Abstract
There is increasing evidence that p38 MAPK, which is classified as a stress activated kinase, also participates in cell cycle regulation, functioning as a suppressor of cell proliferation and tumorigenesis. We conducted a study of p38 MAPK phosphorylation during liver regeneration in mice to determine whether p38 MAPK activation or inactivation may correlate with events that lead to DNA replication after partial hepatectomy (PH), and whether p38 MAPK activation may be required for hepatocyte DNA replication in vivo and in culture. We report that active p38 (Pi-p38 MAPK) is present in normal liver, is rapidly inactivated starting 30 min after PH, and is re-activated by 12h. Although Pi-MKK 3/6 levels, the upstream kinases that activate p38 MAPK increase after PH, the expression of the dual protein phosphatase 1 is also elevated, and may be responsible for Pi-p38 MAPK dephosphorylation after PH. Inactivation and re-activation of p38 MAPK inversely correlates with the stimulation of protein synthesis and translation pathways, as indicated by activation of p70S6 kinase, increases in the phosphorylation of initiation factor elF-4E and translational repressor, 4E-BP. The activity of a p38 MAPK downstream substrate, MAPKAPK2 (MK2), did not reflect the changing levels of Pi-p38 MAPK during liver regeneration. Pi-p38 MAPK may be involved in TNF-stimulated DNA replication of murine hepatocytes in culture, but is not necessary for hepatocyte DNA replication after PH. Our results suggest that p38 MAPK inactivation plays a permissible role in DNA replication during liver regeneration and is consistent with a role for p38 MAPK in the maintenance of hepatocyte cell cycle arrest in adult liver.
Keywords: p38 MAPK, liver, regeneration, hepatocyte, cell cycle
Introduction
Liver regeneration after partial hepatectomy (PH) is a perfectly orchestrated process of compensatory growth that proceeds in stages and involves multiple pathways (Taub 2004; Fausto et al. 2006; Clavien et al. 2007; Michalopoulos 2007). The main event of liver regeneration is hepatocyte replication followed by mitosis, although there are situations in which the liver regains weight by hepatocyte hypertrophy rather than hyperplasia (for review see (Fausto and Campbell 2009). For resections that are larger than 30% of the liver, the extent of hepatocyte replication is proportional to the amount of tissue resected at the time of operation (Bucher and Swaffield 1964). Hepatocyte DNA replication after 2/3 PH is synchronous; in mice it starts around 30h and reaches a peak at 40h after the operation. Endothelial cells, Kupffer cells, and stellate cells replicate 24–48h later than the peak of hepatocyte DNA replication (Grisham 1962). The timing of the wave(s) of DNA replication varies somewhat depending on conditions such as the animal’s age, strain, extent of fasting, and the time of the surgery (Ngala Kenda et al. 1984; Lambotte et al. 1997; Mitchell et al. 2005).
Proto-oncogene activation, cell cycle events, growth factor activation and the involvement of the DNA synthesis machinery that occur after PH have been well described, and it has been shown that mitogen-activated protein kinases (MAPKs or ERK1/2) participate in the intracellular kinase cascades that lead to hepatocyte DNA replication (Talarmin et al. 1999; Rescan et al. 2001; Schwabe and Brenner 2006). MAPKs include the extracellular regulated kinases (ERK 1 and 2), the c-jun N-terminal kinases (JNKs), and the p38 MAP kinases (Graves et al. 1995a; Roux and Blenis 2004; Raman et al. 2007), The last two kinase families are often referred to as stress-activated protein kinases (SAPKs). There is general agreement that during liver regeneration, JNK activation is an early event occurring during the first few hours after PH (Schwabe et al. 2003), while activation of ERK1/2 occurs much later (Talarmin et al. 1999; Rescan et al. 2001).
In contrast to the well delineated increase in ERK phosphorylation during liver regeneration, conflicting results have been reported on the activation of p38 MAPK after PH, the timing of the changes, as well as on the detection of active p38 MAPK in the normal liver. Yamamoto et. al. reported that p38 MAPK phosphorylation increases between 24 and 72 hours after PH in rats and regulates the function of gap and tight junctions in the regenerating liver (Yamamoto et al. 2005). Other reports concluded that p38 MAPK is activated during the first four hours after PH, and shows a prolonged activation in mice in which there is increased reactive oxygen species production (Horimoto et al. 2004). In contrast with these data, Mendelson and co-workers showed that p38 MAPK is constitutively activated in the uninjured or quiescent liver, and is dephosphorylated after oxidative stress (Mendelson et al. 1996). Investigators have also studied the potential relationships between p38 MAPK in DNA replication and mitosis in the regenerating liver. Liao et al. reported that active p38 MAPK is present in the normal liver and is dephosphorylated in the regenerating liver between 2 and 24h after PH and then reactivated at 48 h after PH. Early growth response-1 deficient mice showed hyper-activation of p38 MAPK that was associated with a delay in progression through replication and mitosis (Liao et al. 2004). In contrast, Stepniak et al. reported that induction of p38 MAPK phosphorylation occurs at 12h, and proposed that c-Jun may prevent p38 MAPK mediated accumulation of p21 that could block DNA replication (Stepniak et al. 2006). These data suggest that there may be an inverse relationship between p38 MAPK activity and DNA replication in the regenerating liver. Although p38 MAPK activity has been associated with stress responses and apoptosis, there is increased evidence that p38 MAPK may act on cell cycle checkpoints and function as a suppressor of cell proliferation and tumorigenesis, maintaining tumor cell quiescence (Hui et al. 2007b; Adam et al. 2009; Thornton and Rincon 2009).
Given the uncertainties regarding various aspects of p38 MAPK activation after PH, we performed a detailed analysis of the timing of p38 MAPK activation in mouse liver after PH in comparison with normal liver and sham-operated animals, and investigated whether changes in p38 MAPK phosphorylation in the regenerating liver correlate with alterations in downstream target kinases and pathways involved in protein synthesis and translation, cell cycle progression, and DNA replication.
Materials and Methods
Animals and operative technique
Male mice (C57BL/6) were purchased from Jackson Laboratories, and housed in a specific pathogen free facility with 12-hour light/dark cycles with free access to standard food and water for one week prior to surgery. Mice (n= 2 to 5 per time point or treatment group) were fasted prior to 2/3 PH and sham operations as previously described (Chaisson et al. 2002; Greene and Puder 2003; Campbell et al. 2006; Mitchell and Willenbring 2008). At indicated time points, mice were sacrificed by cervical dislocation or CO2 inhalation, and liver tissue was collected, snap frozen and stored at −80C until analysis. All experiments were approved and performed in accordance with the guidelines of the Institutional Animal Care and Use Committee of the University of Washington, Seattle, WA, which is certified by the Association for Assessment and Accreditation of Laboratory Animal Care.
Treatment of mice with SB220025
For in vivo experiments using the p38 MAPK inhibitor, SB220025 [5-(2-amino-4-pyrimidinyl) - 4-(4-fluorophenyl)-1-(4-piperidinyl) imidazole)] (Calbiochem) cohorts of mice (n = 3 to 5) were injected (ip) with 20 mg/kg SB220025 or vehicle (N, N, dimethyl acetoacetamide (Sigma) /Cremephor El (Sigma)/saline (10:10:80)) using a published method (Jackson et al. 1998) every 12 h after 2/3 PH or sham surgery. Mice were sacrificed at 36 hr after surgery as described above. To assess hepatocyte replication, DNA synthesis was measured by bromodeoxyuridine (BrdU) incorporation where mice were injected with 50 ug/g BrdU 2 h prior to harvest. BrdU staining and quantification was performed as described (Campbell et al. 2006; Riehle et al. 2008).
Hepatocyte cell culture
Mouse hepatocytes cell lines, AML12, NMH, and TAMH, were maintained as previously described (Wu et al. 1994a; Wu et al. 1994b). NIH3T3 fibroblasts were obtained from ATCC and cultured as recommended. For experiments with TNF, AML12 hepatocytes and NIH3T3 fibroblasts were deprived of serum overnight. Cultures were exposed to TNF (20 ng/ml, or the indicated dose) for the indicated lengths of time and then cell lysates were prepared using 1% Trition X lysis buffer as described (Argast et al. 2004; Argast et al. 2005). To measure DNA replication in AML12 cells, [3H]-thymidine was added to the media at a final concentration of 1 uCi/ml for 4hr and incorporation was measured as previously described (Argast et al. 2004). In the experiments using SB220025, the cultures were pre-treated with inhibitor or vehicle (0.1% DMSO) for 30 min prior to TNF stimulation.
