Abstract
Secretogranin II (SgII) is a member of the granin family of proteins found in neuroendocrine and endocrine cells. The expression and storage of SgII in the pituitary gland of Old World primates and rodents have been linked with those of luteinizing hormone (LH). However, New World primates including squirrel monkeys do not express LH in the pituitary gland, but rather CG is expressed. If CG takes on the luteotropic role of LH in New World primates, SgII may be associated with the expression and storage of CG in the pituitary gland. The goal of this study was to evaluate the regulation and distribution of CG and SgII in the squirrel monkey. A DNA fragment containing approximately 750 bp of squirrel monkey SgII promoter was isolated from genomic DNA and found to contain a cyclic AMP response element that is also present in the human SgII promoter and important for GnRH responsiveness. The squirrel monkey and human SgII promoters were similarly activated by GnRH in luciferase reporter gene assays in LβT2 cells. Double immunofluorescence microscopy demonstrated close association of SgII and CG in gonadotrophs of squirrel monkey pituitary gland. These results suggest that CG and SgII have a similar intercellular distribution and are coregulated in squirrel monkey pituitary gland.
1 Introduction
Secretogranin II (SgII) is a member of the granin family of tyrosine-sulfated, acidic proteins found in dense-core granules of neuroendocrine cells. The granin family also includes chromogranin A (CgA), chromogranin B (CgB), and several other secretogranin-like proteins [11, 13, 21, 34]. The physiological function of granins has been widely investigated; they aggregate at low pH and high calcium concentration and may act as mediators of peptide sorting into vesicles and granule formation in the regulated secretion pathway [6, 8, 12]. As the granins are secreted, it is also possible that they have extracellular functions [13, 18].
Luteinizing hormone (LH), one of the critical regulators of reproductive function in Old World primates, is stored in and secreted from dense core granules of the regulated pathway of the anterior pituitary gland. LH has been shown to colocalize with SgII in the bovine, rat, and sheep anterior pituitary [2, 7, 38]. The close association of LH and SgII was also shown in the pituitary glands of gonadotropin-releasing hormone (GnRH)-depleted male mice that resulted in intragranular co-aggregation of LH with SgII [8]. Upon GnRH stimulation, both LH and SgII are secreted and their expression upregulated [4, 10, 20, 31, 32].
Recent studies have shown that New World primates, such as squirrel monkey and the common marmoset, express chorionic gonadotropin (CG) in the anterior pituitary and do not express LH [19, 26]. Because CG assumes the luteotropic role of LH in New World primates, SgII may be involved in the sorting of CG to vesicles in the pituitary gland of these animals. In a companion paper, we show how squirrel monkey CGβ is regulated in a pituitary- or placenta-specific manner using distinct promoter regions [37]. Here, we focus on the nature of CG storage in the squirrel monkey and ask whether the two proteins are coregulated by GnRH and evaluate the distribution of squirrel monkey CG and SgII in the pituitary. We show that SgII and CGβ promoters are both regulated by GnRH in pituitary cells and that CG and SgII are found in gonadotrophs in the squirrel monkey pituitary gland. These results suggest that in squirrel monkey SgII and CG are regulated and distributed in a manner similar to SgII and LH in the pituitary glands of other species.
2 Materials and Methods
2.1 Materials
Culture medium was obtained from Invitrogen (Carlsbad, CA). Fetal bovine serum (FBS) was purchased from Hyclone (Logan, UT). GnRH was obtained from Sigma (St. Louis, MO). pLuc-Link was kindly provided by Dr. Richard Day (University of Virginia). The luciferase reporter gene containing −869/+1 of the human SgII promoter has been described previously [27]. The 518B7 anti-bovine LH monoclonal antibody was provided by Dr. Janet F. Roser (University of California, Davis), and has been shown to react with human LH and marmoset CG [14, 24, 25, 33]. Rabbit polyclonal antibody to human SgII [35] was affinity-purified using a peptide corresponding to the first nineteen N-terminal amino acids of mature SgII protein, QRNQLLGKEPDLRLENVQK (United States Biologicals, Swampscott, MA). Specificity of the SgII antibody was determined by preadsorption with a heat stable fraction of PC12 cells which are abundant in SgII. Prolactin monoclonal antibody 6F11 has been described and its specificity has previously been established [28–30]. Goat anti-mouse fluorescein-conjugated and goat anti-rabbit Texas Red-conjugated antibodies were purchased from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA). Sudan Black B was obtained from Sigma. ProLong Gold anti-fading reagent with 4′, 6-diamidino-2-phenylindole (DAPI) was purchased from Invitrogen.
