Abstract
Nociception modulates heart rate (HR) and mean arterial pressure (MAP), suggesting their use as indicators of pain in animals. We explored this with telemetric recording in unrestrained control and neuropathic (spinal nerve ligation) rats. Plantar stimulation was performed emulating techniques commonly used to measure pain, specifically brush stroke, von Frey fiber application, noxious pin stimulation, acetone for cooling, and radiant heating, while recording MAP, HR, and specific evoked somatomotor behaviors (none; simple withdrawal; or sustained lifting, shaking and grooming representing hyperalgesia). Pin produced elevations in both HR and MAP, and greater responses accompanied hyperalgesia behavior compared to simple withdrawal. Von Frey stimulation depressed MAP, and increased HR only when stimulation produced hyperalgesia behavior, suggesting that minimal nociception occurs without this behavior. Brush increased MAP even when no movement was evoked. Cold elevated both HR and MAP whether or not there was withdrawal, but MAP increased more when withdrawal was triggered. Heating consistently depressed HR and MAP, independent of behavior. Other than a greater HR response to pin in animals made hyperalgesic by injury, cardiovascular events evoked by stimulation did not differ between control and neuropathic animals. We conclude that a) thermoregulation rather than pain may dominate responses to heat and cooling stimuli; b) brush and cooling stimuli may be perceived and produce cardiovascular activation without nocifensive withdrawal; c) sensations that produce hyperalgesia behavior are accompanied by greater cardiovascular activation than those producing simple withdrawal; and d) von Frey stimulation lacks cardiovascular evidence of nociception except when hyperalgesia behavior is evoked.
Keywords: Neuropathic pain, animal pain tests, nociception, pain models, rat, cardiovascular effects of pain
1. Introduction
A fundamental requirement of animal pain research is interpretation of behaviors that are assumed to be triggered by the perception of pain. Although reflex movements are commonly used as a proxy for animal pain, the validity of this approach is uncertain [25,45]. Ultimately, the animal’s experience cannot be known, and assumptions regarding the level of pain must be made on the basis of inferential links. In this context, it would be useful if physiological measures could be used as an additional, quantifiable means to signify pain. Such an experimental strategy echoes early studies by Sherrington and others [46], in which nociception was inferred following cutaneous stimulation that produced somatomotor reactions including “mimetic movements simulating expression of certain affective states” that persisted following decerebration. These behaviors and the accompanying elevation of blood pressure (BP) were termed pseaudaffective reflexes.
Experimentation upon awake humans supports the view that both heart rate (HR) and BP are increased by activation of the pain projection system [20,29,36,42]. Noxious stimuli that are clearly painful in humans also increase HR and BP in anesthetized animals [1,16,32,33,37]. However, general anesthesia may alter the magnitude and even direction of cardiovascular responses to sensory stimulation [11,19,30,34], emphasizing the value of recording from awake animals. Such studies that avoid anesthesia have confirmed a hypertensive and tachycardic response to sustained noxious stimuli in awake animals, for instance following cutaneous formalin injection [43,49], intrapericardial bradykinin [30], or colorectal and duodenal distension [34,35]. The high degree to which HR and BP changes parallel somatomotor manifestations of pain in these models suggests that cardiovascular activation may be employed as a reliable marker of nociception, as suggested by Taylor and others [28].
Hemodynamic activation cannot be assumed to accompany all noxious stimulation, however. For instance, it is recognized that noxious traction or chemical irritation of the mesentery produces immediate BP depression in rats and humans [12,15]. Additionally, only hemodynamic events triggered by noxious stimulation that persists many seconds have so far been examined. Therefore, the goal of the present study is to determine the nature of HR and BP changes that may accompanying brief, escapable sensory stimulation in awake animals, such as the thermal and mechanical stimuli that are now commonly used for testing nociception in animal subjects, in order to determine if cardiovascular measures might be a suitable supplement to somatomotor behavior in these standard tests. It is known that a variety of non-noxious stimuli that alert or stress animal subjects may also activate circulatory parameters [40,50]. To identify the extent to which cardiovascular activation may be nonspecific as an indicator of animal pain, we included stimuli that are non-noxious, and examined animals in their baseline state as well following nerve injury to determine if cardiovascular responses to sensory stimuli are exaggerated in the context of enhanced sensory sensitivity. Finally, since the somatomotor behavior induced by cutaneous stimulation is not uniform, we sought to identify if certain behaviors are particularly associated with cardiovascular activation.
2. Experimental Procedures
2.1 Experimental animals
Complete data across the entire duration of the protocol were obtained from a total of 20 male Sprague-Dawley rats (150–175 g at the initiation of the protocol) that were obtained from a single vendor (Taconic Farms, Inc., Hudson, New York). Animals were housed individually in a room maintained at 22 ± 0.5°C and constant humidity (60 ± 15%) with an alternating 12-h light-dark cycle. Food and water were available ad libitum throughout the experiments. All procedures were approved by the Animal Care and Use Committees of the Zablocki VA Medical Center and Medical College of Wisconsin (Milwaukee, Wisconsin). Baseline HR and BP recordings from some of the rats in this study were used for a prior investigation [Pain manuscript D-08-5230R2, accepted for publication]. No pharmacological interventions in that study preceded the recording of the data reported in this study.
2.2 Surgery
BP was monitored by telemetry using a PA-C10 transmitter (Data Sciences International (DSI), St. Paul, MN), which was implanted during anesthesia with isoflurane (1.5–2.0%) in oxygen. After making a left inguinal incision, the cannula (0.43 mm outer diameter polyethylene) attached to the transmitter was inserted into the left femoral artery, with care taken to avoid manipulation of the adjacent femoral nerve, and the transmitter fixed in a subcutaneous pocket on the left flank of the rat. A redundant loop in the cannula allowed for growth of the rat. The incision was closed with 3-0 silk suture. Following surgery, animals were treated with a single dose of buprenorphine (0.05 mg/kg subcutaneous) for surgical pain.
Four or five days after transmitter implantation surgery, a second surgery was performed. Animals within a cohort that arrived at the laboratory together were randomly allocated to receive either nerve injury or control surgery. For spinal nerve ligation (SNL, [21]), rats were anesthetized with isoflurane (1.5–2.0%) in oxygen, the back was shaved, and the right lumbar paravertebral region was exposed through a midline posterior incision. After subperiosteal removal of the sixth lumbar transverse process, both the right fifth and the sixth lumbar spinal nerves were tightly ligated with 6-0 silk suture and transected distal to the ligature. To minimize non-neural injury, no muscle was removed, muscles and intertransverse fascia were incised only at the site of the two ligations, and articular processes were not removed. The lumbar fascia was closed by 4-0 resorbable polyglactin suture, and the skin was closed with three or four staples. Control surgery consisted of either sham SNL surgery (n=2), which consisted of an identical procedure except that the nerves were not ligated or sectioned after exposure, or skin incision surgery (n=5), which consisted of only anesthesia, a lumbar midline skin incision, and skin closure. Initial analysis of HR and BP responses showed no differences in these control conditions, so these were combined into a single control (C) group for all further analyses. No postoperative analgesic was provided for these procedures in order to avoid possible interference with the development of the chronic pain phenotype after SNL.
