Abstract
Hypertension is a syndrome beyond high blood pressure alone. Hypertension is one of the cardiovascular diseases that may cause cardiovascular remodeling and endothelial dysfunction. Angiotensin II type 1 (AT1R) and type 2 (AT2R) receptors are expressed in most organs and tissues and are implicated in hypertension, endothelial dysfunction, and cardiovascular diseases. Genetic and epigenetic manipulations of the renin angiotensin system play a critical role in programming of cardiovascular diseases, and certain variants of AT1R and AT2R are constitutively predisposed to higher cardiovascular risk and hypertension. The structure-function relationship of angiotensin receptors has been reviewed previously. So, in this review we focused on the structure, expression of angiotensin II receptors, their mode of action, role in cardiovascular pathobiology, and how cardiovascular diseases are programmed in utero. In addition, we described genetic variants of angiotensin receptors, and also discussed possible ways of therapeutic intervensions of Ang II stimulation. Collectively, this information may lead us to future new drug design against cardiovascular diseases.
Introduction
Angiotensin II (Ang II) is a multifunctional peptide hormone that regulates blood pressure (BP), regulates plasma volume, regulates cardiac, renal and neuronal function, and controls thirst responses. This peptide is of central importance in hypertension and myocardial remodeling and is the principle effector molecule of the renin-angiotensin system (RAS). In diseases[E3] or dysfunctional RAS, pathological effects are manifested. The classical effects of Ang II on its target organs are mostly mediated by two membrane receptors, type 1 receptors (AT1Rs) and type 2 receptors (AT2Rs), which mediate tissue-specific functions; however, a widespread non-AT1 and non-AT2 binding site for Ang II was also found in rat, mouse and human brain. Interestingly, this site is not present in rat or mouse liver or adrenal [1–3]. The widespread but discrete distribution of this binding site suggests that it might function as a component of the blood–brain barrier. The AT1R and AT2R cDNAs were cloned almost two decades ago [4–6], and this helped to provide insight into the molecular mechanisms that control the sensitivity of cardiovascular and other target organs to Ang II.
Although acute stimulation with Ang II regulates body fluid homeostasis and vasoconstriction, which regulate BP, chronic stimulation by Ang II promotes migration, hyperplasia and hypertrophy of vascular smooth muscle cells (VSMCs). Chronic exposure to Ang II induces cardiac hypertrophy, remodeling, restenosis and atherosclerosis. Given these diverse functions, it is necessary to understand the role of Ang II receptors in physiological and pathophysiological processes. Studying the varied roles of Ang II is also of tremendous importance given the beneficial effects of angiotensin-converting enzyme (ACE) inhibitors (ACE-Is) and Ang II type I receptor blockers[E4] (ARBs) in reducing morbidity and mortality in diabetes, hypertension, atherosclerosis, heart failure and stroke. The structure–function relationship of angiotensin receptors has already been reviewed extensively [7]; in this review, therefore, we briefly describe their structure, along with the expression of Ang II receptors in the cardiovascular system, their mode of action and the major signaling pathways they participate in. In addition, we describe their role in cardiovascular pathobiology, how they are programmed in utero to cause cardiovascular diseases, and genetic variants of angiotensin receptors, and we discuss possible therapeutic interventions of Ang II stimulation. Collectively, this information might lead us to new drug designs against cardiovascular diseases.
The renin-angiotensin system
The RAS is a hormone system that regulates the BP and fluid balance of the body. A decrease in renal perfusion through the juxtaglomerular apparatus in the kidney’s macula densa region induces the renal juxtaglomerular cells to secrete the enzyme renin, an aspartyl protease [8]. Renin cleaves the inactive zymogen angiotensinogen (secreted mainly by the liver), converting it to angiotensin I (Ang I). Ang I is then converted to Ang II, an octapeptide, by ACE, which is found mainly in lung vascular endothelium. Other cell types in various organs including renal endothelium also possess ACE [9]. Ang II regulates BP and mediates its vasoconstrictor effects by stimulating angiotensin receptors in vascular smooth muscle. Ang II also stimulates the secretion of aldosterone from the adrenal cortex. Aldosterone causes the tubules of the kidneys to increase the reabsorption of sodium and water. This raises the volume of fluid in the body, which also increases BP. If the RAS system is overactive, BP becomes too high. A recently identified ACE homologue (ACE-2, a carboxypeptidase that is insensitive to ACE-I) cleaves one amino acid from either Ang I or Ang II, decreasing circulating Ang II levels, and increases the metabolite Ang (1–7), which is believed to be beneficial in cardiovascular tissues. The ACE2–angiotensin-(1–7)–Mas axis is now seriously considered as a putative target for the development of new cardiovascular drugs [10]. This has been reviewed extensively by Shi et al. [11]. According to Crackower et al. [12], ACE2 is an essential regulator of heart function because there is severe reduction of cardiac functions, including cardiac contractility in ACE2 single and double knockout (KO) mice, with abnormal cardiac structure, despite an apparent lack of hypertrophy and fibrosis. By contrast, the ACE-2 KO mice of Yamamoto et al. [13] and Gurley et al. [14] did not show any cardiac abnormalities. The role of ACE-2 in cardiac functions is thus somewhat controversial. The balance between ACE and ACE-2 could still be an important factor controlling available Ang II levels and may be important in determining the final physiological response in some situations because it has also been reported that the loss of ACE-2 accelerates left ventricular remodeling in response to myocardial infarction and that AT1R-mediated ACE-2 inhibition impairs baroflex function. This supports a crucial role for ACE-2 in the central regulation of BP and the development of hypertension [15–17]. Although, in general, ACE is believed to be the primary enzyme leading to Ang II production, in the heart the majority of Ang I may be converted to Ang II by chymase, a serine protease, which is ACE-I insensitive [9]. Renin inhibitors and ARBs may be able to induce a more complete blockade of the RAS in the heart, therefore, providing a more effective therapeutic intervention in congestive heart failure [18]. Besides ACE-2, several other novel components of RAS have been identified, such as prorenin and the prorenin receptor (PRR). The PRR may bind both renin and prorenin. Receptor-bound prorenin may trigger a profibrotic response and upregulate cyclooxygenase (COX)-2 genes, an Ang-II-independent function. This explains a potential role of PRR in organ damage, particularly in hypertension and diabetes [19]. Nevertheless, definitive proof is still lacking for a role for PRR in disease, by showing improvement of disease by tissue-specific ablation of PRR or a PRR-specific antagonist [20]. Thus, RAS is far more complicated than initially anticipated. The effects of Ang II are primarily manifested via two G-protein-coupled, seven-transmembrane-domain receptors, AT1R and AT2R. They mediate an intricate pattern of function and regulation [9]. These two receptors operate via different signaling pathways and mediate different functions [21]. AT1R activates growth-promoting pathways, mediates major Ang II effects (such as vasoconstriction, increased BP, cardiac contractility, renal tubular sodium absorption and cell proliferation) and mediates detrimental effects (such as oxidative stress, endothelial dysfunction, cardiovascular diseases and inflammation) [22]. By contrast, AT2R is believed to induce opposing effects, namely vasodilatation, hypotension, antigrowth by apoptosis, anti-hypertrophic effects and the possible inhibition of AT1R [21,23–25]. A brief overview of RAS is depicted in Figure 1. Actually, RAS is far more complex that the figure suggests because new members are still being identified. Thus, Ang (1–12) is a newly identified alternate endogenous substrate for Ang II production. Vasoconstrictor effects of Ang (1–12) may be prevented by previous blockade with the ACE-I:captopril or ARB:candesartan in both isolated aorta and in the systemic circulation. The enzyme that cleaves Ang (1–12) to Ang I has yet to be identified. Because of space limitations, we are unable to review information about Ang (1–12) but refer interested readers to an excellent review by Ferrario [26]. Based on the knowledge of RAS, various drugs (e.g. ARBs, such as candesartan, losartan, valsartan and so on, and ACE-Is, such as captopril, enalapril, trandolapril and so on) have been created that interrupt at different steps in this system to control BP. These drugs are our main armaments in the management of cardiovascular diseases, kidney failure and diabetes.
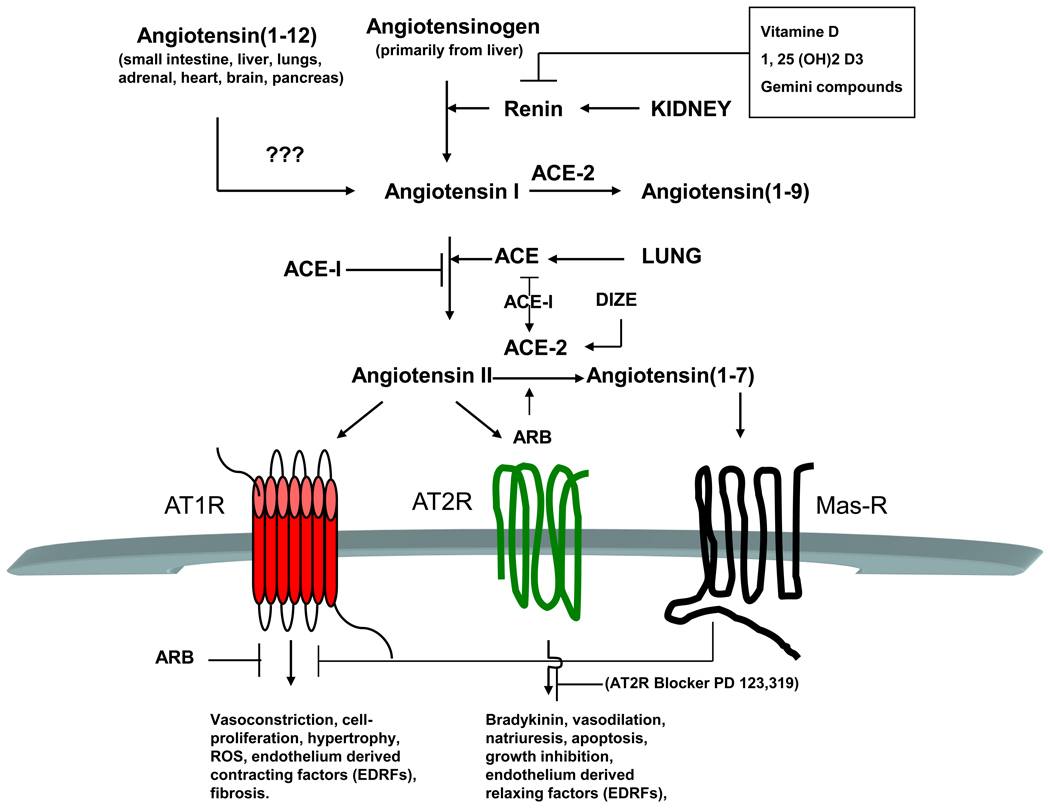
Figure 1.
The renin-angiogenesis system (RAS) and long-term effects of angiotensin II receptor stimulation. Angiotensinogen, secreted mainly from liver, is converted to Ang I by renin. ACE, primarily from lung, subsequently converts Ang I into Ang II. ARB, ACE-I or DIZE, an experimental drug, can activate ACE-2, a homolog of ACE, which cleaves Ang II to Ang (1–7) that acts through Mas receptors. ACE-2 and Ang (1–7) are considered to be beneficial components of RAS. Ang II mediated chronic stimulation of AT1R leads to vasoconstriction and other pathophysiological consequences that may blocked by ARBs or ACE-Is. Long-term stimulation of AT2R, by contrast, leads to vasorelaxation and is considered beneficial. Ang (1–12) is a newly identified alternate endogenous substrate for Ang II production. Vasoconstrictor effects of Ang (1–12) may be prevented by previous blockade with captopril or candesartan in both isolated aorta and the systemic circulation. The enzyme that cleaves Ang (1–12) to Ang I is yet to be identified. Red, AT1R; green, AT2R; black, MasR. ARB, Ang II receptor blocker; ACE-I, ACE inhibitor; DIZE, diminazene aceturate, an experimental drug and activator of ACE-2; vitamin D, [1, 25 (OH)2 D3], 1, 25-dihydroxyvitamin D3, the most active metabolite of vitamin D; Gemini compounds, vitamin D analogs, being screened for rennin[E23]-suppressing activity; ???, not identified yet; arrow in any direction, conversion or activation; T in any direction, inhibition.