2.2 Cell Culture
LβT2 mouse pituitary gonadotroph cells [1] were kindly provided by Dr. Pamela Mellon (University of California, San Diego) and were grown in DMEM, supplemented with 10% FBS, 50 U/mL penicillin G, and 0.05 mg/mL streptomycin. Epstein-Barr virus-transformed squirrel monkey lymphoblast cells (SML) [22] were grown in RPMI 1640, 10% FBS, 4 mM L-glutamine, and antibiotics. Cells were grown at 37°C in a humidified atmosphere of 95% air-5% CO2.
2.3 Isolation of the squirrel monkey SgII promoter and construction of luciferase plasmid
Total genomic DNA was isolated from SML using the QIAGEN DNeasy Blood and Tissue Kit or the Flexigene Kit (QIAGEN, Valencia, CA). A DNA fragment containing the squirrel monkey SgII promoter was amplified from genomic DNA using forward primer 5′-TCT TCA ACC CAG CAT TTG ATC ATG CC-3′, corresponding to a sequence in the human SgII promoter approximately 1700 bp upstream of the transcription initiation site (224468853 to 224468876, NCBI Reference Sequence NC_000002.11). The reverse primer, 5′-TTC AGT TAT CCT ATC GAC ATC AGC TTG-3′, corresponds to a sequence in intron 1 of the squirrel monkey SgII gene that was obtained after PCR amplification of squirrel monkey genomic DNA with primers corresponding to sequences in exons 1 and 2 of the human SgII gene. The nucleotide sequences of squirrel monkey SgII promoter, exon 1 and exon 2 were submitted to GenBank with accession number GU132857. A KpnI linker sequence (underlined) was inserted using a primer, 5′-TAT GGT ACC TGT AAA GCA GTC ACA-3′, corresponding to −745 to −731 of the squirrel monkey SgII promoter (GU132857). A HindIII restriction site (underlined) was added using primer 5′-ATA AAG CTT GGC AGA GGA GCT C-3′, corresponding to +35 to +23 of the squirrel monkey SgII gene (GU132857). The fragment (−745 to +35) was digested with KpnI and HindIII and inserted into the KpnI- and HindIII-cut luciferase reporter vector, pLuc-Link [9].
2.4 Luciferase reporter gene assays
Cells were plated at a density of 3 × 105 cells/well in 6-well dishes. The next day, cells were transfected with 2 μg DNA/well of either promoterless pLuc-Link luciferase plasmid or luciferase plasmids containing fragments of either the squirrel monkey or human SgII promoter or the squirrel monkey CGβ promoter using Superfect Transfection Reagent (QIAGEN) in complete medium for 3 h. Cells were washed, and fresh medium was added. The following day, after treatment with 100 nM GnRH for 6 h, cells were harvested in 300 μL ice-cold Monolight lysis buffer (BD Biosciences, San Jose, CA), and luciferase activity measured in a Monolight 2010 luminometer (Analytical Luminesence Laboratory, San Diego, CA). Results were normalized to the promoterless luciferase plasmid control. Experiments were performed at least three times.
