Abstract
Neuronal nicotinic acetylcholine receptors (nAChRs) are a family of ligand-gated ion channels which are widely distributed in the human brain. Several lines of evidence suggest that two major subtypes (α4β2 and α7) of nAChRs play an important role in the pathophysiology of Alzheimer's disease (AD). Postmortem studies demonstrated alterations in the density of these subtypes of nAChRs in the brain of patients with AD. Currently, nAChRs are one of the most attractive therapeutic targets for AD. Therefore, several researchers have made an effort to develop novel radioligands that can be used to study quantitatively the distribution of these two subtypes in the human brain with positron emission tomography (PET) and single-photon emission computed tomography (SPECT). In this paper, we discuss the current topics on in vivo imaging of two subtypes of nAChRs in the brain of patients with AD.
1. Introduction
Alzheimer's disease (AD) is the most common neurodegenerative disorder in the elderly and has become a major worldwide health problem. Several reports indicated that it is affecting almost 1 in 10 individuals over the age of 65 [1], and as life expectancy increases, over 37 million people suffer with AD, and it is projected to quadruple by 2050 [2]. AD accounts for over 50% of senile dementia and the majority of presenile dementia cases and is characterized by progressive deterioration of higher cognitive functions including the loss of memory [3, 4].
van Duijn and Hofman [5] reported the inverse relationship between smoking history and early onset AD, suggesting that smoking may protect against AD [6]. Furthermore, Rusted and Trawley [7] reported acute improvements in prospective memory following nicotine administration. Although Swan and Lessov-Schlaggar [8] discuss the effects of tobacco smoke and nicotine on cognition in their review, smoking is associated with increased risk for negative preclinical and cognitive outcomes in younger people as well as in older adults. More recently, a meta-analysis including longitudinal studies published between 1995 and 2007 reported that current smokers relative to never-smokers were at increased risk of AD, vascular dementia, any dementia, and cognitive decline, in over the age of 65 [9]. Several lines of evidence demonstrated that smoking almost doubled the risk of AD and that smoking cessation might contribute to a reduction of risk factors for AD and cardiovascular disease [10, 11]. Noteworthy, the later is also known as a risk factor for AD. These results suggest that smoking cessation may play an important role in not only primary but also secondary prevention of AD. In contrast, although the discussion about neuroprotection by smoking has been continued, it is possible that nicotinic acetylcholine receptors (nAChRs) in the brain might play a role in the pathophysiology of AD.
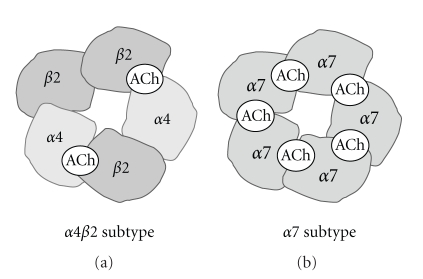
The nAChRs are one of the main classes of AChRs, which have a pentameric structure composed of five membrane spanning subunits, of which nine different types have thus far been identified and cloned. To date, twelve neuronal nAChR subunits have been described [12]; nine (α2–α10) code for subunits [12] based on the presence of adjacent cysteine residues in the predicted protein sequences, in a region homologous to the putative agonist-binding site of the muscle, a subunit (α1) and three referred to as non-α or β-subunits (β2–β4). Among the several nAChR subtypes in the human central nervous system (CNS), the heteromeric α4β2 and homomeric α7 subtypes (Figure 1) are predominant in the brain [13, 14]. It has been reported that other subtypes (e.g., α3, α6) exist in the brain [15, 16] and that α6 subtype might be mainly involved in the pathophysiology of Parkinson's disease [16]. Furthermore, studies using postmortem human brain samples have demonstrated alterations in the levels of α4 and α7 nAChR in the brains of patients with AD [15, 17–19]. Despite its lower number, loss of α3 subtype consistent with α4 and α7 nAChR subtypes was also observed in the brains of patients with AD [15]. Taken together, it is likely that these two subtypes (α4β2 and α7) of nAChR might play a role in the pathogenesis of AD. Therefore, it is of great interest to examine whether these two subtypes of nAChR are altered in the living brain of patients with AD using brain imaging techniques.
Figure 1.
Structures of α4β2 nAChR (a) and α7 nAChR (b).
In this paper, we discuss the recent findings on imaging of these two nAChRs (α4β2 and α7) in the brain with AD using positron emission tomography (PET) and single-photon emission computed tomography (SPECT).
2. α4β2 nAChRs Subtype
2.1. Relationship between Amyloid-β and α4β2 nAChR
Amyloid β protein (Aβ) is a major constituent of senile plaques and one of the candidates for the cause of the neurodegeneration found in AD. It has been shown that the accumulation of Aβ precedes other pathological changes and causes neurodegeneration or neuronal death in vitro and in vivo [20, 21]. The loss of memory seen in AD is thought to be associated with Aβ-induced impairment of synaptic plasticity such as long-term potentiation (LTP) in the hippocampus. There are lines of evidence suggesting that nAChR activation provides protection against Aβ-induced neurotoxicity in cultured cortical neurons [22, 23]. These results indicated that nicotine protects against Aβ-induced neuronal death, and similar effect has been also observed in those selective α4β2 nAChR agonists such as cytosine and epibatidine, but this neuroprotection is blocked by the selective α4β2 nAChR antagonist dihydro-β-erythroidine (DHβE). Moreover, recently, Wu et al. [24] investigated a possible role of α4β2 nAChR in mediating the impairment of long-term potentiation (LTP) by various forms of Aβ in in vivo. They reported that intracerebroventricular injection of Aβ 40, Aβ 25–35, or Aβ 31–35 significantly suppressed high-frequency stimulation-induced LTP. Similarly, epibatidine dose dependently suppressed the induction of LTP. Whereas DHβE showed no effect on the induction of LTP, it significantly reversed Aβ 31–35-induced LTP impairment. These findings suggest that α4β2 nAChR, which can be directly activated by Aβ, is required for Aβ suppression of LTP in vivo. The mechanisms by which nicotine enhanced the inhibition of LTP by Aβ were not clear. A possible explanation is that nicotine could activate nAChRs present in inhibitory interneurons, thereby potentiating inhibitory inputs to hippocampal neurons.
2.2. Cognition and α4β2 nAChR Agonists
It is likely that reduced density of nAChR is related to dementia severity, assessed using a global rating. Nicotine has been postulated to be a possible treatment for AD, improving cognition in humans [25]. Recently, Loughead et al. [26] reported novel evidence that the α4β2 partial agonist varenicline increased working memory-related brain activity after 3 days of nicotine abstinence, particularly at high levels of task difficulty, with associated improvements in cognitive performance among highly dependent smokers.
2.3. Postmortem Studies of α4β2 nAChR in the Brain of Patients with AD
Not only transmitter release but also receptor-binding sites may be altered in the brain of AD patients [27–29]. Postmortem studies showed the reduction (up to 50%) of α4β2 subtype of nAChRs in brain of patients with AD [30], and it may occur very early in the course of AD [31]. Both α4 and α7 subunits are known to be important constituents in α4β2 and α7 receptor subtypes, respectively. Investigation using the autopsy samples of human cerebral cortex has clearly shown that these two subtypes (α4 and α7 isoforms) are significantly decreased in their protein amount in the cortices of AD patients [15, 19, 32].
2.4. Imaging of α4β2 nAChR Subtype
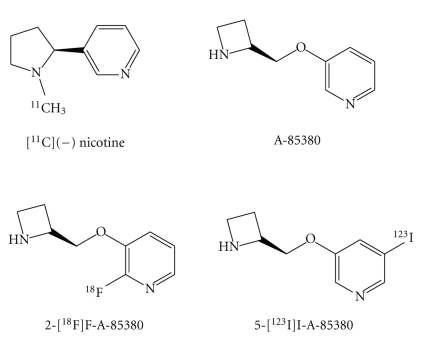
Considering the role of α4β2 nAChR in the pathophysiology of AD, it is of great interest to study α4β2 nAChR in the living human brain using PET/SPECT. Much effort has been devoted to visualize α4β2 nAChR in the brain by PET/SPECT. Currently, two PET ligands, including [11C]nicotine and 2-[18F]fluoro-3-(2 (S)azetidinylmethoxy)pyridine (2-[18F]F-A-85380), and a SPECT ligand, 5-[123I]iodo-3-(2 (S)-2-azetidinylmethoxy)pyridine (5-[123I]I-A-85380), (Figure 2) for in vivo imaging of α4β2 nAChR in the human brain have been used in clinical studies [33–35].