2.3 Measurement of MAP and HR
Telemetric recording of BP was recorded at a sampling rate of 500Hz using the DSI PhysioTel telemetry system connected to a PC computer built in-house, and data were stored for later analysis. Heart rate was determined from the unfiltered BP trace using the DSI Dataquest A.R.T. software system. Recording sessions were scheduled between 10AM and 2PM, in order to minimize diurnal variation and to focus on the daytime interval that is optimal for identifying effects of injury on cardiovascular parameters [18].
2.4 Determination of cardiovascular responses to sensory stimulation
HR and BP responses to plantar cutaneous stimulation were measured using established methods for sensory examination. Testing was performed on the day preceding the second surgery (day -1) and 1st, 3rd, 7th, 14th, and 21st day thereafter. All testing was performed by one of two investigators (M.R. and G.G.), and the complete panel of tests for an individual rat on a given day was performed by only one investigator. With the exception of heat stimulation, testing was performed with the animals placed individually in clear plastic enclosures (10 × 25 cm) upon a 1 4-in wire grid. The examiner performing the sensory testing was unaware of prior sensory testing results or the type of surgery performed on each animal, although there was no means of concealing postural abnormalities of the paw. The examiner did not observe HR and BP during animal handling or testing. All stimuli were applied to the plantar surface of the right foot, and a test stimulus was only applied after the animal ceased exploratory activity. Test modalities were as follows.
Dynamic mechanical stimulation (“brush”)
A camel hair brush 8mm wide was stroked longitudinally along the center of the paw at a rate of approximately 2cm/s. The peak force when measured upon an analytical scale using a similar application was approximately 5g. The test was applied three times separated by intervals of at least one minute.
Cooling stimulation (“cold”)
Acetone was expelled through upright tubing to form a meniscus that was touched to the central skin without contact of the tubing to the skin [6]. In human subjects, this creates a nonpainful cool sensation. Three repetitions were spaced at least 2 minutes apart.
Punctate mechanical stimulation (“von Frey”)
Monofilaments in graded thicknesses (Smith and Nephew Inc, Germantown, WI), hereafter referred to as von Frey fibers, were modified with blunt tungsten tips of 100μm diameter [39], in order to standardize the contact area, which independently modulates the force necessary to produce pain [3]. These were applied taking care to approach the skin slowly to standardize the force/time relationship during stimulation. Contact was made for 1s with a force just adequate to bend the fiber, using the center of the paw but avoiding repeated contact with the same exact site. Fibers with forces ranging from 0.57g to 24.6g were applied using an up-down method [5]. Briefly, the 2.4g fiber was applied and if the animal withdrew the foot, the next weaker fiber was applied, whereas if there was no withdrawal, the next stiffer fiber was applied, until a reversal occurred, defined as a withdrawal after a previous lack of withdrawal, or vice versa. The stiffness of the fiber before the reversal, the one that produced a reversal, and the next four fibers applied according to this continuing schema were used to calculate an approximation to the threshold force for producing withdrawal, according to the method of Dixon [7].
Noxious mechanical stimulation (“pin”)
The point of a 22g spinal anesthesia needle was applied to the center of the paw with enough force to indent the skin but not puncture it. This was applied for 5 applications separated by at least 10s, which was repeated after 2min, making a total of 10 touches [14].
Heating stimulation (“heat”)
Animals were placed on temperature-regulated glass, and exposed to a radiant heat source that produced a circular irradiated area of 8mm in diameter, which induced a withdrawal of the foot [13]. The latency for withdrawal was determined 3 times, separated by 1min.
Scoring response types
For each stimulus application, the presence of a somatomotor response and its type were recorded. Induced somatomotor behaviors were of two types, either a very brisk simple withdrawal with immediate return of the foot to the cage floor, or a sustained elevation with grooming that included licking and chewing, and possibly shaking, which lasted at least 1s [14]. As this is an exaggerated reaction compared to simple withdrawal and includes supraspinally integrated behavior [2]. We hereafter refer to this as a hyperalgesia-type behavior.
Testing protocol and analysis
Our purpose was to record BP while administering stimuli as described above, in a manner that was not additionally stressful beyond that provided by the sensory tests themselves. Each animal’s testing sessions began with a 30min period of accommodation to the environment on the wire grid, after which BP recording was performed for 10min during rest to provide a baseline prior to testing. The receiver for telemetric recording was placed on top of the plastic enclosure to allow access to the plantar surface of the hind paws. Brush testing was carried out first, and then cooling, von Frey fiber testing, and pin testing in succession, each separated by a 5min rest interval. Animals were then placed on the heat testing apparatus, where they rested for 30min prior to testing. Recording of BP was continuous throughout sensory testing at a rate of 500Hz. After generation of accompanying HR traces through analysis of systolic events, the BP data were filtered by moving average to produce 10Hz frequency of BP (mean arterial BP, MAP), in order to have manageable files for analysis.
The effect of sensory testing for each sensory modality was determined as follows. MAP and HR at baseline were measured as the average over 60s during the inactivity period that immediately preceded testing of that modality. Measures were also obtained for the 10s epoch initiated by the first application of the sensory stimulus of that modality, which allowed comparison of the effect of first stimuli for each modality. Additionally, MAP and HR were also measured over the 10s epoch initiated by the last application of that stimulus modality, which was done to identify a possible influence of sensitization produced by repeated stimulation, as well as to limit the contribution of the arousal provided by the novelty of the first presentation. Since presentations of the stimuli were modeled after conventional usage, the last application varied from the third to the tenth for the various modalities. An initial analysis (2-way ANOVA), in which the BP and HR responses triggered by the first and last stimuli were evaluated separately, showed that there were no differences in either the hemodynamic responses or the effects of Day or Group (as defined below). Therefore, subsequent analysis combined these data and included one additional determination in between (the second stimulus for brush, cold, and heat, and the third for von Frey, and the fifth for pin). Analysis of traces was performed in a fashion by which the evaluator was blinded to the identity of the stimulus and injury group.
2.5 Statistical Analysis
Two analyses were performed. In the first, we sought to determine how stimulation affected HR and BP, and if injury-induced hyperalgesia influenced hemodynamic responses to stimulation. We have previously shown that there is variability in pain behavior after SNL [14]. Specifically, while the majority of animals develop mechanical hyperalgesia after SNL, others do not despite being subjected to the same surgery. We therefore assigned SNL animals in this analysis to separate groups according to their type of response to noxious mechanical (pin) stimulation. Those that showed a hyperalgesia-type response in greater than 20% of applications averaged over days 14 and 21 were included in the hyperalgesia (H) group, while the other animals that received SNL but did not develop hyperalgesia at this level were included in the non-hyperalgesia (nonH) group. Data from these days were combined for the purpose of this categorization since there is day-to-day variation in pain behavior, since nonspecific effects may influence behavior initially following surgery, and since post-injury pain behavior is fully evolved by this time [14]. Using this grouping, a two-way ANOVA with repeated measures design (Statistica 8, StatSoft, Tulsa, OK) was used to identify effects of sensory stimulation (the five modalities evaluated separately) on the incremental change of MAP and HR induced by a stimulation event (i.e. ΔMAP and ΔHR, evaluated separately), in which the within factor was Days after surgery and the between factor was Group membership based on injury-induced development of hyperalgesia described above. For each stimulus modality, three values of ΔMAP and ΔHR were derived during a single testing session by subtracting the average MAP or HR value over the 60s baseline interval from the three 10s test intervals defined above. These three values of ΔMAP and ΔHR were averaged for each animal, and the single resulting value was used in statistical analyses. An intercept term (equal to the grand mean squared, multiplied by the overall n) that is significantly different than zero was interpreted as indicating a significant overall main effect of the stimulation upon the measured hemodynamic parameter. There was no attempt to statistically compare responses between different modalities of stimulation.