Immunoblotting analysis
Protein lysates were prepared in a 1% Triton-X lysis buffer and quantified as described (Campbell et al. 2006; Riehle et al. 2008). 30 to 50µg of protein from each sample were separated using SDS-PAGE, and immunoblotting was performed using standard procedures with the following primary antibodies from Cell Signaling: phospho-T180/Y182 p38 MAPK (cat. # 9211), total p38 MAPK (#9212), phospho-ERK 1/2 (#9101), phospho-S473-Akt (#9271), total Akt (#9272), phospho-T222-MK2 (#3316), phospho-T334-MK2 (#3007), total MK2 (#3042), phospho-S189/207-MKK3/6 (#9231), phospho-S6 S240/244 protein (#2215), phospho-S209 eIF-4E (#9741), phospho-S65 4E-BP (#9451), phospho-T70 4E-BP (#9455), and total 4E-BP (#9452). Cyclin D1 antibodies were purchased from Upstate Biotechnology/Millipore),β-actin from Sigma (A5441), and GAPDH and total ERK 1/2 were obtained as previously described (Seger et al. 1994; Campbell et al. 2006; Riehle et al. 2008). Total protein levels were determined by stripping and re-probing blots for total protein levels, or for β-actin or GAPDH to confirm similar amounts of protein were present (i.e. loading controls) using standard procedures. For protein quantification, densitometry analysis was performed using Image J 1.40 software. Data are presented as relative units, which represent the densitometric value for the phosphoprotein of interest that was normalized to the total levels of the same protein after re-probing.
Protein Kinase assays
p70 S6 kinase assays were done using whole liver lysates (5 µg/assay) from 2/3 PH and sham operated mice and 40S ribosomes purified from rat liver as described (Graves et al. 1995b; Bornfeldt and Krebs 1999). MAPKAP kinase 2 (MK2) activity was determined by immuoprecipitation using sheep anti-MAPKAP kinase 2 antibody (Upstate Biotechnology/Millipore) and GSK3 peptide substrate, as described (Stokoe et al. 1992). ERK1/2 and RIP2 kinase activities were measured using myelin basic protein as previously described (Argast et al. 2004; Argast et al. 2005).
Gene expression analysis
Total liver RNA was extracted by homogenizing snap-frozen tissue in TRIzol reagent (Invitrogen) and cDNA was reverse transcribed with the Retroscript kit using random decamer primers (Applied Biosystems). Semi-quantitative real-time polymerase chain reaction was performed using FAM-labeled primers for murine DUSP1 (previously known as MKP-1), DUSP10 (previously known as MKP-5) and 18S (Applied Biosystems). Relative gene expression was normalized to 18S levels and calculated using the ΔΔCt method as previously described (Campbell et al. 2006; Riehle et al. 2008). Relative expression was obtained by normalizing all values to those of non-surgical, but fasted mice.
Results
Dephosphorylation of p38 MAPK during liver regeneration in relationship to the activation of ERK1/2 and expression of cell cycle markers
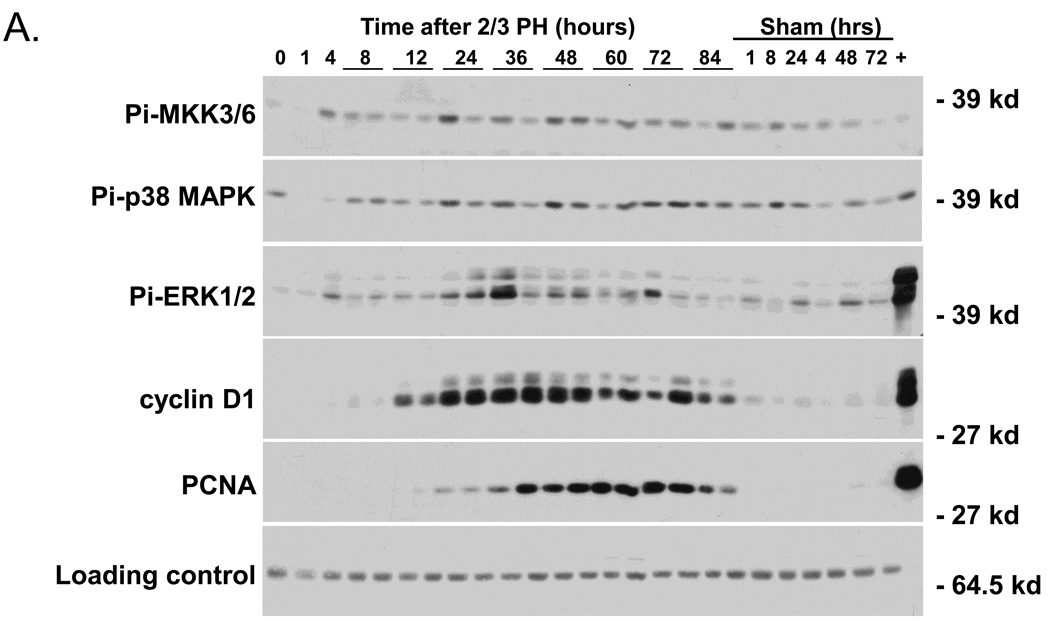
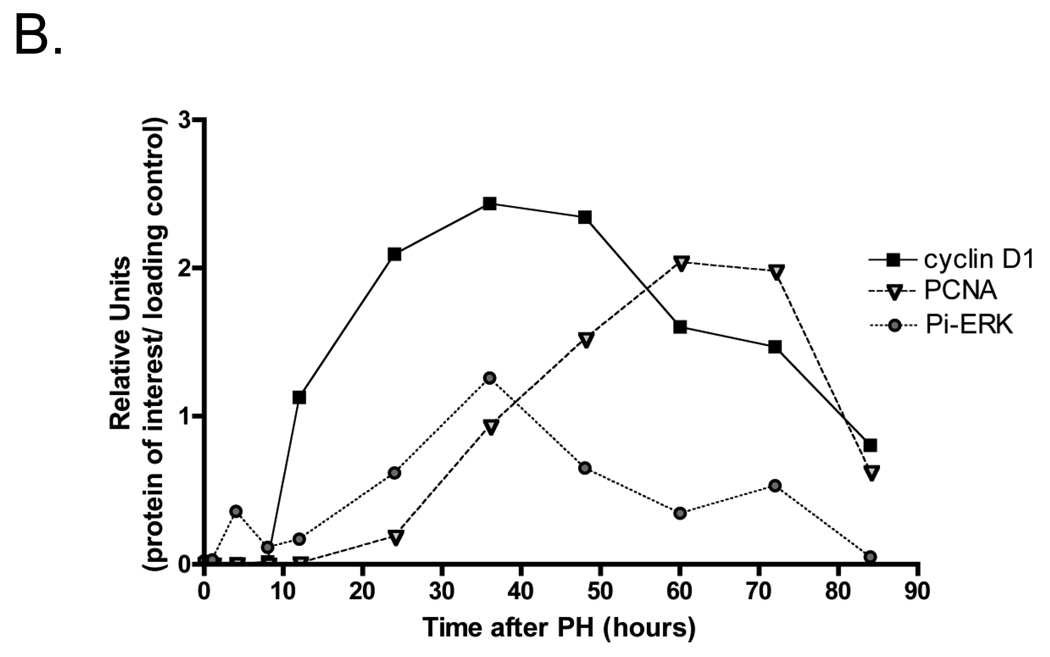
We initially compared the timing of the expression of activated p38 MAPK (Pi-p38 MAPK) after PH and in sham-operated mice, with that of Pi-ERK1/2, cyclin D1, and PCNA, which are well known markers of liver regeneration (Figure 1 A and B). We also examined the phosphorylation of Pi-MKK 3/6, which are the main up-stream activators of p38 MAPK using western blotting techniques. Pi-MKK-3/6 levels were low in non-operated liver, but increased at 4 h after PH, and remained relatively constant up to 84 h. ERK2 (p42) appeared to be preferentially activated over ERK1 (p44) after PH; nevertheless in this presentation we will refer to both forms. Pi-ERK1/2 showed an early peak of activation at 4h after PH, and a more prolonged increase, which started at about 12h, reached a maximum between 24 and 36h, and returned to basal levels by 72h after surgery. Sham-operated mice also showed ERK1/2 phosphorylation, but at much lower levels than those of partially hepatectomized animals. Cyclin D1 up-regulation started at 8–10h after PH, was highest between 24 and 48h after PH, and remained elevated beyond 72h. PCNA was expressed between 24 and 72h after PH, a time that corresponds to the period of DNA replication after PH. Neither cyclin D1 nor PCNA were detectable in the liver extracts from sham-operated mice.
Fig 1.
