Abstract
Environmental estrogens have been the subject of intense research due to their documented detrimental effects on the health of fish and wildlife, and their potential to negatively impact humans. A complete understanding of how these compounds affect health is complicated because environmental estrogens are a structurally heterogeneous group of compounds. In this work, computational molecular dynamics simulations were utilized to predict the binding affinity of different compounds using rainbow trout (Oncorhynchus mykiss) estrogen receptors (ERs) as a model. Specifically, this study presents a comparison of the binding affinity of the natural ligand estradiol-17β to the four rainbow trout ER isoforms with that of three known environmental estrogens 17α-ethinylestradiol, bisphenol A, and raloxifene. Two additional compounds, atrazine and testosterone, were tested that are known to be very weak or non-binders to ERs. The binding affinity of these compounds to the human ERα subtype is also included for comparison. Results of this study suggest that, when compared to estradiol-17 β, bisphenol A binds less strongly to all four receptors, 17α-ethinylestradiol binds more strongly, and raloxifene has a high affinity for the α subtype only. The results also show that atrazine and testosterone are weak or non-binders to the ERs. All of the results are in excellent qualitative agreement with the known in vivo estrogenicity of these compounds in the rainbow trout and other fishes. Computational estimation of binding affinities could be a valuable tool for predicting the impact of environmental estrogens in fish and other animals.
Keywords: molecular dynamics, BPA, EE2, raloxifene, atrazine, estrogen, fish
Introduction
Environmental estrogens comprise a diverse group of human-derived compounds that interact with estrogen receptors (ERs) in vertebrate animals (McLachlan, 1979). Much concern has been raised about environmental estrogens because of their documented detrimental effects on fish and wildlife (Tyler et al., 1998), and potential to impact humans (Toppari et al., 1996). Aquatic animals are particularly targeted because eventually all of these compounds enter the ecosystems they inhabit (Corcoran et al., 2010). Although much is known about the end result of environmental estrogen exposure from both lab and field studies (for reviews see Mathiessen and Sumpter, 1998; Mills and Chichester, 2005), less is known about the precise mechanism(s) of action (Tilghman et al., 2010). A complete understanding is complicated because environmental estrogens, based on their chemical structures, are a heterogeneous group of compounds. Yet all, to varying degrees, appear capable of binding to ERs and initiating an estrogenic response which is their hallmark. The intensity of this estrogenic (biological) response has been equated with the strength of ER binding affinities in target organs (Salum et al., 2008). This provides an important means of comparing different compounds and making predictions on their biological effects.
A key motivation for this study is that purified proteins are often not available to perform assays (as is the case for the rainbow trout ERs studied here), but molecular dynamics (MD) computer simulation can still be used to estimate binding affinities (Oostenbrink et al., 2000; van Lipzig et al., 2004). MD simulations were used in a previous study to determine rainbow trout ER binding affinities for estradiol-17β (E2), the major native estrogen hormone in this fish (Shyu et al., 2010). Currently, use of computational (i.e., in silico) methods for modeling the biological consequences of environmental contaminants is not widespread (Kubli-Garfias, 1998; Sugiyama et al., 2009; Novic and Vracko, 2010) in contrast to direct assays (i.e., in vivo or in vitro) (Klotz et al., 1996). Computational methods such as MD simulation, in which atoms and molecules are allowed to interact consistent with atomistic physics and chemistry based models, provide a view of the motion of the particles. MD simulation permits observation of the dynamics of the individual atoms and, in essence, functions as a virtual microscope with high temporal and spatial resolution (Martínez et al., 2005; Martínez et al., 2006; Sonoda et al., 2007). This unique feature permits the elucidations of the inner workings and atomic interaction of diverse molecules (Allen and Tildesley, 1989; Leach, 2001; Rapaport, 1996).
The purpose of this study was to use MD simulations to determine the binding affinities to rainbow trout ERs for three known environmental estrogens: 17α-ethinylestradiol (EE2), bisphenol A (BPA), and raloxifene (RAL) that differ in chemical structure and reported estrogenicity in fishes (Brian et al., 2005; Caldwell et al., 2008). In addition, the binding affinity of these environmental estrogens with the human ERα subtype was included for comparison. EE2 and BPA are well known environmental estrogens (Arcand-Hoy et al., 1998; Staples et al., 1998). EE2 is a pharmaceutical currently used widely as a human female contraceptive (Shrader and Dickerson, 2008). It is passed from the treated individual in mostly an unconjugated form in the urine and is therefore a prominent estrogen in domestic sewage effluent entering the environment (Kolpin et al., 2002). EE2 stimulates abnormal vitellogenesis (Folmar et al., 2000), causes reproductive problems (Brown et al., 2008), and alters sexual behavior in male fishes (Salierno and Kane 2010). BPA is a component of polycarbonate plastics that leaches from these materials when they degrade (Le et al., 2008). Due to the prevalence of plastics in the environment BPA is a ubiquitous contaminant (Halden, 2010). BPA causes abnormal vitellogenin induction in male fishes (Sohoni et al., 2001). RAL is a pharmaceutical used to prevent bone loss in postmenopausal women and as an anti-estrogen to block breast and uterine cancers (Muchmore, 2000). It is termed a selective estrogen receptor modulator (SERM) that has been reported to almost exclusively interact with the ERα subtype in mammals. While the negative affects of RAL have yet to be demonstrated in fishes, RAL will be an environmental estrogen with its increasing use by the human population (Teeter and Meyerhoff, 2002).