In the second analysis, our goal was to determine the influence of the type of somatomotor Behavior Response (simple withdrawal, hyperalgesia-type behavior, or no withdrawal) upon ΔMAP and ΔHR induced by a stimulation event. For each test modality, the data pool included the three values of ΔMAP and ΔHR derived during each testing session (excluding the baseline day, for which Behavior Response was overwhelmingly of the simple withdrawal type) in each animal, which were not averaged to a single value as in the first analysis. Rather, generalized linear models with generalized estimating equations (GEE) were used in a repeated measures analysis of the effects of Behavioral Response, while controlling for Day and Surgery, on ΔMAP and ΔHR induced by a stimulation event. The mean of each animal’s baseline measurements of MAP and HR (as appropriate) were also considered as covariates, but no significant effects were found that would affect this analysis, or the first analysis above, in all cases but one (rats with a higher baseline HR had a greater ΔHR when stimulated by brush), for which it did not change the conclusions with respect to the other factors considered. For brush stimulation, there was only a single hyperalgesia behavioral response, so this observation was removed from the data for the analysis. Least square means were used to estimate the difference in response between levels of Behavioral Response. No significant interaction effects were found except in the ΔHR model for pin stimulation, for which there was a significant interaction between Surgery group and Behavioral Response (sham surgery animals showing a bigger effect than SNL or skin incision). This effect did not influence other findings and the simple main effects of surgery did not differ significantly in contrast tests. This analysis was performed using SAS version 9.2 (The SAS Institute, Cary, NC).
A significance level of 0.05 was used for all comparisons. Data are reported as mean ± SEM. For ANOVA tests, the F-statistic is provided in the format of F[a,b], in which a is the between-groups degrees of freedom, and b is the within-groups degrees of freedom.
3. Results
3.1 Grouping of subjects
Autopsy confirmed proper anatomical ligation and section in all SNL animals. Taking the SNL animals together, they showed a greater rate of hyperalgesia-type behavior in pin testing averaged over days 14 and 21 when the behavioral phenotype is fully expressed (37±9%, n=13) than the control animals (C group; 7±3%, n=7; t-test P<0.05). Of the 13 SNL animals, 6 animals (nonH group, 46%) failed to develop a hyperalgesia rate greater than 20% averaged over days 14 and 21, and showed an average of 6±3% hyperalgesia-type behavior, while the other 7 SNL animals (H group) developed a 63±7% hyperalgesia behavior rate (ANOVA main effect considering all three groups P<0.001, F[2,17] =434.0; Bonferroni post hoc C vs. H P<0.001, nonH vs. H P<0.001). This incidence of failure to develop sustained hyperalgesia after SNL, as well as the response rates for other stimulation modalities, is similar to that which occurs after SNL in the absence of contralateral femoral artery cannulation or transmitter implantation [14].
3.2 Response to stimulation: general observations
Recordings of MAP and the derived HR traces showed substantial variability in the time domain of seconds to minutes, which was lessened at rest but not eliminated (Fig. 1). Superimposed upon this baseline variability, cardiovascular events triggered by sensory stimulation were nonetheless often clearly identifiable. Moreover, when data were averaged for the animals within a group (Fig. 2 to 6), considerable consistency emerged that showed MAP and HR responses to stimulation that were similar in the three animal groups and similar on sequential days. With the single exception of the effect of pin stimulation upon ΔMAP, Group (C, H, nonH) had no significant overall main effect upon ΔMAP and ΔHR.
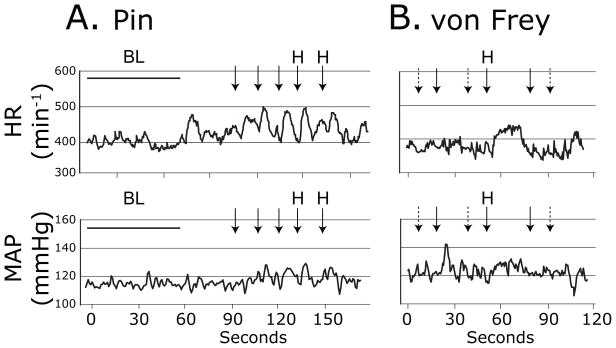
Figure 1.
Sample traces of heart rate (HR, top panels) and mean arterial pressure (MAP, bottom panels) during sensory testing with Pin in an H group animal 3 days following spinal nerve ligation injury (A.), and with von Frey fibers in a non-hyperalgesic animal 3d following spinal nerve ligation (B.). Arrows indicate timing of the application of the stimuli. A dashed arrow represents an application that produced no behavioral response. A solid arrow indicates a withdrawal of the stimulated paw. “H” indicates a hyperalgesia-type withdrawal with sustained elevation with shaking and grooming. “BL” indicates baseline recording interval.
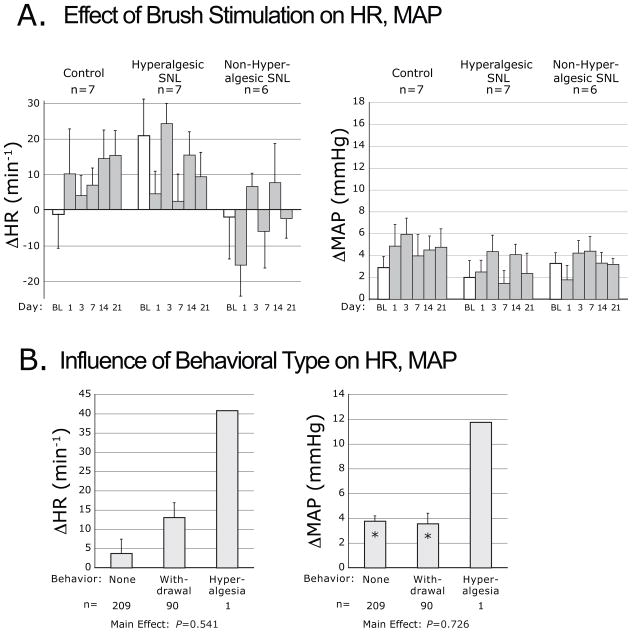
Figure 2.