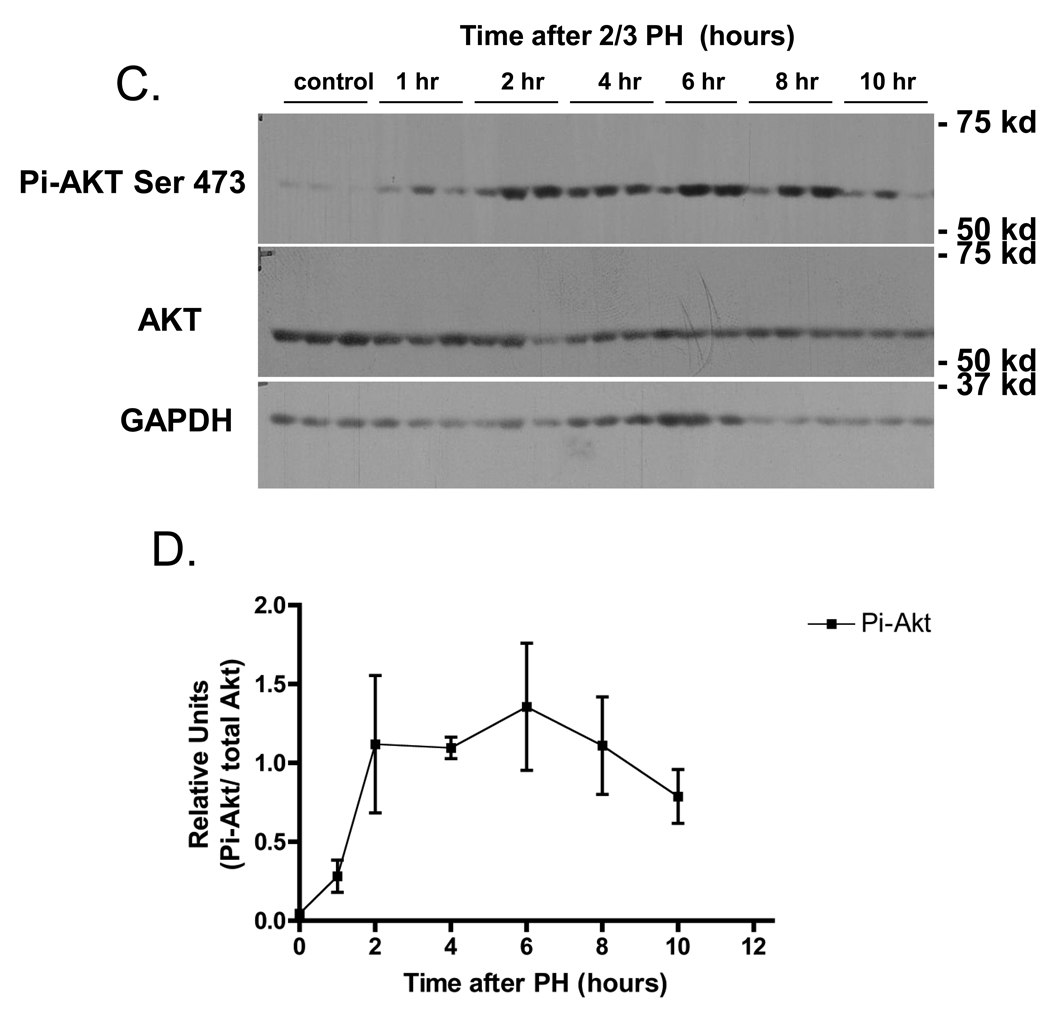
Western blot analysis of cell signaling pathways following partial hepatectomy (PH). A) Expression of activated p38 MAPK with the upstream activator Pi-MKK3/6, and known markers of liver regeneration Pi-ERK1/2, cyclin D1, and PCNA (n=2 mice/PH time points). “+” indicates that lysates from AML12 cells treated for 5’ with EGF were loaded as a positive control. B) Densitometry analysis of Pi-ERK1/2, cyclin D 1, and PCNA. Data points are the average of two independent values. C) Expression of phosphorylated AKT (S473) with total AKT and GAPDH from 3 individual mice were averaged after PH (n=3). D) Densitometry analysis of Pi-AKT normalized to levels of total Akt. Each point represents the mean (+/−SEM) of three mice. These data represent 2 independent experiments. Molecular weight markers are shown on the right.
We also examined the phosphorylation of Akt after 2/3 PH, as this protein kinase is involved in anti-apoptotic and cell proliferation pathways. Akt was not phosphorylated in livers of non-operated mice, but was rapidly phosphorylated, and remained active for up to 12 h after surgery (Figure 1 C& D). However, in contrast to the clearly defined timing of expression of Pi-pERK1/2, Pi-Akt, cyclin D1 and PCNA during liver regeneration shown in Figure 1 A–D, the expression of Pi-p38 MAPK did not appear to increase over basal levels at any time after PH.
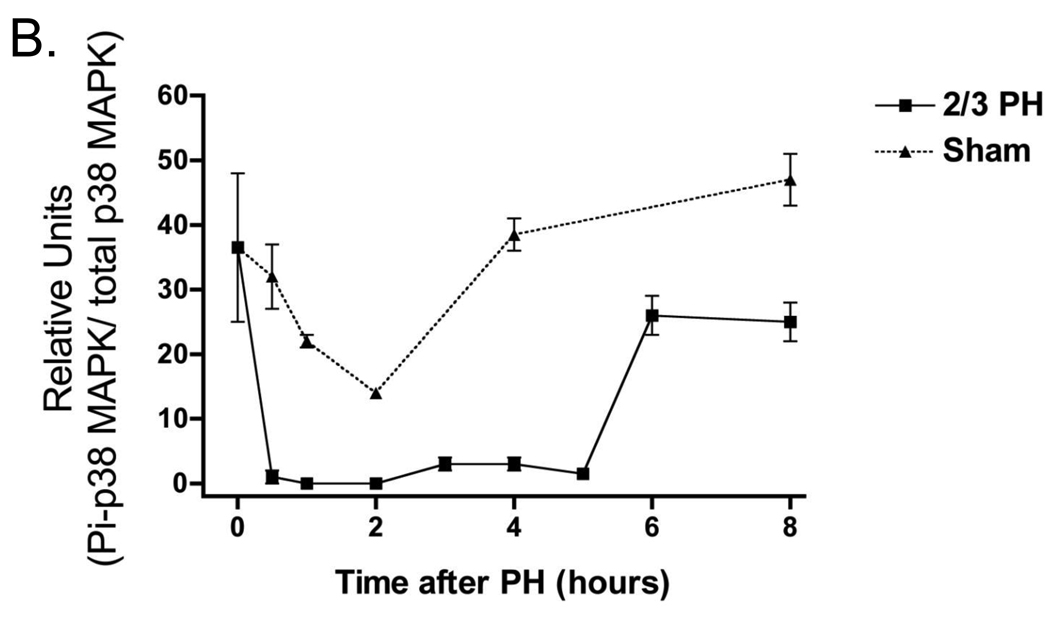
Given these results we examined in more detail the activation of Pi-p38 MAPK after PH and in sham-operated mice. Figure 2A shows western blots of Pi-p38 MAPK with their respective densitometry analysis for the first 8h after PH shown in Figure 2B. Pi-p38 MAPK was activated in non-operated liver but then rapidly inactivated in the regenerating liver between 0.5 and 5h after PH. After 12h, Pi-p38 MAPK levels returned to levels seen in non-operated mice. At later time points during liver regeneration, Pi-p38 MAPK levels were not increased beyond the levels seen in sham-operated mice (data not shown). Interestingly, p38 MAPK was also inactivated and subsequently re-activated in sham-operated mice. However, the inactivation occurring in sham-operated animals was less pronounced, and the duration of the inactivation much shorter than in the regenerating liver, lasting only 2–4 hours. Additional experiments revealed that transient inactivation of Pi-p38 MAPK occurs even after less stressful conditions than sham operation. For example, a simple procedure such as saline injection transiently inactivated Pi-p38 MAPK by 2 to 3 fold around 1 and 2h after the injection (data not shown). These results suggest that p38 MAPK is phosphorylated and active in normal liver, but is rapidly dephosphorylated after PH or other stressful situations.
Fig 2.
Western blot analysis p38 MAPK activation following PH. A) Expression of activated p38 MAPK and total p38 MAPK after PH and sham operations B) Densitometry analysis of the expression of p38 MAPK after PH and sham operations. These data represent 3 independent experiments. Molecular weight markers are shown on the right.
Expression of DUSP 1 and 10 after PH
We next examined the expression of dual specificity phosphatases (DUSP) to evaluate whether they may be responsible for the dephosphorylation of p38 MAPK after PH. MAP kinases are dephosphorylated by a family of dual specificity protein phosphatases (DUSP, previously known as MKPs) whose expression is rapidly induced by activation of MAPKs themselves, forming a negative feedback loop (Liu et al. 2007; Boutros et al. 2008; Patterson et al. 2009). Dephosphorylation of p38 MAPK was observed after conditional over-expression of either DUSP1 and DUSP10 in cell culture (Franklin and Kraft 1997; Tanoue et al. 1999). To determine whether DUSP1 or DUSP10 are induced by PH, we performed real time RT-PCR on RNA isolated from liver after PH or sham operation, and found that only DUSP1 mRNA was induced, increasing at about 1–2h after PH and decreasing by 6h (Figure 3). Thus, DUSP1 might be involved in the dephosphorylation of Pi-p38 MAPK during the initiation of liver regeneration.
Fig 3.
Relative real time PCR expression of Dusp1 and Dusp10 in the liver following PH or sham operations. A) Relative expression of Dusp1 compared to un-operated control. B) Relative expression of Dusp10 compared to un-operated control. Data is presented as the fold change of expression relative to 18s expression. Each point represents the mean (+/−SEM) of three mice and reactions were performed in triplicate.