Ang II type 1 receptor
AT1Rs are expressed in all organs, including heart, kidney, liver, adrenals, brain, lung and vasculature [9,27–29]. There is only one AT1R gene in human, but possible gene duplication gave rise to AT1a receptor (AT1aR) and AT1b receptor (AT1bR) gene subtypes in rat and mouse [4,5]. In contrast to AT1R, rat, mouse and human reportedly have only one AT2R gene, for which no subtypes or splice variants have so far been identified [6,30–32]. In human, the single AT1R coding gene is located on chromosome 3, whereas in rat, the AT1aR and AT1bR gene subtypes are located on chromosomes 17 and 2, respectively. The AT2R genes for human, rat and mouse are localized on the X chromosome [33]. Although most of the well-known actions of Ang II are mediated by the AT1 receptors, the role of AT2R is not yet completely understood.
Structure
AT1aR was originally cloned from rat VSMCs [5], and AT1bR was cloned from rat adrenal gland [4]. These receptors are composed of 359 amino acids, with approximately 40–41 kDa denatured molecular weight and seven-transmembrane domains; they belong to the superfamily of G-protein-coupled receptors. In rat, two isoforms[E5] AT1a and AT1b receptors share 95% amino acid sequence identity in the protein coding region but have significant[E6] differences in the untranslated regions of their mRNAs and respective promoter regions. Pharmacological studies with the expressed cDNAs indicate that these proteins have almost identical ligand-binding affinities and receptor–effector coupling [34,35]. Structurally, AT1 receptors belong to the seven-transmembrane domain superfamily of G-protein-coupled receptors.[E7] The four putative extracellular domains each contains a cysteine residue, which presumably forms two disulfide bonds, one between the first and fourth (last) extracellular domains and the other between the second and third extracellular domains, of which the latter disulfide bond between the second and third is essential for the binding of the nonpeptidic antagonists (e.g. losartan) [36]. The extracellular sequences also contain three consensus N-glycosylation sites [37]. The cytoplasmic tail of AT1 receptors is rich in serine and threonine residues, which are potential phosphorylation sites for several kinases, including G-protein-receptor kinases and protein kinase C (PKC). The cytoplasmic tail also contains consensus sequences for phosphorylation by casein kinase II [33] and a palmitoylation site (Cys355) that may be involved in receptor–effector coupling or in anchoring the tail of the receptor to the plasma membrane [38]. The main differences between the AT1aR and AT1bR genes exist in the protein sequence differences in the c-terminal tail of the molecules giving rise to two additional putative PKC phosphorylation sites, the absence of a possible palmitoylation site in the AT1bR protein and the presence of a low homology (35%) at the 5′ and 3′ untranslated regions of the two mRNAs [33]. These differences indicate possible differential regulation of these two protein subtypes. In addition, although glucocorticoid response elements (GREs) are present in both AT1aR [27] and AT1bR [39] promoter regions, in AT1aR, GREs are positive regulatory, whereas in AT1bR, they are negative regulatory. Because the GRE in AT1aR promoter is functional in vascular smooth muscle [40], it may partially explain the high BP owing to enhanced vasoconstrictor response to Ang II in glucocorticoid-induced hypertension, which is observed in Cushing’s syndrome.
Expression
AT1R is expressed in all cells of the cardiovascular system, namely endothelial cells, smooth muscle cells, fibroblasts, monocytes, macrophages and cardiac myocytes and, thus, is crucial in cardiovascular pathobiology. Ang II and AT1R are also ubiquitous in nervous tissues, including the cardiovascular center of the brain, and control brain cardiovascular, neural and thirst responses [41,42]. In vivo experiments with AT1bR-null mice double mutants show that in kidney and adrenal, AT1aR can take over the role of AT1bR with comparable BP and plasma aldosterone levels. In contrast to AT1aR−/− and angiotensinogen−/− mice, AT1bR−/− mice are devoid of morphological abnormalities in these tissues [43]. Thus, AT1aR isoform could be more important than AT1bR in the regulation of BP. The same might be true in cardiovascular tissues, including brain. In brain, BP increase elicited by centrally administered Ang II can be selectively ascribed to that of AT1aR, but the drinking response requires the presence of AT1bR [44]. AT1aR and AT1bR expression are tissue dependent. AT1aR is more abundant in smooth muscle, liver, kidney, heart and lungs [45], whereas AT1bR is more abundant in pituitary and adrenal glands (i.e. in the hypothalamus–pituitary–adrenal axis) [27]. Studies of the expressed cDNAs indicate that AT1aR and AT1bR mRNAs code for proteins with nearly identical ligand-binding affinities and receptor–effector coupling [33,35]. They differ in hormonal regulation, however [34], and also differ pharmacologically [4]. Thus, estrogen treatment suppressed AT1bR mRNA but not AT1aR mRNA levels in the pituitary gland [34]. Comparative binding studies of these two receptors expressed in COS-7 cells indicated that Ang II was more potent on the expressed AT1bR (IC50 = 5.4 nM) than it is on AT1aR (IC50 = 14 nM). Similarly, Du 753 (now called losartan), an Ang II antagonist, was more potent on the expressed AT1bR (IC50 = 5.4 nM) than it is on AT1aR (IC50 = 15 nM) [4]. Both AT1aR and AT1bR have comparable inhibition constant, however: Ki (50 nM) for losartan and valsartan [33]. In contrast to human, therefore, in rat and mouse the tissue-specific expression of AT1aRs and AT1bRs could selectively mediate different effects of Ang II in the different target tissues, as expressions of two identical proteins are under different promoter controls in different tissue types.
Mode of action
The effect of angiotensin receptor activation by Ang II was described by Glossmann et al. [46]. Activated AT1 receptors can activate multiple signaling pathways. The acute vasoconstrictor function of Ang II is primarily mediated through AT1R by classical G-protein-dependent signaling mechanisms. Depending on cell types, Ang II activates[E8] AT1 receptors can activate at least four different effector pathways – namely, voltage-gated Ca2+ channels, phospholipase C, phospholipase D (PLD) and phospholipase A-2 (PLA-2) – and can inhibit adenylate cyclase [33,47,48]. Activation of phospholipase C produces inositol-1,4,5-trisphosphate and diacylglycerol. Inositol-1,4,5-trisphosphate then binds to its receptor on sarcoplasmic/endoplasmic reticulum[E9], opening a channel that enables Ca2+ efflux into the cytoplasm. Ca2+ binds to calmodulin and activates myosin light chain kinase, which phosphorylates the myosin light chain that enhances the interaction between actin and myosin, inducing smooth muscle cell contractions. Myosin light chain phosphatase maintains equilibrium at this step by dephosphorylating the myosin light chain. In addition, Ang II stimulation of AT1R activates the extracellular-signal- regulated kinase (ERK) cascade, platelet-derived growth factor, epidermal growth factor receptor (EGFR), insulin receptor pathways and non-receptor tyrosine kinases belonging to the c-Src family, proline-rich tyrosine kinase 2, focal adhesion kinase and janus kinases (JAKs) [22]. Besides the G-protein-dependent effects of Ang II-activated AT1R, the activated AT1R can also stimulate G-protein-independent signal transduction mechanisms by directly associating with signaling molecules, such as β-arrestins, JAK, Cdc42 and Src, and has been reviewed extensively by Hunyady and Catt [49]. In brief, the AT1R is a class B G-protein-coupled receptor, which bind β-arrestins tightly [50]. β-arrestins may serve as scaffolds to organize the formation of signaling complexes [51] that mediate AT1R-induced activation of ERK, MAPKs and JNK3 MAPKs [52]. Studies on a mutant AT1R that is fully uncoupled from G proteins can still activate Src tyrosine kinases[E10] [53]. Although additional studies are required to elucidate the exact biological role of these mechanisms, Gq-protein-independent, AT1R-mediated EGFR transactivation was also recently reported to occur in human coronary artery smooth muscle cells [54]. Cumulatively, the effector molecules downstream to AT1R contribute to the vasoconstrictor and growth-promoting properties of Ang II, which is mediated via AT1R. It is these mechanisms that are responsible for vascular lesion formation (owing to migration, proliferation and growth of smooth muscle cells) and vascular dysfunction in hyperglycemia (diabetes) because in hyperglycemia PKC, PLD and free-radical formation are upregulated [55]. Treatment with antioxidants (e.g. probucol and α-tocopherol) attenuates these effects by lowering PKC and PLD activities and by relieving intracellular oxidative stress [56].
AT1R in cardiovascular pathobiology
Oxidative stress
Ang II is also a potent mediator of oxidative stress and reactive oxygen species (ROS)-mediated signaling. Ang II manifests this effect through the activation of AT1R. A large body of evidence indicates that ROS plays a major part in the initiation and progression of cardiovascular dysfunctions associated with diseases such as hyperlipidemia, diabetes mellitus, hypertension, ischemic heart disease and chronic heart failure (CHF) [57]. In addition, Ang II signaling activates membrane NAD(P)H oxidases in VSMCs to produce ROS (e.g. superoxide and hydrogen peroxide), which mediate the pleiotrophic effects of Ang II [58–60]. Ang II signaling mediated activation of NAD(P)H oxidases involves the upstream mediators Src/EGFR/PI3K/Rac-1 and PLD/PKC/p47phox phosphorylation [59,61]. Transcription factors – such as NF-κB, activator protein 1 (AP-1) and Nrf2 – that are implicated in the pathogenesis of atherosclerosis are activated by ROS [62,63]. ROS, in turn, can reversibly modify cysteine residues and inactivate many controlling molecules (e.g. tyrosine phosphatase). One of the most detrimental consequences of AT1R-induced production of superoxides and ROS is the inactivation of endothelial-derived relaxing factor [64]. ROS may cause vessel inflammation by inducing the release of cytokines and causing an increase of expression of the leukocyte adhesion molecules in the cell membranes. This may increase recruitment of monocytes to the area of endothelial damage [65]. Thus, activated AT1R-induced ROS production can lead to changes in structural and functional properties of the vasculature and is the central aspect of vascular pathobiology in hypertension, diabetes and cardiovascular diseases.
