Abstract
Familial clustering of disparate kidney diseases including clinically diagnosed hypertensive and diabetic nephropathy, idiopathic focal segmental glomerulosclerosis (FSGS) and Human Immunodeficiency Virus-associated nephropathy are often observed in African Americans. Admixture mapping recently identified the non-muscle myosin heavy chain 9 gene (MYH9) as a susceptibility factor strongly associated with several non-diabetic etiologies of end-stage renal disease (ESRD) in African Americans, less strongly with diabetes-associated ESRD. MYH9-associated nephropathies reside in the spectrum of FSGS/focal global glomerulosclerosis. The renal histology in proteinuric African Americans homozygous for MYH9 risk variants with longstanding type 2 diabetes mellitus is unknown. We report a case of coincident idiopathic FSGS, collapsing variant; and diabetic nephropathy in an African American homozygous for the MYH9 E1 risk haplotype. This case demonstrates that diabetic African Americans with overt proteinuria can have mixed renal lesions, including those in the spectrum of MYH9-associated nephropathy. Careful interpretation of kidney biopsies in proteinuric African Americans with diabetes is necessary to exclude coincident non-diabetic forms of nephropathy, precisely define etiologies of kidney disease, and determine the natural history and treatment response in mixed lesions of diabetes-associated and MYH9-associated kidney disease.
Summary
We report a case of coincident idiopathic FSGS, collapsing variant; and diabetic nephropathy in an African American homozygous for the MYH9 E1 risk haplotype.
Keywords: African American, collapsing variant focal segmental glomerulosclerosis, diabetes, diabetic nephropathy, MYH9
Introduction
The impressive genetic association observed between variants in the non-muscle myosin heavy chain 9 gene (MYH9) and non-diabetic forms of nephropathy has fundamentally altered our understanding of the epidemiology of chronic kidney disease (CKD), particularly in African-Americans. Approximately 36% of African Americans are homozygous for the major MYH9 E1 risk haplotype, compared to <1% of European Americans. 1, 2 This disparity accounts for much of the observed ethnic variation in incidence rates of non-diabetic end-stage renal disease (ESRD). The odds ratio (OR) for MYH9 association with idiopathic focal segmental glomerulosclerosis (FSGS) and HIV-associated nephropathy (HIVAN) range from 5–8, extremely high for common complex human disease. 1, 2 It is estimated that 70% of African Americans with non-diabetic ESRD have MYH9-associated nephropathy. 2 In contrast, MYH9 is less strongly associated with type 2 diabetic ESRD in African Americans (OR 1.2–1.4) and it may identify a subset of diabetic individuals enriched for non-diabetic renal disease. 2, 3
The renal histology in African American MYH9 risk homozygotes with clinically diagnosed diabetic nephropathy (DN) is unclear. Kidney diseases with proven MYH9 association include focal global glomerulosclerosis (FGGS; the disease historically attributed to hypertension) 4, idiopathic FSGS 1, 2, and the collapsing variant of FSGS as seen in HIVAN and collapsing C1q nephropathy. 4 Although 16% of African Americans with clinically diagnosed type 2 DN have MYH9-associated disease, it is unknown whether their renal pathology is reflective of DN (diffuse thickening of the glomerular basement membranes, mesangial expansion and nodular glomerulosclerosis), FSGS, or combined lesions. 3 This is particularly true in individuals with diabetes who have close relatives with non-diabetic etiologies of ESRD. 4, 5 Diseases in the FSGS/FGGS spectrum likely occur more often in diabetic members of families in whom disparate renal diseases are present. Patients thought to have DN often have non-diabetes-associated kidney diseases, particularly with type 2 diabetes. 4, 6
Herein, we report renal biopsy findings in an African American woman with 2 copies of the MYH9 E1 risk haplotype, longstanding type 2 diabetes mellitus and recently detected heavy proteinuria. She also has a sibling with non-diabetic ESRD.
Materials and Methods
The biopsy specimen obtained was a core of renal cortex including as many as 20 glomeruli. It was prepared for brightfield microscopy in multiple levels of sectioning stained with hematoxylin and eosin, periodic acid schiff, periodic acid methenamine silver, and trichrome stains. Immunofluorescence microscopy was also performed on sections of the paraffin-embedded material, including as many as 10 glomeruli.
Results
The kidney biopsy revealed widespread diffuse and nodular diabetic glomerulosclerosis; with intercurrent FSGS, collapsing variant (Figure 1). Seven glomeruli were obsolescent, one globally sclerotic, and one demonstrated global glomerulosclerosis with features suggestive of the collapsing variant. Hyperplasia and hypertrophy of overlying podocytes were present in several levels, consistent with collapsing glomerulopathy. All remaining glomeruli revealed diffuse mesangial hyperplasia with increased mesangial matrix; several contained superimposed segmental nodular sclerosis with variable sized nodules. Segments containing hyaline material consistent with fibrin caps of diabetic nephropathy were present. Moderately advanced tubular atrophy comprised ~20% of the biopsy area. There was muscular hyperplasia and segmental hyalinosis in small interlobular arterioles. Intermediate caliber interlobular arteries demonstrated similar changes and segmental medial scarring. Immunofluorescence microscopy revealed focal mesangial granular deposits of IgM (2+) with superimposed irregular nonspecific mesangial C3 deposits in segments of tuft scarring. Stains for IgG, IgA, C1q, and kappa and lambda immunoglobulin light chains were negative.
