Abstract
Voltage-gated sodium channels are the primary target of pyrethroid insecticides. Numerous point mutations in sodium channel genes have been identified in pyrethroid-resistant insect species, and many have been confirmed to reduce or abolish sensitivity of channels expressed in Xenopus oocytes to pyrethroids. Recently, several novel mutations were reported in sodium channel genes of pyrethroid-resistant Aedes mosquito populations. One of the mutations is a phenylalanine (F) to cysteine (C) change in segment 6 of domain III (IIIS6) of the Aedes mosquito sodium channel. Curiously, a previous study showed that alanine substitution of this F did not alter the action of deltamethrin, a type II pyrethroid, on a cockroach sodium channel. In this study, we changed this F to C in a pyrethroid-sensitive cockroach sodium channel and examined mutant channel sensitivity to permethrin as well as five other type I or type II pyrethroids in Xenopus oocytes. Interestingly, the F to C mutation drastically reduced channel sensitivity to three type I pyrethroids, permethrin, NRDC 157 (a deltamethrin analogue lacking the α-cyano group) and bioresemthrin, but not to three type II pyrethroids, cypermethrin, deltamethrin and cyhalothrin. These results confirm the involvement of the F to C mutation in permethrin resistance, and raise the possibility that rotation of type I and type II pyrethroids might be considered in the control of insect pest populations where this particular mutation is present.
Keywords: Knockdown resistance, sodium channel, pyrethroid resistance, Type I & type II pyrethroids, Xenopus oocyte expression system
1. Introduction
Pyrethroid insecticides are a large class of synthetic analogues of natural pyrethrins from the flower extracts of Chrysanthemum spp. They are broad-spectrum pesticides and are used in the control of virtually all agriculturally and medically important arthropod pests. Pyrethorids are classified as Type I or type II according to chemical structure. Type I pyrethroids, such as bioresmethrin and permethrin, lack an α-cyano group at the phenoxybenzyl alcohol position, whereas type II pyrethroids, such as deltamethrin and cyhalothrin, contain an α-cyano-3-phenoxybenzyl alcohol moiety. Pyrethroids target voltage-gated sodium channels which are responsible for the initiation and propagation of action potentials in excitable cells. They prolong the opening of sodium channels, resulting in repetitive firing (type I pyrethroids) or membrane depolarization leading to conductance block (type II pyrethroids) of the nervous system (Narahashi, 1988, 2000). A major threat for the sustained use of pyrethroids is the development of pest resistance to these compounds. Understanding of the resistance mechanisms and development of simple and accurate diagnostic tools to monitor the presence of resistance gene mutations is critical for effective management of pyrethroid resistance and sustainable use of pyrethroid insecticides in the future.
One of the most important forms of pyrethroid resistance is known as knockdown resistance (kdr), which has been documented in numerous other pest species (Soderlund and Bloomquist, 1990). kdr is caused by point mutations in the sodium channel gene. To date, about 25 sodium channel mutations have been found to be associated with kdr or kdr-like pyrethroid-resistance in various pest species (Soderlund, 2005). Sodium channels are large transmembrane proteins containing four homologous domains (I to IV), each formed by six membrane spanning segments (S1 to S6) connected by intracellular and extracellular loops. Some kdr or kdr-like mutations are clustered in S5 and S6 of domain II or in S6 of domains I and III, whereas others are scattered, such as in intracellular linkers. Many of them have been confirmed to reduce sensitivity of sodium channels expressed in Xenopus oocytes to pyrethroids (Soderlund, 2005, Dong, 2007, Davies et al., 2007). Interestingly, whereas some of these sodium channel mutations are found in multiple species, others are only found in a given species. For example, a leucine to phenylalanine (L to F) change in IIS6 was the first kdr mutation detected in house flies and cockroaches (L1014F in the house fly sodium channel; and L993F in the cockroach sodium channel). Subsequently, the same mutation was detected in other species, including Anopheles gambiae (Martinez-Torres et al., 1999b) and Culex quinquefasciatus (Xu et al., 2005).
