Abstract
The purpose of this study was to determine whether resistance exercise training-induced reductions in inflammation are mediated via melanocortin 3 receptor expression in obese (BMI 32.7 ± 3.7) women (65.6 ± 2.8 yrs) randomized to either a control (N = 11) or resistance training group (N = 12). The resistance trained group performed resistance training 3 days/week for 12 weeks. Resting blood samples were collected before and after the training intervention in both resistance trained and control groups. Resistance training upregulated melanocortin 3 receptor mRNA by 16-fold (P = .035) and decreased monocyte count, without changing leukocyte number, body composition, or body weight. Resistance trained individuals exhibited increased sensitivity to inflammatory stimuli, whereas control individuals exhibited no change. While there was no change in whole blood tumor necrosis factor alpha mRNA between the groups, whole blood interleukin 10 mRNA was higher in the resistance trained group following the intervention period. In summary, it appears that resistance training may modulate melanocortin 3 receptor expression, providing a possible mechanism for the anti-inflammatory effects of exercise training.
1. Introduction
Postmenopausal women exhibit higher concentrations of inflammatory markers and are also at increased risk for many of the age- and inactivity-related diseases that are prevalent in our society today, including cardiovascular disease and type 2 diabetes mellitus [1]. Chronic exercise has been shown to create favorable changes in the inflammatory profile and is a viable means for preventing the onset and slowing the progression of these diseases [2]. While this reduction in inflammation has been linked to a decrease in cardiovascular disease risk [3], understanding the molecular underpinnings associated with this response may provide further support for the use of resistance exercise as an adjunct or primary treatment for postmenopausal women with inflammatory risk factors.
Acute alterations in inflammation immediately after exercise are evidenced by elevated concentrations of inflammatory cytokines, such as interleukin 6 (IL-6), interleukin-1 beta (IL-1β), and tumor necrosis factor alpha (TNF-α), and increased activity of macrophages [4–7]. In conjunction with increased macrophage activation, acute alterations in inflammation after an exercise bout cause downstream increases in circulating levels of macrophages, natural killer cells, monocytes, lymphocytes, granulocytes, T-helper, and T-cytotoxic cells [6, 8]. Conversely, a previous work has shown that chronic, combined resistance, and aerobic exercise leads to a reduction in inflammation within 10–12 weeks [9], and a recent report suggests that resistance training alone may have a significant role in reducing inflammatory markers [10]. Specifically, consistent exercise is associated with a reduction in inflammatory cytokines and an increase in anti-inflammatory cytokines in circulation in conjunction with altered sensitivity of macrophages to inflammatory stimuli [7, 10, 11].
One area of cellular signaling that has been seemingly overlooked in the exploration of the effects of exercise on inflammation is the melanocortin system. Melanocortin receptors are well characterized G-protein-coupled transmembrane receptors (GPCRs) that act to increase cyclic adenosine monophosphate (cAMP) [12–14]. There are five known melanocortin receptor subtypes: melanocortin 1 receptor (MC1R) through melanocortin 5 receptor (MC5R). One of the most well-studied systems with respect to the MC3R is the leptin signaling pathway. Briefly, leptin is an endocrine hormone secreted in proportion to the amount of adipose tissue present. Leptin binds to leptin receptors located on pro-opiomelanocortin (POMC) neurons within the hypothalamus, specifically the arcuate nucleus and paraventricular nucleus [14, 15]. POMC is upregulated in response to leptin binding and is proteolitically cleaved into several active peptides, including alpha melanocyte stimulating hormone (α-MSH) and adrenocorticotrophic hormone (ACTH) [12, 14]. These peptides act as agonists within the CNS for MC3Rs. Activation of CNS MC3Rs leads to increases in lipolysis and energy expenditure [14, 16]. MCRs are also expressed in the periphery where they may play a role in immunomodulation. The melanocortin receptor subtypes, MC1R, MC3R, and MC5R are found on the plasma membranes of monocytes, macrophages, CD4+ T helper cells, granulocytes, and natural killer cells where, separately, they are activated in response to stress [12, 17–19]. We hypothesize that a redundant signaling mechanism involving POMC and its peptide variants may activate and modulate expression of MCR on systemic monocytes. Interestingly, activation of the MC3R by α-MSH and ACTH in the periphery causes secretion of an anti-inflammatory cytokine, interleukin 10 (IL-10) [15, 20, 21]. The MC3R-induced secretion of IL-10 underscores the potential importance of MC3R as an anti-inflammatory modulator due to the ability of IL-10 to inhibit inflammatory cytokines, including IL-6, TNF-α, and IL-1β secretion from leukocytes [22]. Because of its anti-inflammatory properties as well as its role in energy metabolism, the melanocortin system has become an area of robust research as a potential therapeutic target to pharmacologically treat inflammatory-related conditions [12].
