ensure generation of a reproducible islet product. In these and other FDA documents, the use of the verb “should” indicates that this step is recommended but not required at this time.
The FDA request for better characterization of TDEs used in islet transplantation foreshadowed problems encountered when a change was made from Roche to Serva as the vendor for TDE products used in the National Institutes of Health funded Clinical Islet Transplantation consortium trial in March, 2007(3). Several transplant centers found that use of Serva’s NB-1 collagenase (NB-1) and NB Neutral Protease (NB) failed to provide islet yields comparable to those obtained using Roche Liberase™ HI Purified Enzyme Blend (HI)(4–8). In several cases, this led to modifications to islet isolation protocols to increase islet yields, including selecting specific lots of Serva enzymes(4) or modifying the sequence of enzyme addition used during the islet isolation procedure(5). This experience re-confirmed earlier observations that identified the importance of TDEs as a critical factor for successful islet isolation(9–11).
Many reports showed that collagenase and a general protease active at neutral pH (i.e., neutral protease) were required for successful recovery of cells from mammalian organs(12–16). The development of Liberase HI in 1994 confirmed these earlier reports by showing that two collagenase isoforms from Clostridium histolyticum, class I (C1) and class II (C2) collagenase, and one or more neutral proteases gave human islet yields superior to those found when minimally purified products containing these enzymes were used in the isolation procedure(17–19).The two collagenase isoforms are identified by differences in their substrate specificity and work synergistically to degrade collagen(20;21). The mechanism of how these proteases breakdown the connective tissue extracellular matrix (ECM) for cell release is poorly understood. The focus of this article is to review relevant information on enzymatic tissue dissociation, then apply this knowledge to strengths and weaknesses of the assays used to prequalify these enzymes for use in human islet isolation. To address this task, we provide a brief review of pancreatic histology (with an emphasis on the connective tissue that holds the cells in the organ) and summarize the limited data showing how TDEs degrade the ECM. This information together with our current understanding of C. histolyticum collagenase provides a foundation for developing a hypothetical mechanism describing how these enzymes free cells from tissue. The relevance of this mechanism is compared to assays used to assess the functional activity and purity of the TDEs used in islet isolation. We conclude by commenting on the relevance and utility of these analyses to predict successful human islet isolation for islet transplantation.
Pancreatic Histology
The pancreas is an endocrine-exocrine organ that is composed of acinar cells, ductal tissue, and islets that comprise approximately 85, 10 and 1–2% of the volume of the organ, respectively(22;23). The exocrine tissue functions by acinar cells secreting a solution of proenzymes into the pancreatic ducts. This fluid leaves the acini and flows into the intercalated ducts. These ducts merge and increase in size as the fluid transverses through the intralobular to interlobular ducts to the main duct where the proenzymes are discharged into the duodenum. Here, enteropeptidase converts trypsinogen to trypsin that in turn converts the pancreatic proenzymes to active enzymes that digest food in the intestine.
Islets are the endocrine component of the pancreas. These highly vascularized micro-organs range in size from 40 to 900 microns and may contain several thousand cells(24;25). It is estimated that 15–20% of the volume of the blood entering the rabbit pancreas passes through the islets(26). The islet is surrounded by a periinsular islet capsule whose structure varies between species(27). About half of the human islets are “periductal”, lying adjacent to the ductal tissue(28). This has important implications for mammalian islet isolation since retrograde infusion of the TDE solution via the main pancreatic duct is commonly used to provide the highest islet yields(29). In human islet isolations, the pressure for infusing the enzyme solution via the main pancreatic duct is tightly controlled to minimize damage to the ductal structure, ensuring uniform delivery of the TDEs throughout the tissue.
Conventional histological analysis showed that there is little connective tissue in the pancreas with the majority being found in the septa that separate the lobes and the larger ducts (intralobular, interlobular, & main ducts)(23). However, the complexity of the cellular support within the pancreas was shown by using finer analytical methods. Cell maceration/scanning electron microscopic studies using formalin-fixed pancreatic tissue treated with 2M sodium hydroxide to remove the cells revealed an extensive honeycomb structure formed by a fibrous network present in human pancreas(30). These networks contain numerous round and oval spaces that were believed to contain islets, acini, ducts, and blood vessels prior to clearing the cells with sodium hydroxide. This morphological analysis provides insight into the complexity of support provided by the basement membrane, a fibrous portion of the ECM that surrounds the acini-ductal network.
The ECM is created by the cells in the local area (includes fibroblasts) secreting a diverse array of macromolecules into the surrounding space. These molecules include collagen, elastin, and reticular fibers that comprise the fibrous network; the ground substance that contains hydrophilic polysaccharides(e.g., glycosaminoglycans); and multi-adhesive glycoproteins (laminin, fibronectin) that provide strength to the tissue by binding to integrins on the surface of cells in the tissue(31). Laminin is the predominant adhesive protein found in the pancreas(32) and the distribution of different types vary between the endocrine and exocrine tissue(33). Reports from the Oxford group showed that type VI collagen is pervasive throughout the exocrine pancreas and was the predominant collagen type at the exocrine-endocrine interface(34;35). More detailed analysis of the collagen types at the interface showed a rank order of prevalence with types VI > V > I ~ IV > III(34). This study noted that in some islets, collagen V and VI staining was observed in the pericapillary spaces that penetrated the islet interior, an observation confirmed in a subsequent study(35). These data are consistent with an earlier report showing that the parenchymal endocrine cells within the human islet are surrounded by a double basement membrane at the endocrine:vascular tissue interface. It is thought that one basement membrane is contributed by the parenchymal endocrine cells and the other by the vascular endothelial cells(33).
Tissue Dissociation using TDEs
A recent report showed that when collagenase is infused into the main pancreatic duct, it is broadly distributed within the human exocrine and endocrine tissue and the binding is associated with type VI collagen(35). The effect of TDEs digesting pancreatic tissue was best shown in several papers published by van Schilfgaarde’s group in the 1990’s(16;36). These studies analyzed the affect of different mixtures of purified C. histolyticum collagenase with or without C. histolyticum neutral protease (CHNP) at 37°C on the histochemical staining of the ECM of minced rat pancreatic tissue. Specific staining for collagen, glycoprotein, proteoglycan or elastin was analyzed after taking tissue samples from the digest mixture at 15 min intervals for up to 2 hours. The kinetic loss of histochemical staining for the exocrine and endocrine tissue was similar. These results showed that purified collagenase alone decreased the intensity of collagen, glycoprotein, and proteolglycan staining, indicating that collagen is an important component, somehow linked to other macromolecules in the pancreatic ECM. In contrast, elastin staining was unaffected by collagenase treatment. Concomitant addition of neutral protease to collagenase immediately accelerated the loss of collagen, glycoprotein, and proteoglycan staining and led to loss of elastin staining after a delay of 45 min(16).
A similar experimental study using minced rat pancreas was performed but used purified collagenase isoforms, class I (C1) and class II (C2) collagenase from C. histolyticum in place of purified unfractionated collagenase used in the earlier report(36). The affect of C1 or C2 alone or in combination and in the presence or absence of neutral protease were assessed for their ability to reduce collagen or glycoprotein staining. The results showed that the order of effectiveness for reducing collagen histochemical staining was C1 + C2 > C2 > C1 > neutral protease. Comparison of these same enzymes to decrease glycoprotein staining showed C1 + C2 was more effective than either class alone. The addition of neutral protease to the individual or combined collagenase isoforms accelerated the kinetics of loss of collagen staining but did not change the pattern where C1 + C2 > C2 > C1. This difference was not seen in the loss of glycoprotein staining where C1, C2 or C1 +C2 and neutral protease were equally effective in reducing histochemical staining. The least effective enzyme in reducing glycoprotein staining was neutral protease alone, again indicating the importance of collagen as an integral component of the ECM(36).