Plantar dynamic mechanical stimulation-induced changes of heart rate (ΔHR) and mean arterial pressure (ΔMAP), evoked by using a brush. A. Cardiovascular changes categorized by injury group, including control animals that had skin incision only, animals that became hyperalgesic after spinal nerve ligation (SNL) injury, and animals that did not develop hyperalgesia after SNL. Data are shown for measurements at baseline (BL, open bar) and on different days after surgery (shaded bars). On each day, measures for each animal are averaged across three repetitions (see Experimental Procedures). “n” indicates the number of animals in each group. B. Cardiovascular changes categorized by the type of motor behavior induced by stimulation, including no withdrawal (“None”), simple withdrawal, or sustained withdrawal accompanied by lifting, shaking and grooming (“Hyperalgesia”). Since only a single hyperalgesia event was evoked by brush stimulation, this was not included in the statistical analysis. Bars for each behavior include data pooled from different days (all except the BL day) and different injury groups. “n” indicates number of observations. “*” indicates a cardiovascular change significantly different from zero. P indicates probability for the main effect of behavior type. Bars in both panels indicate mean ± SEM. Note that the ordinate scales are the same in Figures 2 through 6.
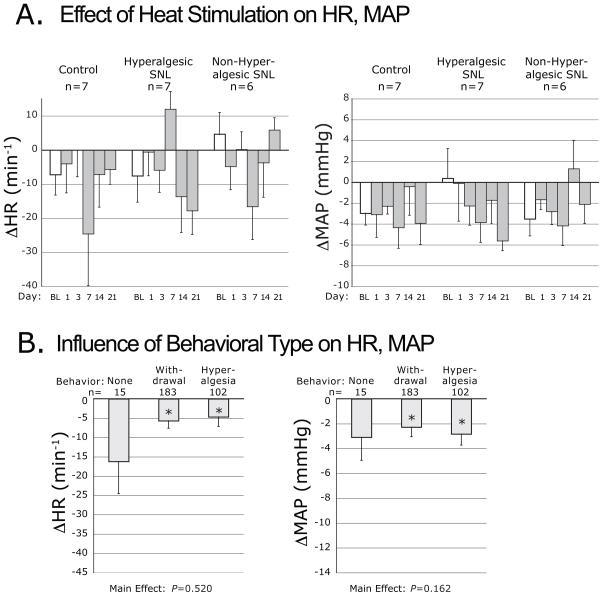
Figure 6.
Plantar heating-induced changes of heart rate (ΔHR) and mean arterial pressure (ΔMAP), evoked by application of radiant heat. Data are categorized by injury group (A.), and by the type of motor behavior induced by heating (B.). Symbols are defined in the legend for Figure 2.
3.3 Response to stimulation: specific modalities
For each modality, a figure is provided (Figs. 2–6) in which the upper panel shows ΔMAP and ΔHR responses to stimulation analyzed according to injury group (C, H, nonH) and day, for which statistical results are presented in Table 1. The bottom panel in each figure shows the influence of the type of somatomotor behavior response (no withdrawal, simple withdrawal, or hyperalgesia-type behavior) upon ΔMAP and ΔHR.
Table 1.
Significance tests for the effects of sensory stimulation (Brush, Cold, von Frey, Pin, and Heat) upon changes in Heart Rate and Mean Arterial Pressure for data shown in the top panels of Figures 2 through 6.
| Heart Rate | Mean Arterial Pressure | |||||||
|---|---|---|---|---|---|---|---|---|
| Intercept | Group | Day | Group*Day | Intercept | Group | Day | Group*Day | |
| df | 1, 119 | 2, 17 | 5, 85 | 10, 85 | 1, 119 | 2, 17 | 5, 85 | 10, 85 |
| Brush | 0.0549 (4.24) | 0.1671 (2.0) | 0.1345 (1.7) | 0.3307 (1.6) | <0.0001 (101.3) | 0.1522 (2.1) | 0.4593 (0.9) | 0.9357 (0.4) |
| Cold | <0.0001 (74.1) | 0.2790 (1.4) | 0.4628 (0.9) | 0.5228 (0.9) | <0.0001 (148.8) | 0.7276 (0.3) | 0.0082 (3.4) | 0.7051 (0.7) |
| von Frey | 0.0159 (7.2, 1) | 0.0867 (2.8) | 0.6835 (0.6) | 0.2949 (1.2) | 0.0102 (8.3) | 0.1424 (2.2) | 0.0253 (2.7) | 0.1727 (1.5) |
| Pin | <0.0001 (35.2) | 0.0442 (3.8) | 0.1134 (1.8) | 0.5034 (0.9) | <0.0001 (64.7) | 0.0701 (3.1) | 0.7928 (0.5) | 0.3818 (1.1) |
| Heat | 0.0315 (5.5) | 0.6118 (0.5) | 0.8009 (1.8) | 0.0682 (1.8) | 0.0012 (15.2) | 0.8772 (0.1) | 0.1695 (1.6) | 0.8256 (0.6) |
P values are shown for each main effect (underlined for P<0.05). The underlying F-statistic is shown in parentheses. A significant intercept term (equal to the grand mean squared, multiplied by the overall n) indicates an overall effect of stimulation on heart rate or mean arterial pressure. “df” row indicates between-group and within-group degrees of freedom for each analysis; “Group*Day” indicates the main effect of the interaction of Group and Day.
Dynamic mechanical stimulation
The probability of simple withdrawal from brush stimulation, averaged over days 14 and 21, was not different between groups (C group 21±14%, nonH group 17±7%, H group 40±10%; ANOVA main effect P=0.305, F[2,17]=1.3). Stroking the foot with a brush had the overall effect of increasing MAP, both before and after injury, without differences between groups (Fig. 2A). The effect on HR was less consistent and did not reach significance. When cardiovascular data were grouped according to the type of somatomotor behavior triggered by brushing (Fig. 2B), it was evident that the increase of MAP was the same whether there was foot withdrawal or not.
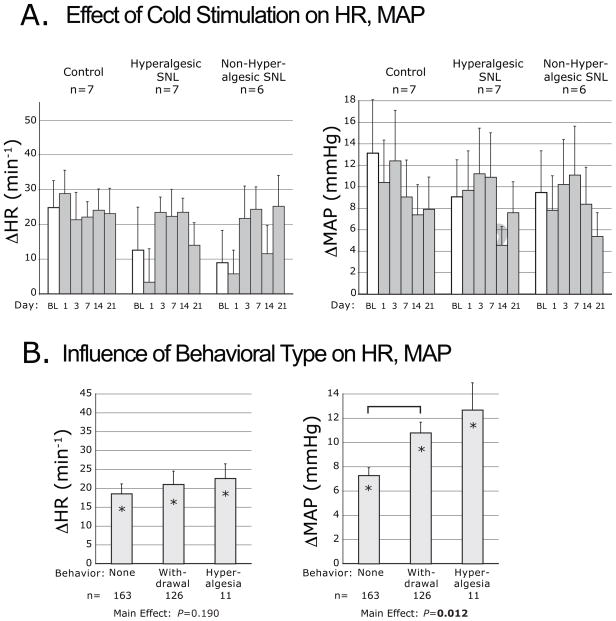
Cooling
The probability of withdrawal (either simple or hyperalgesic) after acetone application, averaged over days 14 and 21, was not different in the various groups (C 21±13%; nonH 28±7%, H 33±10%; ANOVA main effect P=0.73, F[2,17]=0.3). However, hyperalgesia behavior occurred only in the H group (10±5%; ANOVA main effect P=0.057, F[2,17]=3.4). Similar to stroking with a brush, plantar acetone consistently increased MAP, although the magnitude of the effect was greater (Fig. 3A). There was also an effect of Day, in which ΔMAP generally decreased, probably due to an age effect since it was evident in all groups including the control animals. Cooling elevated HR without any difference between groups, and without an influence of Day. Behavior type influenced the MAP response to cooling (Fig. 3B), and post hoc analysis confirmed that foot withdrawal was accompanied by a greater elevation of MAP than when there was no movement.