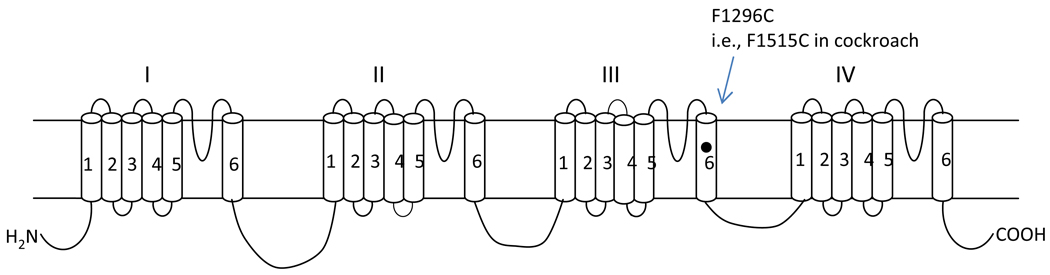
Several novel sodium channel mutations recently have been identified in Aedes aegypti mosquitoes (Brengues et al., 2003; Saavedra-Rodriguez et al., 2009; Chang et al., 2009; Kawada et al., 2009). One of these mutations is a phenylalanine to cysteine change (F1269C) (Fig. 1), identified in permethrin-resistant Aedes populations in Thailand (Yanola et al., 2008) and Vietnam (Kawada et al., 2009). However, we have previously shown that alanine substitution of this F (position 1515) did not alter the action of deltamethrin (Du et al., 2009b) on cockroach sodium channels, even though several neighboring residues in IIIS6 have profound effects on the action of pyrethroids (Du et al., 2009b). To determine whether the F to C substitution has a different effect from the F1515A mutation on pyrethroid action, we examined the sensitivity of the F1515C mutant sodium channel in Xenopus oocytes to six pyrethroids including permethrin.
Fig. 1.
Position of F1296C in the voltage-gated sodium channel protein. The sodium channel protein consists of four homologous domains (I – IV), each having six transmembrane segments (S1–S6). F1269C is detected in Aedes aegypti (Yanola et al., 2008; Kawada et al., 2009). The corresponding amino acid position in the cockroach sodium channel is F1515C according to the deduced amino acid sequence in GenBank (Accession number: U73583).
2. Materials and Methods
2.1 Site-Directed Mutagenesis
cDNA from the cockroach sodium channel, BgNav1-1a (pyrethroid-sensitive), was used to generate the F1515C construct. Site-directed mutagenesis was performed by polymerase chain reaction (PCR) using mutant primers and Pfu Turbo DNA polymerase (Stratagene, La Jolla, CA). The mutagenesis result was verified by DNA sequencing.
2.2 Expression of BgNav Sodium Channels in Xenpous laevis Oocytes
The procedures for oocyte preparation and cRNA injection were identical to those described previously (Du et al. 2009a). For robust expression of BgNav sodium channels, cRNA was co-injected into oocytes with cRNA encoding the Drosophila melanogaster tipE auxiliary subunit (1:1 ratio), which enhances the expression of insect sodium channels in oocytes.
2.3 Electrophysiological Recording and Analysis
Methods for electrophysiological recording and data analysis were identical to those described previously (Du et al. 2009a). The pyrethroid-induced tail current was recorded during a 100-pulse train of 5-ms depolarization from −120 to 0 mV with a 5-ms interpulse interval (Vais et al., 2000). Percentage of channels modified by pyrethroids was calculated using the method by Tatebayashi and Narahashi (1994).
Data are presented as mean ± S.D. Statistical significance was determined by Student’s t-test, and significant values were set at p < 0.05 or as indicated in the table and figure legends.