Assuming the results from the rat studies above can be translated to human islet isolation, the primary conclusion is that collagen is an integral component of the ECM with greatest loss of staining achieved only when collagenase is present in the enzyme mixture. Collagen is intimately involved in the honeycomb fibrous structure that surrounds the acini and ducts, the periinsular islet capsule, and the vascular tissue present in the exocrine or endocrine tissue(35). Type VI collagen is the predominate form of collagen within the pancreas(34;35) and like type IV, forms a “net” like supramolecular structure that provides structural support for holding cells in the tissue(37). This reinforces the observation that collagen degradation is essential for successful tissue dissociation.
Little is known about the exact mechanism by which collagenase and neutral proteases degrade the ECM to release cells from the pancreatic tissue. However, it is clear that both collagenase isoforms and neutral protease(s) are required since each enzyme alone is insufficient to maximize islet yield(16). Prior to proposing a more detailed mechanism of tissue dissociation, the genetic and protein domain structure of C. histolyticum collagenase enzymes will be reviewed.
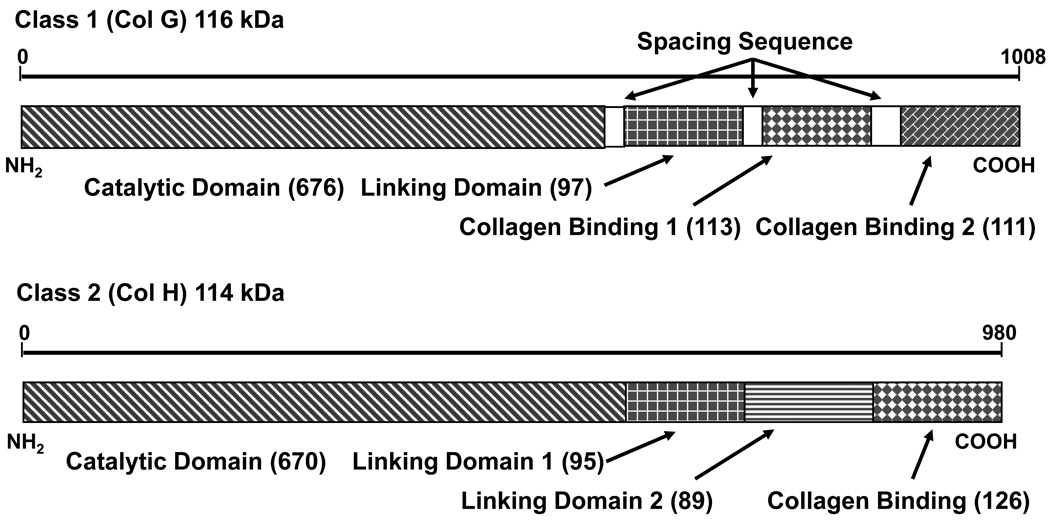
The gene structures of C1 and C2 collagenase from C. histolyticum is shown in Figure 1(38). Nearly identical gene sequences were obtained from two different laboratories with only 13 amino acid differences for C2 and 4 differences for C1(38–41). Expression of these cloned genes led to synthesis of a single polypeptide chain that had biochemical characteristics very similar to the natural forms of the enzyme(42). Both forms contained four domains, with a large catalytic domain (67% of amino acids) followed by a linking domain(s) and collagen binding domain(s) (CBDs) at the carboxy terminal end of the molecule. Intact C1 has a catalytic domain, one linking domain followed by two CBDs (C1116kDa) whereas intact C2 has a catalytic domain, two linking domains followed by one CBD (C2114kDa). The functions of the linking domains are not known(38). The assignment of the domains as CBDs was determined by the ability of these portions of the molecule to bind native collagen independent of any other protein domains(43;44). Three forms of these enzymes contain a catalytic domain and at least one CBD and were found to be effective in degrading native collagen. These enzymes — C1116kDa, C2114kDa, and C1100kDa — will be termed “active collagenase in this article since they possess the unique ability to degrade the triple helical chain structure that is a key characteristic of any collagen molecule. In contrast, collagenase that has a functional catalytic domain and no CBDs is defined as “inactive” collagenase. These enzymes have gelatinase activity, a broad term defining any enzyme that can degrade denatured collagen. Active or inactive collagenase as well as most proteases can degrade gelatin and be termed gelatinases.
Figure 1. Domain structure of C. histolyticum collagenases.
Domain structure of class I (Col G) and class II (Col H) collagenases from C. histolyticum. Numbers in parenthesis after label indicate number of amino acids in domain. Both the catalytic domain and at least one collagen binding domain (CBD) are required for degradation of native collagen. The linking domains does not yet have a known structure or function but in multisequence alignments has regions of homology with similarities to CBD. Spacing sequences are relatively short regions (<12 amino acids) that link domains together. The spacing sequence between the two (CBD) on class 1 collagenase was unstructured in the crystal structure reported by Wilson and co-workers (89).
Hypothetical Mechanism of Tissue Dissociation & Other Factors Influencing Tissue Dissocation
Based on the evidence presented above, one major premise of any hypothesis describing the mechanism of tissue dissociation is the critical role collagen degradation plays in maximizing the breakdown of the ECM that holds cells in tissue. Collagen’s triple helical domain is resistant to proteolytic damage and requires the unique ability of collagenase to initiate the degradation of this structure. Active collagenase binds tightly to the collagen fibers/fibrils and once attached, begins to degrade the collagen structure leading to unraveling of the triple helical structure. Concomitantly, the ECM macromolecules (i.e., glycoproteins, proteoglycans, and reticular fibers) are degraded or released by the neutral protease and gelatinase activities, leading to synergistic attack of multiple enzyme activities to degrade the ECM and release of cells from tissue. An earlier study indicated that C1 and C2 degrade different portions of the collagen macromolecular structure(45). However, there are conflicting reports on the importance of the strength of synergistic response of C1 and C2 to release islets from rat pancreas(46;47). One report that used human tissue showed that when 3 different C1:C2 ratios were tested with a standard dose of thermolysin for their effectiveness to release islets from tissue, the enzyme mixture containing the highest amount of C2 gave the poorest results: longer digestion time, lower islet yield, and higher amount of undigested tissue(48).
Collagenase and neutral protease activity in tissue dissociation can be affected by many different factors as summarized elsewhere(49–53). The majority of enzymes used in tissue dissociation are bacterial metalloproteinases that require divalent cations (zinc and calcium) for optimal enzymatic activity. Hanks balanced salt solution, commonly used for preparation of TDE solutions contains a sufficient amount of calcium to maintain this activity. However, the addition of cation selective chelating agents or any other chemicals that affect calcium or zinc will strongly inhibit enzymatic activity. A simple, potent and biologically compatible inhibitor of both collagenase and neutral protease is a small concentration of adult serum. The addition of 2.5% (v/v) human AB serum to CMRL media supplemented with other substances increased islet survival and improved recovery of human islets after culture(54). This affect may in large part be due to serum inhibition of the endogenous protease activity released from acinar cells. Serum contains α2 macroglobulin, a strong protease inhibitor that rapidly inactivates both the exogeneous and endogeneous proteases(55) that are present in the pancreatic digest mixture.