Figure 2.
Chemical structures of radioligands for nAChRs.
2.5. [11C]Nicotine
The development of radiolabelled nicotine [36, 37] has allowed for evaluating the uptake and distribution of nAChR in the living human brain [38–40]. The data obtained by [11C]nicotine is generally consistent with the known pattern of nAChR measured by in vitro binding in autopsy brain tissue [39]. [11C]nicotine-PET has been used to study α4β2 nAChR in human brain, and a severe loss of the nAChR has been detected in the brain of patients with AD [13]. Cortical nAChRs in mild AD patients are robustly associated with the cognitive function of attention [35] and have revealed a significant negative correlation between severity of cognitive impairment and density of brain nAChR [40]. It will be, therefore, of interest to study an alteration in α4β2 nAChR at a presymptomatic stage of AD. Furthermore, the in vivo cortical AChE inhibition and [11C]nicotine binding were associated with changes in the attention domain of cognition rather than episodic memory when administering galantamine [41]. Thus, [11C]nicotine-PET may be also used for monitoring treatment efficacy in AD patients [41, 42].
Unfortunately, [11C]nicotine displays high levels of nonspecific binding, rapid metabolism, and rapid washout of the brain [43]. The heterogeneity of [11C]nicotine binding in the brain also precludes the identification of a reference region which may be used to accurately determine nonspecific binding. Taken together, it is unlikely that [11C]nicotine might be a suitable PET ligand for in vivo imaging of α4β2 nAChR in human brain.
2.6. 2-[18F]F-A-85380 and 5-[123I]I-A-85380
A-85380 [3-(2(S)-azetidinylmethoxy) pyridine] is a potent and selective agonist with high affinity for α4β2 nAChR subtype and low affinity for other nAChR subtypes [44]. A-85380 is effective in a wide range of preclinical models of CNS disorders [45, 46]. Recently, A-85380 was successfully labeled using 18F or 125/123I with a high affinity (K i = 50 pM for F and K i = 15 pM for I) for α4β2 nAChR [44, 47, 48]. These radioligands have been evaluated in vitro and in vivo as PET/SPECT radioligands to visualize α4β2 nAChR subtype in the brain [49, 50]. In healthy nonsmoking human brain, both 2-[18F]F-A85380 and 5-[123I]I-A85380 have revealed a pattern of highest uptake in the thalamus, intermediate in the midbrain, pons, cerebellum, and cortex, and lowest in white matter [50–52], which is consistent with the regional distribution of α4β2 nAChR.
Furthermore, a study of age-related decline in nicotinic receptor availability showed that regional β2 nAChR availability were inversely correlated with decline ranging from 32% (thalamus) to 18% (occipital cortex) over the adult lifespan, or up to 5% per decade [53]. These results may corroborate postmortem reports of decline in high-affinity nicotine binding with age and may aid in elucidating the role of β2-nAChR in cognitive aging. In addition, 2-[18F]F-A-85380 or 5-[123I]I-A-85380 have been used to evaluate the effect of smoking on occupancy of α4β2 nAChR [54, 55]. Smoking 0.13 (1 to 2 puffs) of a cigarette resulted in 50% occupancy of α4β2 nAChR for 3.1 hours after smoking. Smoking a full cigarette (or more) resulted in more than 88% receptor occupancy and was accompanied by a reduction in cigarette craving. The extent of receptor occupancy found herein suggests that smoking may lead to withdrawal alleviation by maintaining nAChR in the desensitized state.
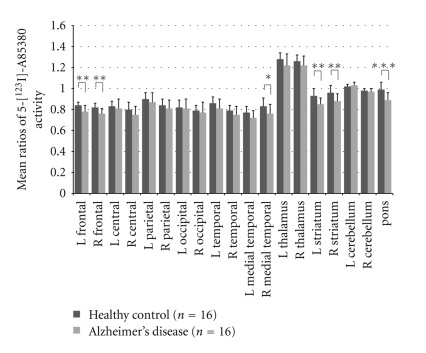
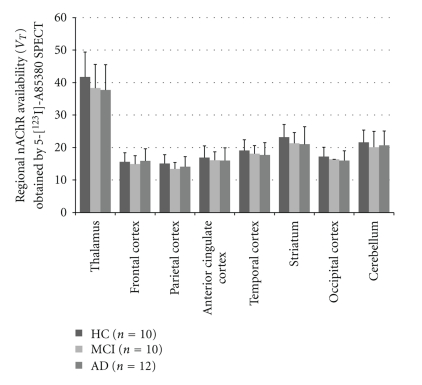
Both 2-[18F]F-A-85380 and 5-[123I]I-A-85380 have been used in AD patients [51, 56–60]. In 17 patients with moderate to severe AD and 6 subjects with amnestic mild cognitive impairment (MCI) compared with 10 healthy control subjects, Sabri et al. [56] found significant reductions of α4β2 nAChR in brain regions (hippocampus, caudate, frontal cortex, temporal cortex, posterior cingulate, anterior cingulate, and parietal cortex) in the brain of AD by using 2-[18F]F-A-85380. Most recently, Kendziorra et al. [57] reported that both patients with AD and those with MCI showed a significant reduction in 2-[18F]F-A-85380 binding potential in typical AD-affected brain regions and that the 2-[18F]F-A-85380 binding potential correlated with the severity of cognitive impairment. In addition, only MCI patients who converted to AD in the later course (n = 5) had a reduction in 2-[18F]F-A-85380 binding potential. Thus, it is likely that 2-[18F]F-A-85380 PET might give prognostic information about a conversion from MCI to AD. Similar findings were also reported by 5-[123I]I-A-85380, showing significant reductions in the activity ratios of the region of interest to cerebellum in the frontal, striatal, right medial temporal, and pontine regions in 16 patients with AD compared with 16 healthy control subjects [59] (Figure 3). These findings suggest that a reduction in α4β2 nAChR occurs during symptomatic stages of AD and that the α4β2 nAChR availability in these regions correlated with the severity of cognitive impairment. In contrast, there were no differences in distribution volume (DV) of nAChR between the healthy controls and early AD patients (Figure 4) [51, 58].
Figure 3.
Comparison with regional uptake values of 5-[123I]I-A85380 in age-matched healthy control and patients with Alzheimer's disease. Significant bilateral reductions in nicotinic receptor binding were identified in frontal, striatal, right medial temporal, and pons in patients with AD compared to controls. (Data is from the paper of O'Brien et al. [59].) *P < .05, **P < .005, ***P < .001.
Figure 4.
Regional α4β2-nAChR availability (V T) of 5-[123I]I-A85380 in age-matched healthy control (HC), mild cognitive impairment (MCI), and Alzheimer's disease (AD) groups. No significant regional differences among the subject groups for any of the 8 regions, including the 4 neocortical regions, were identified. (Data is from the paper of Mitsis et al. [58].)
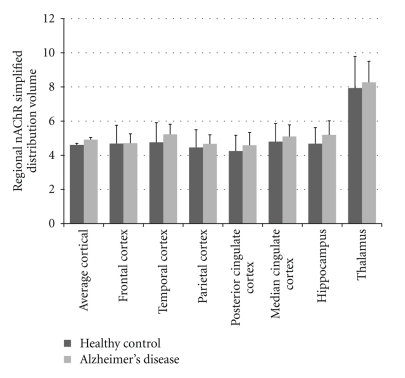
2-[18F]F-A-85380 PET has been used to observe outcome of drug treatment for the improvements of cognition in patients with mild AD [61]. However, no significant correlations were found between cognitive measures and nAChR simplified DV (Figure 5). These results are similar to the results reported by Kadir et al. [41] in their studies using [11C]nicotine. The relationship between cognition in AD and cholinergic dysfunction may be related to a number of factors, including the degree of cholinergic system (or receptor) loss, the other nAChR subtypes, or other neurochemical systems.