Figure 3.
Plantar cooling-induced changes of heart rate (ΔHR) and mean arterial pressure (ΔMAP), evoked by application of acetone. Data are categorized by injury group (A.), and by the type of motor behavior induced by acetone (B.). Symbols are defined in the legend for Figure 2. The bracket indicates a difference by post hoc comparison.
Punctate mechanical stimulation
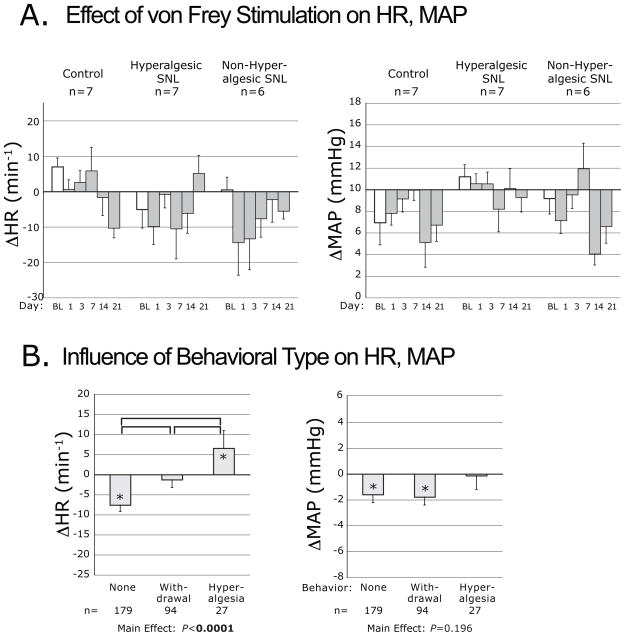
The force threshold for foot withdrawal determined by von Frey stimulation, averaged over days 14 and 21, was decreased in the H group (2.7±0.6g) compared to the nonH group (3.4±0.6g) and C group (5.1±3.0g, ANOVA main effect P<0.05, F[2,17]=4.2, post hoc C vs. H P=0.04), comparable to prior observations [27]. The overall effect of von Frey stimulation was depression of HR and MAP without any influence of Group (Fig. 4A). A significant effect of Day was evident as a generally greater negative ΔMAP with time. Separate consideration of the different somatomotor behavior types (Fig. 4B) showed that HR increased when von Frey stimulation produced a hyperalgesia-type foot withdrawal, whereas HR decreased when stimulation produced a simple withdrawal, and there was no change of HR when there was no movement. The type of behavior induced by von Frey stimulation did not influence the MAP response (Fig. 4B).
Figure 4.
Plantar punctate mechanical stimulation-induced changes of heart rate (ΔHR) and mean arterial pressure (ΔMAP), evoked by application of von Frey fibers. Data are categorized by injury group (A.), and by the type of motor behavior induced by von Frey touch (B.). Symbols are defined in the legend for Figure 2. The brackets indicate differences by post hoc comparison.
Noxious mechanical stimulation
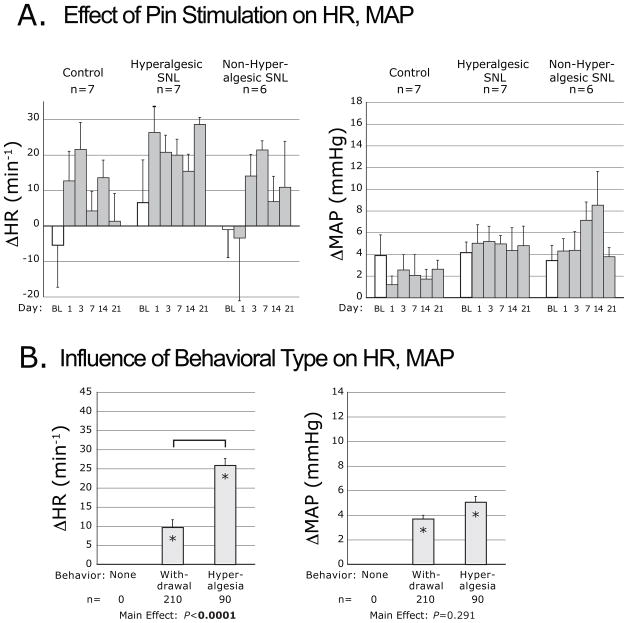
Pin stimulation had an overall effect of increased MAP and HR (Fig. 5A). For HR, there was a significant main effect of Group (Table 1). Pairwise comparisons showed a group mean ΔHR for the H group (19.6±3.4s−1) that was greater than the group mean for the nonH group (8.2±3.7s−1, P<0.05) and the C group (8.0±3.4s−1, P<0.05); each group mean was significantly different from zero. Separate consideration of different somatomotor behavior types (Fig. 5B) showed greater increases of HR when pin produced a hyperalgesia-type behavior than when a simple withdrawal was produced.
Figure 5.
Plantar noxious mechanical stimulation-induced changes of heart rate (ΔHR) and mean arterial pressure (ΔMAP), evoked by pin application. Data are categorized by injury group (A.), and by the type of motor behavior induced by pin (B.). Symbols are defined in the legend for Figure 2. The bracket indicates a difference by post hoc comparison.
Heating
The latency for withdrawal from radiant heating, averaged over days 14 and 21, was not different in the various groups (C 10.5±0.7s, nonH 10.3±0.8s, H 10.6±0.6s; ANOVA main effect P=0.95, F[2,17]=0.1). The frequency of hyperalgesia behavior during heat stimulation was also not affected by group (C 33±8%, nonH 30±7%, H 43±11%; ANOVA main effect P=0.57, F[2,17]=0.6). Plantar radiant heat had an overall depressor effect on HR and MAP, with no influence of group (Fig. 6A). When cardiovascular data were grouped according to the type of somatomotor behavior triggered by heating (Fig. 6B), HR and BP were found to decrease comparably regardless of the type of behavior.
4. Discussion
The regulation of cardiovascular function by sensory activation is readily evident even outside the experimental setting, for instance during routine surgical manipulations, but little work has been done to characterize this relationship for stimuli that are commonly used for cutaneous pain testing. Unlike the predictable elevation in HR and BP evoked by persistent noxious stimulation from which an animal cannot withdraw, our present findings show diverse cardiovascular responses to brief cutaneous stimuli that are modality specific (Table 2). There are several reasons for the divergence of our new observations from previous studies. First, in the present study, animals could terminate the stimulus by withdrawing their limb, such that the duration of stimulation above the noxious threshold was very brief. Secondly, many prior measurements have been obtained during anesthesia, which substantially alters the extent and even direction of cardiovascular events induced by sensory stimulation [11,19,30,34]. Finally, activation of cutaneous primary afferent fibers initiates not only sensory experience, but also triggers processes such as thermoregulation and arousal that together produce cardiovascular response patterns that differ by stimulus modality.