Another factor that may influence the yield and quality of islets recovered after isolation is the generation of endogenous pancreatic protease during the course of the tissue digestion procedure. Exogeneous TDEs have been shown to activate pancreatic proenzymes(56) but the TDEs responsible for this activity have not been identified. Initial studies showed that endogenous pancreatic enzymes generated during the isolation procedure does affect porcine islet yield(57–59) The most definitive studies on the effect of these enzymes on human islet isolation were published several years later by the Edmonton group(60;61). The impact of this factor is unclear since contradictory conclusions are obtained on comparing human islet yields from organs stored cold for different periods of time(60;61). Both studies used HI for tissue dissociation and a serine protease inhibitor (Pefabloc®) to inhibit serine protease activity during the islet isolation procedure. The effect of this treatment was dependent on the cold ischemia time of the organ. If organs were stored > 10 hours, significantly higher mean human islet yields were obtained in Pefabloc treated tissue digests when compared to untreated digests(60). In contrast, the addition of protease inhibitors had no significant effect of islet yield when compared to untreated digests if the organs were stored < 12 hours(61). These studies indicate that the inhibition of endogenous protease does not have a significant effect on islet quality and yield as long as cold storage of the organ is not prolonged. Increased cold ischemia time likely leads to increased endogeneous protease activity that in turn impacts islet yield. This activity may have a negative or positive affect depending on the amount of exogeneous neutral protease used in the tissue dissociation procedure.
A recent report from Edmonton confirmed that endogenous neutral protease activity can influence the islet isolation process. Here, these investigators modified their islet isolation protocol by adding collagenase prior to adding exogeneous neutral protease. In 8 of 24 isolations, the release of free islets from the tissue with collagenase alone led to the omission of exogeneous neutral protease in the isolation protocol(62). In a subsequent report they showed this affect was often seen when there was a long cold ischemia time (> 10 hours) and NB-1 collagenase containing contaminating neutral protease activity were used in the isolation procedure. These results suggested that generation of a sufficient amount of endogeneous neutral protease activity during a digest was sufficient to release islets from human tissue(63).
A recent report may provide an explanation for Edmonton’s results. The Uppsala group has shown that high trypsin like activity (TLA) in selected lots of NB-1 can improve islet yields(4). Clostripain is usually responsible for the TLA/neutral protease activity. Clostripain has a slightly different specificity than trypsin(53;64) and we assume that it works synergistically with other proteases to degrade ECM proteins. It is also possible that some of clostripain’s affect may be indirect since theoretically it should cut human proelastase and convert it to elastase (XXR↓VXX), leading to increased elastase activity during the cell isolation process.
C. histolyticum Collagenase: Functional and Physical Analysis
Tables 1 and 2 summarize the enzymatic and physical assays, respectively, currently used to assess collagenase. The majority of assays used to assess collagenase activity measure both active and inactive enzyme (Table 1). The most commonly used assay is the Wunsch assay because the assay is precise and consistent results are obtained between laboratories if a standardized assay procedure is used(65). The amount of Wunsch activity per organ or per g tissue is commonly used to report the dose of collagenase used in an islet isolation procedure. However, the activity detected by peptide substrates (e.g., Wunsch or FALGPA) is strongly biased to detect C2. The specific Wunsch activity of purified C2 is greater than 50× that found with purified C1 (FE Dwulet, unpublished observation). This activity reflects only the function of the catalytic domain since both the C2114kDa and degraded forms of C2 (no CBD) are detected by this assay(39). This was confirmed by showing that digestion of purified C2 by chymotrypsin led to greater than 95% loss of specific collagen degradation activity (CDA Units/mg protein) but only 7% of the specific Wunsch activity(66). Several studies analyzing the impact of different factors on the success of human islet isolation have shown that Wunsch activity was not predictive of successful isolation(67).
Table 1.
Assays used to assess collagenase enzymatic activity Enzymatic assays
| Assay | Ref. | Substrate | Isoform Bias |
Collagenase | Advantages | Disadvantages | |
|---|---|---|---|---|---|---|---|
| Active | Inactive | ||||||
| Peptide activity |
65,82 | Pz peptide FALGPA |
C2 >> C1 | + | + |
|
|
| Azocoll | 83,84 | Dye impregnated cow hide |
C2 ~ C1 | + | + |
|
|
| Gelatinase | 83 | Gelatin | C1 > C2 | + | + |
|
|
| CDA | 20,68 | Collagen fibers | C2 ~ C1 | + | − |
|
|
| CDA | 66 | FITC collagen fibers |
C1 > C2 ~ C1b ~ C1c |
+ | − |
|
|
Table 2.
Physical assays used to assess collagenase quality
| Assay | Ref | Cost to implement |
Advantages | Disadvanatages |
|---|---|---|---|---|
| SDS-PAGE | 72 | Low |
|
|
| Analytical anion exchange HPLC | 66,69 | Medium-high |
|
|
| Reversed phase HPLC | 70,85 | Medium-high |
|
|
| Capillary electrophoresis | 71 | High |
|
|
The best assay to measure active collagenase is the assessment of collagen degradation activity (CDA). The Mandl assay or its variants used collagen fibers to detect collagenase activity(20;68). However, these assays are time consuming (> 3 hours), non-linear and have poor precision. A recently described fluorescent microplate CDA using fluorescein isothiocyanate coupled to soluble, type I, calf skin collagen fibrils (FITC fibrils) overcomes the linearity and precision problems encountered using the Mandl assay. In addition, the specific CDA correlated to the different molecular forms of C1 and their resolution by analytical anion exchange high pressure liquid chromatography (HPLC)(66). These analyses showed a correlation between the specific CDA and the number of CBDs on the collagenase enzyme: purified C1116kDa with two CBDs has ~ 10× higher specific CDA (CDA U/mg protein) when compared to other purified forms with a single CBD (i.e., C1100kDa and C2114kDa).
Analytical anion exchange HPLC is currently the best physical method to analyze purified collagenase(69) (Table 2). This technique can resolve C2 and three predominant molecular forms of C1 (C1, C1b, C1c). The first peak eluted from the column contains primarily C2 followed by the C1 peak (C1116kDa) followed in rapid succession by the C1b and C1c peaks. The latter two peaks are C1100kDa forms of the collagenase(66). Mass spectrophotometric analysis of purified C1b and C1c has shown that there is only a 12 amino acid sequence difference between C1b and C1c (AG Breite et al., unpublished results). This short sequence imparts a charge difference that accounts for the separation of the forms by anion exchange chromatography and leaves the kinetic activity of the enzyme essentially unchanged. However, this HPLC method cannot provide sensitive discrimination of the different molecular forms of C2 (C2114kDa vs degraded C2 with no CBD).
The other physical assays summarized in Table 2 do not provide additional value to the physical analysis of collagenase by analytical anion exchange HPLC since reversed phase HPLC is unable to discriminate between the different molecular forms of C1(70); microcapillary electrophoresis is an expensive analysis that provides only confirmatory information and is unable to separate the C1b from the C1c molecular form(71); and SDS-PAGE is qualitative and prone to artifacts(72).