Activation of p38 MAPK downstream pathways during liver regeneration
We investigated whether the inactivation of Pi-p38 MAPK observed after PH is associated with alterations in proteins or pathways previously shown to be activated by p38 MAPK (Gaestel 2006), We analyzed the activity of MAPKAPK2 (MK2), and the phosphorylation levels of proteins involved in the formation of elF-4F transcription initiation complex (Kotlyarov et al. 1999; Winzen et al. 1999), and compared the results with the activation of p70S6 kinase.
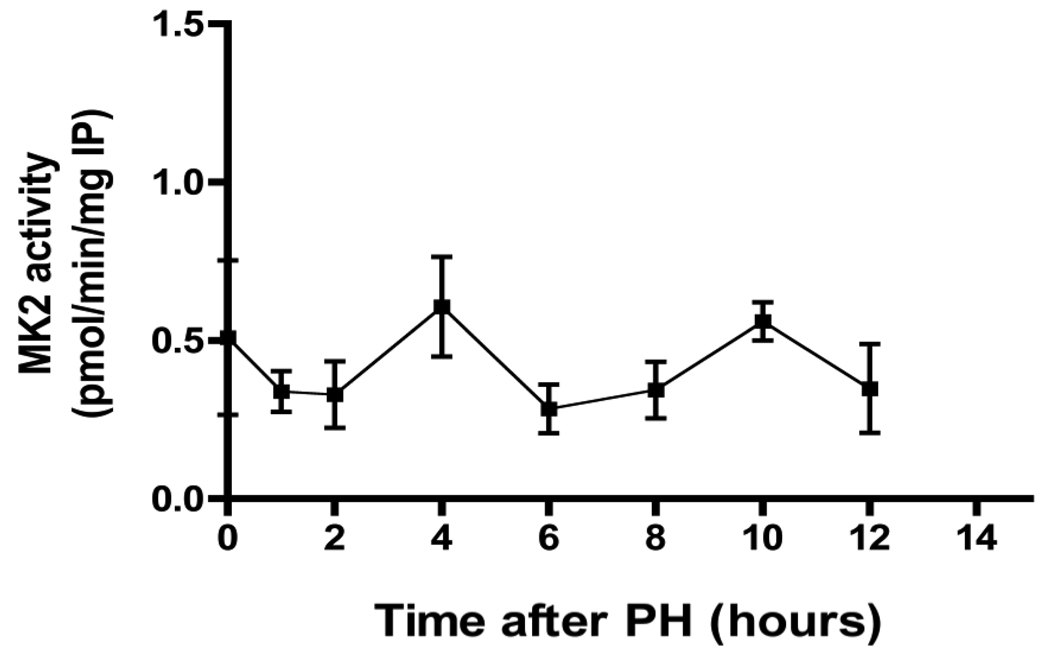
MK2 phosphorylates tristetraprolin, which binds to AU-rich elements (AREs) in TNF and other cytokine RNAs, preventing decay of the RNAs (Sandler and Stoecklin 2008). MK2 kinase activity, as measured by an immunoprecipitation assay, was not activated after either PH or sham-operation (Figure 4). In contrast to p38 MAPK, liver MK2 activity is essentially constant up to 12 h after PH. We also determined MK2 activity in protein lysates from PH and sham-operated livers immediately after surgery, but detected no activation of this protein kinase within the first 60 min after surgery (data not shown). Thus, there is no correlation between p38 MAPK inactivation and re-activation and the activity of a downstream substrate, the protein kinase MK2 after PH in mice.
Fig 4.
MK2 activity after PH. Activity of MK2 during the first 12 hours following PH. MK2 activity was determined by an immunoprecipitation assay as described in the Methods. (For comparison, MK2 activity from TNF-stimulated AML12 cells is approx. 2.2 pmol/min/mg).
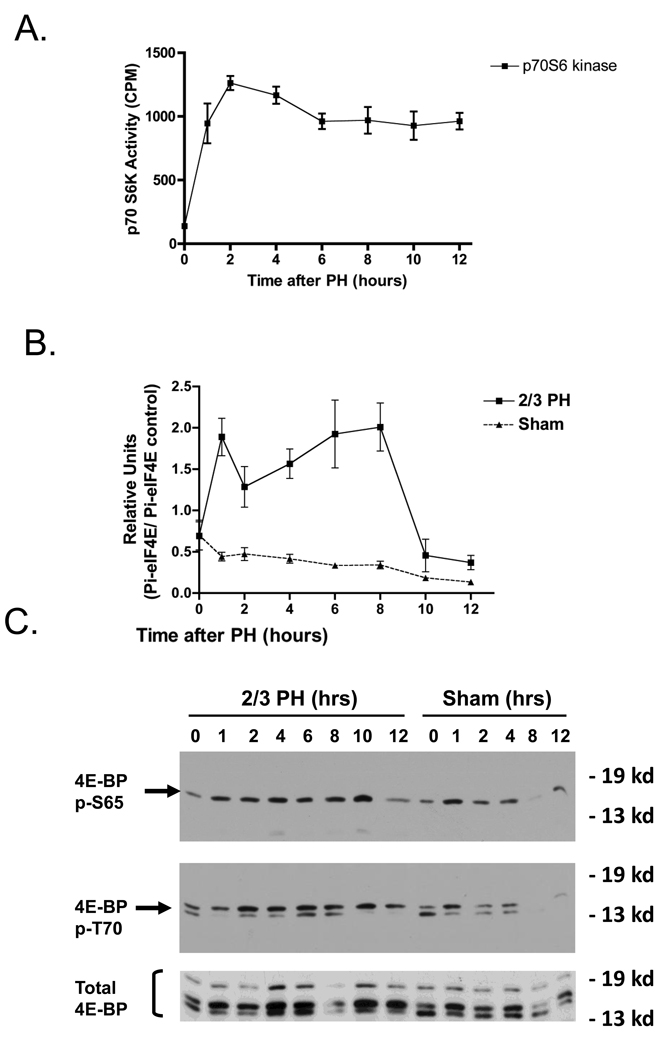
Activation of p38 MAPK affects protein translation through activation of MAP kinase signal integrating kinase 1 (MNK 1/2). These kinases phosphorylate and inactivate 4E–BP, leading to activation of the elF-4F translation initiation complex. p38 MAPK may also indirectly regulate the formation of elF-4E, which in turn regulates protein synthesis. Data shown in Figure 5B demonstrates that there was a rapid increase in elF-4E phosphorylation after PH, with an early peak at 2h and a late peak at about 8h. In sham-operated mice, elF-4E phosphorylation showed a small but progressive decrease after the operation. Similarly to elF-4E phosphorylation, p70S6 kinase activity increased rapidly after PH and remained elevated up to 12.5h after the operation (Figure 5A). In parallel with elF-4E activation, phosphorylation (inactivation) of 4E-BP increased after PH, as measured with two phosphospecific antibodies (Figure 5C). Thus p38 MAPK inactivation after PH inversely correlated with increased activation of p70S6 and Akt kinases, and of the protein complexes, eIF-4E and 4E-BP, that stimulate protein synthesis after PH.
Fig 5.
PH activates protein translation signaling pathways. A) Activity of p70S6K. B) Densitometry analysis of eIF-4 following PH and sham operations. C) Western blots analysis of phosphorylation of 4E-BP at S65 and T70, following PH and sham operations.
The p38 inhibitor SB220025 blocks TNF stimulation of DNA replication of cultured murine hepatocytes
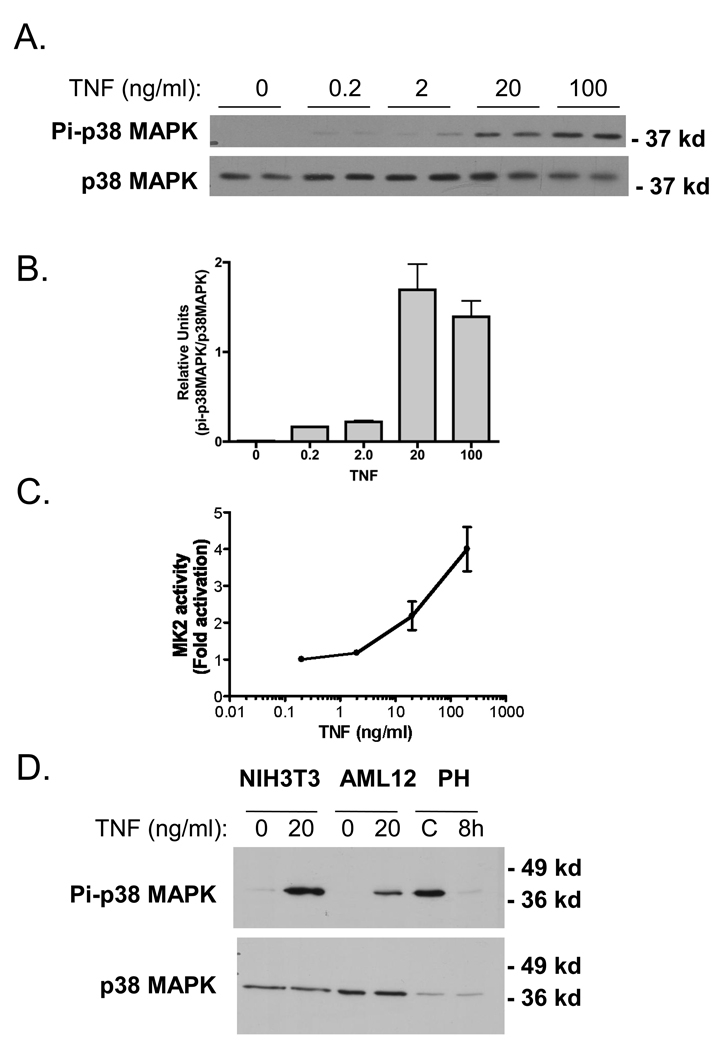
We examined whether p38 MAPK was constitutively phosphorylated in vitro using AML12 cells,0020a murine hepatocyte cell line developed in our laboratory (Wu et al. 1994a; Wu et al. 1994b). Basal levels of Pi-p38 MAPK were low or un-detectable in quiescent cultures that were deprived of serum for 16hrs, but TNF treatment robustly stimulated p38 MAPK phosphorylation after 10 min of cytokine exposure (Fig. 6A and B). MK2 was also robustly activated by TNF treatment in vitro, in a dose-dependent manner (Fig. 6C). These results indicate that p38 MAPK activity in vitro is minimal in hepatocytes, and TNF treatment robustly activates both p38 MAPK and MK2. To corroborate these in vitro observations, we next compared Pi-p38 MAPK levels in a murine fibroblast cell line, NIH3T3 cells, and AML12 hepatocytes with and without TNF treatment. Protein lysates from mice 8 hr after 2/3 PH and mice that did not undergo surgery were included (Figure 6D). TNF induced p38 MAPK phosphorylation in both AML12 and NIH 3T3 cells, while 2/3PH decreased the phosphorylation of this protein kinase. In separate experiments, treatment with TNF increased p38 MAPK phosphorylation in two other hepatocyte cells lines, NMH and TAMH when compared to protein lysates from unstimulated cultures (data not shown). Taken together, these results indicate that in vivo hepatic p38 MAPK activity is regulated in a different manner than in hepatocyte or fibroblast cell lines in vitro.