Endothelial cell dysfunction
Endothelial dysfunction in the context of hypertension and cardiovascular diseases is partly dependent on the production of ROS. AT1R-induced ROS not only destroys nitric oxide (NO) but also reduces NO formation in the endothelium by oxidizing tetrahydrobiopterin, a crucial co-factor for endothelial NO synthase [66]. Superoxide anion, a highly reactive ROS, is known to cause breakdown of endothelial-derived relaxing factor and concomitant breakdown of endothelium [64]. Regenerated endothelium has an impaired ability to release endothelial-derived relaxing factors, in particular NO. Activation of angiotensin receptors triggers an efflux of cytosolic calcium (see the section ‘Mode of action’) in endothelial cells. A rise in calcium efflux activates PLA-2 to release arachidonic acid, which is metabolized by COX to generate various eicosanoids including endoperoxides and various prostaglandins that activate thromboxane A2/prostanoid receptors located on the VSMCs causing contractions [67]. Endothelial COX activity also produces ROS, which in turn stimulates COX in the smooth muscle cell, further initiating thromboxane A2/prostanoid-receptor-mediated contractions in smooth muscle cells and thereby initiating a vicious circle [67]. In addition, a recent report described that in vitro, uric acid – despite its known antioxidant effects – may cause human vascular endothelial cell dysfunction by local activation of RAS and inducing oxidative stress (i.e. ROS)[E11] [68]. Nevertheless, physiological relevance of such in vitro study requires further confirmation. ROS may, in turn, activate local RAS of whole vasculature, including EC, smooth muscle cells, fibroblast-enhancing Ang II production and concomitant stimulation of AT1R to create a positive feedback loop between local RAS and ROS. Overall in hypertensive cardiovascular diseases, endothelial dysfunction is characterized by blunted endothelium-dependent vasodilations or enhanced endothelium-dependent contractions [67].
Insulin resistance
Patients with an imbalance in the RAS exhibit insulin resistance [69]. Insulin resistance develops in various metabolic syndromes, including type 2 diabetes, obesity and hypertension [70]. In vivo studies in rat demonstrated that Ang II infusion confers insulin resistance [71,72] and hyperactive Ang II signaling may overwhelm the insulin signaling [73]. Normally, insulin actions are mediated through binding of insulin to insulin receptor (IR) that has an intrinsic tyrosine kinase activity, which results in tyrosine phosphorylation of the insulin receptor substrates (IRS), Src homology collagen and JAK2 [70]. These phosphorylated proteins dock downstream effector molecules, which are able to activate different signaling cascades. Of these, the ERK pathway is involved in growth and mitogenesis, whereas the activation of phosphatidylinositol-3 kinase (PI3K), primarily through IRS-1, deploys the metabolic actions of insulin[E12] [70]. In view of the important association between cardiovascular diseases and insulin resistance, cross-talk mechanisms between insulin and RAS pathway are known. Thus, Velloso et al. [73] demonstrated that in either insulin or Ang II stimulated rat heart, there is tyrosine phosphorylation of both IRS-1 and IRS-2. These phosphorylations are mediated by JAK2, which associates with AT1R, IRS-1 and IRS-2 in Ang II stimulation. Despite binding of PI3K to IRS-1 and IRS-2, however, there is acute but severe inhibition of PI3K activity in Ang II stimulation in both the basal- and the insulin-stimulated state. These effects of Ang II are inhibited by AT1R antagonists. Folli et al. [74] found that pretreatment of rat aortic smooth muscle cells with Ang II inhibited insulin-stimulated IRS-1 associated PI3K activity by 60%, insulin-stimulated tyrosine phosphorylation of IRS-1 by 50% and the insulin-stimulated association of IRS-1 and the p85 subunit of PI3K activity by 30%–50%. In addition, Ang II increased serine phosphorylation of both the IR-β subunit and IRS-1. Furthermore, Ang II induced ROS induced[E13] serine phosphorylation of IRS-1 for proteasome-dependent degradation in rat aortic smooth muscle cells [75]. Thus, hyperactive RAS may severely perturb normal insulin signaling at multiple levels. Evidently, ACE inhibitors and ARBs can ameliorate the risk of type 2 diabetes and restore insulin sensitivity in hypertension [69].
Cardiovascular remodeling
Pressure overload induced by hypertension and myocardial infarction causes hearts to undergo remodeling. This includes cardiomyocyte hypertrophy, extracellular matrix synthesis, fibrosis and loss of compliance, leading to fatal outcomes. At the molecular level, the RAS, TGF-β and β-adrenergic system network mediates this myocardial remodeling. TGF-β1 mRNA and protein expression is augmented in cardiomyocytes, cardiac fibroblasts[E14], which in these situations transdifferentiate to myofibroblast phenotype [76]. As a consequence, progressive diastolic dysfunction sets in. Ang II caused a significant increase in TGFβ1 mRNA. Chronic administration of Ang II induced TGF-β1 protein expression in myocardium [77]. The direct relationship between the RAS and TGF-β1 in cardiac hypertrophy was demonstrated by Jo El Schultz et al. [78], who showed that Ang II-induced hypertrophic growth of cardiomyocytes is mediated by TGF-β1, which is downstream to Ang II in network. Mice expressing constitutively hyperactive TGF-β1 had a chronic hypertrophy, fibrosis and cardiac dysfunction that was resistant to the ARB telmisartan but blocked by TGF-β antagonist [79]. The Ang II-mediated cardiac remodeling mechanism is thus dependent upon TGF-β1. From all the studies done so far, it is clear that Ang II/TGF-β1 induction, autocrine-paracrine cellular responses in cardiac fibroblasts, the myocardial interstitium and cardiomyocytes cause cardiac hypertrophy.[E15] Ang II-activated TGF-β1 signaling also induces translocation of Smad proteins into the nucleus to drive transcription of fibrotic marker proteins such as collagen, fibronectin and connective tissue growth factor (CTGF) [80]. CTGF is a profibrotic factor that stimulates TGF-β-1 responses mediating fibrosis and apoptosis [81,82]. Because AT1R hyperactivity is implicated in ischemic injury and coronary artery disease, expression profiling studies on coronary artery biopsies were done to relate end point gene expression profile with AT1R activation. This procedure detected high CTGF mRNA expression in chronic ischemic myocardium with a high degree of correlation with thrombospondin 4, collagen type Iα2, versican, adlican, latent TGF-β binding protein 2 and fibronectin, which are involved in extracellular matrix remodeling. These proteins, along with inflammatory cytokines (e.g. IL-8, IL-6, VCAM-1 and MCP-1), are believed to have important roles in the coupling acute ischemic episodes and chronic myocardial remodeling with chronic angiotensin receptor stimulation [83].
Role of AT1R and AT2R in brain
The cardiovascular center of brain (in the medulla) is responsible for the regulation of the rate at which the heart beats. Ang II and its receptors are important determinants of this autonomic tone. Several aspects of AT1R signaling in CHF have emerged in recent years and have been reviewed excellently by Zucker et al. [41]. There is now good evidence that AT1R expression in neurons is mediated by the activation of the transcription factor AP-1. AP-1 and its component proteins are upregulated in the rostral ventrolateral medulla (RVLM) of animals with CHF. Because the increase in AT1R expression and AP-1 activation can be blocked by losartan, a positive feedback mechanism of AT1R expression in CHF is suggested. In addition, whereas AT1R expression in RVLM is upregulated in CHF, the AT2R expression is downregulated. Stimulating RVLM AT1R in the presence of PD123,319 induced a larger response in sham rats but not in CHF rats, indicating that AT2R in RVLM exhibits an inhibitory effect on sympathetic outflow and it is the downregulation of AT2R that contributes to sympathetic overactivity in CHF [42].
Ang II type 2 receptor
Structure
Like AT1R, AT2R also belongs to the superfamily of G-protein-coupled receptors. The AT2R was originally cloned from a rat fetal cDNA library and rat PC12 cells [30,31,84]. Like the AT1R, AT2R also has a seven-transmembrane domain, with a molecular weight of 41 kDa, with 363 aminos, which is only 34% identical to AT1 receptors [30,84]. Subsequently, mouse and human AT2R cDNAs were also cloned, and they show more than 92% homology with the rat homolog [32,85]. Structural comparisons between AT1 and AT2 receptors indicate some important differences. In AT2R, there are five (as opposed to three in AT1R) potential glycosylation sites at the N terminus of protein. Because of possible post-translational modification at these sites, AT2R protein in transfected COS-7 cells shows a molecular weight of 80 kDa, as determined by gel electrophoresis, but can be converted to approximately 40 kDa by treatment with N-glycosidase [84]. The second intracellular loop of AT2R contains a potential PKC phosphorylation site and the cytoplasmic tail contains three consensus PKC phosphorylation sites and one for phosphorylation by cyclic AMP-dependent protein kinase [33].
Like AT1aR and AT1bR promoters, rat AT2R promoter also contains GREs [86]. Like AT1bR, these GREs are also negative regulatory, which explains the downregulation of AT2R expression by growth factors and glucocorticoids [87,88]. Although the human AT2R gene has been cloned, only limited information has been published about it so far [6,32].
Expression
AT2R is highly expressed in fetal heart and fetal aorta and is expressed at a modest level in kidney, lung and liver [89,90]. AT2R expression declines fast after birth, suggesting that it might play an important part in fetal development [90], but can be induced later in adult life under pathological conditions (e.g. fibrosis) [91]. Cardiac-specific overexpression of AT2R, however, led to fatal consequences, showing why AT2R expression is turned off in postnatal life (details in section 4.4.3[E16]). Contrary to these developmental changes in most of the tissues, in brainstem AT2R expression was seen to be increased significantly with age [92]. This finding, along with the downregulation of AT2R in RVLM [42] and in cold-induced hypertension [93], raises new questions about the role of AT2R in neuronal tissues.
Mode of action
Although the AT1R mediates most of the well-known effects of Ang II, the modes of action of the AT2R are still emerging. At a glance, AT2R seems to counterbalance many actions of the AT1R; for example, whereas phosphorylation of IR and IRS-1 are induced in AT1R stimulation, the same could be attenuated by AT2R activation [94]. Because of its intrinsic pro-apoptotic, antiproliferative and vasorelaxing properties, AT2R may control other physiological processes such as tissue remodeling (by inhibiting cell growth and stimulating apoptosis), BP (by vasodilatation), natriuresis and neuronal activity. AT2R induced anti-proliferative and pro-apoptotic changes in VSMCs antagonize that of AT1R. According to one study, AT2R might do so by forming a heterodimer with AT1R and so is considered as a G-protein-coupled AT1R-specific antagonist[E17] [24]. By contrast, a more recent study reported that AT2Rs cause hypertrophy of isolated cardiomyocytes and do not block AT1R-mediated hypertrophy [95]. This hypertrophic response is mediated by direct binding of the transcription factor promyelocytic leukemia zinc finger protein to the tail of the AT2R, leading to nuclear translocation and enhanced transcription of the p85 subunit of PI3K [96]. This might indicate that the AT2R is capable of participating in multiple signaling pathways, based on tissue types or environment. The exact role of AT2R thus remains debatable. Three major pathways have been described for AT2R signaling, however, namely protein phosphatase pathway causing protein dephosphorylation, activation of NO/cGMP pathway and stimulation of PLA-2 with release of arachidonic acid [97].
Cardiovascular effects of AT2R
Vasodilatation
It is generally accepted that AT2R promotes apoptosis and inhibits proliferation and hypertrophy. Unlike AT1Rs, the AT2R contributes to maintenance of BP by controlling the vascular tone through vasodilatation. Available evidence indicates links between AT2R and bradykinin B2 receptors (B2Rs) in NO production in vasodilation [98–100]. AT2R stimulation by Ang II leads to an increase in cGMP levels through a mechanism involving B2R, causing NO release [99,101]. AT2R activation stimulates bradykinin formation by activating kininogenases [98]. These investigators found that chronic infusion of Ang II into AT2R overexpressing mice completely abolished the AT1-mediated pressor effect, suggesting vasodilatation by AT2R. This was blocked by inhibitors of B2R (icatibant) and NO synthase inhibitor Nω-nitro-L-arginine methyl ester. In addition, aortic explants from these transgenic mice overexpressing AT2R showed greatly increased cGMP/NO production and diminished Ang I-induced vascular constriction. Removal of endothelium or treatment with icatibant and Nω-nitro-L-arginine methyl ester abolished these AT2-mediated effects, indicating endothelium is the primary site of effect manifestation by AT2R. AT2R in aortic VSM cells also stimulates the production of bradykinin, which stimulates the NO/cGMP system to promote vasodilation. In contrast to the wild-type mice, in AT2R KO mice, bradykinin-induced dilation is seriously impaired. Bradykinin-induced dilation is inhibited by AT2R antagonist PD123,319 and B2R antagonist HOE-140 but not by Losartan. Thus, AT2R and B2R may form functional heterodimers, as recently shown, and this might lead to increased NO and cGMP production [102]. The physical association between the dimerized AT2R-B2R[E18] initiates changes in intracellular phosphoprotein signaling activities leading to phosphorylation of c-Jun terminal kinase, phosphotyrosine phosphatase activity, inhibitory protein κβα, activating transcription factor 2, dephosphorylation of p38 and p42/44 MAP kinase and signal transducer inhibitor of transcription 3, and enhanced production of NO and cGMP [97].