Bacterial Neutral Protease: Protein Structure & Biochemical Analysis
The bacterial neutral proteases commonly found in TDE products are clostripain or the metalloproteases, including thermolysin, Bacillus polymyxa protease, or CHNP. These metalloproteinases contain a zinc atom that is essential for catalysis‥ In contrast, clostripain is a cysteine protease that requires a thiol group for catalysis.
CHNP, thermolysin and BP protease share similar specificity, cutting peptides on the amino terminal side of hydrophobic amino acid residues. All three enzymes share significant sequence homology across species and strains with the HEXXH catalytic residues absolutely conserved(73). Crystal structures have been reported for thermolysin in the MEROPS database(73). The high degree of sequence homology among these enzymes suggests that all these proteases have a compact structure similar to thermolysin. A reasonable assumption is that all of these neutral protease enzymes contain one functional catalytic domain with a single zinc atom in the active site and with molecular weights in the range of 32–35 kDa.
Clostripain is maximally active under reducing conditions and cuts protein on the carboxy terminal side of arginine(53). As noted above, clostripain is responsible for the TLA found in purified collagenase(64). It is termed TLA since it is detected using the same peptide substrate as trypsin. However, trypsin has broader specificity, cutting protein on carboxy terminal side of arginine or lysine residues and has a 100-fold higher specific activity than clostripain (FE Dwulet, unpublished observation).
The best attribute to assess the consistency of a purified neutral protease preparation is the specific activity (enzyme Units/mg protein). This value will vary depending on the amount of active enzyme in a purified enzyme preparation, the substrate used in the assay, and the assay conditions. This assumes that highly purified enzymes are used in the analysis and that optimal conditions are used to assay for enzyme activity (e.g., activation of clostripain by pretreatment under reducing conditions). Here, accurate determination of a specific activity requires that a consistent value is obtained across an optimal dilution range of enzyme used in the assay. This range often depends on the substrate used in the assay. Prior reports have shown that casein substrates often have a 2 fold range(50;74) whereas our internally developed fluorescent microplate assay for neutral protease activity has a 4–8 fold range(75). This difference may reflect the design of the assay. Table 3 provides a summary of the assays used by the current manufacturers to characterize neutral protease activity. It is important to note that all of the assays, except those performed by VitaCyte, are endpoint assays that use casein or derivatized casein as substrate. In most, but not all cases(74), trichloroacetic acid is used to separate the peptides from the intact protein. The major advantages of the VitaCyte assay are that it is a kinetic assay requiring no separation step and it is easily adaptable for the incorporation of inhibitors to further characterize the enzymes or perform high throughput screening.
Table 3.
Neutral protease assays used by different vendors to assess enzymatic activity
| Vendor | Ref | Separation Step |
Assay (Substrate) | Characteristics |
|---|---|---|---|---|
| Roche | 86 | TCA | FITC casein | Endpoint, low thoughput |
| Roche | 50 | TCA | Casein (absorbance release equivalent to tyrosine) | Endpoint, low thoughput |
| Sigma | 87 | TCA | Casein (absorbance release equivalent to tyrosine) | Endpoint, low thoughput |
| Worthington | 88 | TCA | Caseinase (Folin positive amino acids equivalent to tyrosine) | Endpoint, low thoughput |
| Serva | 74 | N/A | Dimethylcasein (Trinitrobenzene Sulfonic Acid) | Endpoint, low thoughput |
| VitaCyte | 75 | N/A | FITC-BSA | Kinetic, high throughput |
Context, Conclusions and Recommendations
Scientists performing human islet isolation face several challenges to achieve a sufficient yield of islets for transplantation. Assuming the team is experienced in human islet isolation, two primary factors that influence islet yield are donor variables and the enzyme quality. Numerous reports have noted correlation of different donor factors to successful and unsuccessful human islet isolation(76–79). Notable among these factors is the common observation that lower islet yields are obtained when organs are obtained from younger donors when compared to older donors. The reasons for this result are not known and there are no studies that compared the histological features of pancreata obtained from young (<30 year old) versus older donors.
This report reviews the second issue, how can we improve the selection of TDEs used in human islet isolation so appropriate enzyme compositions (i.e., best quality) are used in the isolation procedure. For most scientists, enzyme activity analysis presented on the certificates of analysis for specific lots of product is used for reference, not for lot selection. The best guide for choosing a lot of product is its performance in human islet isolation. These are expensive experiments to perform so often scientists will consult their colleagues for recommendations of a specific TDE lot(s) for purchase. The information presented above summarizes the limitations of using only Wunsch activity analysis to assess the quality of collagenase. Recent evidence indicates the use of several biochemical assays for collagenase may provide more valuable information to select an enzyme for purchase. Assessment of purified collagenases from Serva (NB-1), Roche (HI) and VitaCyte (CIzyme™ Collagenase HA - HA) showed that these products contained different amounts of C1116kDa as detected by increased specific CDA and by the percentage of C1116kDa found after analysis by anion exchange analytical HPLC. HA contained the highest amount of C1116kDa followed by HI then NB-1. This report showed that when post purification islet yields were compared for isolations using HA/thermolysin or NB-1/NB, the HA/thermolysin gave significantly higher islet equivalents (IEQ) per organ or per g tissue (n=14) when compared to those results obtained with NB-1/NB (n =27). There was no significant difference in the functional activities of these islets after assessment by oxygen consumption rate, islet viability or glucose stimulated insulin response(70).
Recent data from the Edmonton group also showed that NB-1 contains predominantly dgraded C1. However, they found that there was no significant difference in human islet yields when using either NB-1/NB or C1116kDa, C2, and thermolysin (Liberase MTF, Roche Applied Sciences). However, a higher proportion of islet isolations using MTF gave yields > 400,000 IEQ (9/17) when compared to those with NB-1 (8/24)(62).
If it is assumed that the biochemical characteristics of NB-1 and HI collagenase have been consistent over the last 6 years, then results from two reports in the literature comparing the performance of NB-1/NB to HI in a large series of human islet isolation indicates that TDEs containing C1116kDa, C2, and thermolysin (i.e., HI) gave superior islet yields when compared to NB-1/NB. Results from data collected by the Islet Cell Resource Centers showed a significantly higher proportion of human islet isolations using HI (n=272) gave islet yields (> 315,000 IEQ/organ) when compared to those isolations using NB-1/NB (n=88, p = 0.048)(80). Similarly, the Uppsala group showed a significantly higher recovery of human islets after isolation using HI (n=101) when compared to those isolations using NB-1/NB (n=96). However, the Serva enzymes gave significantly higher purity and glucose stimulated insulin secretion when compared to islets isolated with HI(8). This latter difference may in part be explained by the choice of neutral protease used in the isolation procedure. Recently, the University of Minnesota group showed that increased numbers of high quality human islets were recovered when CHNP was used in place of thermolysin in enzyme mixtures containing a high proportion of C1116kDa and C2(7). These data are compelling since these conclusions were made after comparing the performance of these enzymes to recover islets from the same organ. Here, the human pancreas was split in two with one portion using a TDE mixture that contained an optimal dose of thermolysin and HA while the other using the same dose of HA and an optimal dose of CHNP in place of thermolysin. The superiority of this new TDE mixture was confirmed when results from 76 human islet isolations were compared. This mixture gave significantly higher islet yields (n=4) when compared to islet isolations using enzyme mixtures that contained either C1116kDa and C2 and thermolysin (n=47) or C1100kDa and C2 and CHNP (n=25).
