Abstract
The human fungal pathogen Candida glabrata is related to Saccharomyces cerevisiae but has developed high resistance against reactive oxygen species. We find that induction of conserved genes encoding antioxidant functions is dependent on the transcription factors CgYap1 and CgSkn7 which cooperate for promoter recognition. Superoxide stress resistance of C. glabrata is provided by superoxide dismutase CgSod1, which is not dependent on CgYap1/Skn7. Only double mutants lacking both CgSod1 and CgYap1 were efficiently killed by primary mouse macrophages. Our results suggest that in C. glabrata the regulation of key genes providing stress protection is adopted to meet a host–pathogen situation.
Keywords: Oxidative stress, Gene regulation, Promoter recognition, Transcript profiling, Human fungal pathogen, Yap1
1. Introduction
The human fungal pathogen Candida glabrata is a common commensal in gastrointestinal and genitourinary tracts, but can turn into an opportunistic fungal pathogen in immunocompromised patients and elderly people [1–4]. C. glabrata lives mostly on mucosal surfaces and does not penetrate tissue efficiently. It is much more related to Saccharomyces cerevisiae than to Candida albicans [5,6]. C. glabrata is obviously adapted to a mammalian environment. In contrast to S. cerevisiae, its optimal growth temperature is near the human body temperature and a number of adhesins allow C. glabrata to avidly adhere to various surfaces and to mammalian cells. These cells have adapted to withstand host defense and the competing microbes on mucosal surfaces. Here we investigate the regulatory basis for the oxidative stress resistance of C. glabrata.
In the mammalian host, cell-mediated immunity, based on phagocytic cells is crucial to counteract fungal infections [7]. In the phagolysosome of phagocytic cells, one of the key defense mechanisms is destruction of the engulfed microorganisms by reactive oxygen species produced by the NADPH oxidase complex [8,9]. The complex catalyzes the production of superoxide anions , serving as initial source for reactive oxygen species (ROS). For successful dissemination, pathogenic fungi have to counteract a broad spectrum of reactive oxidants during the oxidative burst. Therefore, commensal and pathogenic microbial fungal organisms carry a number of antioxidant systems (reviewed in [8,10]), such as catalases, superoxide dismutases, thioredoxins and glutathione-dependent peroxidases and reductases. Several of these enzymes are highly relevant for fungal pathogen virulence. The loss of thioredoxin proteins and superoxide dismutases decreased virulence of Cryptococcus neoformans in mice [11,12]. Unique cell surface superoxide dismutases CaSod4 and CaSod5 are essential for survival of C. albicans in macrophages [13].
Oxidative stress causes rapid changes of transcription of many genes. In S. cerevisiae, the induction of the oxidative stress regulon is largely under control of the conserved transcription factors Yap1 and Skn7 [14–17]. In contrast, the transcription factor Sfp1 and the TORC1 complex are repressed by superoxide anions leading to down-regulation of genes encoding components required for protein biosynthesis [18,19]. Activation of Yap1 involves the inhibition of a nuclear export signal (NES). Cysteine residues become oxidized by the thiol peroxidase Hyr1/Gpx3 which leads to the formation of disulfide bonds, causing Yap1 to accumulate in the nucleus and activate its target genes [20–22]. The role of Yap1 in other stress responses has been reported in several other fungi, such as Kluyveromyces lactis, Ustilago maydis, C. albicans and Aspergillus fumigatus [23–25]. In S. cerevisiae, the presence of both Yap1 and Skn7 is necessary for efficient induction of many oxidative stress response genes [15,26,27]. Skn7 is a nuclear response regulator and part of a two-component system. Mutations in the receiver domain of Skn7 reduce its oxidative stress induced phosphorylation and the in vitro formation of a ternary complex comprising promoter sequences and Yap1 [27]. However, the exact interaction mechanism between Yap1 and Sn7 is not fully understood.
C. glabrata contains genes encoding putative orthologues of Yap1 and Skn7 [28]. CgYap1 has undergone a recent mutation causing a shift of its preferred recognition site [29]. Similar to other fungi, CgYap1 and CgSkn7 are required for full resistance to hydrogen peroxide stress [28,30,39]. The role of Yap1 and Skn7 for virulence in pathogenic fungi varies between species. In C. albicans, the lack of CaSkn7 caused a slight attenuation of virulence [31]. In the fungal plant pathogen U. maydis, loss of the Yap1 orthologue led to reduced virulence [24]. In A. fumigatus, mutant strains lacking AfYap1 or AfSkn7 retained virulence in a mouse infection model [32,33]. The importance of Yap1 and Skn7 for virulence may depend on particular host niche exploited by the pathogen. The high conservation of these factors in fungi underlines their important role.
Here we addressed the function and regulatory interaction of CgYap1 and CgSkn7 for the regulation of the C. glabrata oxidative stress response. We show interdependence of CgYap1 and CgSkn7 for a set of genes which is caused by cooperative promoter recognition. We show that efficient cross-protection between carbon source starvation and oxidative stress is accompanied by parallel regulation of key protective genes. In contrast to S. cerevisiae, the copper–zinc superoxide dismutase CgSOD1 is not under the control of CgYap1. This suggests why, the Cgyap1ΔCgsod1Δ double mutant but not the single mutants had a diminished survival rate during macrophage infection. Thus we propose that C. glabrata has adapted transcription of oxidative stress protective genes to meet a host–pathogen situation.
2. Materials and methods
2.1. Yeast strains and plasmids
Strains and plasmids are listed in Tables 1 and 2. Oligonucleotides used are listed in Table S1. Additional information is available as Supplementary data. C. glabrata strains ARCgskn7Δ, ARCgyap1Δ, ARCgsod1Δ, ARCgyap1Δskn7Δ, and ARCgyap1Δsod1Δ were obtained by replacing the ORFs in strain Δhtu [34] with the S. cerevisiae URA3 or HIS3 genes using fusion PCR [35] from the plasmids pRS316 and pRS313 [36] with the oligonucleotides SKN7-1 to 6, YAP1-1 to 6, and SOD1-1 to 6 and tested by southern analysis (Fig. S1). Gradient plates were prepared as described [37]. All PCR fragments were sequenced. Cells were grown for four generations in YPD at 30 °C to OD600 of 1 before menadione or H2O2 was added for 20 min. The Microarray dataset has been deposited at array express (http://www.ebi.ac.uk/arrayexpress/ E-MEXP-2915). GFP was visualized in live cells without fixation as described [38].