2.5 Chemicals
Pyrethroids were generous gifts from Ralf Nauen (Bayer CropScience AG, Monheim, Germany) and Bhupinder Khambay (Rothamsted Research Ltd). The purities of different pyrethroids range from 99.3% to 99.8%. Stock solutions of pyrethroids (100 mM) were dissolved in dimethyl sulfoxide (DMSO). The working concentration was prepared in ND96 recording solution just prior to the experiments. The concentration of DMSO in the final solution was < 0.5%, which had no effect on the function of sodium channels in the experiments. The method for application of chemicals in the recording system was identical to that described by Tatebayashi and Narahashi (1994). The effects of pyrethroids were measured 10 min after application.
3. Results and Discussion
We introduced F1515C into a pyrethroid-sensitive cockroach sodium channel construct (BgNav1-1a) by site-directed mutagenesis. The mutant channel produced sufficient sodium current in Xenopus oocytes for functional and pharmacological analysis. The F1515C substitution did not alter the voltage-dependences of activation or inactivation (Table 1).
Table 1.
Voltage-dependences of activation and inactivation of BgNav1-1a and F1515C channels
| Na+ Channel Type |
Activation | Inactivation | |||
|---|---|---|---|---|---|
| V1/2 (mV) | k (mV) | V1/2 (mV) | k (mV) | n | |
| BgNav1-1a | −28.23 ± 0.54 | 5.09 ± 0.94 | −48.57 ± 0.13 | 5.02 ± 0.18 | 8 |
| F1515C | −30.72 ± 1.38 | 4.41 ± 1.08 | −48.98 ± 1.58 | 4.79 ± 0.18 | 8 |
The voltage dependences of conductance and inactivation were fitted with a two-state Boltzmann equation to determine V1/2, the voltage for half- maximal conductance or inactivation, and k, the slope factor for conductance or inactivation. The values in the table represent the mean ± S.D. and n is the number of oocytes used.
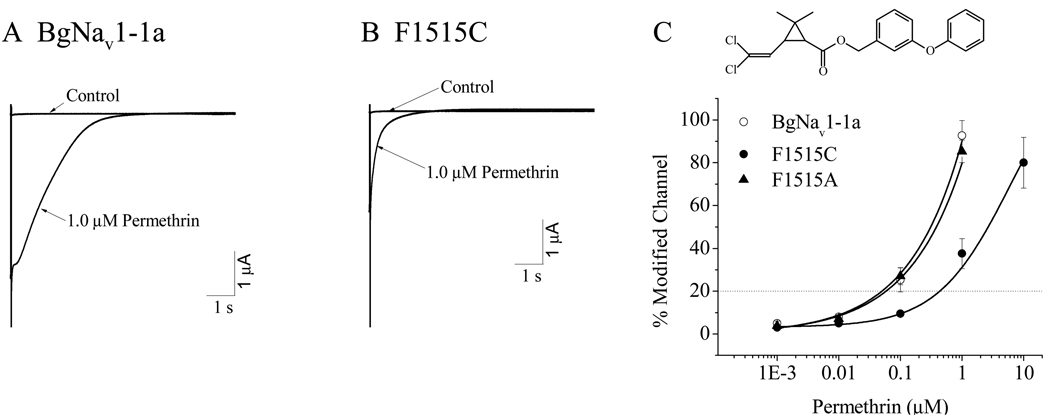
Since the mutation was detected in permethrin-resistant populations, we first evaluated the action of permethrin on the mutant channel. The amplitudes of tail currents induced by 1.0 µM permethrin were recorded for BgNav1-1a (Fig. 2A) and F1515C mutant channel (Fig. 2B) and the percentages of channel modification were plotted (Fig. 2C). The mutation reduced channel sensitivity to permethrin by 10- fold with the EC20 values of 0.05 µM and 0.5 µM, for BgNav1-1a and F1515C mutant channels, respectively. Interestingly, we have previously shown that the F1515A substitution did not alter sodium channel sensitivity to deltamethrin (Du et al., 2009b). We therefore tested the sensitivity of the F1515A channel to permethrin. The F1515A substitution did not alter the channel sensitivity to permethrin either (Fig. 2C).