Fig 6.
Stimulation of p38 MAPK and MK2 by TNF in vitro. A) Western blot analysis of p38 MAPK activation with increasing concentrations of TNF for 10 min. in AML12 cells. B) Densitometry analysis of activated p38 MAPK with increasing concentrations of TNF. C) MK2 activation with increasing concentrations of TNF using the MK2 immunoprecipitation kinase assay described in Materials and Methods. MK2 activity is presented as fold activation (to represent the results of 3 independent experiments.). D) Western blot analysis of p38 MAPK activation in AML12 and NIH3T3 treated with 20 ng/ml for 10 min. Protein lysates from whole mouse liver from non-surgical (−) and 8hr post 2/3 PH (8h) are included for comparison.
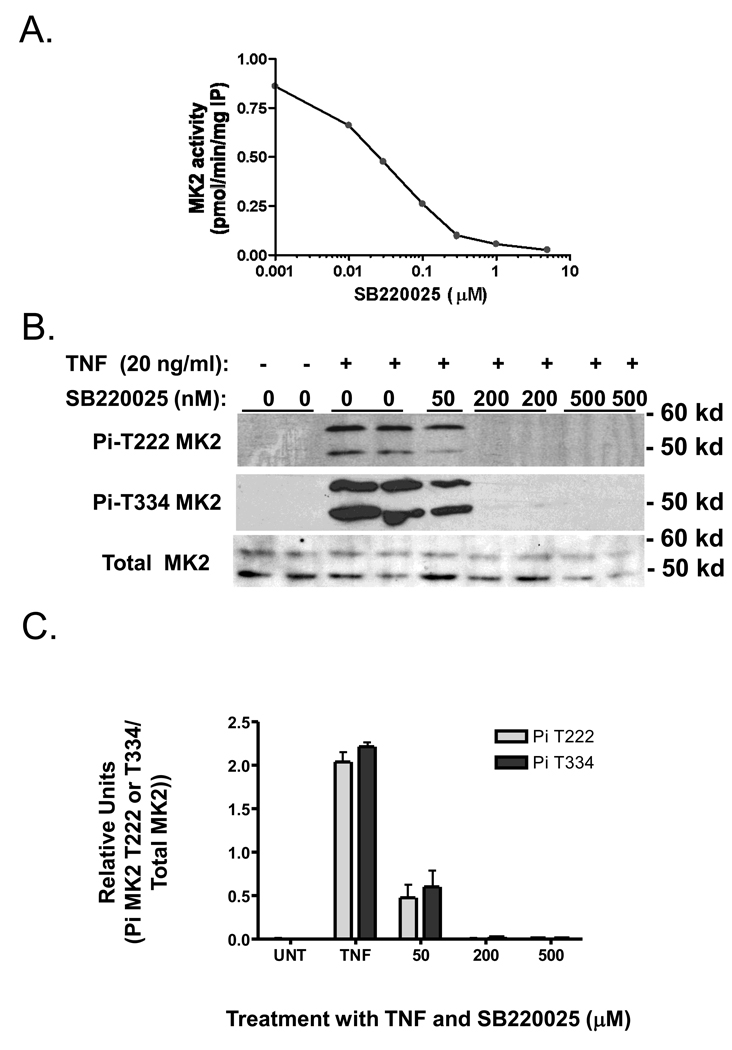
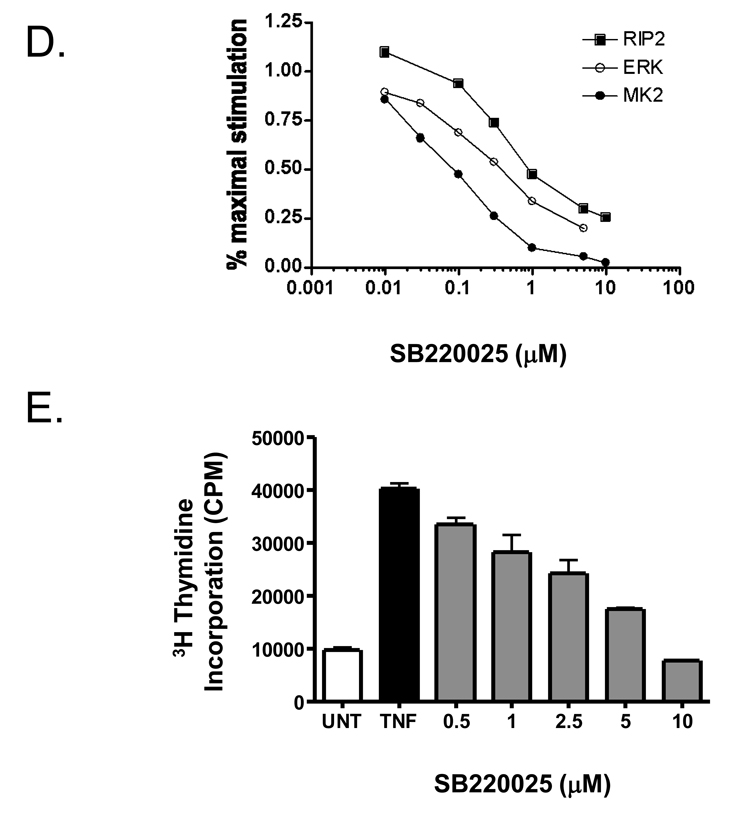
We used the p38 MAPK inhibitor, SB220025, to examine p38 MAPK’s role in hepatocyte proliferation in vitro and in vivo. SB220025 is a member of a class of pyrimidyl imidazole compounds developed to block cytokine production, and found to be highly selective for p38 MAPK inhibition (Jackson et al. 1998). We treated AML12 cell cultures with increasing doses of SB220025 followed by TNF stimulation for 10 min, and measured the extent of MK2 activation (Figure 7A). SB220025 potently inhibited MK2 activity with an apparent IC50 of 10 nM. Using western blotting and phospho-specific antibodies for T222- and T334-MK2, SB220025 inhibited TNF-stimulated phosphorylation of MK2 in a dose dependent manner (Figure 7B&C). We also determined ERK1/2 and RIP2 kinase activities, as these kinases are both reported targets for pryimidyl imidazole compounds. SB220025 also inhibited both ERK1/2 and RIP2 kinases, but the IC50 values were higher than that for MK2 (Figure 7D). Interestingly, SB220025 did not block TNF stimulated JNK activity as measured by a GST-jun pull down assay (Franklin and Kraft 1997) (data not shown). We next examined whether SB220025 would block TNF-stimulated proliferation of AML12 cells. SB220025 blocked TNF-stimulated DNA replication (Fig. 7E) as measured by tritiated thymidine incorporation assay. Taken together these results suggest that p38 MAPK activity is inhibited by SB220025 in vitro as determined by MK2 inhibition, and that this inhibitor blocks TNF- induced proliferation in AML12 cells, suggesting that p38 MAPK may, in part, be necessary for proliferation for hepatocytes in culture.
Fig 7.