Figure 5.
Regional nAChR simplified distribution volume (DV(s)) of 2-[18F]F-A85380 in healthy control (HC) and Alzheimer's disease (AD) groups. No significant difference in nAChR DV(s) was found between both groups. (Data is from the paper of Ellis et al. [51].)
3. α7 nAChR Subtype
3.1. Relationship between Aβ and α7 nAChR
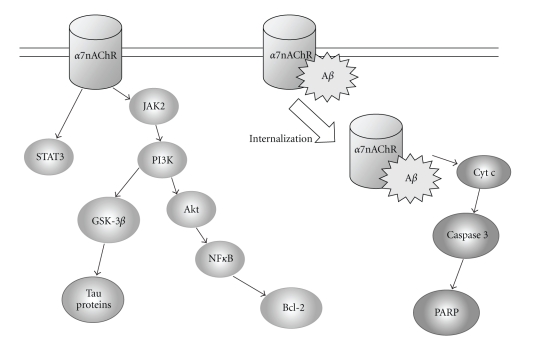
Of the two major subtypes of nAChRs in the CNS, α7 subtype has lower affinity for ACh compared to α4β2 subtype [62]. Accumulating evidence suggests that α7 nAChR plays a role in the pathophysiology of AD. Aβ has picomolar affinity for α7 nAChR [63, 64], which results in the formation of Aβ-α7 nAChR complex. This complex is known to move intracellularly and cause neurotoxicity [63–65]. Interestingly, this neurotoxicity is not present in transgenic mouse model of AD overexpressing a mutated form of the human amyloid precursor protein (APP) and lacking the α7 nAChR [66]. Recently, Bencherif and Lippiello [67] pointed out that the α7-JAK2-(NF-κB; STAT3)-Bcl2 prosurvival pathway is important for the neuroprotective role of α7 nAChR (Figure 6). By blocking cytosolic cytochrome C, which is released from the mitochondria via Aβ 1–42, Bcl2 fully counteracts the Aβ 1–42-induced apoptosis of cells [68]. The fact that this antiapoptotic pathway is further related with ApoE4 [69], GSK-3β-activated tau phosphorylation [70], and Wnt signaling pathways [71] denotes the critical role of α7 nAChR in pathophysiology of AD.
Figure 6.
Schematic representation of neuroprotective role of α7 nAChR.
The 3xTg-AD mice [72], which are triple transgenic mice expressing APP, presinilin-1, and Tau, were shown to have an age-dependent reduction of α7 nAChR. This reduction was limited to brain regions where intraneuronal Aβ 42 accumulation occurred [73]. The early cognitive deficits of 3xTG-AD mice also correlate with intracellular Aβ accumulation, and the clearing of this Aβ accumulation by immunotherapy reverses the early cognitive impairment [74].
Tg2576 transgenic mice (APPswe) dramatically reduced Aβ plaque expression with chronic administration of nicotine for 5.5 months [75]. It is further reported that a 10-day administration of nicotine reduced the guanidinium-soluble Aβ levels by 46 to 66%, whereas the intracellular Aβ levels remained unchanged [76]. This treatment with nicotine also resulted in less glial fibrillary acidic protein- (GFAP-) immunoreactive astrocytes around the amyloid plaques and increased numbers of α7 nAChR in the cortex of APPswe mice [76]. Bencherif [68] points out the importance of these data, as reduction of Aβ with anti-Aβ antibody treatment is reported to rapidly recover the associated neuritic dystrophy in living animals [77].
Orr-Urtreger et al. [78] generated α7 nAChR gene knock-out (KO) mice, and the resulting α7 nAChR KO mice did not show any morphological central nervous system abnormalities [78, 79], but behavioral tests point out some cognitive deficits in KO mice, such as impaired sustained attention [80, 81], impairment in working memory [82], and impairment in performance under high attentional demand [83]. The cognitive deficits seen in APP transgenic mice worsen when α7 nAChR is absent at the same time [84]. These α7 nAChR KO APP mice showed significant reduction in hippocampal and basal forebrain choline acetyltransferase activity and loss of hippocampal neurons and markers; stereological analyses indicated more pronounced loss of hippocampal pyramidal neurons and volume loss compared with APP mice [84]. Taken all together, it is likely that α7 nAChR might play an important role in the process of Aβ disposition which was detected in the brain of patients with AD.
3.2. Cognition and α7 nAChR Agonists
A number of α7 nAChR agonists are reported to improve recognition memory in rodents. These agonists include tropisetron [85], ABBF [86], AR-R 17779 [87], SSR180711 [88, 89], A-582941 [90], and SEN123333 [91]. In nonhuman primates, improvements in long-delay performance of delayed matching tasks are reported by α7 nAChR agonists GTS-21 [92] and A-582941 [93].
It is reported that nicotine inhibits Aβ deposition and aggregation in the cortex and hippocampus of APP transgenic mice [94]. RNA interference experiments indicated that these nicotine-mediated effects require α7 nAChR. In another study [70], the selective α7 nAChR agonist A-582941 led to increased phosphorylation of the inhibitory regulating amino acid residue Ser-9 on glycogen synthase kinase 3β (GSK3β), a major kinase responsible for tau hyperphosphorylation in AD neuropathology. This was observed in mouse cingulate cortex and hippocampus and was not observed in α7 nAChR KO mice. S9-GSK3β phosphorylation was also seen in the hippocampus of Tg2576 (APP), as well as wild-type mice by steady-state exposure of A-582941. Moreover, continuous infusions of A-582941 decreased phosphorylation of tau in hippocampal CA3 Mossy fibers in a hypothermia-induced tau hyperphosphorylation mouse model and also decreased spinal motoneurons in AD double transgenic APP/tau mouse line. This group points out that α7 nAChR agonists may have therapeutic potential through GSK3β inhibition followed by reduction of tau hyperphosphorylation and further suggest that this pharmacology may have the potential to provide disease modifying benefit in the treatment of AD.
It is reported that the α7 nAChR agonist GTS-21 prevented Aβ 25–35-induced impairment of acquisition performance and probe trail test in Morris water maze [95]. Their study showed first in vivo evidence that treatment with GTS-21 ameliorates the Aβ-induced deficit in spatial cognition through not only activating α7 nAChR but also preventing the Aβ-impaired α7 nAChR.
Using a novel selective α7 nAChR partial agonist S 24795, Wang et al. [96] showed that, in contrast to anti-AD drugs, galantamine (a cholinesterase inhibitor) and memantine (an N-methyl-D-aspartate (NMDA) receptor antagonist), S 24795 reduced or limited Aβ 42-α7 nAChR association, Aβ 42-induced tau phosphorylation, Aβ 42 accumulations, and Aβ 42-mediated inhibition of α7 nAChR Ca2+ influx in rodent brain [96]. S 24795 more importantly restored α7 nAChR functional deficits which had resulted from continued exposure to exogenous Aβ 42.
Taken all together, α7 nAChR is one of the therapeutic targets for AD [97, 98].
3.3. Postmortem Studies of α7 nAChR in the Brain of Patients with AD
In the postmortem brain of patients with AD, decline of α7 nAChR appears early in the disease and was associated with the progression of cognitive deficits [99–101]. Although the protein levels are reduced in the cortex and hippocampus of AD patients [15, 19, 32, 100, 102], contradictions arise at the level of gene transcription. For example, levels of α7 nAChR protein were reduced by 36% in the hippocampus of AD patients [15], but α7 nAChR mRNA expression is increased by 65% [18]. Furthermore, no differences in [125I]α-bungarotoxin binding were found in the frontal cortex of AD patients [103] and negative reduction of the α7 nAChR protein levels [104].