Anti-inflammation and apoptosis
Growth and apoptosis are also two opposite components of tissue remodeling. Cardiac remodeling happens in certain physiological or pathological circumstances, such as exercise, aging, hypertension and myocardial infarction. It is known that although AT2R expression decreases sharply after birth, it can increase again in some pathophysiological conditions. Stimulation of de novo AT2R expression may inhibit neointima formation, cell proliferation, inflammation in vascular injury, myocardial infarction and ischemic diseases, suggesting its protective role. Conversely, in AT2R KO mice, recovery from acute myocardial infarction was very much reduced compared to the wild-type mice [103]. Whereas neointima formation and smooth muscle cell proliferation after vascular injury were exaggerated in AT2R KO mice, they were suppressed in AT1aR KO mice. AT2R possibly exerts anti-proliferative effects and pro-apoptotic effects in VSMC by controlling AT1aR [104]. The number of apoptotic cells in the injured artery was increased significantly in AT1aR KO mice but decreased in AT2R KO mice. This indicates that AT2R mediates apoptosis, as was originally demonstrated in PC12W cells by Yamada et al. [23]. Selective AT2R stimulation also inhibited restenosis after balloon angioplasty [105]. In agreement, thus, experimental studies have shown a beneficial role for the re-expression of the AT2R after vascular injury leading to inhibition of cell growth and promotion of apoptosis. Gene transfer studies in vivo have also supported that AT2R expression attenuated neointima accumulation in injured carotid arteries by antagonizing growth-promoting effects of AT1R [25]. Vascular injury studies using mice with disrupted AT2R expression showed enhanced neointima development compared with wild-type mice, suggesting a protective role for AT2R [106].
Ectopic expression
Because de novo AT2R expression is upregulated in several disease conditions, the pathological implication of de novo AT2R overexpression was addressed. Left ventricle (LV)-specific overexpression of AT2R promoted the onset of dilated cardiomyopathy phenotype and heart failure in vivo. Analysis of in vivo LV parameters showed reduced systolic pressure, aberrant left ventricular end diastolic pressure and Doppler echocardiography, which is an indicator of dialated cardiomyopathy. In addition, aberrant phosphorylation of several cardiac signaling proteins, disarrayed z-bands, intercalated discs and contractile fibers were seen [107]. In vivo ectopic AT2R overexpression in postnatal LV myocardium is thus detrimental, explaining the sharp decline of AT2R after birth. If prolonged ARB treatment turns out to be completely beneficial through clinical trials, then either it is a matter of dosage effect (normal level of AT2R expression versus ectopic overexpression of AT2R) or it depends on the time and site of AT2R expression. Hence, the implications of overstimulation of AT2R by long-term use of ARB is being investigated in clinical trials [21].
Acute vs. chronic effects of AT1R and AT2R inhibition
Although the long-term use of ARBs are considered to prevent the deleterious consequences of ischemia-reperfusion injury, reduce cardiovascular remodeling and promote vasorelaxation, the short-term or acute effects of AT1R or AT2R antagonism led to opposing results. Ford et al. [108] first evaluated the consequences of short-term (acute) administration of either AT1R or AT2R antagonists. In their model, the effects of acute administration of losartan (selective AT1R antagonist) and PD123,319 (selective AT2R antagonist) on the recovery of LV mechanical function during reperfusion after 30 minutes of global, no-flow ischemia were studied. Interestingly, whereas PD123319 given before ischemia improved the postischemic recovery of LV function significantly, antagonism by losartan inhibited the recovery of LV function, despite the fact that no antagonists altered coronary vascular conductance. This finding is radically opposite to the prevailing notion and indicates that short-term AT1R agonism (not antagonism) and AT2R antagonism might be cardioprotective. Further studies in isolated hearts demonstrated that PD123319 upregulated AT2R protein and mRNA abundance, along with cGMP and PKCε expressions and its membrane translocation and activation [109]. In ischemic preconditioning (PC) of the heart, membrane translocation of PKCε plays an important part. NO donors can also induce PC by activating PKC isozymes of which PKCε is specifically involved [110]. Augmentation of cGMP levels, along with AT2R protein expression, could mean the involvement of AT2R-B2R-dependent activation of bradykinin pathway in cardioprotection [97]. These studies possibly indicate that the ratio of AT1R/AT2R protein at any point in time dictates the outcome, the signaling mechanism predominant in acute LV-IR recovery, observed by Ford et al. [108]. Because IR + PD123319 augmented expression of AT2R protein and mRNA, based on most current concept of inhibitory heterodimeric association between AT1R and AT2R [24,97,111], it may be extrapolated that a higher proportion of AT1R is present as AT1R-AT2R heterodimer in IR + PD 123319. This remaining free AT1R may perform controlled beneficial cell/tissue regeneration/proliferation[E19] required to rebuild cardiomyocyte population. In addition, this process does not accompany any myocyte apoptosis in the presence of PD123319. Evidently, this procedure might offer a novel approach for the treatment of mechanical dysfunction of heart after ischemia. Figure 2 summarizes acute and chronic effects of selective Ang II receptor stimulation.
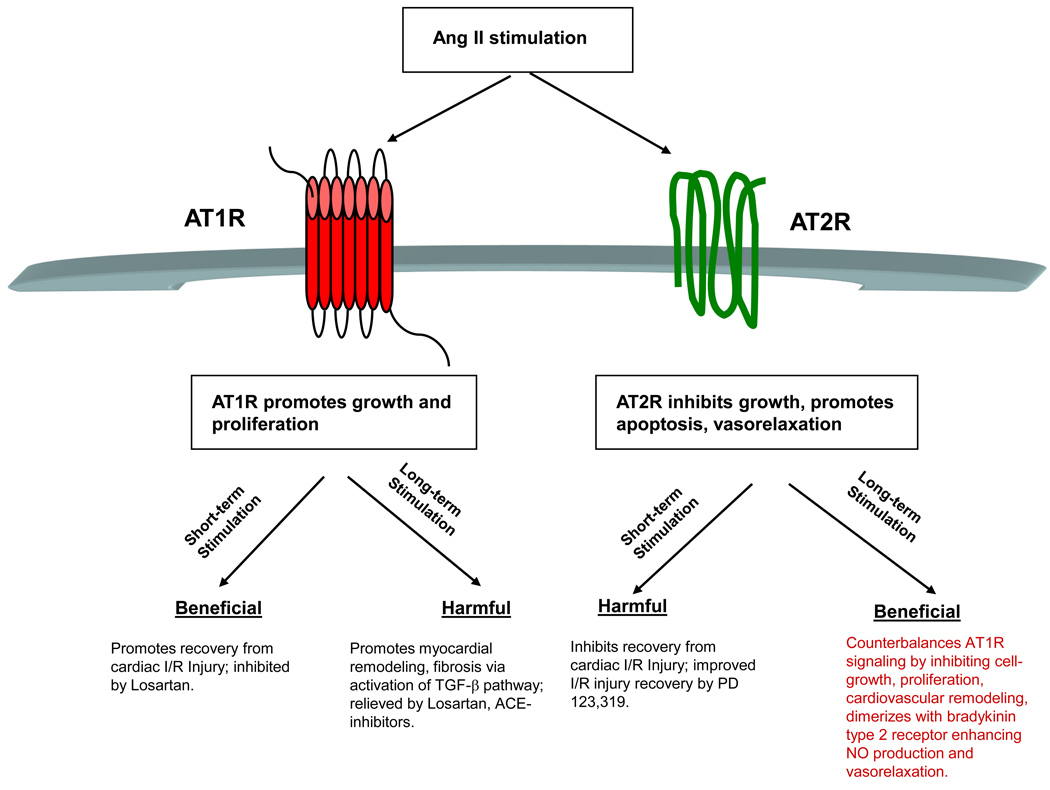
Figure 2.
Summary of acute and chronic stimulation of angiotensin II receptors. Whereas chronic stimulation of AT1R promotes hypertension, cardiovascular remodeling and fibrosis, its acute stimulation may promote recovery of left ventricular function from ischemia-reperfusion (I/R) injury. By contrast, on a short-term basis, AT2R activity inhibits the recovery of left ventricular function from I/R injury, although chronic stimulation of AT2R activity when AT1R function is blunted by ARBs promotes vasodilation and endothelial relaxation and alleviates hypertension. Red, AT1R; green, AT2R.
Epigenetic regulation of Ang II receptors: programming hypertension in utero
Adverse fetal growth environment may program cardiovascular diseases in adults, so increased cardiovascular disease risk in adult life might have its origins in fetal life [112,113]. Barker et al. [113] first documented that adverse intrauterine environment could be a strong risk factor for cardiovascular diseases, and diabetes in adult life [113,114]. Babies who are born small or thin with large placenta at birth have higher than normal rates of ischemia, coronary heart disease (CHD), stroke, hypertension and diabetes in adult life. If the growth of a fetus is constrained by lack of nutrients, there are persistent changes in the physiology of the fetus, which lead to reduced fetal growth and elevated BP. Collectively, reduced fetal growth caused either by lack of maternofetal nutrition or other hostile stimuli (e.g. hypoxia and drug abuse) promote a number of chronic conditions later in life, as described above. This phenomenon was termed ‘programming’ by Barker and has been validated in animal models. The suboptimal fetal growth environment is collectively called ‘intrauterine growth restriction’ (IUGR). Maternal low-protein (MLP) diet in rat pregnancy perturbs fetal growth and caused high BP in postnatal life [115]. This programming has a global impact: it is not restricted to cardio-vasculature only but affects kidney and brain similarly. It is now known that maternal glucocorticoids might play a key part in programming hypertension, causing overstimulation of RAS and increasing vascular resistance and hypertension [115]. Studies on kidney in MLP conditions indicate that hypertension may be programmed in utero by involvement of glucocorticoids and angiotensin receptors [116,117]. There is substantial upregulation of AT2R mRNA expression in MLP and glucocorticoid exposed adult female rat kidneys [117]. Similarly, fetal hypoxia caused upregulation of AT2R mRNA and protein expression in both male and female hearts by participation of glucocorticoid receptor (GR) [118]. MLP diet caused global change in many components of the RAS in different fetal mice tissues. In fetal kidneys, renin mRNA and protein levels were increased significantly. In the lungs, there was a decrease in both ACE I and ACE II mRNA expression. In fetal heart, there is a significant increase in the expression of both AT1aR and AT2R mRNA [119]. Although the implications of many of these changes require further investigation, at least it may be derived that many components of the RAS, including the Ang II receptors of cardiovascular system, may be programmed in an adverse uterine environment. It is known that MLP causes the upregulation of AT1b mRNA and protein, possibly because of hypo-methylation of AT1b gene promoter in adrenal gland [28]. A similar mechanism may be functional in cardiovascular tissues. Role of glucocorticoids in control of Ang II receptor expression in cardiovasculature is also a distinct possibility because both AT1aR and AT1bR promoters contain GREs [27,39]. We have recently identified multiple GREs in the rat AT2R promoter and have demonstrated that fetal hypoxia may program increased AT2R gene expression in the heart by downregulating the GR expression. This may cause increased cardiac vulnerability to ischemic injury in male rat offspring [118]. Although the mechanism of downregulation of GR in hypoxia is a matter for investigation, and hypoxia downregulates GR in human cells too [120], the AT2R expression is epigenetically modified here. Placental 11 β-hydroxysteroid dehydrogenase type 2, an enzyme that inactivates maternal cortisol and thus protects the fetus, is decreased in pregnancies complicated by IUGR. Prenatal exposure to glucocorticoids or the 11 β-hydroxysteroid dehydrogenase type 2 inhibitor carbenoxolone may program low birth weight, glucose intolerance, hypertension and cardiovascular diseases [121]. This programming can be reversed, at least in animal models, by the pharmacological blockade of maternal glucocorticoid synthesis by metyrapone [122]. Furthermore, persistence of such in utero programming effects through several generations might indicate the potential involvement of epigenetic mechanisms in the intergenerational inheritance of the ‘programmed phenotype’ and provides a molecular basis for the inherited association between low birth weight, angiotensin receptors and cardiovascular diseases [123].