SB220025 inhibits TNF-stimulated p38MAPK, ERK1/2 and MK2 activity and TNF-stimulated proliferation in vitro. Serum deprived AML12 were pre-treated with SB22002 at the indicated doses and then stimulated with TNF (20ng/ml) for 10 min. A) TNF-stimulated MK2 activity is inhibited with increasing concentrations of SB220025 from 0.01 to 5 µM. B) TNF-stimulated MK2 phosphorylation at T222 and T334 is inhibited by SB220025. C) Densitometry analysis of phospho-T222-MK2 and T334-MK2 relative to total levels of MK-2. Replicate samples (n=4–5) were analyzed. D) TNF-stimulated RIP2, ERK, and MK2 with increasing concentrations of SB220025. C) SB220025 inhibited TNF-stimulated cell proliferation as determined by thymidine incorporation as described in Materials and Methods.
Inhibition of p38 MAPK activity and DNA replication in the regenerating liver
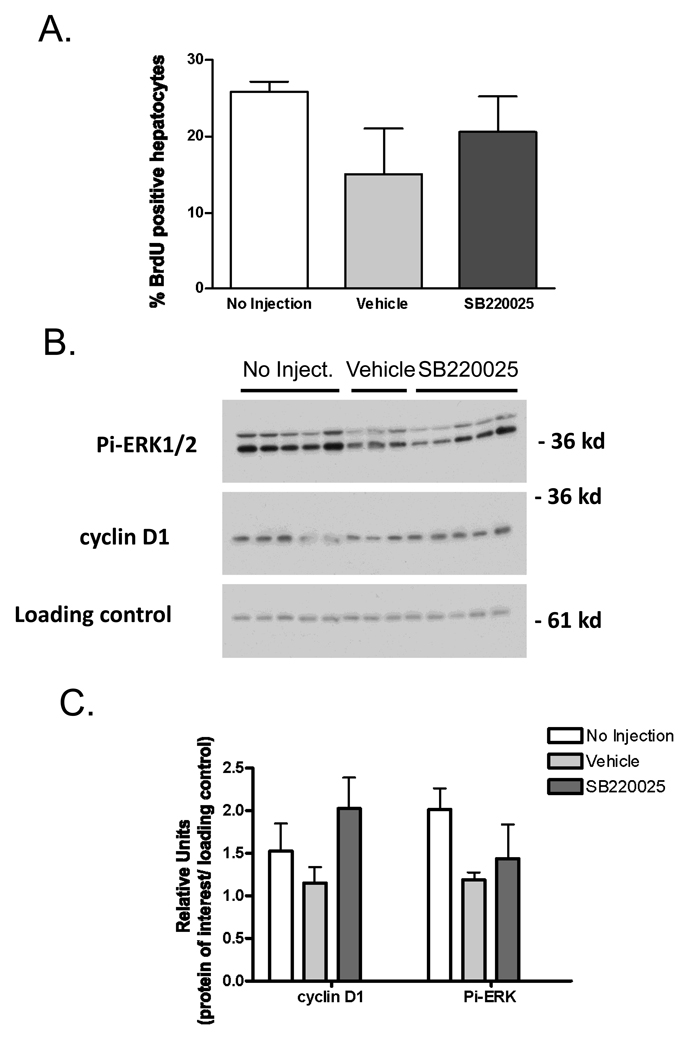
Given that SB220025 inhibited TNF-induced proliferation and p38 MAPK activity in hepatocyte cultures, we next investigated whether blockade of p38 MAPK activity that occurs between 24 and 36h after PH would affect DNA replication. Mice were divided into three treatment groups (Figure 8). One group received SB220025 every 12 h (20 mg/kg, i.p. “SB”), a second group received injections of the vehicle without inhibitor every 12 h (i.p. “vehicle”) and the third group did not receive any injection in order to leave p38 MAPK activity unaltered (“no injection”). Figure 8A shows that SB220025 treatment did not inhibit DNA replication as measured by BrdU labeling at 36h after surgery. The levels of cyclin D1, another marker of proliferation, were also unchanged by SB220025 treatment (Fig 8.B&C). To determine whether p38 MAPK activity was inhibited by SB220025 treatment, we measured MK2 activity using an immunoprecipitation kinase assay at 36 h after surgery. There were no significant differences in MK2 activity in any of the treatment groups (data not shown). We also determined whether SB22025 treatment inhibited ERK1/2 phosphorylation at 36h after surgery since these kinases are activated during liver regeneration (Figure 1A&B), and SB220025 can inhibit ERK1/2 in vitro at a higher IC50 than p38 MAPK (Fig. 7B). At 36h after surgery, ERK1/2 phosphorylation was not blocked by SB220025 treatment (Fig. 8 B&C). Together, the results of these experiments suggest that p38 MAPK activity does not appear to be required for DNA replication in vivo during liver regeneration.
Fig 8.
SB22025 does not inhibit hepatocyte DNA replication, p38 MAPK, ERK1/2 activity or cyclin D1 expression at 36 h after PH. A) BrdU incorporation at 36 hours after partial hepatectomy in mice receiving no injection (n=5), vehicle- control (n=3), or the inhibitor SB22025, (20 mg/kg every 12hr, n=5) was done as described in Materials and Methods. B). Cyclin D1 expression, and ERK1/2 and p38 MAPK activation was determined by western blot analysis. C) Densitometry analysis of Pi-ERK1/2 and cyclin D1 relative to loading control. Treatment groups are the same as in panel A.
Discussion
p38 MAPK was initially identified as a stress kinase in yeast (known as the osmosense gene, HOG1) activated by osmotic shock, and shown to respond to hyperosmolarity and LPS treatment in mammalian cells (Han et al. 1994). Since then, there has been a large number of reports of p38 MAPK activation after extracellular stressors that include osmotic shock, hypoxia, oxidative damage, cytokines, and UV radiation (Raman et al. 2007). Four isoforms of p38 MAPK (α, β, δ, and γ) have been identified thus far with p38 MAPK α/β isoforms being the best studied (Cobb 2007). Activation of p38 MAPK involves the phosphorylation of the Thr-Gly-Tyr (TGY) dual phosphorylation motif. MKK3/6 are the primary p38 MAPK activators, and these kinases are themselves activated by several upstream kinases that respond to various extracellular stimuli (Hui et al. 2007b; Thornton and Rincon 2009).
In addition to its role as a stress kinase, p38 MAPK has effects in the immune system, where it is activated during the stimulation of CD4+ and CD8+ cells, and in some autoimmune diseases (Rincon and Davis 2009). Other data suggests that p38 MAPK may act as a cell cycle regulator and as a suppressor of cell proliferation and tumorigenesis (Hui et al. 2007b). Given the discrepant reports in the literature about p38 MAPK activation during liver regeneration, and its activity in normal liver (Mendelson et al. 1996; Liao et al. 2004; Yamamoto et al. 2005; Stepniak et al. 2006), we investigated the timing of activation of p38 MAPK in partially hepatectomized and sham-operated mice. We also looked for correlations between p38 MAPK activation and changes in downstream pathways and functions, focusing on the role of p38 MAPK in hepatocyte proliferation and protein translation.
p38 MAPK was active in the livers of non-operated mice, despite being classified primarily as a stress kinase. It decreased drastically within 30 min after the operation, and remained at low or undetectable levels for 5–8h. Full recovery of p38 MAPK activity did not occur until approximately 24h post hepatectomy. The levels of Pi-p38 MAPK also decreased after sham-operation, but recovery was rapid, with restoration of normal levels at about 4h after the operation. The timing of Pi-p38 MAPK dephosphorylation after PH we have described is similar to data from Liao et al. (Liao et al. 2004). We observed that induction of Dusp1 mRNA correlated with dephosphorylation of Pi-p38 MAPK, suggesting that this dual specificity phosphatase may be involved in the rapid inactivation of p38 MAPK. Mendelson and co-workers showed that p38 MAPK activity was constitutively active in mouse liver, and was dephosphorylated after oxidative stress and DNA replication produced by CCl4, at the same time that JNK activity was greatly increased (Mendelson et al. 1996). Thus, it appears that hepatic Pi-p38 MAPK is rapidly dephosphorylated, and presumably inactivated, in conditions that lead to hepatocyte replication, regardless of the level of oxidative stress.
During embryonic development, p38 MAPK activity is low, but increases postnatally to reach adult levels after the first month of life, showing an inverse relationship with hepatocyte proliferation (Awad et al. 2000). It is possible that the transient inactivation of p38 MAPK after PH may be a permissible factor for DNA replication. A role for p38 MAPK as a suppressor of DNA replication was demonstrated in experiments in which various cell types, including hepatoblasts, obtained from mice with genetic deletion of p38 MAPK showed increased proliferation (Hui et al. 2007a; Hui et al. 2007b; Wada et al. 2008). Based on these and other experiments, Wada et al. suggested that there is an antagonistic relationship between p38 MAPK and JNK in controlling DNA replication, as well as in senescence and oncogenic transformation (Wada et al. 2008). However, the mechanisms by which p38 MAPK activity may regulate DNA replication and cell cycle check points are not completely defined. p38 MAPK may regulate both the G1/S and G2/M cell cycle checkpoints. It appears that p38 MAPK phosphorylation and activation of p53, leading to the enhancement of p21 transcription may be the most important mechanism blocking cell cycle progression and DNA replication (Hui et al. 2007b). In addition p38 MAPK activity may block JNK, leading to downstream inhibition of c-Jun, Cdk2, cyclinD1 and other genes involved in cell proliferation, as well as inhibition of EGF receptor signaling.