This study also includes binding affinity results for two additional compounds, atrazine (ATZ) and testosterone (TES). Both ATZ and TES have been reported as very weak or non-binders to fish ERs (Denny et al., 2005; Latonnelle et al., 2002). ATZ is an organic compound widely used as herbicide. It has been shown that ATZ acts as an endocrine disruptor, specifically by altering estradiol signaling via increased aromatase activity (Roberge et al., 2004). Several studies have linked ATZ with the abnormal development of a number of species (Ashby et al., 2002; Wiegand et al., 2001; Dianna et al., 2000). It was suggested that ATZ induced aromatase, the enzyme that controls the rate-limiting step in the conversion of androgens into estrogens and increased the concentration of cyclic adenosine monophosphate (cAMP) in the H295R human adrenocortical carcinoma cell line (Sanderson et al., 2000; 2002).
Materials and Methods
Receptor Structures
While there are no purified proteins available, the amino acid sequence for the complete ER gene family is known for the rainbow trout with two ERα isoforms (i.e., ERα1, ERα2) and two ERβ isoforms (i.e., ERβ1, and ERβ2; Nagler et al., 2007), also known as NR3A1a, NR3A1b, NR3A2a and NR3A2b (Nuclear Receptors Nomenclature Committee, 1999). Since no experimental structures are available, the rainbow trout ER holo structures for the ligand-binding domains were generated by SWISS-MODEL (Arnold et al., 2006; Kiefer et al., 2009) using human ER as template (Protein Data Bank entries 1A52 for both ERα isoforms and 3ERT for both ERβ isoforms). Sequence identities between the rainbow trout and human ligand-binding domains were within the range of 75–85%, and thus we expect that the ER structures determined by SWISS-MODEL are reasonable. Moreover, Shyu et al (2010) have shown that different rainbow trout ER isoforms share a common evolutionary origin, and structure tends to be well conserved. The different estrogenic compound topologies, needed for MD simulations, were generated by the PRODRG server (Schüttelkopf and van Aalten, 2004) with the options of full charges and no energy minimization. Estrogenic compounds were then individually docked into the binding pocket of the receptor holo structures with AutoDock (Morris et al., 1998). The Lamarckian genetic algorithm was employed to search for the most probable binding poses for each estrogenic compound and receptor. The number of genetic algorithm runs was set to 5,000 with a population size of 250,000 individuals and 5,000,000 generations. The number of evaluations was set to 2,500,000 for each individual in the population to ensure thorough exploration of the search space. The mutation rate was set to 0.05 and the crossover 0.8. A two- point crossover was used to generate the offspring at each successive generation. The genetic algorithm automatically preserved the 20 best-fit individuals to the next generation and the 20 least-fit individuals were not used to generate offspring. For this study the five compounds EE2, BPA, RAL, ATZ, and TES, were tested for the best-fit binding pose. Data for E2 was taken from Shyu et al (2010).
Computer Simulation Protocols
The computer simulations were performed using a protocol reported previously by Shyu et al. (2010) to estimate the binding affinity for all rainbow trout isoforms to the compounds. The binding free energy calculations were decomposed into several steps in which the compounds are annihilated (i.e., decoupled) from its bound state in the receptor complex and then made to reappear in solution to complete the thermodynamic cycle (Shyu et al., 2010). Harmonic restraints were applied to each compound to the receptors in order to accelerate computer simulation convergence and minimize the detrimental effects of end-point singularities commonly reported in computational alchemical simulations (Shirts et al., 2003).