3.4. Imaging of α7 nAChR in the Brain
Given the role of α7 nAChR in the pathogenesis of AD, it is of great interest to study α7 nAChR in the living human brain using PET/SPECT. Much effort has been devoted to visualize α7 nAChR in the brain by PET/SPECT, but the development of a radioligand that depicts α7 nAChR specifically has been problematic due to its relatively low amount in the brain [105–108]. Generally, α-bungarotoxin and MLA are well known as specific α7 nAChR antagonists. However, due to their large molecular weights, they have difficulty passing through the blood-brain barrier which makes them unfavorable for radioligands [109–111]. Consequently, a number of radioligands for α7 nAChR are being developed and evaluated as PET/SPECT radioligand. However, all radioligands except [11C]CHIBA-1001 were unsuccessful [112].
3.5. [11C]CHIBA-1001 as a Novel PET Ligand for α7 nAChR
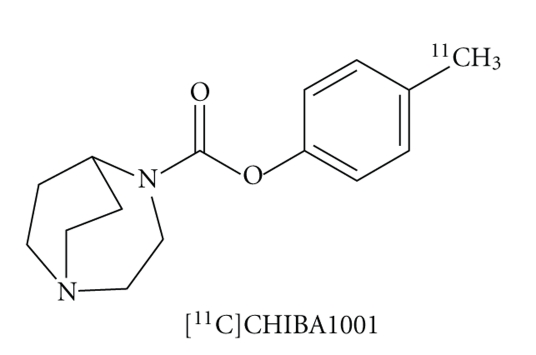
We developed a novel PET ligand, 4-[11C]methylphenyl 1,4-diazabicyclo[3.2.2.]nonane-4-carboxylate ([11C]CHIBA-1001) (Figure 7). A PET study using conscious monkeys demonstrated that the distribution of radioactivity in the brain regions after intravenous administration of [11C]CHIBA-1001 was blocked by pretreatment with the selective α7 nAChR agonist SSR180711 (5.0 mg/kg), but not the selective α4β2 nAChR agonist A85380 (1.0 mg/kg) [89]. In addition, we reported that the order of drugs for the inhibition of [3H]CHIBA-1001 binding to rat brain membranes was similar to α7 nAChR pharmacological profiles [113]. We also reported a preliminary PET study of [11C]CHIBA-1001 in a healthy human [114, 115]. Very recently, we reported that [125I]CHIBA-1006, an iodine derivative of SSR180711, has a high affinity for α7 nAChR as compared with CHIBA-1001 [116]. Considering the good brain permeability of derivatives (e.g., SSR180711 and CHIBA-1001) of CHIBA-1006, it would be of great interest to examine whether [123I]CHIBA-1006 and [124I]CHIBA-1006 are suitable radioligands for in vivo labeling of α7 nAChRs in the brain using SPECT and PET, respectively [116].
Figure 7.
Chemical structure of [11C]CHIBA-1001.
At present, [11C]CHIBA-1001 is the only PET ligand which can be available for in vivo study of α7 nAChRs in intact human brain [114]. PET studies of [11C]CHIBA-1001 in patients with AD are currently underway with [11C]Pittsburgh compound D ([11C]PiB)-PET and [18F]fluorodeoxyglucose ([18F]FDG)-PET. This study aims to evaluate the relationship between the distribution of α7 nAChR (assessed by [11C]CHIBA-1001) and Aβ disposition (assessed by [11C]PiB), while estimating the stage and cognitive levels (assessed by [18F]FDG-PET and neuropsychological examinations) for each AD patient.
4. Conclusions
Considering the importance of early prevention of onset of AD, it is very important to detect alternations in nAChRs at the presymptomatic stage of AD. In patients with MCI, the early detection and early therapeutic intervention would be beneficial. Therefore, brain imaging of nAChRs using PET and SPECT will be a powerful tool to study the mechanisms underlying pathological brain processes of cognitive disturbances in these patients. Currently, some PET and SPECT ligands for both subtypes (α4β2 nAChR and α7 nAChR) have been used to investigate the changes in receptor densities and functions of patients with AD. Gaining a better understanding of the role of nAChRs in the pathophysiology of AD is expected to provide new perspectives for treating this disorder.
Conflict of Interests
The authors have no conflict of interests.
Acknowledgments
This study was supported in part by a grant from the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation of Japan (Grant ID: 06-46, to K. Hashimoto). The authors would like to thank their collaborators who are listed as the coauthors of their papers in the reference list.
Abbreviations
- AD:
Alzheimer's disease
- DV:
Distribution volume
- GFAP:
Glial fibrillary acidic protein
- LTP:
Long-term potentiation
- nAChR:
Nicotinic acetylcholine receptor
- PET:
Positron emission tomography
- SPECT:
Single-photon emission tomography.
References
- 1.Evans DA, Funkenstein HH, Albert MS, et al. Prevalence of Alzheimer’s disease in a community population of older persons. Higher than previously reported. Journal of the American Medical Association. 1989;262(18):2551–2556. [PubMed] [Google Scholar]
- 2.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer’s disease. Alzheimer’s and Dementia. 2007;3(3):186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 3.Octave JN. The amyloid peptide and its precursor in Alzheimer’s disease. Reviews in the Neurosciences. 1995;6(4):287–316. [PubMed] [Google Scholar]
- 4.Grober E, Hall CB, Lipton RB, Zonderman AB, Resnick SM, Kawas C. Memory impairment, executive dysfunction, and intellectual decline in preclinical Alzheimer’s disease. Journal of the International Neuropsychological Society. 2008;14(2):266–278. doi: 10.1017/S1355617708080302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Duijn CM, Hofman A. Relation between nicotine intake and Alzheimer’s disease. British Medical Journal. 1991;302(6791):1491–1494. doi: 10.1136/bmj.302.6791.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graves AB, van Duijn CM, Chandra V, et al. Alcohol and tobacco consumption as risk factors for Alzheimer's disease: a collaborative re-analysis of case-control studies. International Journal of Epidemiology. 1991;20(supplement 2):S48–S57. doi: 10.1093/ije/20.supplement_2.s48. [DOI] [PubMed] [Google Scholar]
- 7.Rusted JM, Trawley S. Comparable effects of nicotine in smokers and nonsmokers on a prospective memory task. Neuropsychopharmacology. 2006;31(7):1545–1549. doi: 10.1038/sj.npp.1300965. [DOI] [PubMed] [Google Scholar]
- 8.Swan GE, Lessov-Schlaggar CN. The effects of tobacco smoke and nicotine on cognition and the brain. Neuropsychology Review. 2007;17(3):259–273. doi: 10.1007/s11065-007-9035-9. [DOI] [PubMed] [Google Scholar]
- 9.Peters R, Poulter R, Warner J, Beckett N, Burch L, Bulpitt C. Smoking, dementia and cognitive decline in the elderly, a systematic review. BMC Geriatrics. 2008;8, article 36 doi: 10.1186/1471-2318-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cataldo JK, Prochaska JJ, Glantz SA. Cigarette smoking is a risk factor for Alzheimer’s disease: an analysis controlling for tobacco industry affiliation. Journal of Alzheimer’s Disease. 2010;19(2):465–480. doi: 10.3233/JAD-2010-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cataldo JK, Glantz SA. Smoking cessation and Alzheimer’s disease: facts, fallacies and promise. Expert Review of Neurotherapeutics. 2010;10(5):629–631. doi: 10.1586/ern.10.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends in Pharmacological Sciences. 2006;27(9):482–491. doi: 10.1016/j.tips.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Paterson D, Nordberg A. Neuronal nicotinic receptors in the human brain. Progress in Neurobiology. 2000;61(1):75–111. doi: 10.1016/s0301-0082(99)00045-3. [DOI] [PubMed] [Google Scholar]