Given the multiple replication-related pathways that are p38 MAPK targets, it is perhaps surprising that mice with p38 alpha MAPK genetic deficiency showed only a small (but significant) increase in DNA replication after PH (Hui et al. 2007b), and that we found no alterations in hepatocyte proliferation at 36h after PH in animals that received injections of the p38 MAPK inhibitor SB220025 every 12h after the operation. These results indicate that loss or inhibition of p38 MAPK activity has only a minor, if any effect, in stimulating DNA replication in the regenerating liver. However these results do not provide information on whether the prolonged but transient inactivation of p38 MAPK after PH has a permissive effect on hepatocyte DNA replication and mitosis. Thus, it would be interesting to determine whether constitutive and specific activation of p38 MAPK in a transgenic mouse model would inhibit liver regeneration.
We also show that p38 MAPK activity in the regenerating liver does not correlate with MK2 activity, a p38 MAPK target that stabilizes mRNAs, particularly mRNAs for TNF and other cytokines, and that p38 MAPK activity has an inverse relationship with the phosphorylation of the translational initiator elf-4E, and p70S6 kinase activity that act on protein elongation and translation. The phosphorylation of a number of other putative p38 MAPK downstream targets did not correlate with p38 MAPK dephosphorylation and re-activation during liver regeneration, suggesting that unidentified p38 MAPK substrates may exist in the liver. Further studies are needed to determine the physiological substrates for p38 MAPK in the liver
Contrary to the results of in vivo experiments, we found that both p38 MAPK and MK2 activity were low in hepatocyte cell lines in vitro, and could be robustly activated after TNF stimulation. Furthermore, inhibition of p38 MAPK using a chemical inhibitor SB220025 correlated with inhibition of MK2 activity in these experiment, corroborating previous published data suggesting that MK2 is a p38 MAPK substrate (Gaestel 2006). In contrast, MK2 activity did not change after PH or with SB220025 treatment in vivo. The reasons for these discrepancies between in vivo and in vitro experiments is not clear at this time, but the in vitro experiments involved TNF-mediated DNA replication, which may not have been the case in the animal experiments. In summary, in contrast to hepatic inflammatory conditions, p38 MAPK activity rapidly decreases in the regenerating liver and does not return to normal levels for at least 24h. Our data is consistent with the notion that p38 MAPK inactivation may be a permissive event in hepatocyte growth and replication during liver regeneration, but this role still remains to be firmly established.
Acknowledgments
The authors would like to thanks Lisa Prichard and Cynthia Yost for technical assistance, and Renay Bauer for critical reading of the manuscript. NIH grants, CA-23226 and CA-74131 NF), and CA-127228 (JSC) supported this work
Abbreviations
- PH
two-thirds partial hepatectomy
- ERK1/2
extracellular regulated kinase
- MAPK
mitogen-activated protein kinase
- Pi-p38 MAPK
active, phosphorylated p38 MAPK
- MKK
MAPK/ERK kinase
- JNK
c-jun N-terminal kinases
- PKB
protein kinase B (also known as Akt)
- MKK3/6
mitogen-activated Protein Kinase Kinase 3 or 6
- DUSP
dual specificity protein phosphatases (previously known as MKP, MAPK phosphatases)
- TNF
tumor necrosis factor
- EGF
epidermal growth factor
- MK2
MAPKAPK2 (mitogen-activated protein kinase-activated protein kinase 2)
- BrdU
bromodeoxyuridine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adam AP, George A, Schewe D, Bragado P, Iglesias BV, Ranganathan AC, Kourtidis A, Conklin DS, Aguirre-Ghiso JA. Computational identification of a p38SAPK-regulated transcription factor network required for tumor cell quiescence. Cancer Res. 2009;69:5664–5672. doi: 10.1158/0008-5472.CAN-08-3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argast GM, Campbell JS, Brooling JT, Fausto N. Epidermal growth factor receptor transactivation mediates tumor necrosis factor-induced hepatocyte replication. J Biol Chem. 2004;279:34530–34536. doi: 10.1074/jbc.M405703200. [DOI] [PubMed] [Google Scholar]
- Argast GM, Fausto N, Campbell JS. Inhibition of RIP2/RIck/CARDIAK activity by pyridinyl imidazole inhibitors of p38 MAPK. Mol Cell Biochem. 2005;268:129–140. doi: 10.1007/s11010-005-3701-0. [DOI] [PubMed] [Google Scholar]
- Awad MM, Enslen H, Boylan JM, Davis RJ, Gruppuso PA. Growth regulation via p38 mitogen-activated protein kinase in developing liver. J Biol Chem. 2000;275:38716–38721. doi: 10.1074/jbc.M008040200. [DOI] [PubMed] [Google Scholar]
- Bornfeldt KE, Krebs EG. Crosstalk between protein kinase A and growth factor receptor signaling pathways in arterial smooth muscle. Cell Signal. 1999;11:465–477. doi: 10.1016/s0898-6568(99)00020-0. [DOI] [PubMed] [Google Scholar]
- Boutros T, Chevet E, Metrakos P. Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase regulation: roles in cell growth, death, and cancer. Pharmacol Rev. 2008;60:261–310. doi: 10.1124/pr.107.00106. [DOI] [PubMed] [Google Scholar]
- Bucher NL, Swaffield MN. The Rate of Incorporation of Labeled Thymidine into the Deoxyribonucleic Acid of Regenerating Rat Liver in Relation to the Amount of Liver Excised. Cancer Res. 1964;24:1611–1625. [PubMed] [Google Scholar]
- Campbell JS, Riehle KJ, Brooling JT, Bauer RL, Mitchell C, Fausto N. Proinflammatory cytokine production in liver regeneration is Myd88-dependent, but independent of Cd14, Tlr2, and Tlr4. J Immunol. 2006;176:2522–2528. doi: 10.4049/jimmunol.176.4.2522. [DOI] [PubMed] [Google Scholar]
- Chaisson ML, Brooling JT, Ladiges W, Tsai S, Fausto N. Hepatocyte-specific inhibition of NF-kappaB leads to apoptosis after TNF treatment, but not after partial hepatectomy. J Clin Invest. 2002;110:193–202. doi: 10.1172/JCI15295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavien PA, Petrowsky H, DeOliveira ML, Graf R. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007;356:1545–1559. doi: 10.1056/NEJMra065156. [DOI] [PubMed] [Google Scholar]
- Fausto N, Campbell JS. Liver Regeneration. Wiley; 2009. [Google Scholar]
- Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45–S53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- Franklin CC, Kraft AS. Conditional expression of the mitogen-activated protein kinase (MAPK) phosphatase MKP-1 preferentially inhibits p38 MAPK and stress-activated protein kinase in U937 cells. J Biol Chem. 1997;272:16917–16923. doi: 10.1074/jbc.272.27.16917. [DOI] [PubMed] [Google Scholar]
- Gaestel M. MAPKAP kinases - MKs - two's company, three's a crowd. Nat Rev Mol Cell Biol. 2006;7:120–130. doi: 10.1038/nrm1834. [DOI] [PubMed] [Google Scholar]
- Graves JD, Campbell JS, Krebs EG. Protein serine/threonine kinases of the MAPK cascade. Ann N Y Acad Sci. 1995a;766:320–343. doi: 10.1111/j.1749-6632.1995.tb26684.x. [DOI] [PubMed] [Google Scholar]
- Graves LM, Bornfeldt KE, Argast GM, Krebs EG, Kong X, Lin TA, Lawrence JC., Jr cAMP- and rapamycin-sensitive regulation of the association of eukaryotic initiation factor 4E and the translational regulator PHAS-I in aortic smooth muscle cells. Proc Natl Acad Sci U S A. 1995b;92:7222–7226. doi: 10.1073/pnas.92.16.7222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene AK, Puder M. Partial hepatectomy in the mouse: technique and perioperative management. J Invest Surg. 2003;16:99–102. [PubMed] [Google Scholar]
- Grisham JW. A morphologic study of deoxyribonucleic acid synthesis and cell proliferation in regenerating rat liver; autoradiography with thymidine-H3. Cancer Res. 1962;22:842–849. [PubMed] [Google Scholar]
- Han J, Lee JD, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- Horimoto M, Fulop P, Derdak Z, Wands JR, Baffy G. Uncoupling protein-2 deficiency promotes oxidant stress and delays liver regeneration in mice. Hepatology. 2004;39:386–392. doi: 10.1002/hep.20047. [DOI] [PubMed] [Google Scholar]
