Abstract
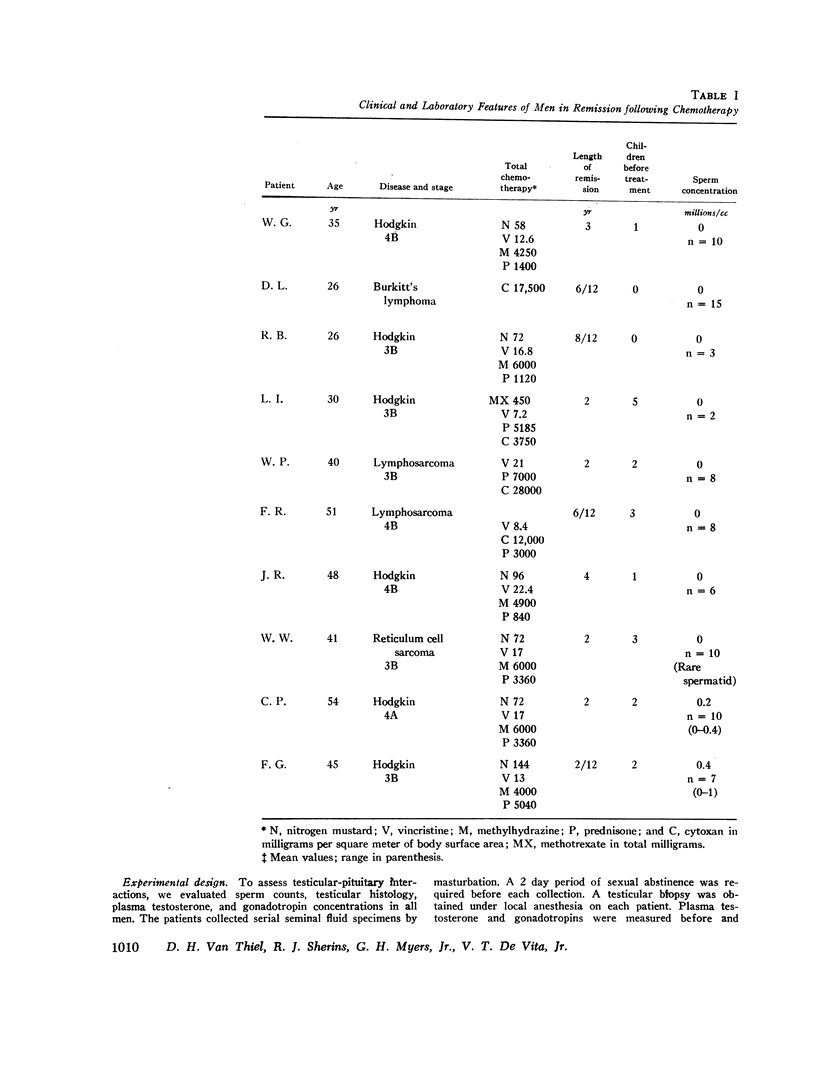
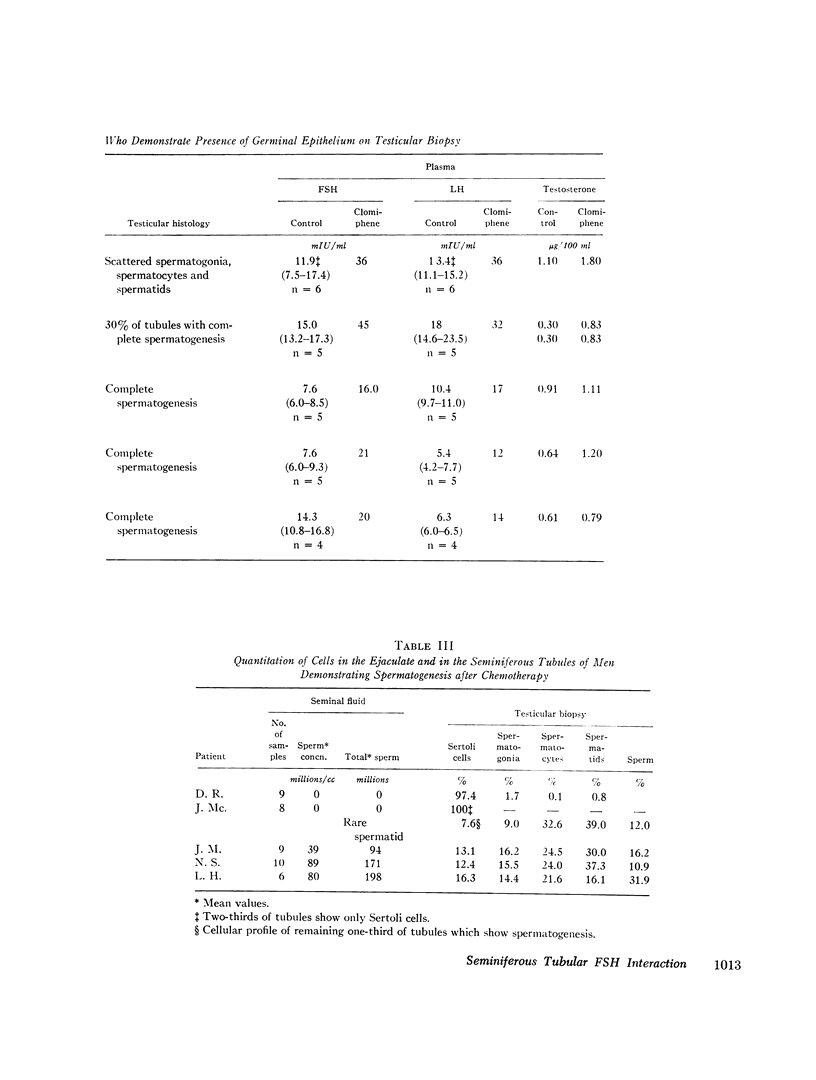
The interaction of the testis and gonadotropin secretion was studied in 15 men surviving chemotherapy for lymphoma. Azoospermia and complete destruction of all testicular germinal elements were present in 10 of the 15 men; however, Sertoli cells and Leydig cells were present. In these 10 men plasma follicle-stimulating hormone (FSH) levels were fourfold higher than in normal men of similar age whereas luteinizing hormone (LH) levels were normal. In contrast, both FSH and LH were normal in the remaining five men. Three had a full complement of spermatogenic tissue on biopsy and normal sperm concentrations. The other two men were azoospermic; one demonstrated full spermatogenesis in 30% of his tubules; the other had only a few spermatogonia in all tubules. In those patients with lower levels of gonadotropins pituitary insufficiency was excluded by the demonstration of appropriate responsiveness of FSH and LH to clomiphene administration. Similarly, Leydig cell function was normal since plasma testosterone was within the normal range in 13 of the 15 men and only slightly decreased in two. Thus, following chemotherapy, testicular damage was restricted to the germinal tissue, and this in turn was associated with a selective increase in FSH. The source of the FSH inhibitor is either the Sertoli cell or early germinal elements. However, since FSH levels are only half as high as those reported for castrate men, other testicular factors may modify FSH secretion.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cargille C. M., Rayford P. L. Characterization of antisera for human follicle-stimulating hormone radioimmunoassay. J Lab Clin Med. 1970 Jun;75(6):1030–1040. [PubMed] [Google Scholar]
- Devita V. T., Jr, Serpick A. A., Carbone P. P. Combination chemotherapy in the treatment of advanced Hodgkin's disease. Ann Intern Med. 1970 Dec;73(6):881–895. doi: 10.7326/0003-4819-73-6-881. [DOI] [PubMed] [Google Scholar]
- Gay V. L., Bogdanove E. M. Plasma and pituitary LH and FSH in the castrated rat following short-term steroid treatment. Endocrinology. 1969 May;84(5):1132–1142. doi: 10.1210/endo-84-5-1132. [DOI] [PubMed] [Google Scholar]
- JACKSON H., FOX B. W., CRAIG A. W. Antifertility substances and their assessment in the male rodent. J Reprod Fertil. 1961 Nov;2:447–465. doi: 10.1530/jrf.0.0020447. [DOI] [PubMed] [Google Scholar]