Translating the current data and information on collagenase and neutral protease activity detected by biochemical assays to the requirements for accepting specific lots for use in clinical human islet isolation procedures is challenging because of the complexity of ECM macromolecular structure. TDE manufacturers will continue to use a combination of enzymatic and physical assays to assess and control the quality of their products. However, a broader view must be taken to put these results in perspective of the end user who must decide on whether or not to use a specific lot of enzyme for human islet isolation. Adopting this perspective, several key conclusions can be made from this review and other factors noted in the scientific literature. First, the appropriate biochemical characterization of collagenase enzymes is critical since they are essential enzymes that degrade the ECM that hold cells to tissue. We recommend that Wunsch, CDA, and analytical anion exchange HPLC be used to characterize collagenase enzymes. Wunsch activity provides the best assessment of C2 catalytic activity; CDA provides the best assessment of active collagenase enzymes; and lastly analytical anion exchange HPLC provides a qualitative assessment of the different molecular forms of C1 and may provide some insight on the quality of C2. Second, neutral protease plays a critical role in the yield and quality of human islets recovered after islet isolation but insufficient information exists to predict the effectiveness of the type or dose of protease enzyme required for a successful isolation procedure. Additional studies need to be performed to confirm Minnesota’s and Uppsala’s results showing the benefit of using CHNP and adding clostripain (i.e., TLA) to the TDE mixtures, respectively. And third, qualification of TDEs for use in clinical islet isolations is an empirical science based on the expertise of individual laboratories performing the procedure. The initiative to standardize the islet isolation procedure (including enzyme composition) for the Immune Tolerance Network trials and later for the current Clinical Islet Transplantation consortium trial illustrates the problems encountered when using heterogeneous pancreata to isolate islets. Results from the Immune Tolerance trial noted that experience counts: only those centers with prior experience were able to achieve a high proportion of patients achieving insulin independence(81). For the Consortium trial, a cGMP protocol was used to standardize the islet isolation procedure but problems were encountered when a switch was made from HI to the Serva enzymes(4;62). This emphasizes the need for more rigorous correlation of the biochemical characteristics of TDEs to the islet yield, viability, and function. However, the linkage of the islet isolation results to the biochemical characterization of the TDEs will not happen without the participation of the relevant stakeholders to develop a standardized methodology for characterizing collagenase and neutral protease enzymes used in human islet isolation. As islet transplantation moves toward the path of a biological license application in the United States, the need to take this step becomes critical to ensure development of a consistent, cost effective treatment for patients with type 1 diabetes.
Summary
The success of islet transplantation is dependent upon the isolation of a sufficient number of high quality islets for use in the transplantation procedure. Successful isolation requires using a reliable source of tissue dissociating enzymes to free the islets from tissue. The FDA has finalized a set of guidelines to consider in manufacture of a cGMP islet product. A critical factor for controlling the manufacturing process is setting acceptance specifications for selecting lots of purified tissue dissociation enzyme products for use in the isolation procedure. This is a challenging problem because of the heterogeneity of the organ donor population and the inability to correlate enzyme activities defined by biochemical analysis to those required to digest the tissue substrates found in the pancreatic extracellular matrix. This article reviews the relevant histological characteristics of the pancreas for tissue dissociation; proposes a mechanism of how the tissue dissociation enzymes degrade the tissue substrate leading to release of cells from tissue; reviews relevant characteristics and assays used to assess tissue dissociation enzymes for cell isolation; and comments on the predictability of results from these assays on successful human islet isolation. The major conclusion from this analysis is that enzyme and physical assays are useful to ensure manufacture of consistent product but the translation of these results to predict successful islet isolation will require improved knowledge sharing among the key stakeholders in this area and development of new assays that are more predictive of use of tissue dissociation enzymes in islet isolation.
Acknowledgements
The project described was supported by Grant Numbers R43DK065467 and R43DK070402 and R43DK079413 and R44DK065467 from the National Institute of Diabetes and Digestive, and Kidney Disease. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Insitute of Diabetes and Digestive, and Kidney Disease or the National Institutes of Health. This research was also supported by SBIR Match Awards and a 21st Century Research and Technology Award from the State of Indiana.
Abbreviations
- C1
class I collagenase from C. histolyticum
- C1100kDa
Degraded form of class I collagenase with molecular weight of 100 kilo Daltons
- C1116kDa
Intact form of class I collagenase with molecular weight of 116 kilo Daltons
- C2
class II collagenase from C. histolyticum
- C2114kDa
Intact form of class II collagenase with a molecular weight of 114 kilo Daltons
- CBD
Collagen binding domain
- CDA
collagen degrading activity
- cGMP
current good manufacturing practice
- CHNP
C. histolyticum neutral protease
- ECM
extracellular matrix
- FALGPA
N-[3-(2-Furyl)acryloyl]-L-leucyl-glycyl-L-prolyl-L-alanine, a peptide substrate that detects collagenase activity
- FDA
United States Food and Drug Administration
- FITC
fluorescein isothiocyanate
- FITC-fibrils
fluorescein isothiocyanate coupled to type I calf skin collagen fibrils
- HA
CIzyme™ Collagenase HA manufactured by VitaCyte LLC
- HI
Liberase™ HI Purified Enzyme Blend manufactured by Roche Applied Sciences
- HPLC
High pressure liquid chromatography
- MTF
Liberase Mammalian Tissue Free
- NB
NB neutral protease from C. histolyticum manufactured by Serva GmbH
- NB-1
NB-1 purified collagenase from C. histolyticum manufactured by Serva GmbH
- TDE
tissue dissociation enzyme
- TLA
trypsin like activity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Participated in research design, data analysis, and writing of the paper, RCM has a financial interest in VitaCyte as cofounder of company
Participated in performance of the research, in data analysis, and writing of the paper, AGB has a financial interest in VitaCyte as employee of company
Participated in data analysis and writing of the paper, MLG has a financial interest in VitaCyte as employee of company
Participated in performance of the research, in data analysis, and writing of the paper, FED has a financial interest in VitaCyte as cofounder of company
Reference List
- 1.Bellin M, Barton F, Hering B, et al. Induction immunosuppression with T cell depleting antibodies facilitates long term insulin independence after islet allotransplantation in Type 1 diabetes. Xenotransplantation. 2009;16:541. [Google Scholar]
- 2.U.S. Food and Drug Administration. [Accessed August 23, 2010];Guidance for industry: considerations for allogenic pancreatic islet cell products. 2009 Available at: http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/ucm182440.htm.
- 3.International Society for Cellular Therapy. [Accessed August 23, 2010];Risk of Bovine Spongiform Encephalopathy (BSE) in Collagenase Enzymes. 2007 Available at: http://www.celltherapysociety.org/files/PDF/Resources/Risk_BSE_in_Collagenase_Enzymes.pdf.