- Hui L, Bakiri L, Mairhorfer A, Schweifer N, Haslinger C, Kenner L, Komnenovic V, Scheuch H, Beug H, Wagner EF. p38alpha suppresses normal and cancer cell proliferation by antagonizing the JNK-c-Jun pathway. Nat Genet. 2007a;39:741–749. doi: 10.1038/ng2033. [DOI] [PubMed] [Google Scholar]
- Hui L, Bakiri L, Stepniak E, Wagner EF. p38alpha: a suppressor of cell proliferation and tumorigenesis. Cell Cycle. 2007b;6:2429–2433. doi: 10.4161/cc.6.20.4774. [DOI] [PubMed] [Google Scholar]
- Jackson JR, Bolognese B, Hillegass L, Kassis S, Adams J, Griswold DE, Winkler JD. Pharmacological effects of SB 220025, a selective inhibitor of P38 mitogen-activated protein kinase, in angiogenesis and chronic inflammatory disease models. J Pharmacol Exp Ther. 1998;284:687–692. [PubMed] [Google Scholar]
- Kotlyarov A, Neininger A, Schubert C, Eckert R, Birchmeier C, Volk HD, Gaestel M. MAPKAP kinase 2 is essential for LPS-induced TNF-alpha biosynthesis. Nat Cell Biol. 1999;1:94–97. doi: 10.1038/10061. [DOI] [PubMed] [Google Scholar]
- Lambotte L, Saliez A, Triest S, Tagliaferri EM, Barker AP, Baranski AG. Control of rate and extent of the proliferative response after partial hepatectomy. Am J Physiol. 1997;273:G905–G912. doi: 10.1152/ajpgi.1997.273.4.G905. [DOI] [PubMed] [Google Scholar]
- Liao Y, Shikapwashya ON, Shteyer E, Dieckgraefe BK, Hruz PW, Rudnick DA. Delayed hepatocellular mitotic progression and impaired liver regeneration in early growth response-1-deficient mice. J Biol Chem. 2004;279:43107–43116. doi: 10.1074/jbc.M407969200. [DOI] [PubMed] [Google Scholar]
- Liu Y, Shepherd EG, Nelin LD. MAPK phosphatases--regulating the immune response. Nat Rev Immunol. 2007;7:202–212. doi: 10.1038/nri2035. [DOI] [PubMed] [Google Scholar]
- Mendelson KG, Contois LR, Tevosian SG, Davis RJ, Paulson KE. Independent regulation of JNK/p38 mitogen-activated protein kinases by metabolic oxidative stress in the liver. Proc Natl Acad Sci U S A. 1996;93:12908–12913. doi: 10.1073/pnas.93.23.12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213:286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C, Nivison M, Jackson LF, Fox R, Lee DC, Campbell JS, Fausto N. Heparin-binding epidermal growth factor-like growth factor links hepatocyte priming with cell cycle progression during liver regeneration. J Biol Chem. 2005;280:2562–2568. doi: 10.1074/jbc.M412372200. [DOI] [PubMed] [Google Scholar]
- Mitchell C, Willenbring H. A reproducible and well-tolerated method for 2/3 partial hepatectomy in mice. Nat Protoc. 2008;3:1167–1170. doi: 10.1038/nprot.2008.80. [DOI] [PubMed] [Google Scholar]
- Ngala Kenda JF, de Hemptinne B, Lambotte L. Role of metabolic overload in the initiation of DNA synthesis following partial hepatectomy in the rat. Eur Surg Res. 1984;16:294–302. doi: 10.1159/000128422. [DOI] [PubMed] [Google Scholar]
- Patterson KI, Brummer T, O'Brien PM, Daly RJ. Dual-specificity phosphatases: critical regulators with diverse cellular targets. Biochem J. 2009;418:475–489. doi: 10.1042/bj20082234. [DOI] [PubMed] [Google Scholar]
- Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene. 2007;26:3100–3112. doi: 10.1038/sj.onc.1210392. [DOI] [PubMed] [Google Scholar]
- Rescan C, Coutant A, Talarmin H, Theret N, Glaise D, Guguen-Guillouzo C, Baffet G. Mechanism in the sequential control of cell morphology and S phase entry by epidermal growth factor involves distinct MEK/ERK activations. Mol Biol Cell. 2001;12:725–738. doi: 10.1091/mbc.12.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riehle KJ, Campbell JS, McMahan RS, Johnson MM, Beyer RP, Bammler TK, Fausto N. Regulation of liver regeneration and hepatocarcinogenesis by suppressor of cytokine signaling 3. J Exp Med. 2008;205:91–103. doi: 10.1084/jem.20070820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincon M, Davis RJ. Regulation of the immune response by stress-activated protein kinases. Immunol Rev. 2009;228:212–224. doi: 10.1111/j.1600-065X.2008.00744.x. [DOI] [PubMed] [Google Scholar]
- Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68:320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler H, Stoecklin G. Control of mRNA decay by phosphorylation of tristetraprolin. Biochem Soc Trans. 2008;36:491–496. doi: 10.1042/BST0360491. [DOI] [PubMed] [Google Scholar]
- Schwabe RF, Bradham CA, Uehara T, Hatano E, Bennett BL, Schoonhoven R, Brenner DA. c-Jun-N-terminal kinase drives cyclin D1 expression and proliferation during liver regeneration. Hepatology. 2003;37:824–832. doi: 10.1053/jhep.2003.50135. [DOI] [PubMed] [Google Scholar]
- Schwabe RF, Brenner DA. Mechanisms of Liver Injury. I. TNF-alpha-induced liver injury: role of IKK, JNK, and ROS pathways. Am J Physiol Gastrointest Liver Physiol. 2006;290:G583–G589. doi: 10.1152/ajpgi.00422.2005. [DOI] [PubMed] [Google Scholar]
- Seger R, Seger D, Reszka AA, Munar ES, Eldar-Finkelman H, Dobrowolska G, Jensen AM, Campbell JS, Fischer EH, Krebs EG. Overexpression of mitogen-activated protein kinase kinase (MAPKK) and its mutants in NIH 3T3 cells. Evidence that MAPKK involvement in cellular proliferation is regulated by phosphorylation of serine residues in its kinase subdomains VII and VIII. J Biol Chem. 1994;269:25699–25709. [PubMed] [Google Scholar]
- Stepniak E, Ricci R, Eferl R, Sumara G, Sumara I, Rath M, Hui L, Wagner EF. c- Jun/AP-1 controls liver regeneration by repressing p53/p21 and p38 MAPK activity. Genes Dev. 2006;20:2306–2314. doi: 10.1101/gad.390506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokoe D, Campbell DG, Nakielny S, Hidaka H, Leevers SJ, Marshall C, Cohen P. MAPKAP kinase-2; a novel protein kinase activated by mitogen-activated protein kinase. Embo J. 1992;11:3985–3994. doi: 10.1002/j.1460-2075.1992.tb05492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talarmin H, Rescan C, Cariou S, Glaise D, Zanninelli G, Bilodeau M, Loyer P, Guguen-Guillouzo C, Baffet G. The mitogen-activated protein kinase kinase/extracellular signal-regulated kinase cascade activation is a key signalling pathway involved in the regulation of G(1) phase progression in proliferating hepatocytes. Mol Cell Biol. 1999;19:6003–6011. doi: 10.1128/mcb.19.9.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanoue T, Moriguchi T, Nishida E. Molecular cloning and characterization of a novel dual specificity phosphatase, MKP-5. J Biol Chem. 1999;274:19949–19956. doi: 10.1074/jbc.274.28.19949. [DOI] [PubMed] [Google Scholar]
- Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004;5:836–847. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- Thornton TM, Rincon M. Non-classical p38 map kinase functions: cell cycle checkpoints and survival. Int J Biol Sci. 2009;5:44–51. doi: 10.7150/ijbs.5.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada T, Stepniak E, Hui L, Leibbrandt A, Katada T, Nishina H, Wagner EF, Penninger JM. Antagonistic control of cell fates by JNK and p38-MAPK signaling. Cell Death Differ. 2008;15:89–93. doi: 10.1038/sj.cdd.4402222. [DOI] [PubMed] [Google Scholar]
- Winzen R, Kracht M, Ritter B, Wilhelm A, Chen CY, Shyu AB, Muller M, Gaestel M, Resch K, Holtmann H. The p38 MAP kinase pathway signals for cytokine-induced mRNA stabilization via MAP kinase-activated protein kinase 2 and an AU-rich region-targeted mechanism. Embo J. 1999;18:4969–4980. doi: 10.1093/emboj/18.18.4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JC, Merlino G, Cveklova K, Mosinger B, Jr, Fausto N. Autonomous growth in serum-free medium and production of hepatocellular carcinomas by differentiated hepatocyte lines that overexpress transforming growth factor alpha 1. Cancer Res. 1997a;54:5964–5973. [PubMed] [Google Scholar]
- Wu JC, Merlino G, Fausto N. Establishment and characterization of differentiated, nontransformed hepatocyte cell lines derived from mice transgenic for transforming growth factor alpha. Proc Natl Acad Sci U S A. 1994b;91:674–678. doi: 10.1073/pnas.91.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Kojima T, Murata M, Takano K, Go M, Hatakeyama N, Chiba H, Sawada N. p38 MAP-kinase regulates function of gap and tight junctions during regeneration of rat hepatocytes. J Hepatol. 2005;42:707–718. doi: 10.1016/j.jhep.2004.12.033. [DOI] [PubMed] [Google Scholar]