It is possible that MC3R may play a novel role in exercise-mediated changes in inflammation. Using chronic resistance training in overweight, postmenopausal women, we hypothesized that RT may increase MC3R expression, reduce inflammation, and change the sensitivity of leukocytes to inflammatory stimuli.
2. Materials and Methods
2.1. Subjects
Obese (BMI 32.7 ± 3.7) women aged 65.6 ± 2.8 years, not having participated in consistent exercise for the previous 6 months, signed an informed consent, completed a medical history form, and obtained written approval from their personal physician. Potential subjects underwent a medical screening (physical exam and dementia screening) by our study physician and completed a sub-maximal treadmill test while blood pressure and ECG were monitored. Any subject meeting the exclusion criteria of the American College of Sports Medicine for exercise testing was excluded. NSAID users were asked to refrain from taking their medication until after the experimental trials on test days. Other exclusion criteria were severe arthritis, central or peripheral nervous system disorders, previous stroke, acute or chronic infection, major affective disorder, HIV infection or autoimmune disorders, metabolic disorders (type 1 or type 2 diabetes mellitus), smokers or smokeless tobacco users, regular aerobic or resistive exercise within the previous six months, oral steroid use, and alcohol intake greater than “moderate” (1 drink/day). Subjects were asked to maintain their “normal” diet regimen throughout the intervention period and to consume no alcohol the days prior to any blood sampling. Subjects recorded all food consumed during the 24 hours prior to the pretraining blood sample which was to represent a “normal” day's diet. After the 12-week intervention, they were given a copy of that food log and asked to record and consume the same meals during the 24 hours prior to the posttraining blood sample. Several subjects were taking general multivitamins, but no one was taking supplemental fish oil or omega-3. Subjects were randomized to either a control (CON: N = 11) or exercise group (EX: N = 12). This project was approved by Institutional Review Boards at Texas Christian University and John Peter Smith Hospital.
2.2. Anthropometrics
To assess the effects of resistance training (RT) on body mass and composition, body mass and percent fat were measured in all participants prior to the first and following the last exercise or control session. Body mass was measured to the nearest 0.1 kg with a calibrated digital scale, and height was measured to the nearest 1 mm with a stadiometer. Body mass index (BMI) was then calculated by dividing body weight (kg) by height squared (m2). In addition, body composition was estimated using a seven-site skinfold procedure [23, 24] by a trained technician. Body composition was obtained in the morning after an overnight fast. Percent body fat was estimated from body density using the Siri equation [25].
2.3. Intervention Protocol
EX underwent progressive RT 3 days per week on nonconsecutive days for 12 weeks while CON attended health education and craft classes twice per week to control for social interaction. All exercise sessions were directly supervised by trained technicians. EX performed 3 sets of the following exercises at their 8-repetition maximum (8RM): chest press, “lat” pull-down, shoulder press, seated row, leg abduction, leg adduction, leg extension, leg flexion, chest flys, and leg press. Subjects performed 8 repetitions in the first two sets and exercised to exhaustion/failure in the third set. When the subject was able to complete 12 repetitions in one set, a technician increased the resistance for the exercise to match her new 8RM on the following training day.