Table 1.
Yeast strains used in this study.
| Genotype | Source | |
|---|---|---|
| C. glabratastrain | ||
| ΔHTU | his3Δ trp1Δ ura3Δ | [33] |
| ΔHT6 | his3Δ trp1Δ | [33] |
| ARCg skn7Δ | his3Δ trp1Δ ura3Δ skn7Δ::ScHIS3 | This study |
| ARCg yap1Δ | his3Δ trp1Δ ura3Δ yap1Δ::ScURA3 | This study |
| ARCg skn7Δyap1Δ | his3Δ trp1Δ ura3Δ skn7Δ::ScHIS3 yap1Δ::ScURA3 | This study |
| ARCg sod1Δ | his3Δ trp1Δ ura3Δ sod1Δ::ScHIS3 | This study |
| ARCg sod1Δyap1Δ | his3Δ trp1Δ ura3Δ sod1Δ::ScHIS3 yap1Δ::ScURA3 | This study |
| S. cerevisiaestrain | ||
| BY4741 | MATa; his3Δ1; leu2Δ0; met15Δ0; ura3Δ0 | Euroscarf |
| BY4741 sod1Δ | MATa; his3Δ1; leu2Δ0; met15Δ0; ura3Δ0 YJR104c::kanMX4 | Euroscarf |
| BY4741 yap1Δ | MATa; his3Δ1; leu2Δ0; met15Δ0; ura3Δ0 YML007w::kanMX4 | Euroscarf |
Table 2.
Plasmids used in this study.
| Plamid | Genotype | Source |
|---|---|---|
| pRS316 | CEN6, ARSH4, ScURA3 | [35] |
| pRS313 | CEN6, ARSH4, ScHIS3 | [35] |
| pGEM-ACT | ARS, CEN and TRP1 marker from C. glabrata | [40] |
| pCgADH1-CgMSN2-CFP | CgADH1-CgMSN2-CFP (SphI/SacII and SacII/NsiI); CgTRP1 | [40] |
| pCgADH1-CgSKN7-CFP | CgADH1-CgSKN7-CFP (SacII and NcoI); CgTRP1 | This study |
| pCgSCgSKN7 | CgSKN7-CgSKN7 (native promoter SphI and SacII); CgTRP1 | This study |
| pGEM-ACT-CgYAP1 | Native promoter inserted via SphI/NotI; CgTRP1 | This study |
| pCgYCgYAP1 | CgYAP1-CgYAP1 (NotI and NsiI); CgTRP1 | This study |
| pCgADH1-GFP-CgYAP1 | CgADH1-GFP-CgYAP1 (NotII/NsiI); GFP inserted via NotI/NotI; CgTRP1 | [40] |
| pCgSKN7-HA | CgSKN7-CgSKN7-HA (HA tag inserted with NcoI); CgTRP1 | This study |
| pHA-CgYAP1 | CgYAP1-HA-CgYAP1 (HA tag inserted with NotI); CgTRP1 | This study |
| pCgYAP1-CgSKN7 | CgSKN7-CgSKN7 inserted with NsiI into pCgYAP1CgYAP1; CgTRP1 marker | This study |
2.2. Macrophage cell culture
Primary bone marrow derived macrophages (BMDMs) were obtained from the femur bone marrow of 6–10 weeks old C57Bl/6 mice. Cells were cultivated in DMEM supplemented with 10% FCS in the presence of L cell-derived CSF-1 as described C. glabrata macrophage infection assays were done as described previously [38]. For infection assays, BMDMs were seeded at 5 × 105 cells/dish in 3.5-cm dishes containing medium without antibiotics.
2.3. Chromatin immunoprecipitation assay
Primer pairs: TRR2 (−639/−511) for CgTRR2 (CAGL0I01166g) and GPX2 (−787/−617) for CgGPX2 (CAGL0C01705g). A centromeric region of Chromosome B was used as a negative control.
3. Results
3.1. C. glabrata reacts differently to various oxidative stress causing agents
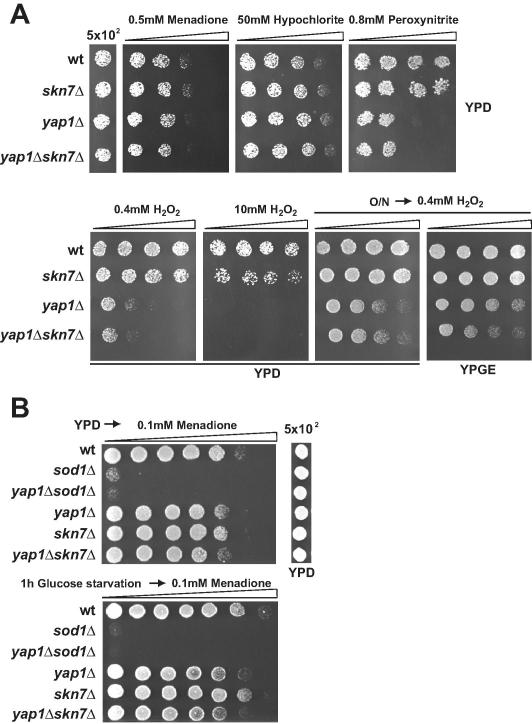
To explore the high oxidative stress resistance of C. glabrata [16], we investigated the role of the transcription factors CgYap1 and CgSkn7. We generated strains lacking either CgYap1 (CAGL0H04631g), CgSkn7 (CAGL0F09097g). To test the susceptibility spectrum of the mutants, we used plates with gradients of hypochlorite, peroxynitrite, hydrogen peroxide, and the superoxide generating compound menadione (Fig. 1A). On plates containing menadione, we observed a small growth difference. In contrast, growth of Cgyap1Δ and Cgyap1Δskn7Δ mutants was severely diminished in the presence of peroxynitrite. Both Cgyap1Δ and Cgyap1Δskn7Δ mutants had reduced survival on plates containing hydrogen peroxide (Fig. 1A, lower left panel). The Cgskn7Δ mutant was sensitive only to high level of hydrogen peroxide consistent with an earlier report [39]. Similar to S. cerevisiae [40], we observed that C. glabrata and Cgyap1Δskn7Δ mutant cells from over-night grown cultures which had switched to fermentative metabolism were highly resistant to hydrogen peroxide (Fig. 1A, lower panel). This is consistent with an earlier report on high level resistance of stationary phase C. glabrata cells [28]. Cells retained this high resistance also in the presence of glucose (Fig. 1A, lower right panel). The Cgsod1Δ mutant lacking the cytosolic copper–zinc superoxide dismutase CgSOD1 (CAGL0C04741g) was highly sensitive to superoxide and additional deletion of CgYAP1 did not enhance its sensitivity. Carbon source starvation slightly elevated the resistance against menadione-caused oxidative stress (Fig. 1B).