All computer simulations were performed using GROMACS 4.0 (Hess et al., 2008) and the default GROMOS-96 43A1 forcefield (van Gunsteren et al., 1996). For equilibration, the systems were first minimized using 1,000 steps of L-BFGS (Broyden-Fletcher-Goldfarb-Shanno) (Broyden, 1970), followed by 1,000 steps of steepest descent minimization. The system was then subject to 1.0 ns of simulation using isothermal molecular dynamics. This was followed by another 1.0 ns of simulation using isothermal-isobaric molecular dynamics with the Berendsen barostat with a time constant of 1.0 ns. All production simulations were performed with isothermal-isobaric conditions at the temperature of 277 K (van Gunsteren and Berendsen, 1988) and a pressure of 1.0 atm (Liao and Parrinello, 2002). Separate simulations were performed for changes in the Lennard-Jones with 21 values of the scaling parameter λ and the electrostatics with 11 (Shyu et al., 2010). For simulations with only Lennard-Jones, all partial charges were set to zero. Simulations were performed at 2.0 ns for each λ value, and the first 1.0 ns was discarded for equilibration and the last 1.0 ns used to calculate the binding affinity. To minimize the numerical integration errors, we employed the polynomial and regression fitting techniques to calculate the free energy difference (Shyu and Ytreberg, 2009; Shyu and Ytreberg, 2010). The Bennett acceptance ratio approach was used to estimate the free energy associated with the restraints (Bennett, 1976).
It is worth noting that the MD simulations produce an estimate for the free energy of binding (ΔG) in kJ/mol. For this report these results were converted to pKd for the standard state (i.e., 1 mol/L concentration) pKd = −log10Kd, where Kd = e−ΔG/RT is the dissociation constant, R = 8.314472 JK−1mol−1 is the universal gas constant, and T = 277 K is the absolute system temperature.
Results
A total of 5,000 independent docking trials were performed for each of the four rainbow trout ERs. The best binding pose from each trial was collected and then ranked based on a scoring function. Figure 1 shows the best-fit binding pose for E2, EE2, BPA, RAL, ATZ, and TES in the ERα1 ligand-binding domain. The best-fit binding poses of these six compounds were similar for the other three ERs (i.e., ERα2, ERβ1, ERβ2), and thus are not shown.
Figure 1.
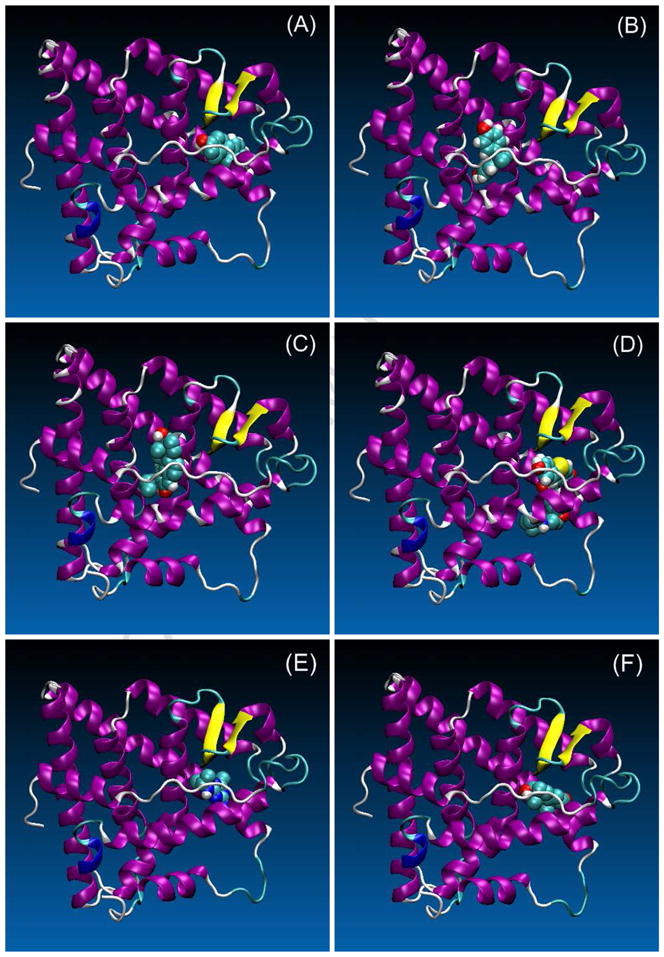
Computationally determined binding poses for (A) estradiol-17β (E2), (B) 17α-ethinylestradiol (EE2), (C) bisphenol A (BPA), (D) raloxifene (RAL), (E) atrazine (ATZ), and (F) testosterone (TES) in the rainbow trout ERα1 ligand-binding domain. The other rainbow trout isoforms are not shown since the binding poses are similar. Images were rendered using the Visual Molecular Dynamics software (Humphrey et al., 1996). The ligands are drawn based on the chemical elements and receptors the secondary structures of the proteins. Specifically, the ligands are shown as beads representing oxygen (red), carbon (cyan), hydrogen (grey), and sulfur (yellow). For the receptor, helices are shown as coiled ribbons (purple), β-sheets as solid arrows (yellow), π-helix as coiled ribbons (blue), and loop structures as tubes (cyan, gray) (Frishman and Argos, 1995).