2.4. Blood Collection/Analyses
Resting blood samples were collected from CON and EX groups before (PRE) and after (PO) the intervention period. Subjects reported to the exercise physiology lab at Texas Christian University after an overnight fast (10 hr) and assumed a supine position for 20 minutes prior to blood collection. Blood samples were collected into Na+ heparin and K+ EDTA tubes. Whole blood from heparinized tubes was diluted 1 : 10 into culture medium (RPMI cell culture medium (100 mL; Sigma Diagnostics, St. Louis, MO) supplemented with 2 ml penicillin (100 U·ml−1), 2 ml streptomycin (100 μg·ml−1), and 1 ml glutamine (2 mM)). Cultured cells were stimulated with lipopolysaccharide (LPS from s. enteriditis, final concentration 25 μg·mL−1, Sigma Diagnostics, St. Louis). After 24 h incubation (37°C, 5%CO2), culture supernatants were harvested and analyzed by ELISA for TNF-α production, following the manufacturer's protocol (Invitrogen, Carlsbad, CA). Total leukocyte number and 5-part differentials were assessed using the AcTDiff 5 hematology analyzer (Beckman-Coulter, Brea, CA) using K+ EDTA-treated blood. LPS-stimulated cytokine production was expressed as per monocyte (fg·monocyte−1).
2.5. Real-Time PCR
Total RNA was isolated from whole blood samples using Trizol LS per the manufacturer's protocol followed by column purification (RNeasy mini kit, Qiagen). cDNA was synthesized using M-MLV reverse transcriptase (Promega). Real-time PCR was completed on each sample in duplicate using commercially available MC3R and cyclophilin B primers and probes (Applied Biosystems) and IL-10, TNF-α, and cyclophilin B primers designed to span exon-exon boundaries (IDT) with Taqman or SyBR Green based detection (Applied Biosystems) on the ABI 7900 HT platform. IL-10, TNF-α, and MC3R Ct values were normalized to cyclophilin Ct values, and mRNA differences were determined using the delta-delta Ct method.
2.6. Statistical Analysis
Mean and standard deviations were calculated for descriptive data, including age, height, body weight, body fat percentage, and estimated BMI. Dependent variables are expressed as mean ± standard error. Age, height, BMI, body composition, LPS-stimulated production of TNF-α, whole blood monocyte and leukocyte number, and IL-10, TNF-α, and MC3R mRNA were analyzed using a 2 × 2 ANOVA with a Tukey post hoc. Pearson stepwise correlation analysis was used to determine relationships between all measurements. Significance was set at P < .05.
3. Results
This was a supervised exercise intervention, and data presented here represent a subset of subjects from a larger study. Adherence to the exercise protocol was excellent as all EX subjects completed the prescribed 36 resistance exercise sessions. CON participants attended an average of 91% (22 of 24) of their control sessions. There was no difference in body weight, BMI, or percent body fat between groups prior to the intervention period. No differences were observed after the intervention period for the descriptive variables above in resistance trained or untrained control postmenopausal women. (Table 1). Percent change in strength for each exercise was significantly greater for EX compared to CON (P < .01). Strength improvements ranged from 21 to 69% for the various exercises in EX with no significant changes in CON.
Table 1.
Descriptive data for the subjects before (PRE) and after (PO) the 12-week resistance training or educational period (values are means ± SD). (CON: educational control group; EX: resistance training group). There were no significant differences between the groups.