Figure 1.
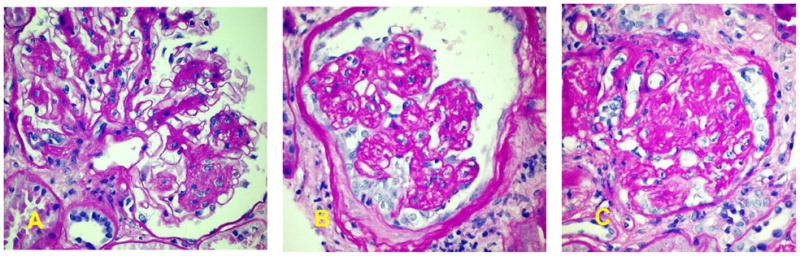
A. Glomerulus showing diffuse and nodular diabetic glomerulosclerosis.
B. Glomerulus showing pattern of collapsing glomerulopathy with individual lobules collapsed, yet retaining their individuality with hypertrophy/hyperplasia of overlying podocytes displaying intracytoplasmic vesicles containing protein reabsorption droplets, more evident on one aspect of the consolidated tuft than on the other.
C. Globally sclerotic glomerulus displaying residual evidence of collapsing glomerulopathy. All PAS stain. Original magnification 400X
Four MYH9 E1 risk haplotype single nucleotide polymorphisms (SNPs) rs4821480, rs2032487, rs4821481, and rs3752462 were genotyped on the Sequenom Mass Array (www.sequenom.com). The patient was homozygous for risk alleles in all 4 MYH9 E1 SNPs (GCCT).
Discussion
It is clear that a subset of patients with biopsy proven diabetic nephropathy have concurrent non-diabetic renal diseases.7–10 In a study conducted in 30 African Americans with clinically diagnosed type 2 DN, DN alone was present in 41.9% of patients, DN with superimposed glomerulonephritis was present in 38.7%, and non-diabetic renal diseases were seen in 19.4%. 10 The present case demonstrates that MYH9-associated nephropathy in African Americans with longstanding diabetes can include glomerular lesions typical of both FSGS and DN. This observation may be particularly relevant in multiply affected families with members having nephropathy from disparate causes, as in this case. It is unclear how often co-existing FSGS and DN occur in sporadic cases of clinically diagnosed DN among those lacking family members with non-diabetic kidney disease. This case revealed idiopathic FSGS, collapsing variant in an MYH9 risk homozygote; a finding often reported in secondary forms of collapsing FSGS such as HIVAN and collapsing C1q nephropathy. 1, 2, 4 Focal global glomerulosclerosis and collapsing FSGS in this case likely represented primary kidney diseases within the MYH9-spectrum and unrelated to diabetes. However, it remains possible that secondary forms of FSGS could have been present as the patient had co-existing hypertension and intrarenal small vessel disease.
This single case does not prove that MYH9 was associated with the presence of idiopathic collapsing FSGS, nor does it demonstrate a role for this gene in development of the typical histologic changes observed in DN. Approximately 36% of African Americans inherit two copies of MYH9 risk variants, so it could have been an incidental finding. However, the strong association between FSGS and MYH9 in several reports makes this less likely. The effects of MYH9 risk variants on diabetes-associated mesangial matrix expansion and GBM thickening will require additional study. Although a sizeable proportion of subjects with type 2 diabetes and proteinuria have etiologies of CKD other than diabetes, this observation has not yet been extended toMYH9-associated nephropathy in African-derived populations. 4, 6
The clinical relevance of coexisting FSGS collapsing variant and DN is immense. Treatment trials and natural history studies evaluating large numbers of diabetic subjects with proteinuria assume that participants have relatively consistent phenotypes. 11 FSGS is a primary renal disease that does not respond to the same therapies used in DN, although renin-angiotensin system blocking agents may benefit both of these proteinuric kidney diseases. This case report suggests the potential to genetically dissect complex phenotypes such as DN. 12 MYH9 genotyping in African Americans with type 2 diabetes and proteinuria holds promise for stratifying individuals with CKD into more homogeneous groups. For example, the effect of reducing serum glucose concentrations on slowing progression of nephropathy could be more impressive in African Americans with pure DN (lacking MYH9 risk alleles and co-existing renal lesions in the FSGS spectrum). Prospective trials will be necessary to test this hypothesis.
MYH9, and other major genes underlying susceptibility to FSGS and FGGS (α-actinin-4 [ACTN4]; transient receptor potential cation channel, subfamily C, member 6 [TRPC6]; and podocin [NPHS2]) have the potential to alter the existing classification of FSGS, as well. 13 The current FSGS diagnostic schema is based solely on renal histology, but might benefit from incorporation of genotypic data. 14
It remains vital to exclude non-diabetic forms of nephropathy in proteinuric individuals with type 2 diabetes; especially those with atypical clinical courses such as short durations of diabetes or the absence of diabetic retinopathy. Careful attention to the examination of renal biopsies by experienced nephropathologists is required to identify subtle forms of nephropathy on a background of DN. Genotyping for MYH9, and other major kidney failure susceptibility genes, appear likely to improve our ability to detect non-diabetic forms of nephropathy in subjects with type 2 diabetes. Evaluating homogeneous subsets of patients with diabetes and kidney disease would allow for more precise determination of the effects of specific treatments in the era of personalized medicine.