Obtaining experimental binding affinity results is not possible for the four rainbow trout ERs because purified proteins are not currently available. Therefore, human ERα was first used to validate the methodology for estimating the binding affinities for the rainbow trout ERs. MD simulations using the human ERα-E2 complex were performed at 300 K and the resulting binding affinity was compared to experimental results reported by Petit et al. (1995). The computational estimate of binding affinity for hER-E2 complex at 300 K was pKd = 11.0 compared to the experimental value of 9.1. This computational estimate is sufficiently accurate because the discrepancy is within the expected error due to the atomic models (Hess et al., 2008; Shyu et al., 2010). Subsequent MD simulations for human and rainbow trout ERs followed exactly the same procedure as human, beginning with docking the compounds into the ERs.
Table 1 shows results for the computational binding affinities for the various compounds and the rainbow trout ER isoforms and human ERα. Among the known estrogenic compounds tested, the highest binding affinity occurred with EE2 and ERβ1, while the lowest binding affinity occurred with BPA and ER β1. In general, across all rainbow trout ER isoforms and the human ERα, EE2 had the highest binding affinities, while BPA had the lowest binding affinities. RAL had significantly stronger affinity to the rainbow trout ERα isoforms, compared to the ERβ isoforms. Very similar binding affinities for RAL and the rainbow trout ERα isoforms and human ERα were determined. Our simulation results for ATZ and TES show that the affinity is small or negative, consistent with the fact that these compounds are known to be weak or non-binders to the ERs.
Table 1.
Binding affinities for estradiol (E2), ethinylestradiol (EE2), bisphenol A (BPA), raloxifene (RAL), atrazine (ATZ), and testosterone (TES) to rainbow trout estrogen receptor (ER) isoforms, ERα1, ERα2, ERβ1, and ERβ2. The affinities are expressed as pKd = −log10 Kd where Kd is the dissociation constant. Results from the human ERα (hERα) are included for comparison (Shyu et al., 2010). Larger positive pKd values correspond to stronger binding and negative values indicate that binding will not occur at all. Note that, as expected, ATZ and TES show very weak or negative affinity for all the ERs.
| Receptors | |||||
|---|---|---|---|---|---|
| ERα1 | ERα2 | ERβ1 | ERβ2 | hERα | |
| E2 | 9.6 | 6.7 | 8.0 | 9.4 | 9.4 |
| EE2 | 10.0 | 9.7 | 13.7 | 11.0 | 11.6 |
| BPA | 4.3 | 6.0 | 3.5 | 3.9 | 4.7 |
| RAL | 11.7 | 11.9 | 6.5 | 4.5 | 11.7 |
| ATZ | 1.9 | 2.0 | −4.7 | 0.1 | 3.2 |
| TES | 2.3 | −2.2 | −1.8 | −1.8 | −3.7 |
Discussion
To our knowledge this study represents the first MD simulation analysis of ER binding affinities for environmental estrogens in a fish. It provides a unique opportunity to relate computationally derived binding affinities with previous studies in fish that have examined the in vivo and in vitro estrogenic effects of these compounds (Klotz et al., 1996; Matthews et al., 2000; Parrot and Blunt, 2005; Bjerregaard et al., 2007). A review of the literature on EE2 shows it to be a potent estrogen in rainbow trout and other fishes (Caldwell et al., 2008), with an efficacy greater than E2 (Brian et al., 2005). The results of this study support those findings. For each rainbow trout ER isoform EE2 has a greater estimated binding affinity compared to E2. BPA has been reported to be a weaker estrogen than E2 in fishes, for example (Brian et al., 2005), and the results of this analysis are in line with these reports also. The binding affinities for BPA with ERs from either the rainbow trout or human are the lowest indicated. It is predicted that much more BPA would be required, compared to EE2 or E2, to achieve a similar estrogenic response in the rainbow trout. RAL has not been extensively studied in fishes, but based on the results from this study it would be predicted to have a strong estrogenic response and be more potent than EE2 at equivalent molar concentrations. This suggests that even low levels of RAL release into the environment will pose a threat to fishes.
RAL is a mammalian SERM, acting primarily through the ERα subtype, which is substantiated in this work by comparison between rainbow trout ER subtypes and the human ERα subtype (see Table 1). The binding affinities for RAL with either the rainbow trout ERα isoforms or human ERα subtype are approximately double that of RAL with the ERβ isoforms. Therefore, RAL should be considered a SERM in the rainbow trout, with the majority of its biological activity predicted to function through the ERα a subtype.