Fig. 1.
C. glabrata survival during different chronic oxidative stress types. (A) C. glabrata resistance against oxidative stress causing agents. C. glabrata wild type, Cgsod1Δ, Cgyap1Δ, Cgyap1Δsod1Δ, Cgskn7Δ, and Cgyap1Δskn7Δ mutant cells were grown to an OD600 of 1 in YPD. 5 × 102 cells were dropped on gradient plates containing menadione, hypochlorite, peroxynitrite and hydrogen peroxide. In addition 5 × 102 cells from stationary over-night cultures were dropped on plates containing hydrogen peroxide (lower right panels).
3.2. The core oxidative stress response of C. glabrata is similar to S. cerevisiae
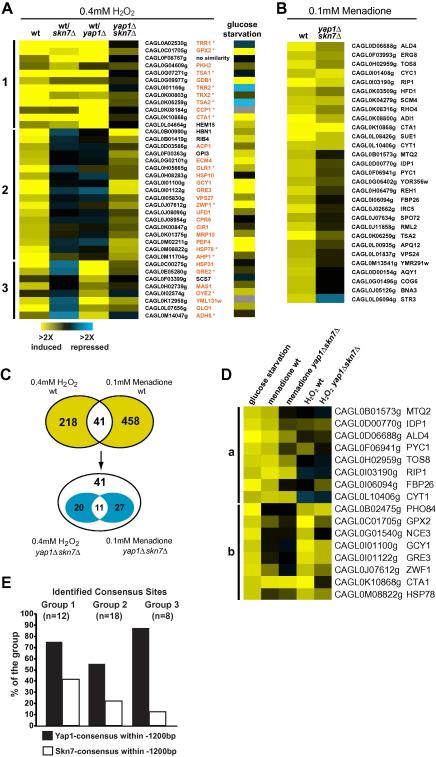
To define the oxidative stress regulon of C. glabrata, the transcriptional response to 0.4 mM hydrogen peroxide was determined by microarray analysis (Fig. 2A). cDNAs from stressed Cgyap1Δ and Cgskn7Δ mutants were co-hybridized with the stressed wild type. Genes dependent on CgYap1, CgSkn7 or both were classified into three groups. Group 1 comprised genes dependent on both CgSkn7 and CgYap1 and included many generic oxidative stress response genes similarly regulated in S. cerevisiae (CgTRR1/2, CgTRX2, CgTSA1/2, CgGPX2, and CgCTA1) [14,41]. Based on this congruence, we designated this group of genes as “core oxidative stress response” (COR; Table 3a). Furthermore, a group of genes was largely dependent on CgYap1 (Fig. 2A, Group 3). We identified enrichment of Yap1 and Skn7 consensus sites in the promoters of the C. glabrata induced genes of the different groups (Fig. 2E). A group of 18 induced genes was not dependent on either CgYap1 or CgSkn7 (Fig. 2A, Group 2). Group 2 contained genes associated to mitochondrial processes (CgACP1, CgOPI3, CgHSP10 and CgMRP10). Transcription of these genes might be redundantly regulated by CgYap1 or CgSkn7. However, most genes of Group 2 were highly induced in the Cgyap1Δskn7Δ double mutant (Fig. 2A), suggesting the involvement of additional oxidative stress responsive factors. We found an overlap of 26 genes induced by oxidative stress to glucose starvation [42] (Fig. 2A, right panel). The majority of these belonged to Group 2, but also included core oxidative stress genes CgCTA1, CgGPX2 and CgTRX2.
Fig. 2.
Comparison of genome-wide expression levels in response to oxidative stress. (A) Transcript sets represent average inductions and comparison of wild type strain versus Cgyap1Δ and Cgskn7Δ mutant strains, and the Cgyap1Δskn7Δ double mutant strain. All treatments were done at 30 °C for 20 min. Genes were clustered after selection (at least once >3-fold induction). Group 1: dependent on CgSkn7 and CgYap1; Group 2: independent of CgSkn7 or CgYap1; Group 3: dependent on CgYap1. Genes found to be upregulated in S. cerevisiae upon oxidative stress are highlighted in red, asterisk indicates those dependent on Skn7/Yap1 or Yap1 alone in S. cerevisiae. Profile of C. glabrata wild type during glucose depletion is included. (B) Genes induced (>4-fold) in menadione-associated oxidative stress response. (C) The overlap between the hydrogen peroxide and menadione stress patterns. Genes were selected with an induction >2-fold. Among 41 overlapping genes, Cgyap1 and Cgskn7Δ dependent genes (>2-fold) were determined. (D) Identification of genes upregulated upon glucose starvation and either menadione stress (Group a) or hydrogen peroxide stress (Group b) (>4-fold induction). Gene names correspond to C. glabrata systematic ORF designations and S. cerevisiae orthologues. (E) Number of Skn7 and Yap1 consensus sites present within −1200 bp upstream regions of C. glabrata genes of Groups 1, 2 and 3.
Table 3a.
Genes of the core response to H2O2-associated oxidative stress (COR).
| Gene name | Systematic name | Function | wta | ΔyΔsa |
|---|---|---|---|---|
| CgTRR1 | CAGL0A02530g | Thioredoxin reductase | 22.8 | 1.1 |
| CgTRR2 | CAGL0I01166g | Thioredoxin reductase | 30.2 | 0.0 |
| CgTRX2 | CAGL0K00803g | Thioredoxin | 16.6 | 1.7 |
| CgTSA1 | CAGL0G07271g | Thioredoxin peroxidase | 11.4 | 0.0 |
| CgTSA2 | CAGL0K06259g | Thioredoxin peroxidase | 22.3 | 1.6 |
| CgGPX2 | CAGL0C01705g | Glutathione peroxidase | 22.4 | 2.1 |
| CgCTA1 | CAGL0K10868g | Catalase | 17.2 | 1.3 |
Fold induction.