- 4.Brandhorst H, Friberg A, Andersson HH, et al. The importance of tryptic-like activity in purified enzyme blends for efficient islet isolation. Transplantation. 2009;87:370. doi: 10.1097/TP.0b013e31819499f0. [DOI] [PubMed] [Google Scholar]
- 5.Kin T, O'Gorman D, Zhai X, et al. Nonsimultaneous administration of pancreas dissociation enzymes during islet isolation. Transplantation. 2009;87:1700. doi: 10.1097/TP.0b013e3181a60240. [DOI] [PubMed] [Google Scholar]
- 6.Szot GL, Lee MR, Tavakol MM, et al. Successful clinical islet isolation using a GMP-manufactured collagenase and neutral protease. Transplantation. 2009;88:753. doi: 10.1097/TP.0b013e3181b443ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balamurugan AN, Loganathan G, Anazawa T, et al. Improved method of human islet isolation for clinical transplantation using combination of Clostridium histolyticum neutral protease (Serva) and high proportion of intact C1 collagenase (VitaCyte) Xenotransplantation. 2009;16:545. [Google Scholar]
- 8.Brandhorst H, Friberg A, Nilsson B, et al. Large-scale comparison of Liberase HI and collagenase NB1 utilized for human islet isolation. Cell Transplant. 2010;19:3. doi: 10.3727/096368909X477507. [DOI] [PubMed] [Google Scholar]
- 9.Scharp DW. Commentary. Cell Transplant. 2003;2:299. [Google Scholar]
- 10.Johnson PR, White SA, London NJ. Collagenase and human islet isolation. Cell Transplantation. 1996;5:437. doi: 10.1177/096368979600500403. [DOI] [PubMed] [Google Scholar]
- 11.Kin T, Johnson PRV, Shapiro AMJ, Lakey JRT. Factors influencing the collagenase digestions phase of human islet isolation. Transplantation. 2007;83:7. doi: 10.1097/01.tp.0000243169.09644.e6. [DOI] [PubMed] [Google Scholar]
- 12.Kono T. Role of collagenases and other proteolytic enzymes in the dispersal of animal tissues. Biochim Biophys Acta. 1969;178:397. doi: 10.1016/0005-2744(69)90410-0. [DOI] [PubMed] [Google Scholar]
- 13.Hatton MW, Berry LR, Krestynski F, Sweeney GD, Regoeczi E. The role of proteolytic enzymes derived from crude bacterial collagenase in the liberation of hepatocytes from rat liver. Identification of two cell-liberating mechanisms. Eur J Biochem. 1983;137:311. doi: 10.1111/j.1432-1033.1983.tb07830.x. [DOI] [PubMed] [Google Scholar]
- 14.Hefley TJ, Stern PH, Brand JS. Enzymatic isolation of cells from neonatal calvaria using two purified enzymes from Clostridium histolyticum. Exp Cell Res. 1983;149:227. doi: 10.1016/0014-4827(83)90394-4. [DOI] [PubMed] [Google Scholar]
- 15.Suggs W, Van Wart H, Sharefkin JB. Enzymatic harvesting of adult human saphenous vein endothelial cells: use of a chemically defined combination of two purified enzymes to attain viable cell yields equal to those attained by crude bacterial collagenase preparations. J Vasc Surg. 1992;15:205. doi: 10.1067/mva.1992.30863. [DOI] [PubMed] [Google Scholar]
- 16.Wolters GH, Vos-Scheperkeuter GH, van Deijnen JH, van Schilfgaarde R. An analysis of the role of collagenase and protease in the enzymatic dissociation of the rat pancreas for islet isolation. Diabetologia. 1992;35:735. doi: 10.1007/BF00429093. [DOI] [PubMed] [Google Scholar]
- 17.Fetterhoff TJ, Cavanagh TJ, Wile KJ, et al. Human pancreatic dissociation using a purified enzyme blend. Transplant Proc. 1995;27:3282. [PubMed] [Google Scholar]
- 18.Linetsky E, Bottino R, Lehmann R, Alejandro R, Inverardi L, Ricordi C. Improved human islet isolation using a new enzyme blend, liberase. Diabetes. 1997;46:1120. doi: 10.2337/diab.46.7.1120. [DOI] [PubMed] [Google Scholar]
- 19.Olack BJ, Swanson CJ, Howard TK, Mohanakumar T. Improved method for the isolation and purification of human islets of langerhans using Liberase enzyme blend. Hum Immunol. 1999;60:1303. doi: 10.1016/s0198-8859(99)00118-4. [DOI] [PubMed] [Google Scholar]
- 20.Peterkofsky B. Bacterial Collagenase. Methods Enzymol. 1982;82:453. [Google Scholar]
- 21.Mookhtiar KA, Van Wart HE. Clostridium histolyticum collagenases: a new look at some old enzymes. Matrix Suppl. 1992;1:116. [PubMed] [Google Scholar]
- 22.Brockman DE. Histology and fine structure. In: Beger HG, Warshaw AL, Bucheler MW, Carr-Locke DL, Neoptolemos JP, Russell C, Sarr MG, editors. The Pancreas Volume 1. Malden MA: Blackwell Science; 1998. p. 19. [Google Scholar]
- 23.Klimstra DS, Hruban RH, Pitman MB. In: Histology for Pathologists. Third Edition. Mills SE, editor. Philadelphia: Lippincott, Williams & Wilkins; 2007. p. 723. [Google Scholar]
- 24.Carroll PB. Anatomy and Physiology of Islets of Langerhans. In: Ricordi C, editor. Pancreatic Islet Cell Transplantation. Austin: RG Landes; 1992. p. 7. [Google Scholar]
- 25.Moldovan S, Brunicardi FC. Endocrine pancreas: summary of observations generated by surgical fellows. World J Surg. 2001;25:468. doi: 10.1007/s002680020339. [DOI] [PubMed] [Google Scholar]
- 26.Lifson N, Kramlinger KG, Mayrand RR, Lender EJ. Blood flow to the rabbit pancreas with special reference to the islets of Langerhans. Gastroenterology. 1980;79:466. [PubMed] [Google Scholar]
- 27.van Deijnen JH, Hulstaert CE, Wolters GH, van Schilfgaarde R. Significance of the peri-insular extracellular matrix for islet isolation from the pancreas of rat, dog, pig, and man. Cell Tissue Res. 1992;267:139. doi: 10.1007/BF00318700. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe T, Yaegashi H, Koizumi M, Toyota T, Takahashi T. Changing distribution of islets in the developing human pancreas: a computer-assisted three-dimensional reconstruction study. Pancreas. 1999;18:349. doi: 10.1097/00006676-199905000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Lakey JR, Warnock GL, Shapiro AM, Korbutt GS, Ao Z, Kneteman NM, Rajotte RV. Intraductal collagenase delivery into the human pancreas using syringe loading or controlled perfusion. Cell Transplant. 1999;8:285. doi: 10.1177/096368979900800309. [DOI] [PubMed] [Google Scholar]
- 30.Ohtani O. Three-dimensional organization of the connective tissue fibers of the human pancreas: a scanning electron microscopic study of NaOH treated-tissues. Arch Histol Jpn. 1987;50:557. doi: 10.1679/aohc.50.557. [DOI] [PubMed] [Google Scholar]
- 31.Martinez-Hernadez A. Electron microscopy of the extracellular matrix: an overview. Methods Enzymol. 1987;145:78. doi: 10.1016/0076-6879(87)45004-0. [DOI] [PubMed] [Google Scholar]