The expression of many genes may be altered by epigenetic mechanisms such as DNA methylation or histone acetylation in promoters. The density of methylation is important because a weak promoter can be silenced by only a few methylated CpGs (5′-CpG-3′≳, whereas a higher density of methylation is required to repress a strong promoter. The window of establishment of the DNA methylation pattern formation possibly lies entirely within the early developmental stage of life (i.e. in utero), so factors – including nutrition, hypoxia and maternal glucocorticoids – that affect this machinery early in uterine life may have permanent effects on gene expression in coming generations. Methylation of DNA is catalyzed by the DNA methyltransferases and utilizes S-adenosylmethionine as the principal methyl donor. As a result, DNA methylation is dependent on two biochemical pathways, namely the folate and the methionine–homocysteine cycle [124]. MLP may cause disturbances in the methionine–homocysteine cycle that hence impact upon the supply of methyl donors for methylation of DNA. Indeed, the decreased folic acid in MLP may play a crucial part in the hypo-methylation of AT1b gene promoter resulting in increased AT1bR expression in the adrenal gland in offspring. A consistent MLP diet supplemented with folic acid, glycine or urea inhibited the detrimental effects of the MLP diet [125,126].[E20] Folic acid (a B vitamin) plays a crucial part in cell proliferation and division, including the production of red blood cells. Folic acid is crucially required in pregnancy. In the fetus, folic acid helps formation of the neural tube and confers protection against various birth defects. Besides folic acid, several dietary nitrogen supplements (e.g. glycine and urea) might also play a protective part in normal cardiovascular development and the maintenance of normal BP in IUGR [126].
Genetic variants of Ang II receptors
Genetic variants of the RAS have been implicated in cardiovascular diseases. Whereas IUGR programs cardiovascular diseases, certain genetic variants of the RAS and Ang II receptors are genetically predisposed to increased sensitivity to Ang II, higher risk of CHD or ischemic events. An adenine/cytosine base substitution at position 1166 in the human AT1R gene is associated with the increased incidence of essential hypertension and coronary artery vasoconstriction, owing to enhanced response to Ang II in patients with the AT1R double mutant (CC) genotype (AT1RCC1166) [127]. The enhanced Ang II responsiveness of AT1RCC1166 variant may explain the described relation between this polymorphism and cardiovascular abnormalities [128]. The CHD event rate was also higher in AT1R-CC1166variants than in AT1R-AC1166 or AT1RAA1166 genotypes. This was further confirmed with decreased survival among the AT1RCC1166 genotype compared to the corresponding A alleles [129]. Furthermore, AT1R-CC1166 and ACE-DD genotypes interact to increase the risk of ischemic heart disease [130]. The role of AT1R polymorphisms has also been evaluated in hyperlipidemia. In patients with familial hypercholesterolemia, the AT1RCC1166 variant may increase the risk of CHD [131,132]. Single nucleotide polymorphic (SNP) variants of human AT2R are also known. There is no association between CHD risk and AT2R1675A vs. AT2R1675G SNP variants. Kaplan-Meier curve revealed no difference in survival between AT2R1675A and AT2R1675G alleles at normotensive systolic BP. In contrast to the AT2R1675G carriers (who are protected from hypertension), the AT2R1675A genotype is associated with a higher risk of CHD in subjects with systolic hypertension. Furthermore, individuals carrying both AT2R3123A and AT2R1675A SNPs have a tenfold greater risk of CHD than AT2R3123A and AT2R1675G haplotypes.
Therapeutic intervention of Ang II stimulation
Pharmacological blockade of the RAS with ACE-I and ARBs restores insulin sensitivity, alleviates diabetic complications and controls hypertension. ACE-I can decrease the incidence of type 2 diabetes in hypertensive patients and, therefore, is widely used to prevent renal and cardiovascular diabetic complications [132]. ACE-I improve glucose metabolism through effects on kinin-NO pathways. ACE-I and ARBs also improve endothelial dysfunction. Mechanisms include increases in bradykinin levels [133] and Ang (1–7) levels [134]. Acting on Mas receptors, Ang (1–7) may attenuate the ACE–AngII–AT1R axis [11]. Ang (1–7) presents a newly recognized Ang II metabolite derived by the action of ACE-2 that contributes to the beneficial cardiorenal and vasculature control of BP [135,136]. Further recent recognition of Ang (1–12), a newly identified endogenous substrate for Ang II production, might be another target for the control of RAS stimulation [137]. It is now appreciated that RAS contains both a pressor and a depressor arm in exerting regulatory functions on vascular tone and cellular signaling, which paved the way for the generation of an alternate hypothesis as to how an imbalance of their functions contributes to cardiovascular diseases [26].[E21] At the same time, it may lead to the design of the synthetic RAS-related peptide derivatives or analogs that may be the RAS therapeutics of the future. However, a new generation ARB, telmisartan, has been shown to effectively activate the peroxisome proliferator activated receptor γ (PPAR-γ), a well-known anti-diabetic component. Thus, the beneficial effects of some new generation ACE-I and ARBs might go well beyond their conventional effects on the RAS. Identification of telmisartan as a unique Ang II receptor antagonist with selective PPARγ-activating ability opens a new frontier for developing next-generation ARBs containing PPARγ-activating properties, with enhanced potential for treating hypertension, diabetes and the metabolic syndrome simultaneously [69]. ACE2, an ACE homolog that promotes degradation of Ang II to Ang (1–7), is a recently recognized therapeutic target for intervention in cardiovascular diseases [11]. The human trypanocide diminazene aceturate [138], an activator of ACE2, is currently being tested. This could be a novel antihypertensive drug in the near future. Understanding mechanisms of IUGR-induced programming of the RAS components calls for a potential therapeutic approach with dietary supplements such as folate and nitrogen-enriched diets in protecting the fetus from an adverse intrauterine environment. AT2R may also be a potential new drug target as AT2R dimerizes with bradykinin receptors inducing vasorelaxation. Controlling the expression of AT2R-B2R and influencing their biologically active dimerization potential presents a novel therapeutic strategy for the treatment of hypertension, cardiovascular and renal disorders [105]. Vitamin D is a potent suppressor of renin biosynthesis and can regulate RAS. Vitamin D deficiency stimulates renin expression and can be reversed by vitamin D injection. In addition, mice lacking vitamin D receptor have elevated production of renin and Ang II. Furthermore, in cell cultures, 1, 25 (OH)2 D3 directly suppresses renin gene transcription in a vitamin-D-receptor-dependent mechanism. Gemini-vitamin D3 analogs have more potent renin-suppressing activity than 1, 25 (OH)2 D3. Thus, vitamin D analogs present yet another novel class of anti-hypertensive agents for the future [139]. Until now, intervention of the RAS has been done by ACE-I and ARBs. New studies done on the RAS, angiotensin receptors and their epigenetic programming mechanism have led to the identification of newer strategies for future drug development and newer ways to better manage hypertensive cardiovascular diseases.
Concluding remarks
The RAS is connected to multiple cellular signaling systems that are crucially balanced under normal conditions. One mechanism for the pathogenesis of hypertensive cardiovascular diseases is aberrant signaling that is initiated by the RAS. Thus, for example, insulin-mediated signaling in smooth muscle cells and EC and oxidative stress in the vessel wall are two major stimulators of cardiovascular diseases.[E22] At present, most interventions are focused on the RAS level, by ACE-I or ARB. Future studies might identify cardiovascular-cell-specific signaling aberrations that can be corrected. Expression level and thus activity of many RAS components are programmed during organism development. Although nutrient supplements might protect future generations from IUGR, an in-depth understanding of the molecules and mechanisms involved in epigenetic modification might identify targets, which might be amenable to correction by therapeutic intervention. An additional level of epigenetic control is mediated by microRNA, which binds to the 3′ end of mRNA to control protein expression. Using similar technology, in future it might be possible to moderate expression of many RAS proteins that are aberrantly programmed in utero. Presently, information on AT2R is still incomplete. With further understanding about the mode of action of AT2R and mapping of its active regions, it might be possible in future to design AT2R peptidomimetics as a potential AT1R antagonist. In addition, because simultaneous AT2R antagonism and AT1R agonism is potentially cardioprotective in acute ischemia, a novel treatment procedure to save lives from acute heart attacks might be designed. Finally, as certain genetic variants of the RAS and Ang II receptors are genetically predisposed to increased sensitivity to Ang II, or suffer higher risk of CHD or ischemic events, in future it might be possible to identify the molecular basis of this hypersensitivity and rectify it. Much work, therefore, remains to be done to perfect our knowledge about pathogenesis of the RAS in cardiovascular diseases to develop corrective remedies beyond ACE-I and ARB.
Acknowledgements
This work was supported in part by NIH grants HL82779 (L.Z.), HL83966 (L.Z.), HL89012 (L.Z.) and HD31226 (L.Z.). We apologize to all authors whose work could not be cited due to space limitations.
Biographies

Lubo Zhang
Dr. Zhang is professor of Pharmacology and Physiology at Loma Linda University School of Medicine, and is visiting professor at Fetal-Origin Diseases Institute, First Affiliated Hospital of Soochow University, Suzhou, China. He received his PhD in Pharmacology from Iowa State University in 1990, and served as the President of the Western Pharmacology Society in the US in 2008. He has been members in the various study sections for grant review for US National Institutes of Health and American Heart Association for more than 15 years. Dr. Zhang is the author/coauthor of over 450 scientific articles, book chapters and abstracts. His research focuses on the epigenetic mechanisms in fetal programming of adult cardiovascular disease.

Chiranjib Dasgupta
Graduated from St. Xavier’s College, Calcutta. He earned his Masters and PhD degrees from Calcutta University, India. He did his post doctoral research work at University of California, Los Angeles and at University of Southern California. He is an ex-faculty research scientist of Los Angeles Biomedical Research Institute, Torrance, California, where he identified mechanism of lung injury in neonatal rats caused by hyperoxia, a component of bronchopulmonary dysplasia. At present he is a senior investigator at Loma Linda University School of Medicine. For last few years, his primary research interest has focused on the modulation of renin-angiotensin-system (RAS) in antenatal hypoxia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Teaser: Intervention of RAS is currently done by ACE-I and ARBs. Identification of additional targets and mode of action of RAS presents new areas for future drug development.