Fig. 2.
The F1515C mutation reduced the BgNav1-1a channel sensitivity to permethrin. A and B. Permethrin-induced tail currents in BgNav1-1a (A) and F1515C (B) cockroach sodium channels. Tail currents were elicited by a 67-Hz train of 100 5-ms depolarization from −120 to 0 mV. C. Dose-response curves of permethrin on BgNav1-1a, F1515A and F1515C cockroach sodium channels. The chemical structure of permethrin is shown at the top. Percentage of channel modification by permethrin was calculated using the equation M = {[Itail/(Eh – ENa)]/[INa/(Et – ENa)]} × 100 (Tatebayashi and Narahashi, 1994), where Itail is the maximal tail current amplitude, Eh is the potential to which the membrane is repolarized, ENa is the reversal potential for sodium current determined from the current-voltage curve, INa is the amplitude of the peak current during depolarization before permethrin exposure, and Et is the potential of step depolarization.
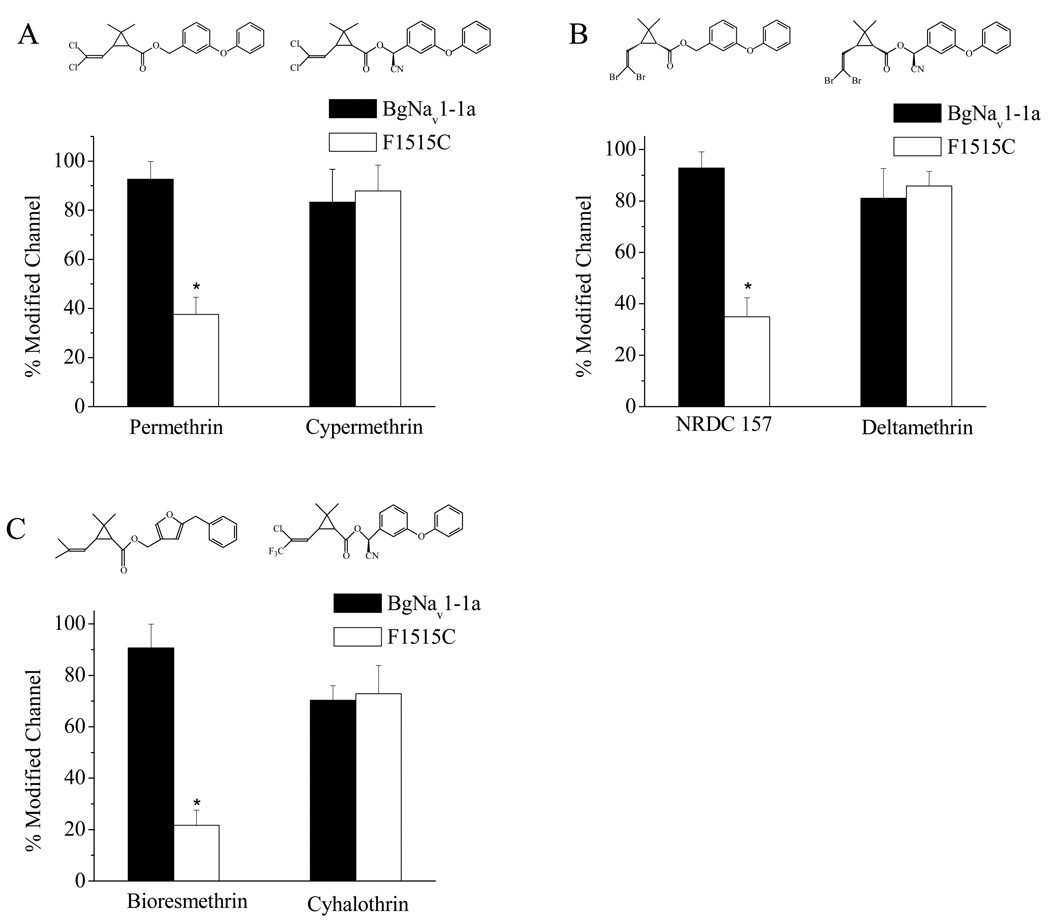
To determine whether this mutation also alters sodium channel sensitivity to other pyrethroids, we tested two more type I pyrethroids, bioresmethrin and NRDC157, and three type II pyrethroids, cypermethrin, cyhalothrin and deltamethrin. Interestingly, F1515C only reduced sodium channel sensitivity to bioresmethrin and NRDC157, but not to cypermethrin, cyhalothrin and deltamethrin (Fig. 3). Permethrin differs structurally to cypermethrin only by the absence of the α-cyano group. NRDC157 is a deltamethrin analogue lacking the α-cyano group. Our results here demonstrate that the F1515C mutation reduces the cockroach channel sensitivity to only type I pyrethroids, but not type II pyrethroids.