The sensitivity tests suggested differences between the response to peroxide and superoxide stress. Therefore, we determined the transcriptional profile to 0.1 mM menadione (Fig. 2B). In wild type cells we observed 29 genes up-regulated more than 4-fold upon menadione stress. The comparison of at least 2-fold induced genes after hydrogen peroxide and superoxide stress revealed an overlap of 41 genes (Fig. 2C). The expression level of only 11 genes was dependent on CgYap1/CgSkn7 during both stresses (Table 3b). We compared genes upregulated by starvation, hydrogen peroxide or menadione stress and found two rather independent groups (Fig. 2D, Groups a and b). These data suggested a separation of specific oxidative stress regulons in C. glabrata.
Table 3b.
Genes dependent on CgYap1 and CgSkn7 during H2O2 and stress.
| Gene name | Systematic name | Function | H2O2 |
|
||
|---|---|---|---|---|---|---|
| wta | ΔyΔsa | wta | ΔyΔsa | |||
| CgYAH1 | CAGL0H00660g | Ferredoxin | 2.4 | 1.3 | 2.4 | 1.3 |
| CgGPX2 | CAGL0C01705g | Glutathione peroxidase | 22.4 | 2.1 | 2.3 | 1.3 |
| CgADH6 | CAGL0M14047g | NADPH dehydrogenase | 2.1 | 0.0 | 3.8 | 1.5 |
| CgPYC1 | CAGL0M14047g | Pyruvate carboxylase | 2.7 | 1.2 | 4.9 | 1.7 |
| CgSMD2 | CAGL0F04961g | Core Sm protein | 2.8 | 0.0 | 2.2 | 1.3 |
| CgTSA2 | CAGL0K06259g | Thioredoxin peroxidase | 22.3 | 1.6 | 8.8 | 2.2 |
| CgGRX7 | CAGL0I04554g | Monothiol glutaredoxin | 3.0 | 1.3 | 2.1 | 1.4 |
| YOR111W | CAGL0I02882g | Unknown function | 2.2 | 1.1 | 2.2 | 0.0 |
| CgTRR2 | CAGL0I01166g | Thioredoxin reductase | 30.2 | 0.0 | 3.4 | 0.0 |
| YJR111c | CAGL0C04873g | Unknown function | 2.4 | 1.4 | 2.3 | 0.0 |
| CgOAZ1 | CAGL0M07403g | Regulator of Spe1p | 2.0 | 0.0 | 2.9 | 0.0 |
Fold induction.
3.3. Expression of important key enzymes to overcome oxidative stress is dependent on CgYap1 and CgSkn7
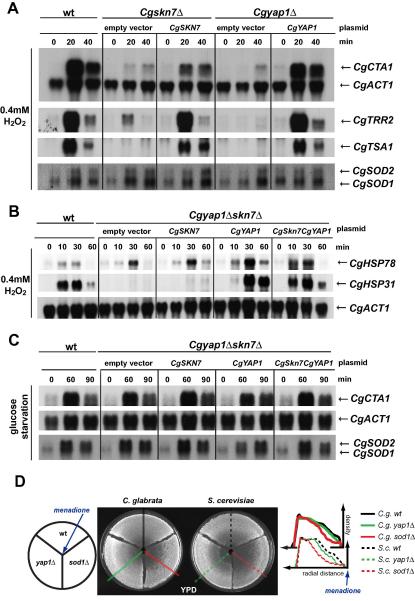
To confirm the functions of CgYap1 and CgSkn7 for C. glabrata, we measured the expression levels of exemplary genes of the defined groups (Fig. 3A and B). For genes dependent both on CgYap1 and CgSkn7, we chose CgCTA1, CgTRR2, and CgTSA1 (Fig. 3A). In C. glabrata wild type cells, expression levels were rapidly and strongly induced upon treatment with 0.4 mM hydrogen peroxide and highly dependent on CgSKN7 and CgYAP1. To confirm the mutant data we included strains complementing the mutations with plasmids carrying the CgYAP1 and CgSKN7 genes regulated by their own promoters. We investigated if the expression of the superoxide dismutases CgSOD1 and CgSOD2 is dependent on the CgSKN7 and CgYAP1. We found that CgSOD1 and was constitutively expressed, and levels of both CgSOD1 and CgSOD2 were not changed during peroxide stress in the CgYap1 and CgSkn7 mutant strains (Fig. 3A).
Fig. 3.
Dual control of transcription of oxidative stress regulon in C. glabrata. (A and B) Northern blot analysis of indicated transcripts during 0.4 mM H2O2 induced oxidative stress. C. glabrata wild type, Cgskn7Δ transformed with pCgSKN7 and Cgyap1Δ transformed with pCgYAP1 were grown to exponential phase before 0.4 mM H2O2 was added. (B) Wild type and Cgskn7Δyap1Δ were transformed with empty vector, pCgSKN7, pCgYAP1 or pCgYAP1-CgSKN7 (A). (C) Northern analysis of CgCTA1, CgSOD1 and CgSOD2 during glucose starvation. (D) Zone inhibition assay of Candida glabrata and S. cerevisiae strains with menadione (1.5 mM). 7 × 106 cells were spread onto YPD. Plates were incubated at 30 °C for 24 h. Density dependent on the radial distance is indicated.
Group 2 comprised genes independent from CgYap1 and CgSkn7. The reason for this could also be a redundant function of CgYap1 and CgSkn7. Expression of CgHSP78 was not dependent on CgYap1 and CgSkn7 or both (Fig. 3B). Therefore, other factors are involved in the regulation of Group 2 genes. Finally, Group 3 predicted a group of genes solely dependent on CgYap1 and we observed a high dependency of CgHSP31 on CgYap1.
Carbon source starvation induces resistance to oxidative stress. In addition, genes induced by carbon source starvation are up-regulated during phagocytosis [43]. Therefore, we investigated expression levels of oxidative stress genes during glucose depletion (Fig. 3C). After 1 h growth in medium lacking glucose CgCTA1 was strongly up-regulated in a CgSkn7 and CgYap1 and also CgMsn2/4 (not shown) independent manner. Furthermore, expression of the superoxide dismutases CgSOD1 and CgSOD2 was similar in Cgyap1Δskn7Δ mutant cells (Fig. 3C).
We compared susceptibility of C. glabrata and S. cerevisiae strains to menadione (Fig. 3D). In S. cerevisiae, expression of ScSOD1 is regulated by ScYap1 during oxidative stress [14]. Correspondingly, we observe increased sensitivity of S. cerevisiae Scyap1Δ mutants. In contrast, C. glabrata Cgyap1Δ mutants had similar sensitivity to menadione as the wild type. C. glabrata and S. cerevisiae sod1Δ mutants were both highly sensitive. We conclude that protection against superoxide by superoxide dismutase seems to be uncoupled from CgYap1 control in C. glabrata.