- 14.Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annual Review of Pharmacology and Toxicology. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- 15.Guan ZZ, Zhang X, Ravid R, Nordberg A. Decreased protein levels of nicotinic receptor subunits in the hippocampus and temporal cortex of patients with Alzheimer’s disease. Journal of Neurochemistry. 2000;74(1):237–243. doi: 10.1046/j.1471-4159.2000.0740237.x. [DOI] [PubMed] [Google Scholar]
- 16.Perez XA, Bordia T, McIntosh JM, Quik M. α6β2* and α4β2* nicotinic receptors both regulate dopamine signaling with increased nigrostriatal damage: relevance to Parkinson's disease. Molecular Pharmacology. 2010;78(5):971–980. doi: 10.1124/mol.110.067561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perry EK, Morris CM, Court JA, et al. Alteration in nicotine binding sites in Parkinson's disease, Lewy body dementia and Alzheimer's disease: possible index of early neuropathology. Neuroscience. 1995;64(2):385–395. doi: 10.1016/0306-4522(94)00410-7. [DOI] [PubMed] [Google Scholar]
- 18.Hellström-Lindahl E, Mousavi M, Zhang X, Ravid R, Nordberg A. Regional distribution of nicotinic receptor subunit mRNAs in human brain: comparison between Alzheimer and normal brain. Molecular Brain Research. 1999;66(1-2):94–103. doi: 10.1016/s0169-328x(99)00030-3. [DOI] [PubMed] [Google Scholar]
- 19.Burghaus L, Schütz U, Krempel U, et al. Quantitative assessment of nicotinic acetylcholine receptor proteins in the cerebral cortex of Alzheimer patients. Molecular Brain Research. 2000;76(2):385–388. doi: 10.1016/s0169-328x(00)00031-0. [DOI] [PubMed] [Google Scholar]
- 20.Yankner BA, Duffy LK, Kirschner DA. Neurotrophic and neurotoxic effects of amyloid β protein: reversal by tachykinin neuropeptides. Science. 1990;250(4978):279–282. doi: 10.1126/science.2218531. [DOI] [PubMed] [Google Scholar]
- 21.Kowall NW, Beal MF, Busciglio J, Duffy LK, Yankner BA. An in vivo model for the neurodegenerative effects of β amyloid and protection by substance P. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(16):7247–7251. doi: 10.1073/pnas.88.16.7247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kihara T, Shimohama S, Urushitani M, et al. Stimulation of α4β2 nicotinic acetylcholine receptors inhibits β- amyloid toxicity. Brain Research. 1998;792(2):331–334. doi: 10.1016/s0006-8993(98)00138-3. [DOI] [PubMed] [Google Scholar]
- 23.Fu W, Jhamandas JH. β-amyloid peptide activates non-α7 nicotinic acetylcholine receptors in rat basal forebrain neurons. Journal of Neurophysiology. 2003;90(5):3130–3136. doi: 10.1152/jn.00616.2003. [DOI] [PubMed] [Google Scholar]
- 24.Wu MN, He YX, Guo F, Qi JS. α4β2 nicotinic acetylcholine receptors are required for the amyloid β protein-induced suppression of long-term potentiation in rat hippocampal CA1 region in vivo . Brain Research Bulletin. 2008;77(2-3):84–90. doi: 10.1016/j.brainresbull.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Newhouse PA, Potter A, Singh A. Effects of nicotinic stimulation on cognitive performance. Current Opinion in Pharmacology. 2004;4(1):36–46. doi: 10.1016/j.coph.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Loughead J, Ray R, Wileyto EP, et al. Effects of the α4β2 partial agonist varenicline on brain activity and working memory in abstinent smokers. Biological Psychiatry. 2010;67(8):715–721. doi: 10.1016/j.biopsych.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 27.Flynn DD, Mash DC. Characterization of L-[3H]nicotine binding in human cerebral cortex: comparison between Alzheimer’s disease and the normal. Journal of Neurochemistry. 1986;47(6):1948–1954. doi: 10.1111/j.1471-4159.1986.tb13113.x. [DOI] [PubMed] [Google Scholar]
- 28.Whitehouse PJ, Martino AM, Antuono PG, et al. Nicotinic acetylcholine binding sites in Alzheimer's disease. Brain Research. 1986;371(1):146–151. doi: 10.1016/0006-8993(86)90819-x. [DOI] [PubMed] [Google Scholar]
- 29.Whitehouse PJ, Martino AM, Wagster MV, et al. Reductions in [3H]nicotinic acetylcholine binding in Alzheimer’s disease and Parkinson’s disease: an autoradiographic study. Neurology. 1988;38(5):720–723. doi: 10.1212/wnl.38.5.720. [DOI] [PubMed] [Google Scholar]
- 30.Warpman U, Nordberg A. Epibatidine and ABT 418 reveal selective losses of α4β2 nicotinic receptors in Alzheimer brains. NeuroReport. 1995;6(17):2419–2423. doi: 10.1097/00001756-199511270-00033. [DOI] [PubMed] [Google Scholar]
- 31.Marutle A, Warpman U, Bogdanovic N, Lannfelt L, Nordberg A. Neuronal nicotinic receptor deficits in Alzheimer patients with the Swedish amyloid precursor protein 670/671 mutation. Journal of Neurochemistry. 1999;72(3):1161–1169. doi: 10.1046/j.1471-4159.2000.0721161.x. [DOI] [PubMed] [Google Scholar]
- 32.Wevers A, Burghaus L, Moser N, et al. Expression of nicotinic acetylcholine receptors in Alzheimer’s disease: postmortem investigations and experimental approaches. Behavioural Brain Research. 2000;113(1-2):207–215. doi: 10.1016/s0166-4328(00)00215-1. [DOI] [PubMed] [Google Scholar]
- 33.Gallezot JD, Bottlaender M, Grégoire MC, et al. in vivo imaging of human cerebral nicotinic acetylcholine receptors with 2-18F-fluoro-A-85380 and PET. Journal of Nuclear Medicine. 2005;46(2):240–247. [PubMed] [Google Scholar]
- 34.Brody AL, Mandelkern MA, London ED, et al. Cigarette smoking saturates brain α4β2 nicotinic acetylcholine receptors. Archives of General Psychiatry. 2006;63(8):907–915. doi: 10.1001/archpsyc.63.8.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kadir A, Almkvist O, Wall A, Långström B, Nordberg A. PET imaging of cortical C-nicotine binding correlates with the cognitive function of attention in Alzheimer’s disease. Psychopharmacology. 2006;188(4):509–520. doi: 10.1007/s00213-006-0447-7. [DOI] [PubMed] [Google Scholar]
- 36.Maziere M, Comar D, Marazano C, Berger G. Nicotine 11C: synthesis and distribution kinetics in animals. European Journal of Nuclear Medicine. 1976;1(4):255–258. doi: 10.1007/BF00252173. [DOI] [PubMed] [Google Scholar]
- 37.Halldin C, Nagren K, Swahn CG, Langstrom B, Nyback H. (S)- and (R)-[11C]nicotine and the metabolite (R/S)-[11C]cotinine. Preparation, metabolite studies and in vivo distribution in the human brain using PET. International Journal of Radiation Applications and Instrumentation B. 1992;19(8):871–880. doi: 10.1016/0883-2897(92)90173-v. [DOI] [PubMed] [Google Scholar]
- 38.Nyback H, Nordberg A, Langstrom B, et al. Attempts to visualize nicotinic receptors in the brain of monkey and man by positron emission tomography. Progress in Brain Research. 1989;79:313–319. doi: 10.1016/s0079-6123(08)62490-5. [DOI] [PubMed] [Google Scholar]
- 39.Nordberg A, Nilsson-Hakansson L, Adem A, et al. The role of nicotinic receptors in the pathophysiology of Alzheimer’s disease. Progress in Brain Research. 1989;79:353–362. [PubMed] [Google Scholar]
- 40.Nordberg A, Lundqvist H, Hartvig P, Lilja A, Langstrom B. Kinetic analysis of regional (S)(-)11C-nicotine binding in normal and Alzheimer brains—in vivo assessment using positron emission tomography. Alzheimer Disease and Associated Disorders. 1995;9(1):21–27. doi: 10.1097/00002093-199505000-00006. [DOI] [PubMed] [Google Scholar]