2.5 Immunostaining
Formalin-fixed, paraffin-embedded pituitary glands from adult, female Bolivian squirrel monkeys were obtained from the Tissue and Biological Fluids Bank of the Center for Neotropical Primate Research and Resources of the USA. Formalin-fixed, paraffin-embedded human pituitary sections were obtained from the University of South Alabama (USA) Department of Pathology. Tissues were sectioned at 5 μm. Slides were deparaffinized by two xylene washes (5 min each) followed by rehydration through serial steps of 100%, 70%, 50% ethanol and PBS for 2 min each. Antigen retrieval was performed by microwaving slides in 0.01 M sodium citrate buffer (pH 5.0) for 1.5 min at low power followed by 2 min at room temperature. This process was repeated nine times. Finally, slides were washed once in PBS and blocked with 5% normal goat serum (NGS) in PBS. Primary antibodies were incubated on the sections in 1% NGS in PBS. Affinity-purified rabbit anti-SgII antibody was used at a dilution of 1:2000, anti-CG 518B7 antibody was used at 1:100, and anti-prolactin 6F11 antibody was used at 1:25,000. After overnight incubation in a humidified chamber at 4°C, slides were allowed to come to room temperature, and washed three times in 0.02% NGS in PBS. Slides were then incubated for one hour at room temperature with secondary antibodies to rabbit or mouse IgG conjugated to Texas Red or fluorescein, respectively, used at a dilution of 1:50 in 1% NGS in PBS. Slides were washed once in PBS with 0.02% NGS and three times in PBS alone. To block autofluorescence, sections were covered in 0.3% Sudan Black B dissolved in 70% ethanol for 10 min at room temperature, and washed ten times with PBS followed by a light-pressurized PBS wash from a squirt bottle. Slides were cover-slipped using ProLong Gold with DAPI anti-fading reagent, set overnight in the dark, and stored at −20°C. Micrographs were taken using a Nikon 80i upright microscope.
2.6 Statistical analysis
Statistical analysis was performed by using GraphPad Prism version 4.0 software (San Diego, CA). Comparisons between multiple groups were performed by using a one-way ANOVA and the Newman-Keuls post-hoc test. Values were considered significantly different when the P value was less than 0.05.
3 Results
3.1 Isolation and characterization of the squirrel monkey secretoganin II (SgII) promoter
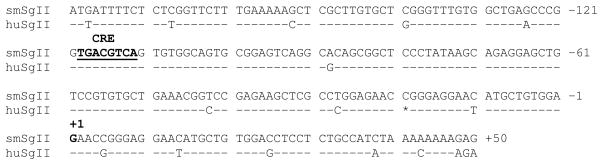
In the squirrel monkey pituitary gland, if CG assumes the role of LH, CG would be expected to be coregulated and costored with the granin protein, SgII. To test this, the regulation of the squirrel monkey SgII and CGβ promoters by GnRH were studied. A DNA fragment containing the squirrel monkey SgII promoter was isolated from genomic DNA. This sequence was compared to the human SgII promoter and was found to contain regions of similarity (86% identity). Comparison of the most proximal 179 bp of the SgII promoters yielded 94% identity between squirrel monkey and human SgII genes (Figure 1). Within this region, a cyclic-AMP response element (CRE) known to be important for GnRH-responsiveness of human SgII [32] is conserved in the squirrel monkey SgII promoter.
Figure 1.
The proximal squirrel monkey SgII promoter (smSgII) shares consensus sequences with the human SgII (huSgII) promoter. The CRE is conserved in the squirrel monkey SgII promoter (underlined and in bold). Dashes indicate nucleotides bases that are identical to the squirrel monkey sequences. Asterisks indicate nucleotide deletions.