Fig. 3.
The F1515C mutation altered the BgNav1-1a channel sensitivity only to type I pyrethroids, but not to type II pyrethroids. Percentages of channel modification by pyrethroids were determined as described in Fig. 2 legend. A. Permethrin and cypermethrin. B. NRDC 157 and deltamethrin. C. Bioresmethrin and cyhalothrin. 1 µM of each pyrethroid was used. Chemical structures of pyrethroids are shown at the top. *Statistically significant differences compared with BgNav1-1a.
In a previous study, we found that several residues (e.g., a glycine and two neighboring positively charged residues) in the second linker connecting domains II and III are critical for the action of type II pyrethroids, but not for the action of type I pyrethroids (Du et al., 2009a). Here we identified an amino acid substitution, F1515C, which alters the action of type I pyrethroids, but not type II pyrethroids. Thus, there appears to be pyrethroid type-specific sodium channel mutations that selectively affect the action of type I or type II pyrethroids. These results are interesting because, although both type I and type II pyrethroids are sodium channel gating modifiers, they can induce distinct symptoms at the whole organism level, and cause some distinct effects on sodium channel gating (Gammon et al, 1981). For example, both types of pyrethroids prolong the opening of sodium channels by inhibiting deactivation and inactivation, resulting in a slow decaying sodium tail current associated with repolarization (Lund and Narahashi, 1981; Vijverberg et al., 1982). However, type II pyrethroids alter gating kinetics more drastically than type I pyrethroids, causing much more slowly decaying tail currents upon repolarization. The underlying basis of pyrethroid type-specific toxicology and sodium channel gating modification are poorly understood at the molecular level. Identification of residues that affect type-specific interactions therefore represents a first step toward comprehension of distinct symptoms and/or modifications of sodium channel gating induced by type I and type II pyrethroids.
Computer modeling of the pyrethroid-binding site, using the crystal structure of the Kv1.2 potassium channel as a template, predicts that the pyrethroid receptor site is located in a hydrophobic cavity delimited by the IIS4-S5 linker and IIS5 and IIIS6 helices (O’Reilly et al., 2006). Recent systematic alanine substitutions of residues in IIIS6 revealed four additional residues, I1514A, G1516A, F1518A and N1522A, that are critical for the action of pyrethroids (Du et al., 2009b), providing additional experimental evidence for the involvement of IIIS6 in the pyrethroid binding and action. It is interesting that alanine substitution of F1515 (F1515A) did not alter the action of both type I and type II pyrethroids (Du et al., 2009b and Fig. 2), but the F1515C substitution caused increased resistance to type I pyrethroids, but not type II pyrethroids. These results suggest that commonly used alanine scanning mutagenesis might be ineffective in determining the involvement of some sodium channel residues in pyrethroid action. In the case of F1515, it appears that F1515 is not critical for the pyrethroid binding; however, a subtle change of amino acid side chain at this position (i.e., those of alanine and phenylalanine vs. that of cysteine) alters the access of type I pyrethroids, but not type II pyrethroids, to the receptor site. The fact that the F1515C mutation is selected in Aedes populations after repeated use of permethrin provides an excellent example of how amazing and powerful natural selection can be.