| Variable | Time | CON (n = 11) | EX (n = 12) |
|---|---|---|---|
| Age | 66.1 ± 3.0 | 65.2 ± 2.6 | |
| Height (cm) | 159.5 ± 8.2 | 161.6 ± 5.3 | |
| Body weight (kg) | PRE | 83.9 ± 13.1 | 84.8 ± 9.1 |
| PO | 84.4 ± 13.5 | 84.2 ± 8.8 | |
| BMI (kg/m2) | PRE | 32.9 ± 4.3 | 32.5 ± 3.3 |
| PO | 33.1± 3.9 | 32.2 ± 3.4 | |
| % Body fat | PRE | 35.7 ± 3.1 | 35.5 ± 3.1 |
| PO | 35.2 ± 2.8 | 35.3 ± 2.8 |
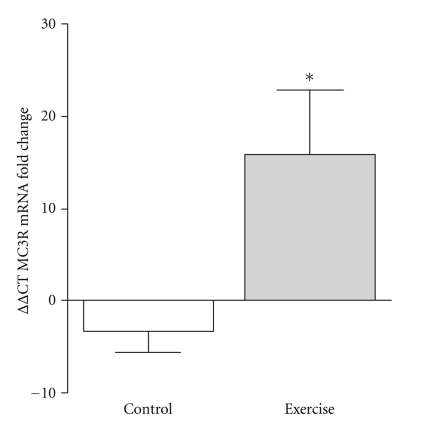
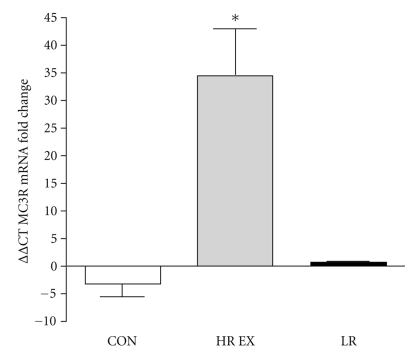
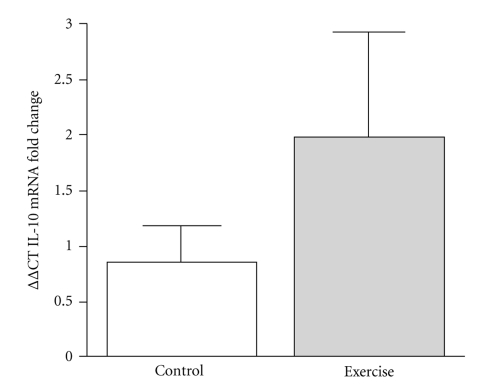
At baseline, there were no differences between CON and EX with respect to MC3R, TNF-α, or IL-10 mRNA. MC3R mRNA from whole blood samples was significantly upregulated (16 fold increase; P = .035) in response to RT. In comparison, control individuals showed no change in MC3R mRNA expression following the intervention period. (Figure 1). Additionally, when the EX group was stratified into groups of low responders (LR: fold change < 2; n = 4) versus high responders (HR: fold change ≥ 2; n = 5), HR EX showed a 34.9-fold increase (P = .002) in MC3R mRNA in response to RT, whereas LR EX showed no change in MC3R mRNA in response to RT when compared to CON (P > .05) (Figure 2). We observed a twofold increase in whole blood IL-10 gene expression in the EX group, although this change was not statistically significant (P = .249, Figure 3). IL-10 mRNA expression did not change in CON, and there was no change in whole blood TNF-α gene expression in either CON or EX groups.
Figure 1.
MC3R gene expression as assessed by real-time PCR in whole blood samples in CON (N = 10) and EX (N = 9). Values are expressed using the delta-delta Ct method to derive relative fold change. *MC3R mRNA was significantly upregulated 15.9-fold in the EX in comparison to CON (P = .035).
Figure 2.
MC3R gene expression stratified into high responders (HR EX: fold change ≥ 2) and low responders (LR EX: fold change < 2) as assessed by real-time PCR in whole blood samples in CON (N = 10) and HR EX (N = 4) and LR EX (N = 5). Values are expressed using the delta-delta Ct method to derive relative fold change. *HR EX was significantly upregulated 34.9-fold in comparison to CON (P = .002).
Figure 3.
IL-10 mRNA fold change, expressed as fold change using the delta-delta Ct method in CON (N = 9) and EX (N = 8) groups. There were no significant differences between the groups.