References
- 1.Karamyan VT, Speth RC. Distribution of the non-AT1, non-AT2 angiotensin-binding site in the rat brain: preliminary characterization. Neuroendocrinology. 2008;88:256–265. doi: 10.1159/000140635. [DOI] [PubMed] [Google Scholar]
- 2.Karamyan VT, Speth RC. Identification of a novel non-AT1, non-AT2 angiotensin binding site in the rat brain. Brain Res. 2007;1143:83–91. doi: 10.1016/j.brainres.2007.01.051. [DOI] [PubMed] [Google Scholar]
- 3.Karamyan VT, et al. Characterization of the brain-specific non-AT(1), non-AT(2) angiotensin binding site in the mouse. Eur. J. Pharmacol. 2008;590:87–92. doi: 10.1016/j.ejphar.2008.05.035. [DOI] [PubMed] [Google Scholar]
- 4.Sandberg K, et al. Cloning and expression of a novel angiotensin II receptor subtype. J. Biol. Chem. 1992;267:9455–9458. [PubMed] [Google Scholar]
- 5.Murphy TJ, et al. Isolation of a cDNA encoding the vascular type-1 angiotensin II receptor. Nature. 1991;351:233–236. doi: 10.1038/351233a0. [DOI] [PubMed] [Google Scholar]
- 6.Koike G, et al. Human type 2 angiotensin II receptor gene: cloned, mapped to the X chromosome, and its mRNA is expressed in the human lung. Biochem. Biophys. Res. Commun. 1994;203:1842–1850. doi: 10.1006/bbrc.1994.2402. [DOI] [PubMed] [Google Scholar]
- 7.Oliveira L, et al. The angiotensin II AT1 receptor structure-activity correlations in the light of rhodopsin structure. Physiol. Rev. 2007;87:565–592. doi: 10.1152/physrev.00040.2005. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen G, et al. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J. Clin. Invest. 2002;109:1417–1427. doi: 10.1172/JCI14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paul M, et al. Physiology of local renin-angiotensin systems. Physiol. Rev. 2006;86:747–803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- 10.Santos RA, Ferreira AJ. Angiotensin-(1–7) and the renin-angiotensin system. Curr. Opin. Nephrol. Hypertens. 2007;16:122–128. doi: 10.1097/MNH.0b013e328031f362. [DOI] [PubMed] [Google Scholar]
- 11.Shi L, et al. Angiotensin-converting enzymes and drug discovery in cardiovascular diseases. Drug Discov. Today. 2010;15:332–341. doi: 10.1016/j.drudis.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crackower MA, et al. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto K, et al. Deletion of angiotensin-converting enzyme 2 accelerates pressure overload-induced cardiac dysfunction by increasing local angiotensin II. Hypertension. 2006;47:718–726. doi: 10.1161/01.HYP.0000205833.89478.5b. [DOI] [PubMed] [Google Scholar]
- 14.Gurley SB, Coffman TM. Angiotensin-converting enzyme 2 gene targeting studies in mice: mixed messages. Exp. Physiol. 2008;93:538–542. doi: 10.1113/expphysiol.2007.040014. [DOI] [PubMed] [Google Scholar]
- 15.Kassiri Z, et al. Loss of angiotensin-converting enzyme 2 accelerates maladaptive left ventricular remodeling in response to myocardial infarction. Circ. Heart Fail. 2009;2:446–455. doi: 10.1161/CIRCHEARTFAILURE.108.840124. [DOI] [PubMed] [Google Scholar]
- 16.Danilczyk U, Penninger JM. Angiotensin-converting enzyme II in the heart and the kidney. Circ. Res. 2006;98:463–471. doi: 10.1161/01.RES.0000205761.22353.5f. [DOI] [PubMed] [Google Scholar]
- 17.Xia H, et al. Angiotensin II type 1 receptor-mediated reduction of angiotensin-converting enzyme 2 activity in the brain impairs baroreflex function in hypertensive mice. Hypertension. 2009;53:210–216. doi: 10.1161/HYPERTENSIONAHA.108.123844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolny A, et al. Functional and biochemical analysis of angiotensin II-forming pathways in the human heart. Circ. Res. 1997;80:219–227. doi: 10.1161/01.res.80.2.219. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen G, Danser AH. Prorenin and (pro)renin receptor: a review of available data from in vitro studies and experimental models in rodents. Exp. Physiol. 2008;93:557–563. doi: 10.1113/expphysiol.2007.040030. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen G, Muller DN. The biology of the (pro)renin receptor. J. Am. Soc. Nephrol. 2009;21:18–23. doi: 10.1681/ASN.2009030300. [DOI] [PubMed] [Google Scholar]
- 21.Levy BI. Can angiotensin II type 2 receptors have deleterious effects in cardiovascular disease? Implications for therapeutic blockade of the renin-angiotensin system. Circulation. 2004;109:8–13. doi: 10.1161/01.CIR.0000096609.73772.C5. [DOI] [PubMed] [Google Scholar]
- 22.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am. J. Physiol. Cell Physiol. 2007;292:C82–C97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 23.Yamada T, et al. Angiotensin II type 2 receptor mediates programmed cell death. Proc. Natl. Acad. Sci. U. S. A. 1996;93:156–160. doi: 10.1073/pnas.93.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.AbdAlla S, et al. The angiotensin II AT2 receptor is an AT1 receptor antagonist. J. Biol. Chem. 2001;276:39721–39726. doi: 10.1074/jbc.M105253200. [DOI] [PubMed] [Google Scholar]
- 25.Nakajima M, et al. The angiotensin II type 2 (AT2) receptor antagonizes the growth effects of the AT1 receptor: gain-of-function study using gene transfer. Proc. Natl. Acad. Sci. U. S. A. 1995;92:10663–10667. doi: 10.1073/pnas.92.23.10663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrario CM. New physiological concepts of the renin-angiotensin system from the investigation of precursors and products of angiotensin I metabolism. Hypertension. 2010;55:445–452. doi: 10.1161/HYPERTENSIONAHA.109.145839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo DF, et al. Identification of a cis-acting glucocorticoid responsive element in the rat angiotensin II type 1A promoter. Circ. Res. 1995;77:249–257. doi: 10.1161/01.res.77.2.249. [DOI] [PubMed] [Google Scholar]
- 28.Bogdarina I, et al. Epigenetic modification of the renin-angiotensin system in the fetal programming of hypertension. Circ. Res. 2007;100:520–526. doi: 10.1161/01.RES.0000258855.60637.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nabeshima Y, et al. Deletion of angiotensin II type I receptor reduces hepatic steatosis. J. Hepatol. 2009;50:1226–1235. doi: 10.1016/j.jhep.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 30.Mukoyama M, et al. Expression cloning of type 2 angiotensin II receptor reveals a unique class of seven-transmembrane receptors. J. Biol. Chem. 1993;268:24539–24542. [PubMed] [Google Scholar]
- 31.Koike G, et al. Cloning, characterization, and genetic mapping of the rat type 2 angiotensin II receptor gene. Hypertension. 1995;26:998–1002. doi: 10.1161/01.hyp.26.6.998. [DOI] [PubMed] [Google Scholar]
- 32.Martin MM, et al. Molecular cloning of the human angiotensin II type 2 receptor cDNA. Biochem. Biophys. Res. Commun. 1994;205:645–651. doi: 10.1006/bbrc.1994.2714. [DOI] [PubMed] [Google Scholar]
- 33.Griendling KK, et al. Angiotensin receptors and their therapeutic implications. Annu. Rev. Pharmacol. Toxicol. 1996;36:281–306. doi: 10.1146/annurev.pa.36.040196.001433. [DOI] [PubMed] [Google Scholar]
- 34.Kakar SS, et al. Angiotensin II type-1 receptor subtype cDNAs: differential tissue expression and hormonal regulation. Biochem. Biophys. Res. Commun. 1992;183:1090–1096. doi: 10.1016/s0006-291x(05)80302-x. [DOI] [PubMed] [Google Scholar]
- 35.Griendling KK, et al. Angiotensin II receptor pharmacology. Adv. Pharmacol. 1994;28:269–306. doi: 10.1016/s1054-3589(08)60498-6. [DOI] [PubMed] [Google Scholar]
- 36.Ohyama K, et al. Disulfide bridges in extracellular domains of angiotensin II receptor type IA. Regul. Pept. 1995;57:141–147. doi: 10.1016/0167-0115(95)00030-f. [DOI] [PubMed] [Google Scholar]
- 37.Desarnaud F, et al. Deglycosylation and fragmentation of purified rat liver angiotensin II receptor: application to the mapping of hormone-binding domains. Biochem. J. 1993;289:289–297. doi: 10.1042/bj2890289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sandberg K. Structural analysis and regulation of angiotensin II receptors. Trends Endocrinol. Metab. 1994;5:28–35. doi: 10.1016/1043-2760(94)90118-x. [DOI] [PubMed] [Google Scholar]
- 39.Bogdarina IG, et al. Characterization of the angiotensin (AT1b) receptor promoter and its regulation by glucocorticoids. J. Mol. Endocrinol. 2009;43:73–80. doi: 10.1677/JME-09-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uno S, et al. Glucocorticoid induction of rat angiotensin II type 1A receptor gene promoter. Biochem. Biophys. Res. Commun. 1994;204:210–215. doi: 10.1006/bbrc.1994.2446. [DOI] [PubMed] [Google Scholar]
- 41.Zucker IH, et al. Regulation of central angiotensin type 1 receptors and sympathetic outflow in heart failure. Am. J. Physiol. Heart Circ. Physiol. 2009;297:H1557–H1566. doi: 10.1152/ajpheart.00073.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao L, et al. Imbalance of angiotensin type 1 receptor and angiotensin II type 2 receptor in the rostral ventrolateral medulla: potential mechanism for sympathetic overactivity in heart failure. Hypertension. 2008;52:708–714. doi: 10.1161/HYPERTENSIONAHA.108.116228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen X, et al. Targeting deletion of angiotensin type 1B receptor gene in the mouse. Am. J. Physiol. 1997;272:F299–F304. doi: 10.1152/ajprenal.1997.272.3.F299. [DOI] [PubMed] [Google Scholar]
- 44.Davisson RL, et al. Divergent functions of angiotensin II receptor isoforms in the brain. J. Clin. Invest. 2000;106:103–106. doi: 10.1172/JCI10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gasc JM, et al. Tissue-specific expression of type 1 angiotensin II receptor subtypes. An in situ hybridization study. Hypertension. 1994;24:531–537. doi: 10.1161/01.hyp.24.5.531. [DOI] [PubMed] [Google Scholar]
- 46.Glossmann H, et al. Properties of angiotensin II receptors in the bovine and rat adrenal cortex. J. Biol. Chem. 1974;249:825–834. [PubMed] [Google Scholar]
- 47.Ushio-Fukai M, et al. Temporal dispersion of activation of phospholipase C-beta1 and -gamma isoforms by angiotensin II in vascular smooth muscle cells. Role of alphaq/11, alpha12, and beta gamma G protein subunits. J. Biol. Chem. 1998;273:19772–19777. doi: 10.1074/jbc.273.31.19772. [DOI] [PubMed] [Google Scholar]
- 48.Ushio-Fukai M, et al. Angiotensin II receptor coupling to phospholipase D is mediated by the betagamma subunits of heterotrimeric G proteins in vascular smooth muscle cells. Mol. Pharmacol. 1999;55:142–149. doi: 10.1124/mol.55.1.142. [DOI] [PubMed] [Google Scholar]