Use of pyrethroid insecticides is a major strategy for controlling mosquitoes, an important vector of many serious human diseases, such as malaria and Dengue fever. Pyrethroids are used in indoor residual spraying, insecticide-impregnated bed nets, and mosquito coils. Prior to this study, all kdr or kdr-like mutations reduced channel sensitivity to both type I and type II pyrethroids (Soderlund, 2005, Dong, 2007). Therefore, once a pest population develops resistance to one pyrethroid, the population is cross-resistant to the entire class of pyrethroids. The F1515C mutation is the first kdr-like mutation that confers resistance to type I, but not type II pyrethroids. An important practical implication of these findings is that use of type II pyrethroids could be recommended to control permethrin-resistant populations in which the F1515C mutation is found.
Acknowledgement
The authors thank Dr. Kris Silver for critical review of the manuscript and Drs. Ralf Nauen (Bayer CropScience AG) and Bhupinder Khambay (Rothamsted Research Ltd) for providing the pyrethroids used in this study. The work is supported by a grant from the National Institutes of Health (GM057440) to KD. Zhaonong Hu is partially supported by the China Scholarship Council.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brengues C, Hawkes NJ, Chandre F, McCaroll L, Duchon S, Guillet P, Manguin S, Morgan JC, Hemingway J. Pyrethroid and DDT cross-resistance in Aedes aegypti is correlated with novel mutations in the voltage-gated sodium channel gene. Med. Vet. Entomol. 2003;17:87–94. doi: 10.1046/j.1365-2915.2003.00412.x. [DOI] [PubMed] [Google Scholar]
- Chang C, Shen WK, Wang TT, Lin YH, Hsu EL, Dai SM. A novel amino acid substitution in a voltage-gated sodium channel is associated with knockdown resistance to permethrin in Aedes aegypti. Insect Biochem. Mol. Biol. 2009;39:272–278. doi: 10.1016/j.ibmb.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Davies TGE, Field LM, Usherwood PNR, Williamson MS. DDT, pyrethrins, pyrethroids and insect sodium channels. IUBMB Life. 2007;59:151–162. doi: 10.1080/15216540701352042. [DOI] [PubMed] [Google Scholar]
- Dong K. Insect sodium channels and insecticide resistance. Invert. Neurosci. 2007;7:17–30. doi: 10.1007/s10158-006-0036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Nomura Y, Luo N, Liu Z, Lee JE, Khambay B, Dong K. Molecular determinants on the insect sodium channel for the specific action of type II pyrethroid insecticides. Toxicol. Appl. Pharmacol. 2009a;234(2):266–272. doi: 10.1016/j.taap.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Lee JE, Nomura Y, Zhang T, Zhorov BS, Dong K. Identification of a cluster of residues in transmembrane segment 6 of domain III of the cockroach sodium channel essential for the action of pyrethroid insecticides. Biochem. J. 2009b;419(2):377–385. doi: 10.1042/BJ20082082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammon DW, Brown MA, Casida JE. Two classes of pyrethroid action in the cockroach, Pestic. Biochem. Physiol. 1981;15:181–191. [Google Scholar]
- Lund AE, Narahashi T. Kinetics of sodium channel modification by the insecticide tetramethrin in squid axon membranes. J. Pharmacol. Exp. Ther. 1981;219:463–473. [PubMed] [Google Scholar]