3.4. CgYap1 and CgSkn7 are interdependent for activation of expression of oxidative stress genes
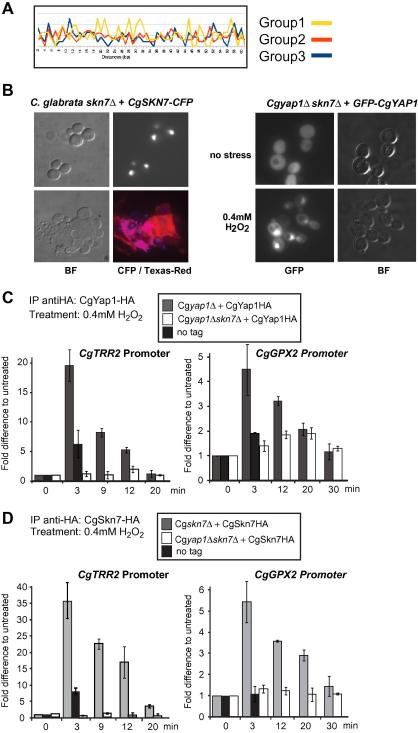
Genes comprising Group 1 were dependent on both CgYap1 and CgSkn7 for full expression. We tested recruitment of CgYap1 and CgSkn7 to oxidative stress gene promoters. In silico analyses of promoter regions of Group 1 genes revealed that Yap1/Skn7 binding site pairs located in close proximity (10–20 nucleotides from centre to centre of each binding site) were significantly enriched (Fischer exact test P < 0.01). Moreover, inter-motif distances in these pairs were not evenly distributed but presented peaks at 10, 14, and 19 nucleotide distances, which correspond to roughly, 1, 1.5 and 2 turns of the DNA double helix (Fig. 4A). CgSkn7-CFP localized constitutively to the nucleus and GFP-Yap1 regulated localization was not dependent on CgSkn7. Fluorescent protein fusions were over expressed (Fig. 4B).
Fig. 4.
CgSkn7 and CgYap1 cooperatively bind to oxidative stress inducible promoters. (A) Analysis of the promoter regions for significantly enriched Yap1/Skn7 binding site pairs located in close proximity. (B) CgSkn7-CFP is constitutively localized in the nucleus. CgSkn7-CFP was visualized by fluorescence microscopy of Cgskn7Δ mutant cells transformed with pCgADH1-CgSKN7-CFP. Upon oxidative stress, GFP-CgYap1 is localized in the nucleus in Cgyap1Δskn7Δ mutant cells. Cgyap1Δskn7Δ cells transformed with pCgADH1-GFP-CgYAP1 were grown in synthetic medium. GFP-CgYap1 was visualized by fluorescence microscopy (0.4 mM hydrogen peroxide, 10 min). (C) Chromatin recruitment assay of CYap1 and CgSkn7. Cgyap1Δ, Cgskn7Δ and Cgyap1Δskn7Δ mutant strains transformed with pHA-CgYAP1 (C) or pCgSKN7-HA (D) were grown to early exponential phase (OD 0.4) and treated with 0.4 mM H2O2 for times indicated. Anti-HA precipitated DNA was analyzed by qPCR.
To elucidate the binding of these factors to the respective promoters, we performed chromatin immune-precipitations. To preserve the nuclear localization signal CgSkn7 was tagged with three HA epitopes at the C-terminus (Fig. 4C), whereas CgYap1 was tagged at the N-terminus. Both constructs were expressed from centromeric plasmids under the control of their native promoter and complemented the respective deletion mutation for hydrogen peroxide induction using catalase activity as a reporter (not shown). As target gene promoters for ChIP we chose CgTRR2 and CgGPX2. HA-CgYap1, expressed in the yap1Δ mutant, was detected at the CgTRR2 promoter within 3 min upon stress induction by treatment with 0.4 mM H2O2 (Fig. 4C, left panel). After initial recruitment, HA-CgYap1 dissociated gradually. Importantly, HA-CgYap1 expressed in absence of CgSkn7 in the Cgskn7Δyap1Δ double mutant, was not detectably recruited to the CgTRR2 promoter (Fig. 4C, left panel).
CgSkn7-HA, similar to HA-CgYap1, was recruited within 3 min to the CgTRR2 promoter. Notably, CgSkn7-HA stayed slightly longer at the CgTRR2 promoter than HA-CgYap1 (Fig. 4D, left panel). CgSkn7-HA was not detectable in the promoter regions in the double mutant Cgskn7Δyap1Δ (Fig. 4, lower panel). CgYap1 and CgSkn7 had very similar binding characteristics to the CgGPX2 promoter (Fig. 4C and D, right panels). Taken together, binding of CgYap1 and CgSkn7 to certain promoters is strongly interdependent upon induction by oxidative stress. These results show that CgYap1 and CgSkn7 cooperate to achieve rapid promoter recruitment and explain why some genes require presence of both factors.
3.5. Both CgYap1 and CgSod1 are required for sustained C. glabrata survival in a primary mouse macrophage infection model
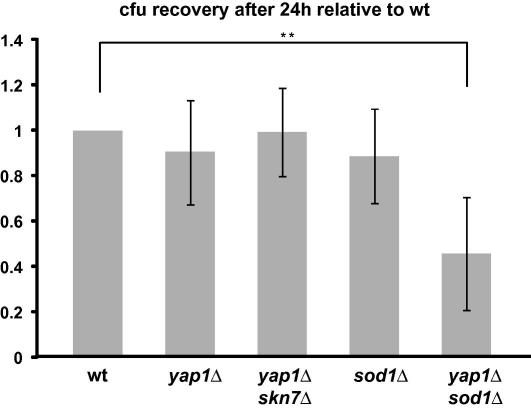
The oxidative burst is part of the strategy of phagocytic cells to erase engulfed cells. Consequently, we analyzed the importance of CgYap1, CgSkn7 and CgSod1 during phagocytosis. We infected murine bone marrow derived macrophages (BMDM) with Cgyap1Δ, Cgyap1Δskn7Δ, Cgsod1Δ and Cgyap1Δsod1Δ mutant cells (Fig. 5). C. glabrata cells were added to macrophages in a 1:1 ratio. After 24 h, engulfed C. glabrata cells were recovered on YPD plates. Cgyap1Δ, Cgsod1Δ and Cgskn7Δyap1Δ mutant cells displayed no diminished survival rate. However, the loss of both Cgsod1Δ and Cgyap1Δ had a major and significant effect on surviving phagocytosis.