Table 2.
Summary of findings.
| Stimulus | Heart Rate | Mean Arterial Pressure | |||
|---|---|---|---|---|---|
| Modality | Agent | Change | Influence of Behavior type | Change | Influence of Behavior type |
| Dynamic Mechanical | Brush | - | NA | ↑ | N |
| Cooling | Acetone | ↑ | N | ↑ | Y |
| Punctate Mechanical | von Frey fibers | ↑/↓ | Y | ↓ | N |
| Noxious Mechanical | Pin | ↑ | Y | ↑ | N |
| Heating | Radiant Heat | ↓ | N | ↓ | N |
Arrows ↑ and ↓ indicate an increase or decrease of heart rate and mean arterial pressure following plantar cutaneous stimulation by the indicated modality and agent. “Influence of Behavior type” indicates whether the phasic cardiovascular change was amplified in accordance with somatomotor behavior; “Y” (yes) indicates a greater cardiovascular change is associated with greater degree of limb movement, “N” (no) indicates no such association, “NA” indicates not applicable.
Interpretation of our cardiovascular observations during noxious mechanical stimulation appears straightforward. Application of a pin increases MAP and HR, similar to prior reports that used sustained noxious mechanical stimulation [1,16]. The amplified response of HR in hyperalgesic animals after SNL supports the validity of this well-accepted model of neuropathic pain, and suggests that HR monitoring could provide additional insight into pain perception. The generation of a greater HR elevation accompanying hyperalgesia-type behavior suggests that this sustained and complex behavior denotes a distinct, more intense nociceptive event, which is compatible with our recent observation that this behavior is selectively associated with aversiveness in an operant model using conditioned place avoidance [47]. Although a gold standard for a pain experience per se is inevitably lacking in animal research, a specific association of hyperalgesia-type behavior with accentuated cardiovascular activation supports the validity of this behavior type as an indicator of pain.
Using a commonly employed paradigm for punctate mechanical stimulation with von Frey fibers, the amplitudes of MAP and HR responses are generally small, consistent with the low intensity of these stimuli that straddle the threshold for triggering a behavioral response. Unexpectedly, MAP is depressed by applications of von Frey fibers. This effect is similar after touches that produce no movement and those that cause withdrawal, which suggests that these stimuli are perceived without producing nociception. Since fully noxious stimuli such as pin application increase MAP, it is possible that most von Frey fiber applications fail to generate nociception and pain, but rather trigger somatomotor and vasomotor events through an arousal mechanism. The few applications (9% in our present data) that result in hyperalgesia-type behavior provoke elevated HR, in contrast to depressed HR in the absence of withdrawal, which likely indicates activation of nociceptive pathways when hyperalgesia-type behavior occurs. These observations raise the possibility that the conventional format for sensory testing with von Frey fibers, in which a simple withdrawal event is equated with nociception, may be an unreliable test for pain. This speculation is supported by the failure of von Frey stimulation to produce conditioned place avoidance, even after nerve injury [47].
Brushing the plantar skin to produce dynamic mechanical stimulation reliably elevates MAP even without nerve injury, and may do so without any form of foot withdrawal. Stroking with a soft brush is not conventionally considered a painful stimulus, but this is open to question since the animal’s experience cannot be known. In most cases, we observed cardiovascular activation by brushing with no accompanying nocifensive somatomotor behavior. While this suggests that these perceptions produced arousal without nociception, this cardiovascular activation contrasts with the depression induced by von Frey touches that likewise produce no movement, for which we have no explanation.
Together, our data show that cutaneous mechanical stimulation affects cardiovascular parameters in divergent patterns that depend on the manner of application. Early studies that used direct electrical stimulation of peripheral nerves in anesthetized animals demonstrated the ability of afferent sensory activity to either depress or elevate BP as well as peripheral sympathetic vasomotor neuronal activity [8,19]. Even when both C-type as well as A-type cutaneous afferent fibers are stimulated, low frequency activity (1–10Hz) decreases MAP within 5 seconds, whereas higher frequency activity (≥50Hz) elevates sympathetic activity and MAP [22]. The frequency of afferent traffic elicited by noxious pin stimulation is likely higher than that evoked by threshold stimulation with von Frey fibers [4,9,23], which may explain the divergent effects of pin and von Frey stimulation upon MAP. Also, since sympathetic activation is proportionate to the total number of neuronal depolarizations in a cutaneous afferent train [38], brush stimulation may thereby produce a vasopressor effect through the sustained nature of the afferent traffic it produces, the spatial summation of stimulating a large cutaneous area, and the particular potency of moving mechanical stimuli in producing afferent pulse trains [17].
Cooling of the plantar skin in our study provoked the greatest increase in MAP and HR of the several modalities tested. Since cardiovascular responses to cooling are comparable in control and injured animals, it is possible that the form of surface cooling we used is noxious in control animals. However, our data reveal cardiovascular responses even when no somatomotor behavior is triggered, making it unlikely that the stimulation is consistently painful. Compared to applications that trigger no somatomotor behavior, cooling that produces foot withdrawal and especially hyperalgesia-type behavior is accompanied by amplified MAP responses, which might suggest a component of nociception in these cases. It is well established that local nonpainful cutaneous cooling may induce global vasoconstriction and elevation of MAP and HR [24,41], which could be the predominating influence upon vasomotor control under the conditions of our study. It is also possible that somatomotor behavior is dictated by central thermoregulatory processing rather than nociceptive systems. Since evaporative cold stimulation cannot be terminated immediately by limb withdrawal, the intensity of the circulatory response may be due in part to persistence of the stimulation.
Plantar heat produces overall depression of MAP and HR, even in control animals. This contrasts with published demonstrations of the pressor effect of sustained cutaneous heating [1,16,32,33], but the method used in the present study allowed the animals to minimize exposure to stimulation above the nociceptive threshold. Notably, we observed comparable cardiovascular depression upon plantar warming even in the absence of paw withdrawal, which further suggests a minimal role of nociceptive activation. The application of non-noxious heat to small areas of skin has long been recognized as a potent trigger of global suppression of sympathetic activity and systemic vasodilatation [10,44], in a fashion opposite to that triggered by local cooling, and this thermoregulatory pathway could account for cardiovascular influences during thermal threshold testing. An alternative explanation for the depressor response stems from the selective activation of C-type nociceptive fibers during the relatively slow pace at which we heated the skin [48]. In contrast to Aδ fiber stimulation, such as that caused by punctate mechanical stimuli or rapid skin warming, preferential stimulation of C-type fibers by slow warming selectively activates a distinct, coordinated passive coping response that includes cardiovascular depression [26].