To determine whether the squirrel monkey SgII promoter is upregulated by GnRH, we transiently transfected LβT2 cells with a reporter gene containing 780 bp of 5′-flanking sequence of the squirrel monkey SgII gene or the promoterless vector and subsequently stimulated the cells with 100 nM GnRH or vehicle control. Because human SgII is known to be upregulated by GnRH, activity of the human SgII promoter was also measured. Stimulation of the squirrel monkey SgII promoter fragment by GnRH led to a 2.5-fold increase in activity that was similar to that found with human SgII promoter construct (Figure 2). In side-by-side experiments, GnRH evoked an approximately four-fold increase in squirrel monkey CGβ promoter activity. These results demonstrate that the squirrel monkey SgII promoter shows similar activity to the human SgII promoter and that the squirrel monkey SgII and CGβ promoters are both regulated by GnRH in pituitary cells.
Figure 2.

Squirrel monkey SgII and CGβ promoters are regulated by GnRH. LβT2 cells were transiently transfected with luciferase-reporter plasmids driven by either the squirrel monkey (sm) SgII (−745/+35), human (h) SgII (−869/+1), or squirrel monkey CGβ (−1898/+9, smCGβ) promoter constructs. After 24 h, cells were incubated in the absence or presence of 100 nM GnRH for 6 h. Cells were harvested and assayed for luciferase activity, and data are shown as percent change in luciferase activity over that achieved in unstimulated cells, set at 100 percent. Each bar represents the mean ± SEM of at least three independent experiments. *, significantly different than control.
3.2 Distribution of CG and SgII in squirrel monkey pituitary gland
Having demonstrated that the squirrel monkey SgII and CGβ promoters are both regulated by GnRH, we investigated the distribution of CG and SgII in the pituitary gland. Cell nuclei were visualized using DAPI stain (Figure 3A). We observed a distinct population of cells exhibiting CG-positive staining that was localized to the perinuclear space and appeared granular in nature (Figure 3B). SgII immunoreactivity produced a similar pattern of granular staining located in the perinuclear space (Figure 3C). A subset of cells stained for SgII only. However, in a distinct population of cells staining for SgII and CG, the composite micrograph shows that CG is found with SgII in the same cells of the squirrel monkey pituitary (Figure 3D). As a positive control, sections of human pituitary gland were stained for LH and SgII, which have been shown to colocalize in human pituitary tissue [36]. As expected, LH and SgII colocalized in cells in the human pituitary gland and a subset of cells stained for SgII only (Figure 3E). Squirrel monkey pituitary tissue was also stained for prolactin and SgII, which normally do not colocalize [5, 23]. As expected, prolactin and SgII were found in distinct and separate cell populations in the squirrel monkey pituitary gland (Figure 3F).
Figure 3.

Distribution of CG and SgII in squirrel monkey pituitary gland. A, DAPI nuclear staining of squirrel monkey pituitary cells. B, The presence of CG in squirrel monkey pituitary gland was detected using 518B7 antibody, visualized with FITC-labeled goat anti-mouse secondary antibody. C, The presence of SgII was detected using an anti-SgII antibody, visualized with Texas Red-labeled goat anti-rabbit secondary antibody. D, Composite micrograph of squirrel monkey pituitary gland showing DAPI, CG, and SgII labeling in squirrel monkey pituitary gland. E, Composite micrograph showing DAPI, LH, and SgII labeling in human pituitary gland. F, Composite micrograph of squirrel monkey pituitary showing separate staining of CG and prolactin, visualized using 6F11 antibody and Texas Red-labeled goat anti-rabbit secondary antibody. Scale bars, 20 μm.
4 Discussion
In this study, we investigated the characteristics of storage of CG and its regulation and distribution with SgII in the pituitary gland of squirrel monkeys. The granins are thought to act as mediators of protein aggregation and sorting into the regulated secretory pathway [6, 11, 13]. Since some pituitary hormones, including LH, are coregulated and costored with SgII, it follows that CG and SgII may be similarly regulated and distributed in the squirrel monkey pituitary, which expresses CG in place of LH. Our results show that the squirrel monkey SgII and CG promoters are both activated by GnRH, and that CG and SgII are similarly distributed in gonadotrophs of the squirrel monkey pituitary gland.