Fig. 5.
Cgyap1Δsod1Δ double mutants are sensitive to phagocytosis. Exponentially growing C. glabrata ht6Δ (wt), Cgyap1Δ, Cgsod1Δ Cgyap1Δskn7Δ, and Cgyap1Δsod1Δ cells were washed (PBS, 0.1% glucose) and added to macrophages in a 1:1 ratio and incubated at 37 °C for 24 h. The viability of engulfed cells was assessed after hypotonic lysis of the macrophages and quantification of C. glabrata colony formation on rich medium. At least three independent experiments with at least four measurements each were analyzed. One-way ANOVA was performed and P values were calculated (∗∗P < 0.005).
4. Discussion
The preferred environments of C. glabrata and S. cerevisiae differ radically. C. glabrata lives preferably on mucosal surfaces competing with the microbial flora and may also encounter phagocytic cells of the innate immune system. These environments may have selected its high resistance against starvation, oxidative and chemical stress. We found that in C. glabrata, like in S. cerevisiae, Yap1 and Skn7 regulate core peroxide stress resistance genes. Different to S. cerevisiae, expression of C. glabrata superoxide dismutases was not regulated by CgYap1 but dependent on carbon source. Both CgYap1 and CgSod1 were required for optimal survival during macrophage phagocytosis.
4.1. The C. glabrata core peroxide stress response is related to S. cerevisiae
In S. cerevisiae, expression of more than 70 genes is increased within minutes upon exposure to hydrogen peroxide [44]. ScYap1 controls about thirty genes of the S. cerevisiae oxidative stress regulon [14,41]. Fifteen of these proteins required both Skn7 and Yap1 for induction. This is in agreement with an earlier report on CgSkn7 regulated genes [39]. Two distinct Yap1 regulons were defined in S. cerevisiae, covering oxidative stress response, the second involved in the metabolic pathways regenerating the main cellular reducing power, GSH and NADPH [14]. In C. glabrata the core response to oxidative stress included thioredoxin peroxidases (CgTsa1, CgTsa2), thioredoxin reductases (CgTrr1, CgTrr2), the thioredoxin cofactor CgTrx2, the glutathione peroxidase CgGpx2, and the catalase CgCta1. Cgyap1Δ mutant cells displayed higher susceptibility to hydrogen peroxide. In contrast to S. cerevisiae, CgYap1 had only a small effect on the susceptibility to superoxide anions [45]. This is in line with the observation that the DNA recognition pattern recognized CgYap1 in C. glabrata is changed due to a point mutation from ScYap1 [29]. Similar to our results, CgSkn7 has been shown recently to be important for peroxide stress protection and for the induction of CgTRX2, CgTRR1, CgTSA1 and CgCTA1 [39].
4.2. CgYap1 and CgSkn7 cooperate for promoter binding
In S. cerevisiae, genetic and in vitro evidence suggested that a direct interaction between Yap1 and Skn7 is necessary for induction of a number of oxidative stress response genes [15,27]. Our ChIP data for two genes showed that cooperation occurs at the level of promoter recognition. This kind of interdependence was previously observed in vitro. Electrophoretic mobility shift assays demonstrated the presence of a Skn7–Yap1 complex with the promoter DNA of TSA1 [14]. Here, we showed the first time in vivo, that Yap1 and Skn7 cooperatively bind to the upstream region of core oxidative stress genes.
4.3. Dual control by oxidative stress and carbon source
Twenty seven genes of the oxidative stress regulon are upregulated during glucose starvation [42]. C. albicans, S. cerevisiae and C. glabrata cells grown to stationary phase exhibit increased resistance against menadione and hydrogen peroxide [28,40,46]. Thus, adaptation to these environmental changes causes a simultaneous upregulation of a set of genes, beneficial for both oxidative stress and glucose starvation. The transcription factor mediating this activation in C. glabrata remains to be uncovered. CgMsn2/4 are most probably not involved since carbon source control of CgCTA1 is also observed in the double mutant (Roetzer and Schüller, unpublished observation). Interestingly, nutrient limitation can also increase resistance to oxidative stress and to pH stress in bacteria such as Staphylococcus aureus and Salmonella typhimurium. An Afsod1Δ/Afsod2Δ/Afsod3Δ triple mutant and AfSkn7 and AfYap1 mutant strains showed no defect in pathogenicity in murine infection models despite being sensitive against menadione and peroxide [47]. This might point to a comparable additive protection mechanism in A. fumigatus.
4.4. The distinct oxidative stress regulons have a synergistic impact on virulence
In a primary mouse BMDM macrophage model Cgyap1Δ and Cgyap1Δskn7Δ mutant cells were similar resistant as the wild type. Accordingly, Cgyap1Δskn7Δ cells can easily overcome 0.4 mM H2O2 stress, the concentration that C. glabrata cells most probably experience inside the mammalian host [28,48]. Cgsod1Δ mutants had no severe decrease of survival upon phagocytosis. However, the combined loss of superoxide protection with loss of hydrogen peroxide response (Cgyap1Δsod1Δ) made cells much more sensitive to BMDM internalization. We suggest that the production of superoxides inside the phagolysosome is intercepted by CgSod1. Only if both CgSod1 and CgYap1 are absent, C. glabrata cells are more sensitive to the oxidative burst. It will be interesting to test whether this relates to the mechanism how C. glabrata cells can suppress ROS production upon internalization by macrophages [49]. In summary, in C. glabrata regulation of oxidative stress protective factors supports survival of phagocytosis conditions.
Acknowledgements
We thank Gustav Ammerer, Wolfgang Reiter and Christa Gregori for discussions. C.S. was supported by the Herzfelder Foundation. This work was supported by the Austrian Research Foundation (FWF) through grants P16726-B14, I27-B03 and SFB F28 to P.K., grant I031-B from the University of Vienna (to C.S. and P.K.), and P19966-B12 to C.S.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.febslet.2010.12.006.