Pain research has been hobbled by the difficulty of drawing inferences relevant to human pain from models employing animal subjects [31]. Although quantifying somatomotor behavior induced by cutaneous stimulation is the accepted standard for sensory testing in animals, this has been adopted largely out of convenience, and doubts have been raised regarding the validity of this approach [25,45]. Our study reveals unexpected complexity in the phasic responses of MAP and HR induced by cutaneous stimulation, probably due to the participation of central circuits serving processes other than sensory discrimination and pain, such as arousal and thermoregulation. Although this restricts the usefulness of cardiovascular measures as independent gauges of nociception and pain, our findings also show that particular escape behaviors are selectively linked to specific patterns of phasic cardiovascular reflexes. Specifically HR activation that is characteristic of nociception is accentuated after noxious pin stimuli when sustained and complex hyperalgesia-type behavior is provoked. Furthermore, punctate von Frey stimulation only triggers cardiovascular activation when hyperalgesia-type behavior occurs. These observations indicate that HR measurement after intense mechanical stimulation may provide additional quantification of the painful experience, and also highlight the value of higher-level integrated behaviors as an outcome measure, rather than relying solely on the threshold or latency for simple withdrawal of the stimulated extremity.
Acknowledgments
Funding was provided by grant NS-42150 to Q.H. and NS-40538 to CS from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, Maryland, USA; VA Medical Research Funds to J.S and Q.H.; and National Science Foundation Grant IOS 0751613 to C.D. The Medical College of Wisconsin Biostatistics Consulting Service is supported by funds from the Division of Biostatistics, Department of Population Health and the Clinical Translational Science Institute (CTSI) of Southeast Wisconsin. The authors have no financial or other relationships that constitute a conflict of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abram SE, Kostreva DR, Hopp FA, Kampine JP. Cardiovascular responses to noxious radiant heat in anesthetized cats. Am J Physiol. 1983;245(4):R576–580. doi: 10.1152/ajpregu.1983.245.4.R576. [DOI] [PubMed] [Google Scholar]
- 2.Berridge KC. Progressive degradation of serial grooming chains by descending decerebration. Behav Brain Res. 1989;33(3):241–253. doi: 10.1016/s0166-4328(89)80119-6. [DOI] [PubMed] [Google Scholar]
- 3.Bishop GH. Relation of pain sensory threshold to form of mechanical stimulator. Journal of neurophysiology. 1949;12(1):51–57. doi: 10.1152/jn.1949.12.1.51. [DOI] [PubMed] [Google Scholar]
- 4.Burgess PR, Perl ER. Myelinated afferent fibres responding specifically to noxious stimulation of the skin. J Physiol. 1967;190(3):541–562. doi: 10.1113/jphysiol.1967.sp008227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. Journal of Neuroscience Methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 6.Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain. 1994;59(3):369–376. doi: 10.1016/0304-3959(94)90023-X. [DOI] [PubMed] [Google Scholar]
- 7.Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- 8.Fedina L, Katunskii AY, Khayutin VM, Mitsanyi A. Responses of renal sympathetic nerves to stimulation of afferent A and C fibres of tibial and mesenterial nerves. Acta Physiol Acad Sci Hung. 1966;29(2):157–175. [PubMed] [Google Scholar]
- 9.Garell PC, McGillis SL, Greenspan JD. Mechanical response properties of nociceptors innervating feline hairy skin. Journal of neurophysiology. 1996;75(3):1177–1189. doi: 10.1152/jn.1996.75.3.1177. [DOI] [PubMed] [Google Scholar]
- 10.Gibbon JH, Landis EM. Vasodilatation in the Lower Extremities in Response to Immersing the Forearms in Warm Water. J Clin Invest. 1932;11(5):1019–1036. doi: 10.1172/JCI100456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gibbs NM, Larach DR, Skeehan TM, Schuler HG. Halothane induces depressor responses to noxious stimuli in the rat. Anesthesiology. 1989;70(3):503–510. doi: 10.1097/00000542-198903000-00022. [DOI] [PubMed] [Google Scholar]
- 12.Gottlieb A, Skrinska VA, O’Hara P, Boutros AR, Melia M, Beck GJ. The role of prostacyclin in the mesenteric traction syndrome during anesthesia for abdominal aortic reconstructive surgery. Ann Surg. 1989;209(3):363–367. doi: 10.1097/00000658-198903000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32(1):77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 14.Hogan Q, Sapunar D, Modric-Jednacak K, McCallum JB. Detection of neuropathic pain in a rat model of peripheral nerve injury. Anesthesiology. 2004;101(2):476–487. doi: 10.1097/00000542-200408000-00030. [DOI] [PubMed] [Google Scholar]
- 15.Holzer-Petsche U, Brodacz B. Traction on the mesentery as a model of visceral nociception. Pain. 1999;80(1–2):319–328. doi: 10.1016/s0304-3959(98)00233-4. [DOI] [PubMed] [Google Scholar]
- 16.Ishide T, Amer A, Maher TJ, Ally A. Nitric oxide within periaqueductal gray modulates glutamatergic neurotransmission and cardiovascular responses during mechanical and thermal stimuli. Neurosci Res. 2005;51(1):93–103. doi: 10.1016/j.neures.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Jarvilehto T, Hamalainen H, Laurinen P. Characteristics of single mechanoreceptive fibres innervating hairy skin of the human hand. Exp Brain Res. 1976;25(1):45–61. doi: 10.1007/BF00237325. [DOI] [PubMed] [Google Scholar]
- 18.Jin Y, Sato J, Yamazaki M, Omura S, Funakubo M, Senoo S, Aoyama M, Mizumura K. Changes in cardiovascular parameters and plasma norepinephrine level in rats after chronic constriction injury on the sciatic nerve. Pain. 2008;135(3):221–231. doi: 10.1016/j.pain.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 19.Johansson B. Circulatory responses to stimulation of somatic afferents with special reference to depressor effects from muscle nerves. Acta Physiol Scand Suppl. 1962;198:1–91. [PubMed] [Google Scholar]
- 20.Kemppainen P, Forster C, Handwerker HO. The importance of stimulus site and intensity in differences of pain-induced vascular reflexes in human orofacial regions. Pain. 2001;91(3):331–338. doi: 10.1016/S0304-3959(00)00462-0. [DOI] [PubMed] [Google Scholar]
- 21.Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50(3):355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- 22.Koizumi K, Sato A, Kaufman A, Brooks CM. Studies of sympathetic neuron discharges modified by central and peripheral excitation. Brain Res. 1968;11(1):212–224. doi: 10.1016/0006-8993(68)90082-6. [DOI] [PubMed] [Google Scholar]