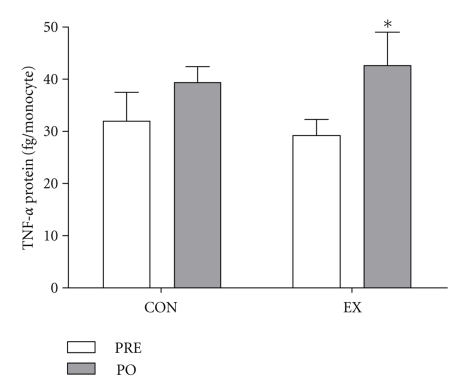
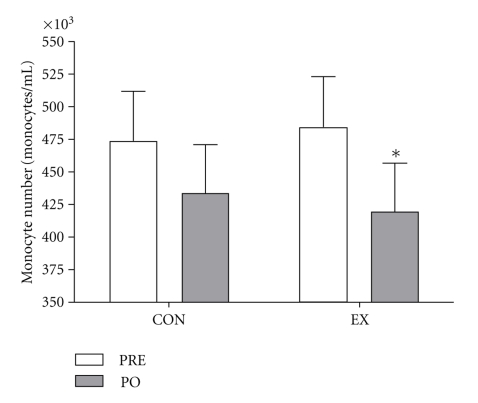
TNF-α concentrations from LPS-stimulated whole blood cultures increased after training (Figure 4) in EX individuals. There was no significant difference in LPS-stimulated TNF-α production in control individuals after the intervention period (Figure 4). There were no significant differences between HR and LR EX MC3R groups with respect to TNF-α or IL-10 mRNA or LPS-stimulated TNF-α production. There was no significant change in leukocyte number from PRE to PO training in either group EX PRE 6.3 ± 0.4103/ul, EX PO 5.9 ±1.8103/ul, CON PRE 6.2 ± 0.4103/ul, CON PO 6.1 ± 0.4 103/ul; (P > .05); however, significant training-induced differences in whole blood monocyte number were observed. Specifically, RT decreased monocyte number, but there was no difference in monocyte number in the CON group after the intervention period (Figure 5). There were no significant correlations between MC3R mRNA and IL-10 or TNF-α mRNA, LPS-stimulated TNF-α production, or monocyte number.
Figure 4.
LPS-stimulated TNF-α expressed in fg/monocyte for control (CON: N = 6) and resistance trained (EX: N = 8), before (PRE) and after (PO) the intervention period. *denotes significance (P < .05).
Figure 5.
Whole blood monocyte number before (PRE) and after (PO) the intervention in CON (N = 6) and EX (N = 8) participants. *denotes significance (P < .05).
4. Discussion
Traditionally, exercise has been used to help prevent or slow the progression of several inflammatory diseases including type 2 diabetes and coronary heart disease [26]. More recently, RT has been found to positively alter inflammatory profiles [9, 10, 27]. Here, we show evidence that MC3R may play a novel role in explaining these improvements. Specifically, our results are the first to suggest that chronic resistance training may be involved in increasing MC3R gene expression in human whole blood. These results are provocative given that there was a slight increase in IL-10 mRNA coupled with a significant increase in MC3R gene expression without a concomitant change in leukocyte number and a reduction in monocyte number. Given the lack of change in leukocyte counts and the reduction in monocyte number that has been shown in other studies [28], these findings point to changes in gene expression and cell function that are not merely reflective of changes in cell population number.
Previous data have suggested that other cell populations or tissues may be involved in controlling inflammation [29, 30]. Adiposity is directly related to inflammatory status, where inflammation is an indication of dysfunction in cellular metabolism and increased adiposity [31]. For example, MC3R KO mice exhibit increased adiposity and nutrient partitioning in addition to impaired immune function, evidenced by decreased macrophage infiltration and decreased monocyte chemoattractant protein 1 (MCP-1) and chemokine of differentiation 68 (CD68) mRNA expression into adipose tissue in comparison to wild-type mice [16, 32]. Conversely, Trevaskis et al. have shown an increase in macrophage infiltration and increases in and CD68 and MCP-1 mRNA expression within the adipose tissue in MC3R KO mice compared to control [33]. Comparisons of the two studies are difficult to make, as the mice in the study by Ellacott et al. were weaned directly onto 40% HF diet for 4 weeks and sacrificed thereafter, whereas the mice in the study by Trevaskis et al. were weaned onto a standard 10% chow diet then switched to an HF 60% diet at 12 to 14 weeks of age until 24 to 26 weeks of age at which time they were sacrificed [32, 33]. Differences in outcome variables may be due to the age of the mice as well as the fat content of the diet, with a dose response occurring due to each. Further support for an anti-inflammatory role of the MC3R occurs in models of vascular inflammation [34] and arthritis [35]. Within the mesenteric artery, it has been shown that stimulation of MC3R by αMSH decreases cell adhesion, emigration, and cytokine expression in response to ischemia and reperfusion injury of the vasculature [34]. Agonist stimulation of MC3R also decreases arthritis incidence and severity, evidenced by decreased nuclear factor kappa B (NFκB) DNA-binding activity in response to receptor activator of NF-kappaB ligand (RANKL) stimulation and decreased chemokine (C-C motif) ligand 2 (CCL2) expression. Additionally, MC3R expression is crucial in mediating the anti-inflammatory effects induced by vascular tissue injury, as MC3R KO mice exhibit increased cell emigration and adhesion and increased monocyte chemotactic protein-1(MCP-1) and keratinocyte-derived chemokine mRNA expression in response to mesenteric artery tissue injury [34]. MC3R KO mice also express increased IL-1β, IL-6, chemokine receptors, and chemokine ligands within isolated osteoclasts from arthritic joints, indicating increased inflammation in response to inflammatory stress, further supporting a role of MC3R in mediating the inflammatory response [35]. While it is well established that adiposity and MC3R expression play a significant role in inflammation [36], it is possible to achieve alterations in inflammation without changes in body composition. For example, exercise training studies have shown that inflammation can be altered, even with short intervention periods without a change in body composition [9, 37]. Thus, it is important to note that while the EX subjects in the current study tended to experience an increase in IL-10 mRNA expression and had increased expression of the MC3R, they did not experience significant alterations in body weight or composition. RT causes changes in gene expression independent of changes in adiposity and despite decreases in monocyte number.