Appendix A. Supplementary data
References
- 1.Pfaller M.A., Diekema D.J. Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev. 2007;20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Presterl E., Daxbock F., Graninger W., Willinger B. Changing pattern of candidaemia 2001–2006 and use of antifungal therapy at the University Hospital of Vienna, Austria. Clin. Microbiol. Infect. 2007;13:1072–1076. doi: 10.1111/j.1469-0691.2007.01812.x. [DOI] [PubMed] [Google Scholar]
- 3.Li L., Redding S., Dongari-Bagtzoglou A. Candida glabrata: an emerging oral opportunistic pathogen. J. Dent. Res. 2007;86:204–215. doi: 10.1177/154405910708600304. [DOI] [PubMed] [Google Scholar]
- 4.Sandven P., Bevanger L., Digranes A., Haukland H.H., Mannsaker T., Gaustad P. Candidemia in Norway (1991–2003): results from a nationwide study. J. Clin. Microbiol. 2006;44:1977–1981. doi: 10.1128/JCM.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaur R., Domergue R., Zupancic M.L., Cormack B.P. A yeast by any other name: Candida glabrata and its interaction with the host. Curr. Opin. Microbiol. 2005;8:378–384. doi: 10.1016/j.mib.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 6.Marcet-Houben M., Gabaldon T. The tree versus the forest: the fungal tree of life and the topological diversity within the yeast phylome. PLoS ONE. 2009;4:e4357. doi: 10.1371/journal.pone.0004357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mansour M.K., Levitz S.M. Interactions of fungi with phagocytes. Curr. Opin. Microbiol. 2002;5:359–365. doi: 10.1016/s1369-5274(02)00342-9. [DOI] [PubMed] [Google Scholar]
- 8.Brown A.J., Haynes K., Quinn J. Nitrosative and oxidative stress responses in fungal pathogenicity. Curr. Opin. Microbiol. 2009;12:384–391. doi: 10.1016/j.mib.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Segal A.W. How neutrophils kill microbes. Annu. Rev. Immunol. 2005;23:197–223. doi: 10.1146/annurev.immunol.23.021704.115653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller R.A., Britigan B.E. Role of oxidants in microbial pathophysiology. Clin. Microbiol. Rev. 1997;10:1–18. doi: 10.1128/cmr.10.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cox G.M., Harrison T.S., McDade H.C., Taborda C.P., Heinrich G., Casadevall A., Perfect J.R. Superoxide dismutase influences the virulence of Cryptococcus neoformans by affecting growth within macrophages. Infect. Immun. 2003;71:173–180. doi: 10.1128/IAI.71.1.173-180.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Missall T.A., Lodge J.K. Function of the thioredoxin proteins in Cryptococcus neoformans during stress or virulence and regulation by putative transcriptional modulators. Mol. Microbiol. 2005;57:847–858. doi: 10.1111/j.1365-2958.2005.04735.x. [DOI] [PubMed] [Google Scholar]
- 13.Frohner I.E., Bourgeois C., Yatsyk K., Majer O., Kuchler K. Candida albicans cell surface superoxide dismutases degrade host-derived reactive oxygen species to escape innate immune surveillance. Mol. Microbiol. 2009;71:240–252. doi: 10.1111/j.1365-2958.2008.06528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee J., Godon C., Lagniel G., Spector D., Garin J., Labarre J., Toledano M.B. Yap1 and Skn7 control two specialized oxidative stress response regulons in yeast. J. Biol. Chem. 1999;274:16040–16046. doi: 10.1074/jbc.274.23.16040. [DOI] [PubMed] [Google Scholar]
- 15.He X.J., Fassler J.S. Identification of novel Yap1p and Skn7p binding sites involved in the oxidative stress response of Saccharomyces cerevisiae. Mol. Microbiol. 2005;58:1454–1467. doi: 10.1111/j.1365-2958.2005.04917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nikolaou E., Agrafioti I., Stumpf M., Quinn J., Stansfield I., Brown A.J. Phylogenetic diversity of stress signalling pathways in fungi. BMC Evol. Biol. 2009;9:44. doi: 10.1186/1471-2148-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodrigues-Pousada C., Menezes R.A., Pimentel C. The Yap family and its role in stress response. Yeast. 2010;27:245–258. doi: 10.1002/yea.1752. [DOI] [PubMed] [Google Scholar]
- 18.Neklesa T.K., Davis R.W. Superoxide anions regulate TORC1 and its ability to bind Fpr1:rapamycin complex. Proc. Natl. Acad. Sci. USA. 2008;105:15166–15171. doi: 10.1073/pnas.0807712105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marion R.M., Regev A., Segal E., Barash Y., Koller D., Friedman N., O’Shea E.K. Sfp1 is a stress- and nutrient-sensitive regulator of ribosomal protein gene expression. Proc. Natl. Acad. Sci. USA. 2004;101:14315–14322. doi: 10.1073/pnas.0405353101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuge S., Jones N., Nomoto A. Regulation of yAP-1 nuclear localization in response to oxidative stress. EMBO J. 1997;16:1710–1720. doi: 10.1093/emboj/16.7.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delaunay A., Pflieger D., Barrault M.B., Vinh J., Toledano M.B. A thiol peroxidase is an H2O2 receptor and redox-transducer in gene activation. Cell. 2002;111:471–481. doi: 10.1016/s0092-8674(02)01048-6. [DOI] [PubMed] [Google Scholar]
- 22.Wood M.J., Storz G., Tjandra N. Structural basis for redox regulation of Yap1 transcription factor localization. Nature. 2004;430:917–921. doi: 10.1038/nature02790. [DOI] [PubMed] [Google Scholar]
- 23.Billard P., Dumond H., Bolotin-Fukuhara M. Characterization of an AP-1-like transcription factor that mediates an oxidative stress response in Kluyveromyces lactis. Mol. Gen. Genet. 1997;257:62–70. doi: 10.1007/s004380050624. [DOI] [PubMed] [Google Scholar]
- 24.Molina L., Kahmann R. An Ustilago maydis gene involved in H2O2 detoxification is required for virulence. Plant Cell. 2007;19:2293–2309. doi: 10.1105/tpc.107.052332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiao J., Kontoyiannis D.P., Calderone R., Li D., Ma Y., Wan Z., Li R., Liu W. Afyap1, encoding a bZip transcriptional factor of Aspergillus fumigatus, contributes to oxidative stress response but is not essential to the virulence of this pathogen in mice immunosuppressed by cyclophosphamide and triamcinolone. Med. Mycol. 2008;46:773–782. doi: 10.1080/13693780802054215. [DOI] [PubMed] [Google Scholar]