The activity of the squirrel monkey SgII promoter was upregulated by GnRH, consistent with the GnRH-induction of SgII in other species [32, 39]. We show that the squirrel monkey SgII promoter contains an intact CRE [3]. The GnRH-responsiveness of the rat SgII promoter is conferred through a canonical CRE via binding of CRE-binding protein (CREB) in pituitary cells [32]. However, other factors such as the activating protein factor-3 and c-Jun are also involved in the GnRH regulation of the SgII promoter [39]. Further studies are needed to determine the specific trans-factors and signaling pathways involved in what is likely to be complex regulation of the squirrel monkey SgII promoter by GnRH.
We show that the storage of CG in squirrel monkey pituitary gonadotrophs appears to be closely associated with that of SgII, like the storage of LH and SgII in other species, although definite evidence for colocalization of SgII and CG will require ultrastructural analysis. It is possible that SgII plays an active and essential role in the formation of LH or CG containing granules, as has been suggested for proopiomelanocortin and CgA [17]. On the other hand, it is possible that LH and squirrel monkey CG have structural features that enhance their sorting to the regulated pathway on their own. The C-terminus of LH is thought to contribute to this sorting to granules [16]. CG is more complex because the carboxy-terminal peptide (CTP) of human CG may sort the protein to the constitutive pathway [15]. However, the CTP of squirrel monkey CG is different [26]. The four O-linked glycosylation sites present on the human CGβ CTP do not exist on the squirrel monkey CGβ. Rather, it is predicted that the squirrel monkey CGβ contains two N-linked glycosylation sites, one of which is on the C-terminal extension. This change in glycosylation sites appears to be common to New World primates, as the common marmoset and owl monkey CGβ peptide sequences also have the same predicted glycosylation patterns as squirrel monkey CGβ [26]. Thus, it is possible that characteristics of the squirrel monkey CGβ C-terminal peptide may contribute to its trafficking to a regulated secretory pathway in the squirrel monkey pituitary. Future studies are necessary to determine the relative roles of the squirrel monkey CGβ CTP and SgII in the storage of CG in vesicles of the pituitary.
Acknowledgments
This study was supported by Grant number 13200 from the National Center for Research Resources (NCRR), a component of the National Institutes of Heath (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH. Ms. Christina Mahanic was supported by REU: Structure and Function of Proteins National Science Foundation Research Experiences for Undergraduates (NSF #0751684). We are grateful to Drs. David Weber, Mark Taylor, Anthony Gard, and Beth Rutland (University of South Alabama) for their help with microscopy and immunohistochemistry. We thank Ms. Sandra Chapman and Subha Pyakurel for their technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alarid ET, Windle JJ, Whyte DB, Mellon PL. Immortalization of pituitary cells at discrete stages of development by directed oncogenesis in transgenic mice. Development. 1996;122:3319–3329. doi: 10.1242/dev.122.10.3319. [DOI] [PubMed] [Google Scholar]
- 2.Bassetti M, Huttner WB, Zanini A, Rosa P. Co-localization of secretogranins/chromogranins with thyrotropin and luteinizing hormone in secretory granules of cow anterior pituitary. J Histochem Cytochem. 1990;38:1353–1363. doi: 10.1177/38.9.2387987. [DOI] [PubMed] [Google Scholar]