There were several instances in which the binding affinities of both rainbow trout ER isoforms within a subtype were very similar for a particular environmental estrogen (see Table 1). For example, both ERα isoforms had nearly identical binding affinities for EE2 or RAL. This was observed for the ERβ isoforms too, with E2 or BPA. The ligand-binding region, within the E-domain of ERs (Tsai and O’Malley, 1994), is the location where ligands interact most intimately with the ER molecule (see Figure 1). The similarity in binding affinities observed reflects the overall similarity in E-domain molecular structure between isoforms within an ER subtype. The very similar RAL binding affinities of the rainbow trout and human ERαs are likely explained by highly convergent molecular structures within the ER ligand-binding region.
In conclusion, MD simulations were used to estimate the binding affinity of three environmental estrogens, EE2, BPA and RAL for the four rainbow trout ERs, and compare them to E2. The results show that BPA binds less strongly to all four receptors, EE2 binds more strongly, and RAL has a very high affinity for the α subtype, but not the β. The results show significant differences between these environmental estrogens that are in concert with their known estrogenic response in biological assays. One test compound, RAL, a known mammalian SERM, is predicted to be highly estrogenic in the rainbow trout, but this will need to be confirmed by the appropriate assay(s). Finally, it is worth noting that MD simulations successfully predicted that both ATZ and TES are weak or non-binders to ERs. The results of this study suggest that when purified forms of the receptors are not available MD simulation could be a useful technique to predict the estrogenic potential of compounds known to be present in the environment.
Acknowledgments
The project described was supported by Award Numbers P20RR016448 and R21GM083827 from the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The research was also supported by Idaho NSF-EPSCoR, and by IBEST and BANTech at the University of Idaho.
Footnotes
Conflict of Interest Statement
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Conrad Shyu, Email: conrads@uidaho.edu.
Timothy D. Cavileer, Email: tcavi@uidaho.edu.
James J. Nagler, Email: jamesn@uidaho.edu.
F. Marty Ytreberg, Email: ytreberg@uidaho.edu.
References
- Allen MP, Tildesley DJ. Computer simulation of liquids. Oxford University Press; Oxford UK: 1989. [Google Scholar]
- Arcand-Hoy LD, Nimrod AC, Benson WH. Endocrine-modulating substances in the environment: Estrogenic effects of pharmaceutical products. Int J Toxicol. 1998;17:139–158. [Google Scholar]
- Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modeling. Bioinformatics. 2006;22:195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- Ashby J, Tinwell H, Stevens J, Pastoor T, Breckenridge CB. The effects of atrazine on the sexual maturation of female rates. Regul Toxicol Pharm. 2002;35:468–473. doi: 10.1006/rtph.2002.1571. [DOI] [PubMed] [Google Scholar]
- Bennett C. Efficient estimation of free energy differences from Monte Carlo data. J Comput Chem. 1976;30:245–268. [Google Scholar]
- Bjerregaard P, Andersen SB, Pedersen KL, Pedersen SN, Korsgaard B. Orally administered bisphenol A in rainbow trout (Oncorhynchus mykiss): estrogenicity, metabolism, and retention. Environ Toxicol Chem. 2007;26:1910–1915. doi: 10.1897/06-645R.1. [DOI] [PubMed] [Google Scholar]
- Brian JV, Harris CA, Scholze M, Backhaus T, Booy P, Lamoree M, Pojana G, Jonkers N, Runnalls T, Bonfà A, Marcomini A, Sumpter JP. Accurate prediction of the response of freshwater fish to a mixture of estrogenic chemicals. Environ Health Perspect. 2005;113:721–728. doi: 10.1289/ehp.7598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KH, Schultz IR, Nagler JJ. Reduced embryonic survival in rainbow trout resulting from paternal exposure to the environmental estrogen 17α–ethynylestradiol during late sexual maturation. Reproduction. 2007;135:659–666. doi: 10.1530/REP-07-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyden C. The convergence of a class of double-rank minimization algorithms 1. General considerations. J Comput Chem. 1970;18:1463–1472. [Google Scholar]