- 23.Koltzenburg M, Stucky CL, Lewin GR. Receptive properties of mouse sensory neurons innervating hairy skin. Journal of neurophysiology. 1997;78(4):1841–1850. doi: 10.1152/jn.1997.78.4.1841. [DOI] [PubMed] [Google Scholar]
- 24.Kregel KC, Seals DR, Callister R. Sympathetic nervous system activity during skin cooling in humans: relationship to stimulus intensity and pain sensation. J Physiol. 1992;454:359–371. doi: 10.1113/jphysiol.1992.sp019268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Bars D, Gozariu M, Cadden SW. Animal models of nociception. Pharmacol Rev. 2001;53(4):597–652. [PubMed] [Google Scholar]
- 26.Lumb BM. Hypothalamic and midbrain circuitry that distinguishes between escapable and inescapable pain. News Physiol Sci. 2004;19:22–26. doi: 10.1152/nips.01467.2003. [DOI] [PubMed] [Google Scholar]
- 27.Ma C, Shu Y, Zheng Z, Chen Y, Yao H, Greenquist KW, White FA, LaMotte RH. Similar electrophysiological changes in axotomized and neighboring intact dorsal root ganglion neurons. Journal of neurophysiology. 2003;89(3):1588–1602. doi: 10.1152/jn.00855.2002. [DOI] [PubMed] [Google Scholar]
- 28.Mahinda TB, Lovell BM, Taylor BK. Morphine-induced analgesia, hypotension, and bradycardia are enhanced in hypertensive rats. Anesth Analg. 2004;98(6):1698–1704. doi: 10.1213/01.ANE.0000115148.03515.56. [DOI] [PubMed] [Google Scholar]
- 29.Maixner W, Gracely RH, Zuniga JR, Humphrey CB, Bloodworth GR. Cardiovascular and sensory responses to forearm ischemia and dynamic hand exercise. Am J Physiol. 1990;259(6 Pt 2):R1156–1163. doi: 10.1152/ajpregu.1990.259.6.R1156. [DOI] [PubMed] [Google Scholar]
- 30.McDermott DA, Meller ST, Gebhart GF, Gutterman DD. Use of an indwelling catheter for examining cardiovascular responses to pericardial administration of bradykinin in rat. Cardiovasc Res. 1995;30(1):39–46. [PubMed] [Google Scholar]
- 31.Mogil JS. Animal models of pain: progress and challenges. Nat Rev Neurosci. 2009;10(4):283–294. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- 32.Nagasaka H, Yaksh TL. Effects of intrathecal mu, delta, and kappa agonists on thermally evoked cardiovascular and nociceptive reflexes in halothane-anesthetized rats. Anesth Analg. 1995;80(3):437–443. doi: 10.1097/00000539-199503000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Nason MW, Jr, Mason P. Modulation of sympathetic and somatomotor function by the ventromedial medulla. Journal of neurophysiology. 2004;92(1):510–522. doi: 10.1152/jn.00089.2004. [DOI] [PubMed] [Google Scholar]
- 34.Ness TJ, Gebhart GF. Colorectal distension as a noxious visceral stimulus: physiologic and pharmacologic characterization of pseudaffective reflexes in the rat. Brain Research. 1988;450(1–2):153–169. doi: 10.1016/0006-8993(88)91555-7. [DOI] [PubMed] [Google Scholar]
- 35.Nijsen MJ, Ongenae NG, Coulie B, Meulemans AL. Telemetric animal model to evaluate visceral pain in the freely moving rat. Pain. 2003;105(1–2):115–123. doi: 10.1016/s0304-3959(03)00170-2. [DOI] [PubMed] [Google Scholar]
- 36.Nordin M, Fagius J. Effect of noxious stimulation on sympathetic vasoconstrictor outflow to human muscles. J Physiol. 1995;489 ( Pt 3):885–894. doi: 10.1113/jphysiol.1995.sp021101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sato A, Sato Y, Schmidt RF. Changes in blood pressure and heart rate induced by movements of normal and inflamed knee joints. Neurosci Lett. 1984;52(1–2):55–60. doi: 10.1016/0304-3940(84)90350-1. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt RF, Weller E. Reflex activity in the cervical and lumbar sympathetic trunk induced by unmyelinated somatic afferents. Brain Res. 1970;24(2):207–218. doi: 10.1016/0006-8993(70)90101-0. [DOI] [PubMed] [Google Scholar]
- 39.Song XJ, Hu SJ, Greenquist KW, Zhang JM, LaMotte RH. Mechanical and thermal hyperalgesia and ectopic neuronal discharge after chronic compression of dorsal root ganglia. Journal of neurophysiology. 1999;82(6):3347–3358. doi: 10.1152/jn.1999.82.6.3347. [DOI] [PubMed] [Google Scholar]
- 40.Stotz-Potter EH, Willis LR, DiMicco JA. Muscimol acts in dorsomedial but not paraventricular hypothalamic nucleus to suppress cardiovascular effects of stress. J Neurosci. 1996;16(3):1173–1179. doi: 10.1523/JNEUROSCI.16-03-01173.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sturup G, Bolton B, Williams DJ, Charmichael WA. Vasomotor responses in hemiplegic patients. Brain. 1935;58:456–469. [Google Scholar]
- 42.Tassorelli C, Micieli G, Osipova V, Rossi F, Nappi G. Pupillary and cardiovascular responses to the cold-pressor test. J Auton Nerv Syst. 1995;55(1–2):45–49. doi: 10.1016/0165-1838(95)00026-t. [DOI] [PubMed] [Google Scholar]
- 43.Taylor BK, Peterson MA, Basbaum AI. Persistent cardiovascular and behavioral nociceptive responses to subcutaneous formalin require peripheral nerve input. J Neurosci. 1995;15(11):7575–7584. doi: 10.1523/JNEUROSCI.15-11-07575.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uprus V, Gaylor JB, Williams DJ, Carmichael EA. Vasodilatation and vasoconstriction in response to warming and cooling the body: A study in patients with hemiplegia. Brain. 1935;58:448–455. [Google Scholar]
- 45.Vierck C. Animal models of pain. In: McMahon S, Koltzenburg M, editors. Wall and Melzack’s Textbook of Pain. Amsterdam: Elsevier Churchill Livingstone; 2005. pp. 175–185. [Google Scholar]
- 46.Woodworth RS, Sherrington CS. A pseudaffective reflex and its spinal path. J Physiol. 1904;31(3–4):234–243. doi: 10.1113/jphysiol.1904.sp001034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu HE, Gemes G, Zoga V, Kawano T, Hogan QH. Learned avoidance from noxious mechanical simulation but not threshold semmes weinstein filament stimulation after nerve injury in rats. J Pain. 2010;11(3):280–286. doi: 10.1016/j.jpain.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yeomans DC, Proudfit HK. Nociceptive responses to high and low rates of noxious cutaneous heating are mediated by different nociceptors in the rat: electrophysiological evidence. Pain. 1996;68(1):141–150. doi: 10.1016/S0304-3959(96)03177-6. [DOI] [PubMed] [Google Scholar]
- 49.Yoon MH, Yaksh TL. The effect of intrathecal gabapentin on pain behavior and hemodynamics on the formalin test in the rat. Anesth Analg. 1999;89(2):434–439. doi: 10.1097/00000539-199908000-00034. [DOI] [PubMed] [Google Scholar]
- 50.Yu YH, Blessing WW. Cutaneous vasoconstriction in conscious rabbits during alerting responses detected by hippocampal theta-rhythm. Am J Physiol. 1997;272(1 Pt 2):R208–216. doi: 10.1152/ajpregu.1997.272.1.R208. [DOI] [PubMed] [Google Scholar]