- 3.Cibelli G, Jungling S, Schoch S, Gerdes HH, Thiel G. Identification of a functional cAMP response element in the secretogranin II gene. Eur J Biochem. 1996;236:171–179. doi: 10.1111/j.1432-1033.1996.00171.x. [DOI] [PubMed] [Google Scholar]
- 4.Clarke IJ, Cummins JT. The temporal relationship between gonadotropin releasing hormone (GnRH) and luteinizing hormone (LH) secretion in ovariectomized ewes. Endocrinology. 1982;111:1737–1739. doi: 10.1210/endo-111-5-1737. [DOI] [PubMed] [Google Scholar]
- 5.Conn PM, Janovick JA, Braden TD, Maurer RA, Jennes L. SIIp: a unique secretogranin/chromogranin of the pituitary released in response to gonadotropin-releasing hormone. Endocrinology. 1992;130:3033–3040. doi: 10.1210/endo.130.5.1572311. [DOI] [PubMed] [Google Scholar]
- 6.Courel M, Vasquez MS, Hook VY, Mahata SK, Taupenot L. Sorting of the neuroendocrine secretory protein Secretogranin II into the regulated secretory pathway: role of N- and C-terminal alpha-helical domains. J Biol Chem. 2008;283:11807–11822. doi: 10.1074/jbc.M709832200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crawford JL, McNeilly AS. Co-localisation of gonadotrophins and granins in gonadotrophs at different stages of the oestrous cycle in sheep. J Endocrinol. 2002;174:179–194. doi: 10.1677/joe.0.1740179. [DOI] [PubMed] [Google Scholar]
- 8.Crawford JL, McNeilly JR, Nicol L, McNeilly AS. Promotion of intragranular co-aggregation with LH by enhancement of secretogranin II storage resulted in increased intracellular granule storage in gonadotrophs of GnRH-deprived male mice. Reproduction. 2002;124:267–277. doi: 10.1530/rep.0.1240267. [DOI] [PubMed] [Google Scholar]
- 9.d’Emden MC, Okimura Y, Maurer RA. Analysis of functional cooperativity between individual transcription-stimulating elements in the proximal region of the rat prolactin gene. Mol Endocrinol. 1992;6:581–588. doi: 10.1210/mend.6.4.1584222. [DOI] [PubMed] [Google Scholar]
- 10.Dorn C, Ou Q, Svaren J, Crawford PA, Sadovsky Y. Activation of luteinizing hormone β gene by gonadotropin-releasing hormone requires the synergy of early growth response-1 and steroidogenic factor-1. J Biol Chem. 1999;274:13870–13876. doi: 10.1074/jbc.274.20.13870. [DOI] [PubMed] [Google Scholar]
- 11.Gerdes HH, Rosa P, Phillips E, Baeuerle PA, Frank R, Argos P, Huttner WB. The primary structure of human secretogranin II, a widespread tyrosine-sulfated secretory granule protein that exhibits low pH- and calcium-induced aggregation. J Biol Chem. 1989;264:12009–12015. [PubMed] [Google Scholar]
- 12.Glombik MM, Gerdes HH. Signal-mediated sorting of neuropeptides and prohormones: secretory granule biogenesis revisited. Biochimie. 2000;82:315–326. doi: 10.1016/s0300-9084(00)00195-4. [DOI] [PubMed] [Google Scholar]
- 13.Huttner WB, Gerdes HH, Rosa P. The granin (chromogranin/secretogranin) family. Trends Biochem Sci. 1991;16:27–30. doi: 10.1016/0968-0004(91)90012-k. [DOI] [PubMed] [Google Scholar]
- 14.Illera JC, Silvan G, Illera MJ, Munro CJ, Lessey BA, Illera M. Measurement of serum and peritoneal fluid LH concentrations as a diagnostic tool for human endometriosis. Reproduction. 2001;121:761–769. doi: 10.1530/rep.0.1210761. [DOI] [PubMed] [Google Scholar]