- 49.Hunyady L, Catt KJ. Pleiotropic AT1 receptor signaling pathways mediating physiological and pathogenic actions of angiotensin II. Mol. Endocrinol. 2006;20:953–970. doi: 10.1210/me.2004-0536. [DOI] [PubMed] [Google Scholar]
- 50.Hunyady L, et al. Identification of a cytoplasmic Ser-Thr-Leu motif that determines agonist-induced internalization of the AT1 angiotensin receptor. J. Biol. Chem. 1994;269:31378–31382. [PubMed] [Google Scholar]
- 51.Luttrell LM, Lefkowitz RJ. The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. J. Cell Sci. 2002;115:455–465. doi: 10.1242/jcs.115.3.455. [DOI] [PubMed] [Google Scholar]
- 52.Thomas WG, Qian H. Arresting angiotensin type 1 receptors. Trends Endocrinol. Metab. 2003;14:130–136. doi: 10.1016/s1043-2760(03)00023-7. [DOI] [PubMed] [Google Scholar]
- 53.Seta K, et al. AT1 receptor mutant lacking heterotrimeric G protein coupling activates the Src-Ras-ERK pathway without nuclear translocation of ERKs. J. Biol. Chem. 2002;277:9268–9277. doi: 10.1074/jbc.M109221200. [DOI] [PubMed] [Google Scholar]
- 54.Miura S, et al. Activation of extracellular signal-activated kinase by angiotensin II-induced Gq-independent epidermal growth factor receptor transactivation. Hypertens. Res. 2004;27:765–770. doi: 10.1291/hypres.27.765. [DOI] [PubMed] [Google Scholar]
- 55.Srivastava AK. High glucose-induced activation of protein kinase signaling pathways in vascular smooth muscle cells: a potential role in the pathogenesis of vascular dysfunction in diabetes. Int. J. Mol. Med. 2002;9:85–89. [PubMed] [Google Scholar]
- 56.Yasunari K, et al. Antioxidants improve impaired insulin-mediated glucose uptake and prevent migration and proliferation of cultured rabbit coronary smooth muscle cells induced by high glucose. Circulation. 1999;99:1370–1378. doi: 10.1161/01.cir.99.10.1370. [DOI] [PubMed] [Google Scholar]
- 57.Taniyama Y, Griendling KK. Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Hypertension. 2003;42:1075–1081. doi: 10.1161/01.HYP.0000100443.09293.4F. [DOI] [PubMed] [Google Scholar]
- 58.Touyz RM. Reactive oxygen species and angiotensin II signaling in vascular cells–implications in cardiovascular disease. Braz. J. Med. Biol. Res. 2004;37:1263–1273. doi: 10.1590/s0100-879x2004000800018. [DOI] [PubMed] [Google Scholar]
- 59.Seshiah PN, et al. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ. Res. 2002;91:406–413. doi: 10.1161/01.res.0000033523.08033.16. [DOI] [PubMed] [Google Scholar]
- 60.Rajagopalan S, et al. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J. Clin. Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Touyz RM, et al. p47phox associates with the cytoskeleton through cortactin in human vascular smooth muscle cells: role in NAD(P)H oxidase regulation by angiotensin II. Arterioscler. Thromb. Vasc. Biol. 2005;25:512–518. doi: 10.1161/01.ATV.0000154141.66879.98. [DOI] [PubMed] [Google Scholar]
- 62.Papaiahgari S, et al. Hyperoxia stimulates an Nrf2-ARE transcriptional response via ROS-EGFR-PI3K-Akt/ERK MAP kinase signaling in pulmonary epithelial cells. Antioxid. Redox Signal. 2006;8:43–52. doi: 10.1089/ars.2006.8.43. [DOI] [PubMed] [Google Scholar]
- 63.Wu S, et al. Activation of AP-1 through reactive oxygen species by angiotensin II in rat cardiomyocytes. Free Radic. Biol. Med. 2005;39:1601–1610. doi: 10.1016/j.freeradbiomed.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 64.Gryglewski RJ, et al. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986;320:454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- 65.Touyz RM. Intracellular mechanisms involved in vascular remodelling of resistance arteries in hypertension: role of angiotensin II. Exp. Physiol. 2005;90:449–455. doi: 10.1113/expphysiol.2005.030080. [DOI] [PubMed] [Google Scholar]
- 66.Schulz E, et al. Nitric oxide, tetrahydrobiopterin, oxidative stress, and endothelial dysfunction in hypertension. Antioxid. Redox Signal. 2008;10:1115–1126. doi: 10.1089/ars.2007.1989. [DOI] [PubMed] [Google Scholar]
- 67.Tang EH, Vanhoutte PM. Endothelial dysfunction: a strategic target in the treatment of hypertension? Pflugers Arch. 2010;459:995–1004. doi: 10.1007/s00424-010-0786-4. [DOI] [PubMed] [Google Scholar]
- 68.Yu MA, et al. Oxidative stress with an activation of the renin-angiotensin system in human vascular endothelial cells as a novel mechanism of uric acid-induced endothelial dysfunction. J. Hypertens. 2010;28:1234–1242. [PubMed] [Google Scholar]
- 69.Kurtz TW, Pravenec M. Antidiabetic mechanisms of angiotensin-converting enzyme inhibitors and angiotensin II receptor antagonists: beyond the renin-angiotensin system. J. Hypertens. 2004;22:2253–2261. doi: 10.1097/00004872-200412000-00003. [DOI] [PubMed] [Google Scholar]
- 70.Velloso LA, et al. The multi-faceted cross-talk between the insulin and angiotensin II signaling systems. Diabetes Metab. Res. Rev. 2006;22:98–107. doi: 10.1002/dmrr.611. [DOI] [PubMed] [Google Scholar]
- 71.Patiag D, et al. Possible interactions between angiotensin II and insulin: effects on glucose and lipid metabolism in vivo and in vitro. J. Endocrinol. 2000;167:525–531. doi: 10.1677/joe.0.1670525. [DOI] [PubMed] [Google Scholar]
- 72.Ogihara T, et al. Angiotensin II-induced insulin resistance is associated with enhanced insulin signaling. Hypertension. 2002;40:872–879. doi: 10.1161/01.hyp.0000040262.48405.a8. [DOI] [PubMed] [Google Scholar]
- 73.Velloso LA, et al. Cross-talk between the insulin and angiotensin signaling systems. Proc. Natl. Acad. Sci. U. S. A. 1996;93:12490–12495. doi: 10.1073/pnas.93.22.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Folli F, et al. Angiotensin II inhibits insulin signaling in aortic smooth muscle cells at multiple levels. A potential role for serine phosphorylation in insulin/angiotensin II crosstalk. J. Clin. Invest. 1997;100:2158–2169. doi: 10.1172/JCI119752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Taniyama Y, et al. Mechanisms of reactive oxygen species-dependent downregulation of insulin receptor substrate-1 by angiotensin II. Arterioscler. Thromb. Vasc. Biol. 2005;25:1142–1147. doi: 10.1161/01.ATV.0000164313.17167.df. [DOI] [PubMed] [Google Scholar]
- 76.Campbell SE, Katwa LC. Angiotensin II stimulated expression of transforming growth factor-beta1 in cardiac fibroblasts and myofibroblasts. J. Mol. Cell. Cardiol. 1997;29:1947–1958. doi: 10.1006/jmcc.1997.0435. [DOI] [PubMed] [Google Scholar]
- 77.Kupfahl C, et al. Angiotensin II directly increases transforming growth factor beta1 and osteopontin and indirectly affects collagen mRNA expression in the human heart. Cardiovasc. Res. 2000;46:463–475. doi: 10.1016/s0008-6363(00)00037-7. [DOI] [PubMed] [Google Scholar]
- 78.Schultz Jel J, et al. TGF-beta1 mediates the hypertrophic cardiomyocyte growth induced by angiotensin II. J. Clin. Invest. 2002;109:787–796. doi: 10.1172/JCI14190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rosenkranz S. TGF-beta1 and angiotensin networking in cardiac remodeling. Cardiovasc. Res. 2004;63:423–432. doi: 10.1016/j.cardiores.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 80.Lim H, Zhu YZ. Role of transforming growth factor-beta in the progression of heart failure. Cell. Mol. Life Sci. 2006;63:2584–2596. doi: 10.1007/s00018-006-6085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Perbal B. CCN proteins: multifunctional signalling regulators. Lancet. 2004;363:62–64. doi: 10.1016/S0140-6736(03)15172-0. [DOI] [PubMed] [Google Scholar]
- 82.Abreu JG, et al. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-beta. Nat. Cell Biol. 2002;4:599–604. doi: 10.1038/ncb826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gabrielsen A, et al. Gene expression signals involved in ischemic injury, extracellular matrix composition and fibrosis defined by global mRNA profiling of the human left ventricular myocardium. J. Mol. Cell. Cardiol. 2007;42:870–883. doi: 10.1016/j.yjmcc.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 84.Kambayashi Y, et al. Molecular cloning of a novel angiotensin II receptor isoform involved in phosphotyrosine phosphatase inhibition. J. Biol. Chem. 1993;268:24543–24546. [PubMed] [Google Scholar]
- 85.Nakajima M, et al. Cloning of cDNA and analysis of the gene for mouse angiotensin II type 2 receptor. Biochem. Biophys. Res. Commun. 1993;197:393–399. doi: 10.1006/bbrc.1993.2492. [DOI] [PubMed] [Google Scholar]
- 86.Ichiki T, et al. Transcription of the rat angiotensin II type 2 receptor gene. Biochem. Biophys. Res. Commun. 1996;222:566–571. doi: 10.1006/bbrc.1996.0784. [DOI] [PubMed] [Google Scholar]
- 87.Kijima K, et al. Gene transcription of angiotensin II type 2 receptor is repressed by growth factors and glucocorticoids in PC12 cells. Biochem. Biophys. Res. Commun. 1995;216:359–366. doi: 10.1006/bbrc.1995.2632. [DOI] [PubMed] [Google Scholar]
- 88.Matsubara H. Pathophysiological role of angiotensin II type 2 receptor in cardiovascular and renal diseases. Circ. Res. 1998;83:1182–1191. doi: 10.1161/01.res.83.12.1182. [DOI] [PubMed] [Google Scholar]
- 89.Shanmugam S, et al. Expression of angiotensin II AT2 receptor mRNA during development of rat kidney and adrenal gland. Am. J. Physiol. 1995;268:F922–F930. doi: 10.1152/ajprenal.1995.268.5.F922. [DOI] [PubMed] [Google Scholar]
- 90.Shanmugam S, Sandberg K. Ontogeny of angiotensin II receptors. Cell Biol. Int. 1996;20:169–176. doi: 10.1006/cbir.1996.0021. [DOI] [PubMed] [Google Scholar]
- 91.Tsutsumi Y, et al. Angiotensin II type 2 receptor is upregulated in human heart with interstitial fibrosis, and cardiac fibroblasts are the major cell type for its expression. Circ. Res. 1998;83:1035–1046. doi: 10.1161/01.res.83.10.1035. [DOI] [PubMed] [Google Scholar]
- 92.Yu L, et al. Developmental changes in AT1 and AT2 receptor-protein expression in rats. J. Renin Angiotensin Aldosterone Syst. doi: 10.1177/1470320310379065. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Peng JF, Phillips MI. Opposite regulation of brain angiotensin type 1 and type 2 receptors in cold-induced hypertension. Regul. Pept. 2001;97:91–102. doi: 10.1016/s0167-0115(00)00218-4. [DOI] [PubMed] [Google Scholar]
- 94.Elbaz N, et al. Functional trans-inactivation of insulin receptor kinase by growth-inhibitory angiotensin II AT2 receptor. Mol. Endocrinol. 2000;14:795–804. doi: 10.1210/mend.14.6.0488. [DOI] [PubMed] [Google Scholar]