The results presented here provide a glimpse into how circulating cell function may be altered with exercise. Specifically, maximal capacity to produce inflammatory cytokines, as evidenced by the LPS-stimulated TNF-a production, was increased in EX compared to CON groups. These data differ from a previous work which has shown a decrease in LPS-stimulated TNF-α after exercise training in postmenopausal women [28, 37]. It is possible that alterations in MC3R expression lead to sensitization of circulating immune cells to inflammatory stimuli. Thus, future studies that involve stimulating whole blood cultures with agonists and antagonists to MC3R and measuring inflammatory markers may provide more information on the activity of these receptors in response to RT. Exercise-induced changes in MC3R may be mediated by ACTH and αMSH [19, 38]. It is known that an increase of ACTH is observed during an acute bout of exercise [39]. Possible mechanisms responsible for these increases in ACTH may be related to the induction of IL-6 or IL-1β observed acutely after exercise [4, 6, 40]. Postexercise increases in IL-6 may be due to changes in macrophages/monocytes or myocyte IL-6 production, whereas IL-1β secretion is more tightly linked to leukocytes [5, 6]. These cytokines may be associated with increased exercise-induced concentrations of ACTH and our observed monocyte/macrophage (whole blood) sensitization and MC3R upregulation. It is also possible that sensitization of the MC3R and expression, indirectly regulated by proinflammatory cytokine production acutely during and following an exercise bout, may be linked to the reductions in circulating inflammatory markers observed in other exercise studies [2].
There were several limitations in this study. This was an exploratory investigation designed to examine the relationship between exercise and inflammation, but it was not specifically focused on the melanocortin receptor. As a result, we acknowledge that our subject pool was somewhat small. Regardless of sample size, however, we observed a statistically significant upregulation (16-fold) of the MC3R in our participants with a statistical power of 0.5811 with the current number of subjects. Yet, when we divided our EX group into LR and HR, we observed a significant upregulation (34.9 fold) of MC3R with a statistical power of 0.902. A larger study will be necessary to explore the reasons for the variation (HR versus LR) in response to the RT program. Furthermore, although MC3R is expressed in a number of immune cell populations, its actions have been most explored in the monocyte population because they are some of the most responsive and largest producers of inflammation in the body [41]. Thus, measuring exercise-induced changes in MC3R cell-surface expression in specific immune cell populations, including monocytes, may be an important direction for future work. Finally, stimulation assays in untrained and trained individuals with known agonists and antagonists to MC3R will help further understand the role of exercise in modulating the sensitivity of this receptor.
In summary, it appears that 12 weeks of moderate-intense resistance training led to a 16-fold upregulation of MC3R gene expression in whole blood from obese postmenopausal women. We are the first to examine the influence of exercise on aspects of the melanocortin system in humans. These findings may help to explain previously observed reductions in inflammation consequent to consistent exercise training.
Funding
This work was supported by a Texas Christian University Research and Creative Activities Fund Grant, the John Peter Smith Hospital Sport Medicine Program, and the Louisiana State University Graduate Student Corbett Award to T. M. Henagan.