- 15.Jablonka-Shariff A, Boime I. Secretory trafficking signal encoded in the carboxyl-terminal region of the CGb-subunit. Mol Endocrinol. 2009;23:316–323. doi: 10.1210/me.2008-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jablonka-Shariff A, Pearl CA, Comstock A, Boime I. A carboxyl-terminal sequence in the lutropin b subunit contributes to the sorting of lutropin to the regulated pathway. J Biol Chem. 2008;283:11485–11492. doi: 10.1074/jbc.M800654200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim T, Tao-Cheng JH, Eiden LE, Loh YP. Large dense-core secretory granule biogenesis is under the control of chromogranin A in neuroendocrine cells. Ann N Y Acad Sci. 2002;971:323–331. doi: 10.1111/j.1749-6632.2002.tb04487.x. [DOI] [PubMed] [Google Scholar]
- 18.Kirchmair R, Hogue-Angeletti R, Gutierrez J, Fischer-Colbrie R, Winkler H. Secretoneurin--a neuropeptide generated in brain, adrenal medulla and other endocrine tissues by proteolytic processing of secretogranin II (chromogranin C) Neuroscience. 1993;53:359–365. doi: 10.1016/0306-4522(93)90200-y. [DOI] [PubMed] [Google Scholar]
- 19.Muller T, Simoni M, Pekel E, Luetjens CM, Chandolia R, Amato F, Norman RJ, Gromoll J. Chorionic gonadotrophin β subunit mRNA but not luteinising hormone β subunit mRNA is expressed in the pituitary of the common marmoset (Callithrix jacchus) J Mol Endocrinol. 2004;32:115–128. doi: 10.1677/jme.0.0320115. [DOI] [PubMed] [Google Scholar]
- 20.Nicol L, McNeilly JR, Stridsberg M, McNeilly AS. Differential secretion of gonadotrophins: investigation of the role of secretogranin II and chromogranin A in the release of LH and FSH in LβT2 cells. J Mol Endocrinol. 2004;32:467–480. doi: 10.1677/jme.0.0320467. [DOI] [PubMed] [Google Scholar]
- 21.Ozawa H, Takata K. The granin family--its role in sorting and secretory granule formation. Cell Struct Funct. 1995;20:415–420. doi: 10.1247/csf.20.415. [DOI] [PubMed] [Google Scholar]
- 22.Reynolds PD, Roveda KP, Tucker JA, Moore CM, Valentine DL, Scammell JG. Glucocorticoid-resistant B-lymphoblast cell line derived from the Bolivian squirrel monkey (Saimiri boliviensis boliviensis) Lab Anim Sci. 1998;48:364–370. [PubMed] [Google Scholar]
- 23.Rundle S, Somogyi P, Fischer-Colbrie R, Hagn C, Winkler H, Chubb IW. Chromogranin A, B and C: immunohistochemical localization in ovine pituitary and the relationship with hormone-containing cells. Regul Pept. 1986;16:217–233. doi: 10.1016/0167-0115(86)90021-2. [DOI] [PubMed] [Google Scholar]
- 24.Saltzman W, Hogan BK, Horman BM, Abbott DH. Social suppression of cortisol in female marmosets: role of luteinizing hormone/chorionic gonadotropin. Gen Comp Endocrinol. 2006;149:90–99. doi: 10.1016/j.ygcen.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Saltzman W, Schultz-Darken NJ, Wegner FH, Wittwer DJ, Abbott DH. Suppression of cortisol levels in subordinate female marmosets: reproductive and social contributions. Horm Behav. 1998;33:58–74. doi: 10.1006/hbeh.1998.1436. [DOI] [PubMed] [Google Scholar]
- 26.Scammell JG, Funkhouser JD, Moyer FS, Gibson SV, Willis DL. Molecular cloning of pituitary glycoprotein β-subunit and follicle stimulating hormone and chorionic gonadotropin β-subunits from New World squirrel monkey and owl monkey. Gen Comp Endocrinol. 2008;155:534–541. doi: 10.1016/j.ygcen.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scammell JG, Reddy S, Valentine DL, Coker TN, Nikolopoulos SN, Ross RA. Isolation and characterization of the human secretogranin II gene promoter. Brain Res Mol Brain Res. 2000;75:8–15. doi: 10.1016/s0169-328x(99)00269-7. [DOI] [PubMed] [Google Scholar]