- 26.Morgan B.A., Banks G.R., Toone W.M., Raitt D., Kuge S., Johnston L.H. The Skn7 response regulator controls gene expression in the oxidative stress response of the budding yeast Saccharomyces cerevisiae. EMBO J. 1997;16:1035–1044. doi: 10.1093/emboj/16.5.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He X.J., Mulford K.E., Fassler J.S. Oxidative stress function of the S. cerevisiae Skn7 receiver domain. Eukaryot. Cell. 2009;8:768–778. doi: 10.1128/EC.00021-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cuellar-Cruz M., Briones-Martin-del-Campo M., Canas-Villamar I., Montalvo-Arredondo J., Riego-Ruiz L., Castano I., De Las Penas A. High resistance to oxidative stress in the fungal pathogen Candida glabrata is mediated by a single catalase, Cta1p, and is controlled by the transcription factors Yap1p, Skn7p, Msn2p, and Msn4p. Eukaryot. Cell. 2008;7:814–825. doi: 10.1128/EC.00011-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuo D. Coevolution within a transcriptional network by compensatory trans and cis mutations. Genome Res. 2010;20:1672–1678. doi: 10.1101/gr.111765.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen K.H., Miyazaki T., Tsai H.F., Bennett J.E. The bZip transcription factor Cgap1p is involved in multidrug resistance and required for activation of multidrug transporter gene CgFLR1 in Candida glabrata. Gene. 2007;386:63–72. doi: 10.1016/j.gene.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 31.Singh P., Chauhan N., Ghosh A., Dixon F., Calderone R. SKN7 of Candida albicans: mutant construction and phenotype analysis. Infect. Immun. 2004;72:2390–2394. doi: 10.1128/IAI.72.4.2390-2394.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lamarre C., Ibrahim-Granet O., Du C., Calderone R., Latge J.P. Characterization of the SKN7 ortholog of Aspergillus fumigatus. Fungal Genet. Biol. 2007;44:682–690. doi: 10.1016/j.fgb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 33.Lessing F., Kniemeyer O., Wozniok I., Loeffler J., Kurzai O., Haertl A., Brakhage A.A. The Aspergillus fumigatus transcriptional regulator AfYap1 represents the major regulator for defense against reactive oxygen intermediates but is dispensable for pathogenicity in an intranasal mouse infection model. Eukaryot. Cell. 2007;6:2290–2302. doi: 10.1128/EC.00267-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kitada K., Yamaguchi E., Arisawa M. Isolation of a Candida glabrata centromere and its use in construction of plasmid vectors. Gene. 1996;175:105–108. doi: 10.1016/0378-1119(96)00132-1. [DOI] [PubMed] [Google Scholar]
- 35.Noble S.M., Johnson A.D. Strains and strategies for large-scale gene deletion studies of the diploid human fungal pathogen Candida albicans. Eukaryot. Cell. 2005;4:298–309. doi: 10.1128/EC.4.2.298-309.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sikorski R.S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szybalski W., Bryson V. Genetic studies on microbial cross resistance to toxic agents. I. Cross resistance of Escherichia coli to fifteen antibiotics. J. Bacteriol. 1952;64:489–499. doi: 10.1128/jb.64.4.489-499.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roetzer A., Gratz N., Kovarik P., Schüller C. Autophagy supports Candida glabrata survival during phagocytosis. Cell. Microbiol. 2009;12:199–216. doi: 10.1111/j.1462-5822.2009.01391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saijo T. Skn7p is involved in oxidative stress response and virulence of Candida glabrata. Mycopathologia. 2010;169:81–90. doi: 10.1007/s11046-009-9233-5. [DOI] [PubMed] [Google Scholar]
- 40.Jamieson D.J. Saccharomyces cerevisiae has distinct adaptive responses to both hydrogen peroxide and menadione. J. Bacteriol. 1992;174:6678–6681. doi: 10.1128/jb.174.20.6678-6681.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inoue Y., Matsuda T., Sugiyama K., Izawa S., Kimura A. Genetic analysis of glutathione peroxidase in oxidative stress response of Saccharomyces cerevisiae. J. Biol. Chem. 1999;274:27002–27009. doi: 10.1074/jbc.274.38.27002. [DOI] [PubMed] [Google Scholar]
- 42.Roetzer A. Candida glabrata environmental stress response involves Saccharomyces cerevisiae Msn2/4 orthologous transcription factors. Mol. Microbiol. 2008;69:603–620. doi: 10.1111/j.1365-2958.2008.06301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaur R., Ma B., Cormack B.P. A family of glycosylphosphatidylinositol-linked aspartyl proteases is required for virulence of Candida glabrata. Proc. Natl. Acad. Sci. USA. 2007;104:7628–7633. doi: 10.1073/pnas.0611195104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Godon C. The H2O2 stimulon in Saccharomyces cerevisiae. J. Biol. Chem. 1998;273:22480–22489. doi: 10.1074/jbc.273.35.22480. [DOI] [PubMed] [Google Scholar]
- 45.Brombacher K., Fischer B.B., Rufenacht K., Eggen R.I. The role of Yap1p and Skn7p-mediated oxidative stress response in the defence of Saccharomyces cerevisiae against singlet oxygen. Yeast. 2006;23:741–750. doi: 10.1002/yea.1392. [DOI] [PubMed] [Google Scholar]
- 46.Kusch H., Engelmann S., Bode R., Albrecht D., Morschhauser J., Hecker M. A proteomic view of Candida albicans yeast cell metabolism in exponential and stationary growth phases. Int. J. Med. Microbiol. 2008;298:291–318. doi: 10.1016/j.ijmm.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 47.Lambou K., Lamarre C., Beau R., Dufour N., Latge J.P. Functional analysis of the superoxide dismutase family in Aspergillus fumigatus. Mol. Microbiol. 2010;75:910–923. doi: 10.1111/j.1365-2958.2009.07024.x. [DOI] [PubMed] [Google Scholar]
- 48.Enjalbert B., MacCallum D.M., Odds F.C., Brown A.J. Niche-specific activation of the oxidative stress response by the pathogenic fungus Candida albicans. Infect. Immun. 2007;75:2143–2151. doi: 10.1128/IAI.01680-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wellington M., Dolan K., Krysan D.J. Live Candida albicans suppresses production of reactive oxygen species in phagocytes. Infect. Immun. 2009;77:405–413. doi: 10.1128/IAI.00860-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.