- 32.Stendahl JC, Kaufman DB, Stupp SI. Extracellular matrix in pancreatic islets: relevance to scaffold design and transplantation. Cell Transplant. 2009;18:1. doi: 10.3727/096368909788237195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Virtanen I, Banerjee M, Palgi J, et al. Blood vessels of human islets of Langerhans are surrounded by a double basement membrane. Diabetologia. 2008;51:1181. doi: 10.1007/s00125-008-0997-9. [DOI] [PubMed] [Google Scholar]
- 34.Hughes SJ, Clark A, McShane P, Contractor HH, Gray DW, Johnson PR. Characterisation of collagen VI within the islet-exocrine interface of the human pancreas: implications for clinical islet isolation? Transplantation. 2006;81:423. doi: 10.1097/01.tp.0000197482.91227.df. [DOI] [PubMed] [Google Scholar]
- 35.Cross SE, Hughes SJ, Partridge CJ, Clark A, Gray DW, Johnson PR. Collagenase penetrates human pancreatic islets following standard intraductal administration. Transplantation. 2008;86:907. doi: 10.1097/TP.0b013e318186df87. [DOI] [PubMed] [Google Scholar]
- 36.Vosscheperkeuter GH, Vansuylichem PTR, Vonk MWA, Wolters GHJ, Vanschilfgaarde R. Histochemical Analysis of the Role of Class I and Class Ii Clostridium histolyticum Collagenase in the Degradation of Rat Pancreatic Extracellular Matrix for Islet Isolation. Cell Transplant. 1997;6:403. doi: 10.1177/096368979700600407. [DOI] [PubMed] [Google Scholar]
- 37.Birk DE, Bruckner P. Collagen suprastructures. Top Curr Chem. 2005;247:185. [Google Scholar]
- 38.Matsushita O, Jung CM, Katayama S, Minami J, Takahashi Y, Okabe A. (1999) Gene duplication and multiplicity of collagenases in Clostridium histolyticum. J Bacteriol. 1999;181:923. doi: 10.1128/jb.181.3.923-933.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshihara K, Matsushita O, Minami J, Okabe A. Cloning and nucleotide sequence analysis of the colH gene from Clostridium histolyticum encoding a collagenase and a gelatinase. J Bacteriol. 1994;176:6489. doi: 10.1128/jb.176.21.6489-6496.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burtscher H, Ambrosius D, Hesse F. Recombinant collagenase type I from Clostridium histolyticum and its use for isolating cells and groups of cells. 6,475,764. US Patent. 2002
- 41.Ambrosius D, Hesse F, Burtscher H. Recombinant collagenase type II from Clostridium histolyticum and its use for the isolation of cellular agglomerations. 1995 EP0677586 (A1). [PubMed] [Google Scholar]
- 42.Matsushita O, Okabe A. Clostridial hydrolytic enzymes degrading extracellular components. Toxicon. 2001;39:1769. doi: 10.1016/s0041-0101(01)00163-5. [DOI] [PubMed] [Google Scholar]
- 43.Matsushita O, Jung CM, Minami J, Katayama S, Nishi N, Okabe A. A study of the collagen-binding domain of a 116-kDa Clostridium histolyticum collagenase. J Biol Chem. 1998;273:3643. doi: 10.1074/jbc.273.6.3643. [DOI] [PubMed] [Google Scholar]
- 44.Matsushita O, Koide T, Kobayashi R, Nagata K, Okabe A. Substrate recognition by the collagen-binding domain of Clostridium histolyticum class I collagenase. J Biol Chem. 2001;276:8761. doi: 10.1074/jbc.M003450200. [DOI] [PubMed] [Google Scholar]
- 45.French MF, Mookhtiar KA, Van Wart HE. Limited proteolysis of type I collagen at hyperreactive sites by class I and II Clostridium histolyticum collagenases: complementary digestion patterns. Biochemistry. 1987;26:681. doi: 10.1021/bi00377a004. [DOI] [PubMed] [Google Scholar]
- 46.Wolters GH, Vos-Scheperkeuter GH, Lin HC, van Schilfgaarde R. Different roles of class I and class II Clostridium histolyticum collagenase in rat pancreatic islet isolation. Diabetes. 1995;44:227. doi: 10.2337/diab.44.2.227. [DOI] [PubMed] [Google Scholar]
- 47.Brandhorst H, Raemsch-Guenther N, Raemsch R. The ratio between collagenase class I and class II influences the efficient islet release from the rat pancreas. Transplantation. 2008;85:456. doi: 10.1097/TP.0b013e31816050c8. [DOI] [PubMed] [Google Scholar]
- 48.Kin T, Zhai X, O'Gorman D, Shapiro Shapiro. Detrimental effect of excessive collagenase class II on human islet isolation outcome. Transpl Int. 2008;21:1059. doi: 10.1111/j.1432-2277.2008.00734.x. [DOI] [PubMed] [Google Scholar]
- 49.Seifter S, Harper E. Collagenases. Methods Enzymol. 1970;19:613. [Google Scholar]
- 50.Matsubara H. Thermolysin. Methods Enzymol. 1970;19:642. [Google Scholar]
- 51.Griffin PJ, Fogarty WM. Physiochemical properties of the native, zinc- and manganese-prepared metalloprotease of Bacillus polymyxa. Appl Microbiol. 1973;26:191. doi: 10.1128/am.26.2.191-195.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sparrow LG, McQuade AB. Isolation by affinity chromatography of neutral proteinase from Clostridium histolyticum. Biochim Biophys Acta. 1973;302:90. doi: 10.1016/0005-2744(73)90011-9. [DOI] [PubMed] [Google Scholar]
- 53.Mitchell W, Harrington W. Clostripain. Methods Enzymol. 1970;19:635. [Google Scholar]
- 54.Kerr-Conte J, Vandewalle B, Moerman E, et al. Upgrading pretransplant human islet culture technology requires human serum combined with media renewal. Transplantation. 2010;89:1154. doi: 10.1097/TP.0b013e3181d154ac. [DOI] [PubMed] [Google Scholar]
- 55.Roberts RC. Protease inhibitors of human plasma. Alpha-2-macroglobulin. J Med. 1985;16:129. [PubMed] [Google Scholar]
- 56.Traverso LW, Abou-Zamzam AM. Activation of pancreatic proteolytic enzymes by commercial collagenases. Transplantation. 1978;25:226. doi: 10.1097/00007890-197804000-00015. [DOI] [PubMed] [Google Scholar]
- 57.Heiser A, Ulrichs K, Muller-Ruchholtz W. Isolation of porcine pancreatic islets: low trypsin activity during the isolation procedure guarantees reproducible high islet yields. J Clin Lab Anal. 1994;8:407. doi: 10.1002/jcla.1860080611. [DOI] [PubMed] [Google Scholar]
- 58.Basir I, van der Burg MP, Scheringa M, Tons A, Bouwman E. Improved outcome of pig islet isolation by Pefabloc inhibition of trypsin. Transplant Proc. 1997;29:1939. doi: 10.1016/s0041-1345(97)00168-1. [DOI] [PubMed] [Google Scholar]
- 59.White SA, Djaballah H, Hughes DP, Roberts DL, Contractor HH, Pathak S, London NJ. A preliminary study of the activation of endogenous pancreatic exocrine enzymes during automated porcine islet isolation. Cell Transplant. 1999;8:265. doi: 10.1177/096368979900800307. [DOI] [PubMed] [Google Scholar]
- 60.Lakey JR, Helms LM, Kin T, Korbutt GS, Rajotte RV, Shapiro AM, Warnock GL. Serine-protease inhibition during islet isolation increases islet yield from human pancreases with prolonged ischemia. Transplantation. 2001;72:565. doi: 10.1097/00007890-200108270-00003. [DOI] [PubMed] [Google Scholar]