- Caldwell DJ, Mastrocco F, Hutchinson TH, Länge R, Heijerick D, Janssen C, Anderson PD, Sumpter JP. Derivation of an aquatic predicted no-effect concentration for the synthetic hormone, 17 alpha-ethinyl estradiol. Environ Sci Technol. 2008;42:7046–7054. doi: 10.1021/es800633q. [DOI] [PubMed] [Google Scholar]
- Corcoran J, Winter MJ, Tyler CR. Pharmaceuticals in the aquatic environment: A critical review of the evidence for health effects in fish. Crit Rev Toxicol. 2010;40:287–304. doi: 10.3109/10408440903373590. [DOI] [PubMed] [Google Scholar]
- Denny JS, Tapper MA, Schmieder PK, Hornung MW, Jensen KM, Ankley GT, Henry TR. Comparison of relative binding affinities of endocrine active compounds to fathead minnow and rainbow trout estrogen receptors. Environ Toxicol Chem. 2005;24:2948–2953. doi: 10.1897/04-595r.1. [DOI] [PubMed] [Google Scholar]
- Diana SG, Resetarits WJ, Schaeffer DJ, Beckman KB, Beasley VR. Effects of atrazine on amphibian growth and survival in artificial aquatic communities. Environ Toxicol Chem. 2000;19:2961–2967. [Google Scholar]
- Folmar LC, Hemmer M, Hemmer R, Bowman C, Kroll K, Denslow ND. Comparative estrogenicity of estradiol, ethynyl estradiol, and diethylstilbestrol in an in vivo, male sheepshead minnow (Cyprinodon variegates), vitellogenin bioassay. Aquat Toxicol. 2000;49:77–88. doi: 10.1016/s0166-445x(99)00076-4. [DOI] [PubMed] [Google Scholar]
- Frishman D, Argos P. Knowledge-based secondary structure assignment. Proteins: Structure, Function and Genetics. 1995;23:566–579. doi: 10.1002/prot.340230412. [DOI] [PubMed] [Google Scholar]
- Halden RU. Plastics and health risks. Annu Rev Public Health. 2010;31:179–194. doi: 10.1146/annurev.publhealth.012809.103714. [DOI] [PubMed] [Google Scholar]
- Hess B, Kutzner C, van der Spoel D, Lindahl E. GROMACS 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation. J Chem Theo and Comput. 2008;4:435–447. doi: 10.1021/ct700301q. [DOI] [PubMed] [Google Scholar]
- Humphrey W, Dalke A, Schulten K. VMD: visual molecular dynamics. J Molec Graphics. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- Kiefer F, Arnold K, Künzli M, Bordoli L, Schwede T. The SWISS-MODEL repository and associated resources. Nucleic Acids Research. 2009;37:D387–D392. doi: 10.1093/nar/gkn750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz DM, Beckman BS, Hill SM, McLachlan JA, Walters MR, Arnold SF. Identification of environmental chemicals with estrogenic activity using a combination of in vitro assays. Environ Health Perspect. 1996;10:1084–1089. doi: 10.1289/ehp.961041084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolpin DW, Edward TF, Meyer MT, Thurman EM, Zaugg SD, Barber JB, Buston HT. Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999–2000: A national reconnaissance. Environ Sci Technol. 2002;36:1202–1211. doi: 10.1021/es011055j. [DOI] [PubMed] [Google Scholar]
- Kubli-Garfias C. Comparative study of the electronic structure of estradiol, epiestradiol and estrone by ab initio theory. Theochem J Mol Struct. 1998;452:175–183. [Google Scholar]
- Latonnelle K, Fostier A, Le Menn F, Bennetau-Pelissero C. Binding affinities of hepatic nuclear estrogen receptors for phytoestrogens in rainbow trout (Oncorhynchus mykiss) and Siberian sturgeon (Acipenser baeri) Gen Comp Endocrinol. 2002;129:69–79. doi: 10.1016/s0016-6480(02)00512-9. [DOI] [PubMed] [Google Scholar]
- Le HH, Carlson EM, Chua JP, Belcher SM. Bisphenol A is released from polycarbonate drinking bottles and mimics the neurotoxic actions of estrogen in developing cerebellar neurons. Toxicol Lett. 2008;176:149–156. doi: 10.1016/j.toxlet.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach A. Molecular modeling: principles and applications. 2. Prentice Hall; New York NY: 2001. [Google Scholar]
- Liao A, Parrinello M. Escaping free-energy minima. Proc Natl Acad Sci USA. 2002;99:12562–12566. doi: 10.1073/pnas.202427399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez L, Sonoda MT, Webb P, Baxter JD, Skaf MS, Polikarpov I. Molecular dynamics simulations reveal multiple pathways of ligand dissociation from thyroid hormone receptors. Biophys J. 2005;89:2011–2023. doi: 10.1529/biophysj.105.063818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez L, Webb P, Polikarpov I, Skaf MS. Molecular dynamics simulations of ligand dissociation from thyroid hormone receptors: evidence of the likeliest escape pathway and its implications for the design of novel ligands. J Med Chem. 2006;49:23–26. doi: 10.1021/jm050805n. [DOI] [PubMed] [Google Scholar]