- LACY D. Certain aspects of testis structure and function. Br Med Bull. 1962 Sep;18:205–208. doi: 10.1093/oxfordjournals.bmb.a069979. [DOI] [PubMed] [Google Scholar]
- Leonard J. M., Leach R. B., Couture M., Paulsen C. A. Plasma and urinary follicle-stimulating hormone levels in oligospermia. J Clin Endocrinol Metab. 1972 Jan;34(1):209–214. doi: 10.1210/jcem-34-1-209. [DOI] [PubMed] [Google Scholar]
- McCULLAGH E. P., SCHAFFENBURG C. A. The role of the seminiferous tubules in the production of hormones. Ann N Y Acad Sci. 1952 Nov 20;55(4):674–684. doi: 10.1111/j.1749-6632.1952.tb26586.x. [DOI] [PubMed] [Google Scholar]
- Odell W. D., Ross G. T., Rayford P. L. Radioimmunoassay for luteinizing hormone in human plasma or serum: physiological studies. J Clin Invest. 1967 Feb;46(2):248–255. doi: 10.1172/JCI105527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter P., Calamera J. C., Morgenfeld M. C., Kierszenbaum A. L., Lavieri J. C., Mancini R. E. Effect of chlorambucil on spermatogenesis in the human with malignant lymphoma. Cancer. 1970 May;25(5):1026–1030. doi: 10.1002/1097-0142(197005)25:5<1026::aid-cncr2820250506>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Rosen S. W., Weintraub B. D. Monotropic increase of serum FSH correlated with low sperm count in young men with idiopathic oligospermia and aspermia. J Clin Endocrinol Metab. 1971 Mar;32(3):410–416. doi: 10.1210/jcem-32-3-410. [DOI] [PubMed] [Google Scholar]
- STEINBERGER E., NELSON W. O., BOCCABELLA A., DIXON W. J. A radiomimetic effect of triethylenemelamine on reproduction in the male rat. Endocrinology. 1959 Jul;65(1):40–50. doi: 10.1210/endo-65-1-40. [DOI] [PubMed] [Google Scholar]
- Sherins R. J., Gandy H. M., Thorslund T. W., Paulsen C. A. Pituitary and testicular function studies. I. Experience with a new gonadal inhibitor, 17-alpha-pregn-4-en-20-yno-(2,3-d)isoxazol-17-ol (Danazol). J Clin Endocrinol Metab. 1971 Apr;32(4):522–531. doi: 10.1210/jcem-32-4-522. [DOI] [PubMed] [Google Scholar]
- Swerdloff R. S., Walsh P. C., Jacobs H. S., Odell W. D. Serum LH and FSH during sexual maturation in the male rat: effect of castration and cryptorchidism. Endocrinology. 1971 Jan;88(1):120–128. doi: 10.1210/endo-88-1-120. [DOI] [PubMed] [Google Scholar]
- de Rooij D. G., Kramer M. F. The effect of three alkylating agents on the seminiferous epithelium of rodents. I. Depletory effect. Virchows Arch B Cell Pathol. 1970;4(4):267–275. doi: 10.1007/BF02906082. [DOI] [PubMed] [Google Scholar]