- 28.Scammell JG, Scott MG, Outlaw KK, Thompson ME, SJO, Belen RB. Characterization of monoclonal antibodies against ovine prolactin: suitability for use in immunocytochemical analysis of rat prolactin. J Histochem Cytochem. 1990;38:117–122. doi: 10.1177/38.1.2403576. [DOI] [PubMed] [Google Scholar]
- 29.Scammell JG, Wear LB, Von Haven R. A monoclonal antibody which inhibits the biological activity of rat prolactin, but not prolactin from other species. Mol Cell Endocrinol. 1990;71:125–131. doi: 10.1016/0303-7207(90)90249-8. [DOI] [PubMed] [Google Scholar]
- 30.Scott MG, Lin WH, Lyle LR, Atkinson PR, Seely JE, Markoff E. Monoclonal antibodies specific for non-glycosylated porcine prolactin and for pituitary porcine prolactin. Biochem Biophys Res Commun. 1988;151:1427–1433. doi: 10.1016/s0006-291x(88)80521-7. [DOI] [PubMed] [Google Scholar]
- 31.Sion B, Chanat E, Duval J, Thieulant ML. Peptides co-released with luteinizing hormone by perifused pituitary cell aggregates. Mol Cell Endocrinol. 1988;60:151–161. doi: 10.1016/0303-7207(88)90174-8. [DOI] [PubMed] [Google Scholar]
- 32.Song SB, Rhee M, Roberson MS, Maurer RA, Kim KE. Gonadotropin-releasing hormone-induced stimulation of the rat secretogranin II promoter involves activation of CREB. Mol Cell Endocrinol. 2003;199:29–36. doi: 10.1016/s0303-7207(02)00334-9. [DOI] [PubMed] [Google Scholar]
- 33.Tannenbaum PL, Schultz-Darken NJ, Woller MJ, Abbott DH. Gonadotrophin-releasing hormone (GnRH) release in marmosets II: pulsatile release of GnRH and pituitary gonadotrophin in adult females. J Neuroendocrinol. 2007;19:354–363. doi: 10.1111/j.1365-2826.2007.01535.x. [DOI] [PubMed] [Google Scholar]
- 34.Taupenot L, Harper KL, O’Connor DT. The chromogranin-secretogranin family. N Engl J Med. 2003;348:1134–1149. doi: 10.1056/NEJMra021405. [DOI] [PubMed] [Google Scholar]
- 35.Thompson ME, Zimmer WE, Wear LB, MacMillan LA, Thompson WJ, Huttner WB, Hidaka H, Scammell JG. Differential regulation of chromogranin B/secretogranin I and secretogranin II by forskolin in PC12 cells. Brain Res Mol Brain Res. 1992;12:195–202. doi: 10.1016/0169-328x(92)90084-o. [DOI] [PubMed] [Google Scholar]
- 36.Vallet VS, Li JY, Duval J. Secretogranin II (SgII) distribution and processing studies in human normal and adenomatous anterior pituitaries using new polyclonal antibodies. Regul Pept. 1997;68:155–163. doi: 10.1016/s0167-0115(96)02110-6. [DOI] [PubMed] [Google Scholar]
- 37.Vasauskas AA, Hubler TR, Boston L, Scammell JG. Tissue-specific expression of squirrel monkey chorionic gonadotropin. Gen Comp Endocrinol. doi: 10.1016/j.ygcen.2010.11.023. (in review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watanabe T, Uchiyama Y, Grube D. Topology of chromogranin A and secretogranin II in the rat anterior pituitary: potential marker proteins for distinct secretory pathways in gonadotrophs. Histochemistry. 1991;96:285–293. doi: 10.1007/BF00271348. [DOI] [PubMed] [Google Scholar]
- 39.Xie J, Roberson MS. 3′, 5′-cyclic adenosine 5′-monophosphate response element-dependent transcriptional regulation of the secretogranin II gene promoter depends on gonadotropin-releasing hormone-induced mitogen-activated protein kinase activation and the transactivator activating transcription factor 3. Endocrinology. 2008;149:783–792. doi: 10.1210/en.2007-0694. [DOI] [PMC free article] [PubMed] [Google Scholar]