Acknowledgments
The authors thank and acknowledge the time and efforts of their enthusiastic participants.
References
- 1.Rizzo M, Corrado E, Coppola G, Muratori I, Novo G, Novo S. Markers of inflammation are strong predictors of subclinical and clinical atherosclerosis in women with hypertension. Coronary Artery Disease. 2009;20(1):15–20. doi: 10.1097/MCA.0b013e3283109065. [DOI] [PubMed] [Google Scholar]
- 2.Pedersen BK. Exercise and cytokines. Immunology and Cell Biology. 2000;78(5):532–535. doi: 10.1111/j.1440-1711.2000.t01-11-.x. [DOI] [PubMed] [Google Scholar]
- 3.Plaisance EP, Grandjean PW. Physical activity and high-sensitivity C-reactive protein. Sports Medicine. 2006;36(5):443–458. doi: 10.2165/00007256-200636050-00006. [DOI] [PubMed] [Google Scholar]
- 4.Ostrowski K, Rohde T, Asp S, Schjerling P, Pedersen BK. Pro- and anti-inflammatory cytokine balance in strenuous exercise in humans. Journal of Physiology. 1999;515(1):287–291. doi: 10.1111/j.1469-7793.1999.287ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ostrowski K, Rohde T, Zacho M, Asp S, Pedersen BK. Evidence that interleukin-6 is produced in human skeletal muscle during prolonged running. Journal of Physiology. 1998;508(3):949–953. doi: 10.1111/j.1469-7793.1998.949bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goebel MU, Mills PJ, Irwin MR, Ziegler MG. Interleukin-6 and tumor necrosis factor-α production after acute psychological stress, exercise, and infused isoproterenol: differential effects and pathways. Psychosomatic Medicine. 2000;62(4):591–598. doi: 10.1097/00006842-200007000-00019. [DOI] [PubMed] [Google Scholar]
- 7.Woods JA, Lu Q, Ceddia MA, Lowder T. Exercise-induced modulation of macrophage function. Immunology and Cell Biology. 2000;78(5):545–553. doi: 10.1111/j.1440-1711.2000.t01-9-.x. [DOI] [PubMed] [Google Scholar]
- 8.Guirao X, Kumar A, Katz J, et al. Catecholamines increase monocyte TNF receptors and inhibit TNF through β-adrenoreceptor activation. American Journal of Physiology. 1997;273(6, part 1):E1203–E1208. doi: 10.1152/ajpendo.1997.273.6.E1203. [DOI] [PubMed] [Google Scholar]
- 9.Stewart LK, Flynn MG, Campbell WW, et al. The influence of exercise training on inflammatory cytokines and C-reactive protein. Medicine and Science in Sports and Exercise. 2007;39(10):1714–1719. doi: 10.1249/mss.0b013e31811ece1c. [DOI] [PubMed] [Google Scholar]
- 10.Phillips MD, Flynn MG, McFarlin BK, Stewart LK, Timmerman KL. Resistance training at eight-repetition maximum reduces the inflammatory milieu in elderly women. Medicine and Science in Sports and Exercise. 2010;42(2):314–325. doi: 10.1249/MSS.0b013e3181b11ab7. [DOI] [PubMed] [Google Scholar]
- 11.Lu Q, Ceddia MA, Price EA, Ye SM, Woods JA. Chronic exercise increases macrophage-mediated tumor cytolysis in young and old mice. American Journal of Physiology. 1999;276(2):R482–R489. doi: 10.1152/ajpregu.1999.276.2.R482. [DOI] [PubMed] [Google Scholar]
- 12.Catania A, Gatti S, Colombo G, Lipton JM. Targeting Melanocortin Receptors as a Novel Strategy to Control Inflammation. Pharmacological Reviews. 2004;56(1):1–29. doi: 10.1124/pr.56.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Konda Y, Gantz I, DelValle J, Shimoto Y, Miwa H, Yamada T. Interaction of dual intracellular signaling pathways activated by the melanocortin-3 receptor. Journal of Biological Chemistry. 1994;269(18):13162–13166. [PubMed] [Google Scholar]