- 61.Rose NL, Palcic MM, Helms LM, Lakey JR. Evaluation of Pefabloc as a serine protease inhibitor during human-islet isolation. Transplantation. 2003;75:462. doi: 10.1097/01.TP.0000046537.47139.CE. [DOI] [PubMed] [Google Scholar]
- 62.O'Gorman D, Kin T, Imes S, Pawlick R, Senior P, Shapiro AM. Comparison of human islet isolation outcomes using a new mammalian tissue-free enzyme versus Collagenase NB-1. Transplantation. 2010;90:255. doi: 10.1097/TP.0b013e3181e117ce. [DOI] [PubMed] [Google Scholar]
- 63.Kin T, O'Gorman D, Senior P, Shapiro AMJ. Experience of islet isolation without neutral protease supplementation. Islets. 2010;2:1. doi: 10.4161/isl.2.5.12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mitchell WM. The contamination of purified collagenase preparations by clostridiopeptidase B (clostripain): the potential effect on studies utilizing collagenase as a highly specific structural tool. John Hopkins Med J. 1970;127:192. [PubMed] [Google Scholar]
- 65.Wünsch E, Heidrich HG. Zur quantitativen bestimmung der kollagenase. Hoppe-Seyler's Z Physiol Chem. 1963;333:149. doi: 10.1515/bchm2.1963.333.1.149. [DOI] [PubMed] [Google Scholar]
- 66.McCarthy RC, Spurlin B, Wright MJ, Breite AG, Sturdevant LK, Dwulet CS, Dwulet FE. Development and characterization of a collagen degradation assay to assess purified collagenase used in islet isolation. Transplant Proc. 2008;40:339. doi: 10.1016/j.transproceed.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 67.Kin T, Zhai X, Murdoch TB, Salam A, Shapiro AM, Lakey JR. Enhancing the success of human islet isolation through optimization and characterization of pancreas dissociation enzyme. Am J Transplant. 2007;7:1233. doi: 10.1111/j.1600-6143.2007.01760.x. [DOI] [PubMed] [Google Scholar]
- 68.Mandl I, MacLennan JD, Howes EL. Isolation and characterization of proteinase and collagenase from Cl. histolyticum. J Clin Invest. 1953;32:1323. doi: 10.1172/JCI102861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hefley TJ. Utilization of FPLC-purified bacterial collagenase for the isolation of cells from bone. J Bone and Mineral Research. 1987;2:505–516. doi: 10.1002/jbmr.5650020607. [DOI] [PubMed] [Google Scholar]
- 70.Balamurugan AN, Breite AG, Anazawa T, et al. Successful human islet isolation and transplantation indicating the importance of class 1 collagenase and collagen degradation activity assay. Transplantation. 2010;89:954. doi: 10.1097/TP.0b013e3181d21e9a. [DOI] [PubMed] [Google Scholar]
- 71.Antonioli B, Fermo I, Cainarca S, et al. Characterization of collagenase blend enzymes for human islet transplantation. Transplantation. 2007;84:568. doi: 10.1097/01.tp.0000295719.88525.60. [DOI] [PubMed] [Google Scholar]
- 72.Bond MD, Van Wart HE. Characterization of the individual collagenases from Clostridium histolyticum. Biochemistry. 1984;23:3085. doi: 10.1021/bi00308a036. [DOI] [PubMed] [Google Scholar]
- 73.Rawlings ND, Barrett AJ, Bateman A. MEROPS: the peptidase database. Nucleic Acids Res. 2010;38:D227. doi: 10.1093/nar/gkp971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin Y, Means GE, Feeney RE. The action of proteolytic enzymes on N,N-dimethyl proteins. Basis for a microassay for proteolytic enzymes. J Biol Chem. 1969;244:789. [PubMed] [Google Scholar]
- 75.Breite AG, Dwulet FE, McCarthy RC. Tissue Dissociation Enzyme Neutral Protease Assessment. Transplant Proc. 2010;42:2052. doi: 10.1016/j.transproceed.2010.05.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lakey JR, Warnock GL, Rajotte RV, et al. Variables in organ donors that affect the recovery of human islets of Langerhans. Transplantation. 1996;61:1047. doi: 10.1097/00007890-199604150-00010. [DOI] [PubMed] [Google Scholar]
- 77.Nano R, Clissi B, Melzi R, et al. Islet isolation for allotransplantation: variables associated with successful islet yield and graft function. Diabetologia. 2005;48:906. doi: 10.1007/s00125-005-1725-3. [DOI] [PubMed] [Google Scholar]
- 78.O'Gorman D, Kin T, Murdoch T. The standardization of pancreatic donors for islet isolation. Transplant Proc. 2005;37:1309. doi: 10.1016/j.transproceed.2004.12.087. [DOI] [PubMed] [Google Scholar]
- 79.Hanley SC, Paraskevas S, Rosenberg L. Donor and isolation variables predicting human islet isolation success. Transplantation. 2008;85:950. doi: 10.1097/TP.0b013e3181683df5. [DOI] [PubMed] [Google Scholar]
- 80.Kaddis JS, Danobeitia JS, Niland JC, Stiller T, Fernandez LA. Multicenter analysis of novel and established variables associated with successful human islet isolation outcomes. Am J Transplant. 2010;10:646. doi: 10.1111/j.1600-6143.2009.02962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shapiro AM, Ricordi C, Hering BJ, et al. International trial of the Edmonton protocol for islet transplantation. New Eng J Med. 2006;355:1318. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 82.Van Wart HE, Steinbrink DR. A continuous spectrophotometric assay for Clostridium histolyticum collagenase. Anal Biochem. 1981;113:356. doi: 10.1016/0003-2697(81)90089-0. [DOI] [PubMed] [Google Scholar]
- 83.Mandl I, Keller S, Manahan J. Multiplicity of Clostridium histolytcum collagenases. Biochemistry. 1964;3:1737. doi: 10.1021/bi00899a026. [DOI] [PubMed] [Google Scholar]
- 84.Chavira R, Burnett TJ, Hageman JH. Assaying proteases with azocoll. Anal Biochem. 1984;136:446. doi: 10.1016/0003-2697(84)90242-2. [DOI] [PubMed] [Google Scholar]
- 85.Bucher P, Mathe Z, Morel P, et al. Assessment of a novel two-component enzyme preparation for human islet isolation and transplantation. Transplantation. 2005;79:91. doi: 10.1097/01.tp.0000147344.73915.c8. [DOI] [PubMed] [Google Scholar]
- 86.Twining SS. Fluorescein isothiocyanate-labeled casein assay for proteolytic enzymes. Anal Biochem. 1984;143:30. doi: 10.1016/0003-2697(84)90553-0. [DOI] [PubMed] [Google Scholar]
- 87. [Accessed August 23, 2010];Sigma neutral protease assay. http://www.sigmaaldrich.com/etc/medialib/docs/Sigma/Enzyme_Assay/p1512enz.Par.0001.File.tmp/p1512enz.pdf.
- 88. [Accessed August 23, 2010];Worthington neutral protease assay. http://www.worthington-biochem.com/DISP/assay.html.
- 89.Wilson JJ, Matsushita O, Okabe A, Sakon J. A bacterial collagen-binidng domain with novel calcium-binding motif controls domain orientation. EMBO J. 2003;8:1743. doi: 10.1093/emboj/cdg172. [DOI] [PMC free article] [PubMed] [Google Scholar]