- 41.Kadir A, Darreh-Shori T, Almkvist O, et al. PET imaging of the in vivo brain acetylcholinesterase activity and nicotine binding in galantamine-treated patients with AD. Neurobiology of Aging. 2008;29(8):1204–1217. doi: 10.1016/j.neurobiolaging.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 42.Nordberg A, Lundqvist H, Hartvig P, et al. Imaging of nicotinic and muscarinic receptors in Alzheimer's disease: effect of tacrine treatment. Dementia and Geriatric Cognitive Disorders. 1997;8(2):78–84. doi: 10.1159/000106611. [DOI] [PubMed] [Google Scholar]
- 43.Grunwald F, Biersack HJ, Kuschinsky W, Sorger D, Kampfer I, Knapp WH. Nicotine receptor mapping. European Journal of Nuclear Medicine. 1996;23(8):1012–1014. doi: 10.1007/BF01084381. [DOI] [PubMed] [Google Scholar]
- 44.Sullivan JP, Donnelly-Roberts D, Briggs CA, et al. A-85380 [3-(2(S)-azetidinylmethoxy) pyridine]: in vitro pharmacological properties of a novel, high affinity α4β2 nicotinic acetylcholine receptor ligand. Neuropharmacology. 1996;35(6):725–734. doi: 10.1016/0028-3908(96)84644-2. [DOI] [PubMed] [Google Scholar]
- 45.Rueter LE, Meyer MD, Decker MW. Spinal mechanisms underlying A-85380-induced effects on acute thermal pain. Brain Research. 2000;872(1-2):93–101. doi: 10.1016/s0006-8993(00)02472-0. [DOI] [PubMed] [Google Scholar]
- 46.Buckley MJ, Surowy C, Meyer M, Curzon P. Mechanism of action of A-85380 in an animal model of depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2004;28(4):723–730. doi: 10.1016/j.pnpbp.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 47.Rueter LE, Donnelly-Roberts DL, Curzon P, Briggs CA, Anderson DJ, Bitner RS. A-85380: a pharmacological probe for the preclinical and clinical investigation of the αβ neuronal nicotinic acetylcholine receptor. CNS Drug Reviews. 2006;12(2):100–112. doi: 10.1111/j.1527-3458.2006.00100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mukhin AG, Gündisch D, Horti AG, et al. 5-iodo-a-85380, an α4β2 subtype-selective ligand for nicotinic acetylcholine receptors. Molecular Pharmacology. 2000;57(3):642–649. doi: 10.1124/mol.57.3.642. [DOI] [PubMed] [Google Scholar]
- 49.Chefer SI, London ED, Koren AO, et al. Graphical analysis of 2-[18F]FA binding to nicotinic acetylcholine receptors in rhesus monkey brain. Synapse. 2003;48(1):25–34. doi: 10.1002/syn.10180. [DOI] [PubMed] [Google Scholar]
- 50.Kimes AS, Horti AG, London ED, et al. 2-[18F]F-A-85380: PET imaging of brain nicotinic acetylcholine receptors and whole body distribution in humans. FASEB Journal. 2003;17(10):1331–1333. doi: 10.1096/fj.02-0492fje. [DOI] [PubMed] [Google Scholar]
- 51.Ellis JR, Villemagne VL, Nathan PJ, et al. Relationship between nicotinic receptors and cognitive function in early Alzheimer’s disease: a 2-[18F]fluoro-A-85380 PET study. Neurobiology of Learning and Memory. 2008;90(2):404–412. doi: 10.1016/j.nlm.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 52.Fujita M, Ichise M, van Dyck CH, et al. Quantification of nicotinic acetylcholine receptors in human brain using [123I]5-I-A-85380 SPET. European Journal of Nuclear Medicine and Molecular Imaging. 2003;30(12):1620–1629. doi: 10.1007/s00259-003-1320-0. [DOI] [PubMed] [Google Scholar]
- 53.Mitsis EM, Cosgrove KP, Staley JK, et al. Age-related decline in nicotinic receptor availability with [123I]5-IA-85380 SPECT. Neurobiology of Aging. 2009;30(9):1490–1497. doi: 10.1016/j.neurobiolaging.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cosgrove KP, Batis J, Bois F, et al. β2-nicotinic acetylcholine receptor availability during acute and prolonged abstinence from tobacco smoking. Archives of General Psychiatry. 2009;66(6):666–676. doi: 10.1001/archgenpsychiatry.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brody AL, Mandelkern MA, Costello MR, et al. Brain nicotinic acetylcholine receptor occupancy: effect of smoking a denicotinized cigarette. International Journal of Neuropsychopharmacology. 2009;12(3):305–316. doi: 10.1017/S146114570800922X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sabri O, Kendziorra K, Wolf H, Gertz HJ, Brust P. Acetylcholine receptors in dementia and mild cognitive impairment. European Journal of Nuclear Medicine and Molecular Imaging. 2008;35(1):S30–S45. doi: 10.1007/s00259-007-0701-1. [DOI] [PubMed] [Google Scholar]
- 57.Kendziorra K, Wolf H, Meyer PM, et al. Decreased cerebral α4β2* nicotinic acetylcholine receptor availability in patients with mild cognitive impairment and Alzheimer's disease assessed with positron emission tomography. doi: 10.1007/s00259-010-1644-5. European Journal of Nuclear Medicine and Molecular Imaging. In press. [DOI] [PubMed] [Google Scholar]
- 58.Mitsis EM, Reech KM, Bois F, et al. 123I-5-IA-85380 SPECT imaging of nicotinic receptors in Alzheimer disease and mild cognitive impairment. Journal of Nuclear Medicine. 2009;50(9):1455–1463. doi: 10.2967/jnumed.109.064030. [DOI] [PubMed] [Google Scholar]
- 59.O’Brien JT, Colloby SJ, Pakrasi S, et al. α4β2 nicotinic receptor status in Alzheimer’s disease using I-5IA-85380 single-photon-emission computed tomography. Journal of Neurology, Neurosurgery and Psychiatry. 2007;78(4):356–361. doi: 10.1136/jnnp.2006.108209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Colloby SJ, Perry EK, Pakrasi S, et al. Nicotinic 123I-5IA-85380 single photon emission computed tomography as a predictor of cognitive progression in alzheimer’s disease and dementia with lewy Bodies. American Journal of Geriatric Psychiatry. 2010;18(1):86–90. doi: 10.1097/JGP.0b013e3181b972aa. [DOI] [PubMed] [Google Scholar]
- 61.Ellis JR, Nathan PJ, Villemagne VL, et al. Galantamine-induced improvements in cognitive function are not related to alterations in α4β2 nicotinic receptors in early Alzheimer’s disease as measured in vivo by 2-[18F]Fluoro-A- 85380 PET. Psychopharmacology. 2009;202(1–3):79–91. doi: 10.1007/s00213-008-1347-9. [DOI] [PubMed] [Google Scholar]
- 62.Clarke PBS. The fall and rise of neuronal α-bungarotoxin binding proteins. Trends in Pharmacological Sciences. 1992;13(11):407–413. doi: 10.1016/0165-6147(92)90125-p. [DOI] [PubMed] [Google Scholar]
- 63.Wang HY, Lee DHS, D’Andrea MR, Peterson PA, Shank RP, Reitz AB. β-Amyloid(1–42) binds to α7 nicotinic acetylcholine receptor with high affinity. Implications for Alzheimer’s disease pathology. Journal of Biological Chemistry. 2000;275(8):5626–5632. doi: 10.1074/jbc.275.8.5626. [DOI] [PubMed] [Google Scholar]
- 64.Wang HY, Lee DHS, Davis CB, Shank RP. Amyloid peptide Aβ(1–42) binds selectively and with picomolar affinity to α7 nicotinic acetylcholine receptors. Journal of Neurochemistry. 2000;75(3):1155–1161. doi: 10.1046/j.1471-4159.2000.0751155.x. [DOI] [PubMed] [Google Scholar]
- 65.D’Andrea MR, Nagele RG, Wang HY, Peterson PA, Lee DHS. Evidence that neurones accumulating amyloid can undergo lysis to form amyloid plaques in Alzheimer’s disease. Histopathology. 2001;38(2):120–134. doi: 10.1046/j.1365-2559.2001.01082.x. [DOI] [PubMed] [Google Scholar]