Clinical Summary.
A 67 year old African American woman with a ten year history of type 2 diabetes mellitus and hypertension was referred after a urinalysis revealed 300 mg/dl proteinuria and trace hematuria. Spot urine protein:creatinine and urine albumin:creatinine ratios were 4.36 g/g and 3.03 g/g, respectively; serum creatinine concentration was 1.2 mg/dL (estimated glomerular filtration rate 58 ml/min). She lacked diabetic retinopathy when evaluated by an ophthalmologist and had negative antinuclear cytoplasmic antibody (ANCA), HIV, hepatitis C and hepatitis B antibody titers, normal C3 and C4 complement levels, and no serum or urine monoclonal proteins. Magnetic Resonance Imaging revealed a left kidney that was 8.3 cm left and a right kidney that was 8.7 cm with bilateral renal artery stenosis. Family history included a brother with hematuria, proteinuria, and ESRD that developed at 50 years of age. He had hepatitis C viremia with biopsy-proven cryoglobulinemia in a membranoproliferative pattern.
Acknowledgments
This work was supported in part by NIH grants RO1 DK53591 (DWB), RO1 DK070941 (BIF) and RO1 DK084149 (BIF).
Footnotes
The authors report no conflicts of interest in this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Kopp JB, Smith MW, Nelson GW, et al. MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat Genet. 2008;40(10):1175–84. doi: 10.1038/ng.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kao WH, Klag MJ, Meoni LA, et al. MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nat Genet. 2008;40(10):1185–92. doi: 10.1038/ng.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freedman BI, Hicks PJ, Bostrom MA, et al. Non-muscle myosin heavy chain 9 gene MYH9 associations in African Americans with clinically diagnosed type 2 diabetes mellitus-associated ESRD. Nephrol Dial Transplant. 2009;11:3366–71. doi: 10.1093/ndt/gfp316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bostrom MA, Freedman BI. The spectrum of MYH9-associated nephropathy. Clinical Journal of the American Society of Nephrology. 2010 doi: 10.2215/CJN.08721209. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freedman BI, Iskandar SS, Appel RG. The link between hypertension and nephrosclerosis. Am J Kidney Dis. 1995;25(2):207–21. doi: 10.1016/0272-6386(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 6.Cordonnier D. Glomerular involvement in type II diabetes - is it all diabetic glomerulosclerosis? Nephrol Dial Transplant. 1996;11:936–8. doi: 10.1093/oxfordjournals.ndt.a027504. [DOI] [PubMed] [Google Scholar]
- 7.Arif M, Arif MK, Arif MS. An evaluation of renal biopsy in type-II diabetic patients. J Coll Physicians Surg Pak. 2009;19(10):627–31. doi: 10.2009/JCPSP.627631. [DOI] [PubMed] [Google Scholar]
- 8.Ghani AA, Al WS, Al SA, Hussain N. Renal biopsy in patients with type 2 diabetes mellitus: indications and nature of the lesions. Ann Saudi Med. 2009;29(6):450–3. doi: 10.4103/0256-4947.57167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nebuloni M, Barbiano di BG, Genderini A, et al. Glomerular lesions in HIV-positive patients: a 20-year biopsy experience from Northern Italy. Clin Nephrol. 2009;72(1):38–45. doi: 10.5414/cnp72038. [DOI] [PubMed] [Google Scholar]
- 10.Nzerue CM, Hewan-Lowe K, Harvey P, et al. Prevalence of non-diabetic renal disease among African-American patients with type II diabetes mellitus. Scand J Urol Nephrol. 2000;34(5):331–5. doi: 10.1080/003655900750048378. [DOI] [PubMed] [Google Scholar]
- 11.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329(20):1456–62. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 12.Freedman BI, Bostrom M, Daeihagh P, Bowden DW. Genetic factors in diabetic nephropathy. Clin J Am Soc Nephrol. 2007;2(6):1306–16. doi: 10.2215/CJN.02560607. [DOI] [PubMed] [Google Scholar]
- 13.Winkler CA, Nelson G, Oleksyk TK, Nava MB, Kopp JB. Genetics of focal segmental glomerulosclerosis and human immunodeficiency virus-associated collapsing glomerulopathy: the role of MYH9 genetic variation. Semin Nephrol. 2010;30(2):111–25. doi: 10.1016/j.semnephrol.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Agati VD, Fogo AB, Bruijn JA, Jennette JC. Pathologic classification of focal segmental glomerulosclerosis: a working proposal. Am J Kidney Dis. 2004;43(2):368–82. doi: 10.1053/j.ajkd.2003.10.024. [DOI] [PubMed] [Google Scholar]