- Matthews J, Celius T, Halgren R, Zacharewski T. Differential estrogen receptor binding of estrogenic substances: a species comparison. J Ster Biochem Mol Biol. 2000;74:223–234. doi: 10.1016/s0960-0760(00)00126-6. [DOI] [PubMed] [Google Scholar]
- Mathiessen P, Sumpter JP. Effects of estrogenic substances in the aquatic environment. EXS. 1998;86:319–335. doi: 10.1007/978-3-0348-8853-0_10. [DOI] [PubMed] [Google Scholar]
- McLachlan JA. Estrogens in the Environment. Elsevier/North-Holland; New York: 1979. [Google Scholar]
- Mills LJ, Chichester C. Review of evidence: are endocrine-disrupting chemicals in the aquatic environment impacting fish populations? Science of the Total Environ. 2005;343:1–34. doi: 10.1016/j.scitotenv.2004.12.070. [DOI] [PubMed] [Google Scholar]
- Morris G, Goodsell D, Halliday R, Huey R, Hart W, Belew RK, Olson AJ. Automated docking using a lamarckian genetic algorithm and empirical binding free energy function. J Comput Chem. 1998;28:1639–1662. [Google Scholar]
- Muchmore DB. Raloxifene: a selective estrogen receptor modulator (SERM) with multiple target system effects. The Oncologist. 2000;5:388–392. doi: 10.1634/theoncologist.5-5-388. [DOI] [PubMed] [Google Scholar]
- Nagler JJ, Cavileer TD, Sullivan J, Cyr DG, Rexroad C., III The complete nuclear estrogen receptor family in the rainbow trout: discovery of the novel ERα2 and both ERβ isoforms. Gene. 2007;392:164–173. doi: 10.1016/j.gene.2006.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novic M, Vracko M. QSAR models for reproductive toxicology and endocrine disruption activity. Molecules. 2010;15:1987–1999. doi: 10.3390/molecules15031987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuclear Receptors No men clature Committee. A unified no men clature system for the nuclear receptor super family. Cell. 1999;97:161–163. [Google Scholar]
- Oostenbrink BC, Pitera JW, vanLipzig MMH, Meerman JHN, vanGusteren WF. Simulations of the estrogen receptor ligand -binding domain: affinity of natural ligands and xenoestrogens. J Med Chem. 2000;43:4594–4605. doi: 10.1021/jm001045d. [DOI] [PubMed] [Google Scholar]
- Parrott JL, Blunt BR. Life-cycle exposure of fathead minnows (Pimephales promelas) to an ethinylestradiol concentration below 1 ng/L reduces egg fertilization success and demasculinizes males. Environ Toxicol. 2005;20:131–141. doi: 10.1002/tox.20087. [DOI] [PubMed] [Google Scholar]
- Petit F, Valotaire Y, Pakdel F. Differential functional activities of rainbow rout and human estrogen receptors expressed in the yeast Saccharomyces cerevisiae. Euro J Biochem. 1995;233:584–592. doi: 10.1111/j.1432-1033.1995.584_2.x. [DOI] [PubMed] [Google Scholar]
- Rapaport DC. The art of molecular dynamics simulations. Cambridge University Press; Cambridge UK: 1996. [Google Scholar]
- Roberg M, Hakk H, Larsen G. Atrazine is a competitive inhibitor of phosphodiesterase but does not affect the estrogen receptor. Toxicol Lett. 154:61–68. doi: 10.1016/j.toxlet.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Salierno JD, Kane AS. 17alpha-ethinylestradiol alters reproductive behaviors, circulating hormones, and sexual morphology in male fathead minnows (Pimephales promelas) Environ Toxicol Chem. 2010;28:953–61. doi: 10.1897/08-111.1. [DOI] [PubMed] [Google Scholar]
- Salum LB, Polikarpov I, Andricopulo AD. Structure-based approach for the study of estrogen receptor binding activity and subtype selectivity. J Chem Inf Model. 2008;48:2243–2253. doi: 10.1021/ci8002182. [DOI] [PubMed] [Google Scholar]
- Sanderson JT, Seinen W, Giesy JP, van den Berg M. 2-chloro-s-triazine herbicides induce aromatase (CYP19) activity in H295R human adrenocortical carcinoma cells: a novel mechanism for estrogenicity. Toxicol Sci. 2000;54:121–127. doi: 10.1093/toxsci/54.1.121. [DOI] [PubMed] [Google Scholar]
- Sanderson JT, Boerma J, Lansbergen GWA, van den Berg M. Induction and inhibition of aromatase (CYP19) activity by various classes of pesticides in H295R human adrenocortical carcinoma cells. Toxicol Appl Pharmacol. 2002;182:44–54. doi: 10.1006/taap.2002.9420. [DOI] [PubMed] [Google Scholar]