- 66.Dziewczapolski G, Glogowski CM, Masliah E, Heinemann SF. Deletion of the α7 nicotinic acetylcholine receptor gene improves cognitive deficits and synaptic pathology in a mouse model of Alzheimer’s disease. Journal of Neuroscience. 2009;29(27):8805–8815. doi: 10.1523/JNEUROSCI.6159-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bencherif M, Lippiello PM. α7 neuronal nicotinic receptors: the missing link to understanding Alzheimer’s etiopathology? Medical Hypotheses. 2010;74(2):281–285. doi: 10.1016/j.mehy.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 68.Bencherif M. Neuronal nicotinic receptors as novel targets for inflammation and neuroprotection: mechanistic considerations and clinical relevance. Acta Pharmacologica Sinica. 2009;30(6):702–714. doi: 10.1038/aps.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eddins D, Klein RC, Yakel JL, Levin ED. Hippocampal infusions of apolipoprotein E peptides induce long-lasting cognitive impairment. Brain Research Bulletin. 2009;79(2):111–115. doi: 10.1016/j.brainresbull.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bitner RS, Nikkel AL, Markosyan S, Otte S, Puttfarcken P, Gopalakrishnan M. Selective α7 nicotinic acetylcholine receptor activation regulates glycogen synthase kinase3β and decreases tau phosphorylation in vivo . Brain Research. 2009;1265(C):65–74. doi: 10.1016/j.brainres.2009.01.069. [DOI] [PubMed] [Google Scholar]
- 71.Farías GG, Vallés AS, Colombres M, et al. Wnt-7a induces presynaptic colocalization of α7-nicotinic acetylcholine receptors and adenomatous polyposis coli in hippocampal neurons. Journal of Neuroscience. 2007;27(20):5313–5325. doi: 10.1523/JNEUROSCI.3934-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oddo S, Caccamo A, Shepherd JD, et al. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Aβ and synaptic dysfunction. Neuron. 2003;39(3):409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 73.Oddo S, Caccamo A, Green KN, et al. Chronic nicotine administration exacerbates tau pathology in a transgenic model of Alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(8):3046–3051. doi: 10.1073/pnas.0408500102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM. Intraneuronal Aβ causes the onset of early Alzheimer’s disease-related cognitive deficits in transgenic mice. Neuron. 2005;45(5):675–688. doi: 10.1016/j.neuron.2005.01.040. [DOI] [PubMed] [Google Scholar]
- 75.Nordberg A, Hellström-Lindahl E, Lee M, et al. Chronic nicotine treatment reduces β-amyloidosis in the brain of a mouse model of Alzheimer’s disease (APPsw) Journal of Neurochemistry. 2002;81(3):655–658. doi: 10.1046/j.1471-4159.2002.00874.x. [DOI] [PubMed] [Google Scholar]
- 76.Unger C, Svedberg MM, Yu WF, Hedberg MM, Nordberg A. Effect of subchronic treatment of memantine, galantamine, and nicotine in the brain of Tg2576 (APPswe) transgenic mice. Journal of Pharmacology and Experimental Therapeutics. 2006;317(1):30–36. doi: 10.1124/jpet.105.098566. [DOI] [PubMed] [Google Scholar]
- 77.Brendza RP, Bacskai BJ, Cirrito JR, et al. Anti-Aβ antibody treatment promotes the rapid recovery of amyloid-associated neuritic dystrophy in PDAPP transgenic mice. Journal of Clinical Investigation. 2005;115(2):428–433. doi: 10.1172/JCI23269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Orr-Urtreger A, Goldner FM, Saeki M, et al. Mice deficient in the α7 neuronal nicotinic acetylcholine receptor lack α-bungarotoxin binding sites and hippocampal fast nicotinic currents. Journal of Neuroscience. 1997;17(23):9165–9171. doi: 10.1523/JNEUROSCI.17-23-09165.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Paylor R, Nguyen M, Crawley JN, Patrick J, Beaudet A, Orr-Urtreger A. α7 nicotinic receptor subunits are not necessary for hippocampal- dependent learning or sensorimotor gating: a behavioral characterization of Acra7-deficient mice. Learning and Memory. 1998;5(4-5):302–316. [PMC free article] [PubMed] [Google Scholar]
- 80.Hoyle E, Genn RF, Fernandes C, Stolerman IP. Impaired performance of α7 nicotinic receptor knockout mice in the five-choice serial reaction time task. Psychopharmacology. 2006;189(2):211–223. doi: 10.1007/s00213-006-0549-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Young JW, Crawford N, Kelly JS, et al. Impaired attention is central to the cognitive deficits observed in alpha 7 deficient mice. European Neuropsychopharmacology. 2007;17(2):145–155. doi: 10.1016/j.euroneuro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 82.Fernandes C, Hoyle E, Dempster E, Schalkwyk LC, Collier DA. Performance deficit of α7 nicotinic receptor knockout mice in a delayed matching-to-place task suggests a mild impairment of working/episodic-like memory. Genes, Brain and Behavior. 2006;5(6):433–440. doi: 10.1111/j.1601-183X.2005.00176.x. [DOI] [PubMed] [Google Scholar]
- 83.Keller JJ, Keller AB, Bowers BJ, Wehner JM. Performance of α7 nicotinic receptor null mutants is impaired in appetitive learning measured in a signaled nose poke task. Behavioural Brain Research. 2005;162(1):143–152. doi: 10.1016/j.bbr.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 84.Hernandez CM, Kayed R, Zheng H, Sweatt JD, Dineley KT. Loss of α7 nicotinic receptors enhances β-amyloid oligomer accumulation, exacerbating early-stage cognitive decline and septohippocampal pathology in a mouse model of Alzheimer’s disease. Journal of Neuroscience. 2010;30(7):2442–2453. doi: 10.1523/JNEUROSCI.5038-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hashimoto K, Fujita Y, Ishima T, Hagiwara H, Iyo M. Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of tropisetron: role of α7 nicotinic receptors. European Journal of Pharmacology. 2006;553(1–3):191–195. doi: 10.1016/j.ejphar.2006.09.055. [DOI] [PubMed] [Google Scholar]
- 86.Boess FG, de Vry J, Erb C, et al. The novel α7 nicotinic acetylcholine receptor agonist N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl]-7-[2-(methoxy)phenyl]-1-benzofuran-2- carboxamide improves working and recognition memory in rodents. Journal of Pharmacology and Experimental Therapeutics. 2007;321(2):716–725. doi: 10.1124/jpet.106.118976. [DOI] [PubMed] [Google Scholar]
- 87.van Kampen M, Selbach K, Schneider R, Schiegel E, Boess F, Schreiber R. AR-R 17779 improves social recognition in rats by activation of nicotinic α7 receptors. Psychopharmacology. 2004;172(4):375–383. doi: 10.1007/s00213-003-1668-7. [DOI] [PubMed] [Google Scholar]
- 88.Pichat P, Bergis OE, Terranova JP, et al. SSR180711, a novel selective α7 nicotinic receptor partial agonist: (II) efficacy in experimental models predictive of activity against cognitive symptoms of schizophrenia. Neuropsychopharmacology. 2007;32(1):17–34. doi: 10.1038/sj.npp.1301188. [DOI] [PubMed] [Google Scholar]
- 89.Hashimoto K, Nishiyama S, Ohba H, et al. [11C]CHIBA-1001 as a novel PET ligand for α7 nicotinic receptors in the brain: a PET study in conscious monkeys. PLoS ONE. 2008;3(9, article e3231) doi: 10.1371/journal.pone.0003231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tietje KR, Anderson DJ, Bitner RS, et al. Preclinical characterization of A-582941: a novel α7 neuronal nicotinic receptor agonist with broad spectrum cognition-enhancing properties. CNS Neuroscience and Therapeutics. 2008;14(1):65–82. doi: 10.1111/j.1527-3458.2008.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Roncarati R, Scali C, Comery TA, et al. Procognitive and neuroprotective activity of a novel α7 nicotinic acetylcholine receptor agonist for treatment of neurodegenerative and cognitive disorders. Journal of Pharmacology and Experimental Therapeutics. 2009;329(2):459–468. doi: 10.1124/jpet.108.150094. [DOI] [PubMed] [Google Scholar]