- Kawada H, Higa Y, Komagata O, Kasai S, Tomita T, Kawada H, Higa Y, Komagata O, Kasai S, Tomita T, Thi Yen N, Loan LL, Sánchez RA, Takagi M. Widespread distribution of a newly found point mutation in voltage-gated sodium channel in pyrethroid-resistant Aedes aegypti populations in Vietnam. PloS. Negl. Trop. Dis. 6. 2009;3(10):e527. doi: 10.1371/journal.pntd.0000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Torres D, Chevillon C, Brun-Barale A, Berge JB, Pasteur N, Pauron D. Voltage-dependent Na+ channels in pyrethroid resistant Culex pipiens L mosquitoes. Pest Sci. 1999b;55:1012–1020. [Google Scholar]
- Narahashi T. Molecular and cellular approaches to neurotoxicology: past, present and future. In: Lunt GG, editor. Neurotox ‘88: molecular basis of drug and pesticide action. New York: Elsevier; 1988. pp. 563–582. [Google Scholar]
- Narahashi T. Neuroreceptors and ion channels as the basis for drug action: past, present and future. J. Pharmacol. Exp. Ther. 2000;294:1–26. [PubMed] [Google Scholar]
- O’Reilly AQ, Khambay BPS, Williamson MS, Field LM, Wallace BA, Davies TGE. Modeling insecticide binding sites at the voltage-gated sodium channel. Biochem. J. 2006;396:255–263. doi: 10.1042/BJ20051925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra-Rodriguez K, Urdaneta-Marquez L, Rajatileka S, Moulton M, Flores AE, Femandez-Salas I, Bisset J, Rodriguea M, McCall PJ, Donnelly MJ, Ranson H, Hemingway J, Black WC., 4th A mutation in the voltage-gated sodium channel gene associated with pyrethroid resistance in Latin American Aedes aegypti. Insect Mol. Biol. 2007;16:785–798. doi: 10.1111/j.1365-2583.2007.00774.x. [DOI] [PubMed] [Google Scholar]
- Soderlund DM, Bloomquist JR. Molecular mechanisms of insecticide resistance. In: Roush RT, Tabashnik BE, editors. Pesticide resistance in arthropods. New York: Chapman and Hall; 1990. pp. 58–96. [Google Scholar]
- Soderlund D. Sodium channels. In: Gilbert LI, Iatrou K, Gill SS, editors. Comprehensive insect science. Pharmacology. vol 5. Amsterdam: Elsevier B.V.; 2005. pp. 1–24. [Google Scholar]
- Tatebayashi H, Narahashi T. Differential mechanism of action of the pyrethroid tetramethrin on tertodotoxin-sensitive and tetrodotoxin-resistant sodium channels. J. Pharmacol. Exp.Ther. 1994;270:595–603. [PubMed] [Google Scholar]
- Vais H, Williamson MS, Goodson SJ, Devonshire AL, Warmke JW, Usherwood PNR, Cohen C. Activation of Drosophila sodium channels promotes modification by deltamethrin: reductions in affinity caused by knock-down resistance mutations. J. Gen. Physiol. 2000a;115:305–318. doi: 10.1085/jgp.115.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijverberg HP, van der Zalm JM, van der Bercken J. Similar mode of action of pyrethroids and DDT on sodium channel gating in myelinated nerves. Nature. 1982;295:601–603. doi: 10.1038/295601a0. [DOI] [PubMed] [Google Scholar]
- Xu Q, Liu H, Zhang L, Liu N. Resistance in the mosquito, Culex quinquefasciatus, and possible mechanisms for resistance. Pest Manag. Sci. 2005;61:1096–1102. doi: 10.1002/ps.1090. [DOI] [PubMed] [Google Scholar]
- Yanola J, Somboon P, Prapanthadara L. A novel point mutation in the Aedes aegypti voltage-gated sodium channel gene associated with permethrin resistance. The 2nd International Conference on Dengue and Dengue Haemorhagic Fever; Oct. 15–17, 2008; Phuket, Thailand. 2008. [Google Scholar]