- Schüettelkopf AW, van Aalten DMF. PROGRG – a tool for high-throughput crystallography for protein-ligand complexes. Acta Crystallographica. 2004;D60:355–1363. doi: 10.1107/S0907444904011679. [DOI] [PubMed] [Google Scholar]
- Shirts M, Pitera J, Swope W, Pande V. Extremely precise free energy calculations of amino acid side chain analogs: comparison of common molecular mechanics force fields proteins. J Chem Phys. 2003;119:5740. [Google Scholar]
- Shrader SP, Dickerson LM. Extended- and continuous-cycle oral contraceptives. Pharmacotherapy. 2008;28:1033–1040. doi: 10.1592/phco.28.8.1033. [DOI] [PubMed] [Google Scholar]
- Shyu C, Ytreberg FM. Reducing the bias and uncertainty of free energy estimates by using regression to fit thermodynamic integration data. J Comput Chem. 2009;30:2297–2304. doi: 10.1002/jcc.21231. [DOI] [PubMed] [Google Scholar]
- Shyu C, Brown CJ, Ytreberg FM. Computational study of evolutionary selection pressure on rainbow trout estrogen receptors. PLoS One. 2010;5(3):e9392. doi: 10.1371/journal.pone.0009392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyu C, Ytreberg FM. Accurate estimation of salvation free energy using polynomial fitting techniques. J Comput Chem. 2010 doi: 10.1002/jcc.21609. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohoni P, Tyler CR, Hurd K, Caunter J, Hetheridge M, Williams T, Woods C, Evans M, Toy R, Gargas M, Sumpter JP. Reproductive effects of long-term exposure to Bisphenol A in the fathead minnow (Pimephales promelas) Environ Sci Technol. 2001;35:2917–2925. doi: 10.1021/es000198n. [DOI] [PubMed] [Google Scholar]
- Sonoda MT, Martínez L, Webb P, Skaf MS, Polikarpov I. Ligand dissociation from estrogen receptor is mediated by receptor dimerization: evidence molecular dynamics simulations. Molec Endocrino. 2007;22:1565–1578. doi: 10.1210/me.2007-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staples CA, Dorn PB, Klecka GM, O’Block ST, Harris LR. A review of the environmental fate, effects, and exposures of bisphenol A. Chemosphere. 1998;36:2149–2173. doi: 10.1016/s0045-6535(97)10133-3. [DOI] [PubMed] [Google Scholar]
- Sugiyama H, Kumamoto T, Suganami A, Nakanishi W, Sowa Y, Takiguchi M, Ishikawa T, Tamura Y. Insight into estrogenicity of phytoestrogens using in silico simulation. Biochem Biophys Research Comm. 2009;379:139–144. doi: 10.1016/j.bbrc.2008.12.046. [DOI] [PubMed] [Google Scholar]
- Teeter JS, Meyerhoff RD. Environmental fate and chemistry of raloxifene hydrochloride. Environ Toxicol Chem. 2002;21:729–736. [PubMed] [Google Scholar]
- Tilghman SL, Nierth-Simpson EN, Wallace R, Burow ME, McLachlan JA. Environmental hormones: Multiple pathways for response may lead to multiple disease outcomes. Steroids. 2010;75:520–523. doi: 10.1016/j.steroids.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Toppari J, Larsen JC, Christiansen P, Giwercman A, Grandjean P, Guillette LJ, Jr, Jégou B, Jensen TK, Jouannet P, Keiding N, Leffers H, McLachlan JA, Meyer O, Müller J, Rajpert-De Meyts E, Scheike T, Sharpe R, Sumpter J, Skakkebaek NE. Male reproductive health and environmental xenoestrogens. Environ Health Perspect. 1996;104 (Suppl 4):741–803. doi: 10.1289/ehp.96104s4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MJ, O’Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- Tyler C, Jobling S, Sumpter JP. Endocrine disruption in wildlife: A critical review of the evidence. Crit Rev Toxicol. 1998;28:319–363. doi: 10.1080/10408449891344236. [DOI] [PubMed] [Google Scholar]
- van Gunstern W, Berendsen H. A leap-frog algorithm for stochastic dynamics. Mol Simul. 1988;1:173–185. [Google Scholar]
- van Gunsteren W, Billeter S, Eising A, Hünenberger P, Krüger P, Mark AE, Scott WRP, Tironi IG. Biomolecular simulation: the GROMOS96 manual and user guide. Zürich, Germany: Vdf Hochschulverlag AG an der ETH Zürich; 1996. [Google Scholar]
- van Lipzig MMH, ter Laak AM, Jongejan A, Vermeulen NPE, Warnelink M, Geerke D, Meerman HN. Prediction of ligand binding affinity and orientation of xenoestrogens to the estrogen receptor by molecular dynamics simulations and the linear interaction energy method. J Med Chem. 2004;47:1018–1030. doi: 10.1021/jm0309607. [DOI] [PubMed] [Google Scholar]
- Wiegand C, Krause E, Steinberg C, Pflugmacher S. Toxicokinetics of atrazine in embryos of the zebrafish (Danio rerio) Excotoxicol Environ Saf. 2001;49:199–205. doi: 10.1006/eesa.2001.2073. [DOI] [PubMed] [Google Scholar]