- 95.D'Amore A, et al. The angiotensin II type 2 receptor causes constitutive growth of cardiomyocytes and does not antagonize angiotensin II type 1 receptor-mediated hypertrophy. Hypertension. 2005;46:1347–1354. doi: 10.1161/01.HYP.0000193504.51489.cf. [DOI] [PubMed] [Google Scholar]
- 96.Senbonmatsu T, et al. A novel angiotensin II type 2 receptor signaling pathway: possible role in cardiac hypertrophy. EMBO J. 2003;22:6471–6482. doi: 10.1093/emboj/cdg637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lemarie CA, Schiffrin EL. The angiotensin II type 2 receptor in cardiovascular disease. J. Renin Angiotensin Aldosterone Syst. 2010;11:19–31. doi: 10.1177/1470320309347785. [DOI] [PubMed] [Google Scholar]
- 98.Tsutsumi Y, et al. Angiotensin II type 2 receptor overexpression activates the vascular kinin system and causes vasodilation. J. Clin. Invest. 1999;104:925–935. doi: 10.1172/JCI7886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Katada J, Majima M. AT(2) receptor-dependent vasodilation is mediated by activation of vascular kinin generation under flow conditions. Br. J. Pharmacol. 2002;136:484–491. doi: 10.1038/sj.bjp.0704731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bergaya S, et al. Flow-dependent dilation mediated by endogenous kinins requires angiotensin AT2 receptors. Circ. Res. 2004;94:1623–1629. doi: 10.1161/01.RES.0000131497.73744.1a. [DOI] [PubMed] [Google Scholar]
- 101.Wiemer G, et al. The possible role of angiotensin II subtype AT2 receptors in endothelial cells and isolated ischemic rat hearts. J. Hypertens. Suppl. 1993;11:S234–S235. [PubMed] [Google Scholar]
- 102.Abadir PM, et al. Angiotensin II type 2 receptor-bradykinin B2 receptor functional heterodimerization. Hypertension. 2006;48:316–322. doi: 10.1161/01.HYP.0000228997.88162.a8. [DOI] [PubMed] [Google Scholar]
- 103.Adachi Y, et al. Angiotensin II type 2 receptor deficiency exacerbates heart failure and reduces survival after acute myocardial infarction in mice. Circulation. 2003;107:2406–2408. doi: 10.1161/01.CIR.0000072763.98069.B4. [DOI] [PubMed] [Google Scholar]
- 104.Suzuki J, et al. Role of angiotensin II-regulated apoptosis through distinct AT1 and AT2 receptors in neointimal formation. Circulation. 2002;106:847–853. doi: 10.1161/01.cir.0000024103.04821.86. [DOI] [PubMed] [Google Scholar]
- 105.Peters S, et al. Valsartan for prevention of restenosis after stenting of type B2/C lesions: the VAL-PREST trial. J. Invasive Cardiol. 2001;13:93–97. [PubMed] [Google Scholar]
- 106.Wu L, et al. Effect of angiotensin II type 1 receptor blockade on cardiac remodeling in angiotensin II type 2 receptor null mice. Arterioscler. Thromb. Vasc. Biol. 2002;22:49–54. doi: 10.1161/hq0102.102277. [DOI] [PubMed] [Google Scholar]
- 107.Yan X, et al. Ventricular-specific expression of angiotensin II type 2 receptors causes dilated cardiomyopathy and heart failure in transgenic mice. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H2179–H2187. doi: 10.1152/ajpheart.00361.2003. [DOI] [PubMed] [Google Scholar]
- 108.Ford WR, et al. Opposite effects of angiotensin AT1 and AT2 receptor antagonists on recovery of mechanical function after ischemia-reperfusion in isolated working rat hearts. Circulation. 1996;94:3087–3089. doi: 10.1161/01.cir.94.12.3087. [DOI] [PubMed] [Google Scholar]
- 109.Xu Y, et al. AT(1) and AT(2) receptor expression and blockade after acute ischemia-reperfusion in isolated working rat hearts. Am. J. Physiol. Heart Circ. Physiol. 2002;282:H1206–H1215. doi: 10.1152/ajpheart.00839.2000. [DOI] [PubMed] [Google Scholar]
- 110.Ping P, et al. Isoform-selective activation of protein kinase C by nitric oxide in the heart of conscious rabbits: a signaling mechanism for both nitric oxide-induced and ischemia-induced preconditioning. Circ. Res. 1999;84:587–604. doi: 10.1161/01.res.84.5.587. [DOI] [PubMed] [Google Scholar]
- 111.Masaki H, et al. Cardiac-specific overexpression of angiotensin II AT2 receptor causes attenuated response to AT1 receptor-mediated pressor and chronotropic effects. J. Clin. Invest. 1998;101:527–535. doi: 10.1172/JCI1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Forsdahl A. Tidsskr. Nor. Laegeforen. 1973;93:661–667. [PubMed] [Google Scholar]
- 113.Barker DJ, et al. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2:577–580. doi: 10.1016/s0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- 114.de Boo HA, Harding JE. The developmental origins of adult disease (Barker) hypothesis. Aust. N. Z. J. Obstet. Gynaecol. 2006;46:4–14. doi: 10.1111/j.1479-828X.2006.00506.x. [DOI] [PubMed] [Google Scholar]
- 115.Langley-Evans SC. Fetal programming of cardiovascular function through exposure to maternal undernutrition. Proc. Nutr. Soc. 2001;60:505–513. doi: 10.1079/pns2001111. [DOI] [PubMed] [Google Scholar]
- 116.McMullen S, Langley-Evans SC. Sex-specific effects of prenatal low-protein and carbenoxolone exposure on renal angiotensin receptor expression in rats. Hypertension. 2005;46:1374–1380. doi: 10.1161/01.HYP.0000188702.96256.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McMullen S, Langley-Evans SC. Maternal low-protein diet in rat pregnancy programs blood pressure through sex-specific mechanisms. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;288:R85–R90. doi: 10.1152/ajpregu.00435.2004. [DOI] [PubMed] [Google Scholar]
- 118.Xue Q, et al. Fetal hypoxia increases cardiac AT2R expression and subsequent vulnerability to adult ischemic injury. Cardiovasc. Res. doi: 10.1093/cvr/cvq303. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Goyal R, et al. Maternal protein deprivation: changes in systemic renin-angiotensin system of the mouse fetus. Reprod. Sci. 2009;16:894–904. doi: 10.1177/1933719109337260. [DOI] [PubMed] [Google Scholar]
- 120.Huang Y, et al. Hypoxia down-regulates glucocorticoid receptor alpha and attenuates the anti-inflammatory actions of dexamethasone in human alveolar epithelial A549 cells. Life Sci. 2009;85:107–112. doi: 10.1016/j.lfs.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 121.Ojeda NB, et al. Intrauterine growth restriction: fetal programming of hypertension and kidney disease. Adv. Chronic Kidney Dis. 2008;15:101–106. doi: 10.1053/j.ackd.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Langley-Evans SC. Metabolic programming in pregnancy: studies in animal models. Genes Nutr. 2007;2:33–38. doi: 10.1007/s12263-007-0005-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Drake AJ, et al. Intergenerational consequences of fetal programming by in utero exposure to glucocorticoids in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;288:R34–R38. doi: 10.1152/ajpregu.00106.2004. [DOI] [PubMed] [Google Scholar]
- 124.Newell-Price J, et al. DNA methylation and silencing of gene expression. Trends Endocrinol. Metab. 2000;11:142–148. doi: 10.1016/s1043-2760(00)00248-4. [DOI] [PubMed] [Google Scholar]
- 125.Lillycrop KA, et al. Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J. Nutr. 2005;135:1382–1386. doi: 10.1093/jn/135.6.1382. [DOI] [PubMed] [Google Scholar]
- 126.Jackson AA, et al. Increased systolic blood pressure in rats induced by a maternal low-protein diet is reversed by dietary supplementation with glycine. Clin. Sci. (Lond.) 2002;103:633–639. doi: 10.1042/cs1030633. [DOI] [PubMed] [Google Scholar]
- 127.Bonnardeaux A, et al. Angiotensin II type 1 receptor gene polymorphisms in human essential hypertension. Hypertension. 1994;24:63–69. doi: 10.1161/01.hyp.24.1.63. [DOI] [PubMed] [Google Scholar]
- 128.van Geel PP, et al. Angiotensin II type 1 receptor A1166C gene polymorphism is associated with an increased response to angiotensin II in human arteries. Hypertension. 2000;35:717–721. doi: 10.1161/01.hyp.35.3.717. [DOI] [PubMed] [Google Scholar]
- 129.Jones A, et al. Genetic variants of angiotensin II receptors and cardiovascular risk in hypertension. Hypertension. 2003;42:500–506. doi: 10.1161/01.HYP.0000088853.27673.D0. [DOI] [PubMed] [Google Scholar]
- 130.van Geel PP, et al. Increased risk for ischaemic events is related to combined RAS polymorphism. Heart. 2001;85:458–462. doi: 10.1136/heart.85.4.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wierzbicki AS, et al. Renin-angiotensin system polymorphisms and coronary events in familial hypercholesterolemia. Hypertension. 2000;36:808–812. doi: 10.1161/01.hyp.36.5.808. [DOI] [PubMed] [Google Scholar]
- 132.Schmieder RE, et al. Renin-angiotensin system and cardiovascular risk. Lancet. 2007;369:1208–1219. doi: 10.1016/S0140-6736(07)60242-6. [DOI] [PubMed] [Google Scholar]
- 133.Weir MR. Effects of renin-angiotensin system inhibition on end-organ protection: can we do better? Clin. Ther. 2007;29:1803–1824. doi: 10.1016/j.clinthera.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 134.Simoes e Silva AC, et al. The therapeutic potential of Angiotensin-(1–7) as a novel renin-angiotensin system mediator. Mini Rev. Med. Chem. 2006;6:603–609. doi: 10.2174/138955706776876203. [DOI] [PubMed] [Google Scholar]
- 135.Santos RA, et al. Expression of an angiotensin-(1–7)-producing fusion protein produces cardioprotective effects in rats. Physiol. Genomics. 2004;17:292–299. doi: 10.1152/physiolgenomics.00227.2003. [DOI] [PubMed] [Google Scholar]
- 136.Ferrario CM, Varagic J. The ANG-(1–7)/ACE2/mas axis in the regulation of nephron function. Am. J. Physiol. Renal Physiol. 2010;298:F1297–F1305. doi: 10.1152/ajprenal.00110.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Arnold AC, et al. Angiotensin-(1–12) requires angiotensin converting enzyme and AT1 receptors for cardiovascular actions within the solitary tract nucleus. Am. J. Physiol. Heart Circ. Physiol. 2010;299:H763–H771. doi: 10.1152/ajpheart.00345.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.de Koning HP, et al. The trypanocide diminazene aceturate is accumulated predominantly through the TbAT1 purine transporter: additional insights on diamidine resistance in African trypanosomes. Antimicrob. Agents Chemother. 2004;48:1515–1519. doi: 10.1128/AAC.48.5.1515-1519.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li YC, et al. Vitamin D: a negative endocrine regulator of the renin-angiotensin system and blood pressure. J. Steroid Biochem. Mol. Biol. 2004;89–90:387–392. doi: 10.1016/j.jsbmb.2004.03.004. [DOI] [PubMed] [Google Scholar]