- 92.Briggs CA, Anderson DJ, Brioni JD, et al. Functional characterization of the novel neuronal nicotinic acetylcholine receptor ligand GTS-21 in vitro and in vivo . Pharmacology Biochemistry and Behavior. 1997;57(1-2):231–241. doi: 10.1016/s0091-3057(96)00354-1. [DOI] [PubMed] [Google Scholar]
- 93.Buccafusco JJ, Terry AV, Jr., Decker MW, Gopalakrishnan M. Profile of nicotinic acetylcholine receptor agonists ABT-594 and A-582941, with differential subtype selectivity, on delayed matching accuracy by young monkeys. Biochemical Pharmacology. 2007;74(8):1202–1211. doi: 10.1016/j.bcp.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 94.Liu Q, Zhang J, Zhu H, Qin C, Chen Q, Zhao B. Dissecting the signaling pathway of nicotine-mediated neuroprotection in a mouse Alzheimer disease model. FASEB Journal. 2007;21(1):61–73. doi: 10.1096/fj.06-5841com. [DOI] [PubMed] [Google Scholar]
- 95.Chen L, Wang H, Zhang Z, et al. DMXB (GTS-21) ameliorates the cognitive deficits in beta amyloid25-35-injected mice through preventing the dysfunction of α7 nicotinic receptor. Journal of Neuroscience Research. 2010;88(8):1784–1794. doi: 10.1002/jnr.22345. [DOI] [PubMed] [Google Scholar]
- 96.Wang HY, Bakshi K, Shen C, Frankfurt M, Trocmé-Thibierge C, Morain P. S 24795 limits β-amyloid-α7 nicotinic receptor interaction and reduces Alzheimer’s disease-like pathologies. Biological Psychiatry. 2010;67(6):522–530. doi: 10.1016/j.biopsych.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 97.Hashimoto K, Iyo M. Amyloid cascade hypothesis of Alzheimer's disease and α7 nicotinic receptor. Nihon Shinkei Seishin Yakurigaku Zasshi. 2002;22(2):9–53. [PubMed] [Google Scholar]
- 98.Toyohara J, Hashimoto K. α7 nicotinic receptor agonists: potential therapeutic drugs for treatment of cognitive impairments in schizophrenia and Alzheimer's disease. Open Medicinal Chemistry Journal. 2010;4:37–56. doi: 10.2174/1874104501004010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nordberg A. Human nicotinic receptors—their role in aging and dementia. Neurochemistry International. 1994;25(1):93–97. doi: 10.1016/0197-0186(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 100.Nordberg A. Nicotinic receptor abnormalities of Alzheimer’s disease: therapeutic implications. Biological Psychiatry. 2001;49(3):200–210. doi: 10.1016/s0006-3223(00)01125-2. [DOI] [PubMed] [Google Scholar]
- 101.Whitehouse PJ, Kalaria RN. Nicotinic receptors and neurodegenerative dementing diseases: basic research and clinical implications. Alzheimer Disease and Associated Disorders. 1995;9(supplement 2):3–5. doi: 10.1097/00002093-199501002-00002. [DOI] [PubMed] [Google Scholar]
- 102.Martin-Ruiz CM, Court JA, Molnar E, et al. α4 but not α3 and α7 nicotinic acetylcholine receptor subunits are lost from the temporal cortex in Alzheimer’s disease. Journal of Neurochemistry. 1999;73(4):1635–1640. doi: 10.1046/j.1471-4159.1999.0731635.x. [DOI] [PubMed] [Google Scholar]
- 103.Davies P, Feisullin S. Postmortem stability of α-bungarotoxin binding sites in mouse and human brain. Brain Research. 1981;216(2):449–454. doi: 10.1016/0006-8993(81)90148-7. [DOI] [PubMed] [Google Scholar]
- 104.Engidawork E, Gulesserian T, Balic N, Cairns N, Lubec G. Changes in nicotinic acetylcholine receptor subunits expression in brain of patients with Down syndrome and Alzheimer's disease. Journal of Neural Transmission, Supplement. 2001;(61):211–222. doi: 10.1007/978-3-7091-6262-0_17. [DOI] [PubMed] [Google Scholar]
- 105.Falk L, Nordberg A, Seiger A, Kjaeldgaard A, Hellström-Lindahl E. Higher expression of α7 nicotinic acetylcholine receptors in human fetal compared to adult brain. Developmental Brain Research. 2003;142(2):151–160. doi: 10.1016/s0165-3806(03)00063-4. [DOI] [PubMed] [Google Scholar]
- 106.Marutle A, Zhang X, Court J, et al. Laminar distribution of nicotinic receptor subtypes in cortical regions in schizophrenia. Journal of Chemical Neuroanatomy. 2001;22(1-2):115–126. doi: 10.1016/s0891-0618(01)00117-x. [DOI] [PubMed] [Google Scholar]
- 107.Court JA, Perry EK, Spurden D, et al. The role of the cholinergic system in the development of the human cerebellum. Developmental Brain Research. 1995;90(1-2):159–167. doi: 10.1016/0165-3806(96)83496-1. [DOI] [PubMed] [Google Scholar]
- 108.Court J, Martin-Ruiz C, Piggott M, Spurden D, Griffiths M, Perry E. Nicotinic receptor abnormalities in Alzheimer’s disease. Biological Psychiatry. 2001;49(3):175–184. doi: 10.1016/s0006-3223(00)01116-1. [DOI] [PubMed] [Google Scholar]
- 109.James RW, Bersinger NA, Schwendimann B, Fulpius BW. Characterization of iodinated derivatives of α-bungarotoxin. Hoppe-Seyler’s Zeitschrift fur Physiologische Chemie. 1980;361(10):1517–1524. doi: 10.1515/bchm2.1980.361.2.1517. [DOI] [PubMed] [Google Scholar]
- 110.Davies ARL, Hardick DJ, Blagbrough IS, Potter BVL, Wolstenholme AJ, Wonnacott S. Characterisation of the binding of [3H]methyllycaconitine: a new radioligand for labelling α7-type neuronal nicotinic acetylcholine receptors. Neuropharmacology. 1999;38(5):679–690. doi: 10.1016/s0028-3908(98)00221-4. [DOI] [PubMed] [Google Scholar]
- 111.Navarro HA, Zhong D, Abraham P, Xu H, Carroll FI. Synthesis and pharmacological characterization of [125I]iodomethyllycaconitine ([125I]iodo-MLA). A new ligand for the α(7) nicotinic acetylcholine receptor. Journal of Medicinal Chemistry. 2000;43(2):142–145. doi: 10.1021/jm990544f. [DOI] [PubMed] [Google Scholar]
- 112.Toyohara J, Wu J, Hashimoto K. Recent development of radioligands for imaging α7 nicotinic acetylcholine receptors in the brain. Current Topics in Medicinal Chemistry. 2010;10(15):1544–1557. doi: 10.2174/156802610793176828. [DOI] [PubMed] [Google Scholar]
- 113.Tanibuchi Y, Wu J, Toyohara J, Fujita Y, Iyo M, Hashimoto K. Characterization of [3H]CHIBA-1001 binding to α7 nicotinic acetylcholine receptors in the brain from rat, monkey, and human. Brain Research. 2010;1348(C):200–208. doi: 10.1016/j.brainres.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 114.Toyohara J, Sakata M, Wu J, et al. Preclinical and the first clinical studies on [11C]CHIBA-1001 for mapping α7 nicotinic receptors by positron emission tomography. Annals of Nuclear Medicine. 2009;23(3):301–309. doi: 10.1007/s12149-009-0240-x. [DOI] [PubMed] [Google Scholar]
- 115.Sakata M, Wu J, Toyohara J, et al. Biodistribution and radiation dosimetry of the α7 nicotinic acetylcholine receptor ligand [11C]CHIBA-1001 in humans. doi: 10.1016/j.nucmedbio.2010.09.007. Nuclear Medicine and Biology. In press. [DOI] [PubMed] [Google Scholar]
- 116.Wu J, Toyohara J, Tanibuchi Y, et al. Pharmacological characterization of [125I]CHIBA-1006 binding, a new radioligand for α7 nicotinic acetylcholine receptors, to rat brain membranes. Brain Research. 2010;1360(C):130–137. doi: 10.1016/j.brainres.2010.08.095. [DOI] [PubMed] [Google Scholar]