- 14.Cone RD. Studies on the physiological functions of the melanocortin system. Endocrine Reviews. 2006;27(7):736–749. doi: 10.1210/er.2006-0034. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG. Identification of targets of leptin action in rat hypothalamus. Journal of Clinical Investigation. 1996;98(5):1101–1106. doi: 10.1172/JCI118891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Kilroy GE, Henagan TM, et al. Targeted deletion of melanocortin receptor subtypes 3 and 4, but not CART, alters nutrient partitioning and compromises behavioral and metabolic responses to leptin. FASEB Journal. 2005;19(11):1482–1491. doi: 10.1096/fj.05-3851com. [DOI] [PubMed] [Google Scholar]
- 17.Andersen GN, Hägglund M, Nagaeva O, et al. Quantitative measurement of the levels of melanocortin receptor subtype 1, 2, 3 and 5 and pro-opio-melanocortin peptide gene expression in subsets of human peripheral blood leucocytes. Scandinavian Journal of Immunology. 2005;61(3):279–284. doi: 10.1111/j.1365-3083.2005.01565.x. [DOI] [PubMed] [Google Scholar]
- 18.Mechanick JI, Levin N, Roberts JL, Autelitano DJ. Proopiomelanocortin gene expression in a distinct population of rat spleen and lung leukocytes. Endocrinology. 1992;131(1):518–525. doi: 10.1210/endo.131.1.1612033. [DOI] [PubMed] [Google Scholar]
- 19.Getting SJ, Gibbs L, Clark AJL, Flower RJ, Perretti M. POMC gene-derived peptides activate melanocortin type 3 receptor on murine macrophages, suppress cytokine release, and inhibit neutrophil migration in acute experimental inflammation. Journal of Immunology. 1999;162(12):7446–7453. [PubMed] [Google Scholar]
- 20.Redondo P, García-Foncillas J, Okroujnov I, Bandrés E. α-MSH regulates interleukin-10 expression by human keratinocytes. Archives of Dermatological Research. 1998;290(8):425–428. doi: 10.1007/s004030050330. [DOI] [PubMed] [Google Scholar]
- 21.Lam CW, Perretti M, Getting SJ. Melanocortin receptor signaling in RAW264.7 macrophage cell line. Peptides. 2006;27(2):404–412. doi: 10.1016/j.peptides.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 22.De Waal Malefyt R, Abrams J, Bennett B, Figdor CG, De Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. Journal of Experimental Medicine. 1991;174(5):1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson AS. Research design and analysis of data procedures for predicting body density. Medicine and Science in Sports and Exercise. 1984;16(6):616–620. [PubMed] [Google Scholar]
- 24.Jackson AS, Pollock ML, Ward A. Generalized equations for predicting body density of women. Medicine and Science in Sports and Exercise. 1980;12(3):175–182. [PubMed] [Google Scholar]
- 25.Siri WE, Lukaski HC. Body composition from fluid spaces and density: analysis of methods. Nutrition. 1993;9(5):480–492. [PubMed] [Google Scholar]
- 26.Rauramaa R, Halonen P, Väisänen SB, et al. Effects of aerobic physical exercise on inflammation and atherosclerosis in men: the DNASCO study. A six-year randomized, controlled trial. Annals of Internal Medicine. 2004;140(12):1007–1014. doi: 10.7326/0003-4819-140-12-200406150-00010. [DOI] [PubMed] [Google Scholar]
- 27.Brooks N, Layne JE, Gordon PL, Roubenoff R, Nelson ME, Castaneda-Sceppa C. Strength training improves muscle quality and insulin sensitivity in Hispanic older adults with type 2 diabetes. International Journal of Medical Sciences. 2007;4(1):19–27. doi: 10.7150/ijms.4.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McFarlin BK, Flynn MG, Campbell WW, et al. Physical activity status, but not age, influences inflammatory biomarkers and toll-like receptor 4. Journals of Gerontology A. 2006;61(4):388–393. doi: 10.1093/gerona/61.4.388. [DOI] [PubMed] [Google Scholar]
- 29.Starkie RL, Rolland J, Angus DJ, Anderson MJ, Febbraio MA. Circulating monocytes are not the source of elevations in plasma IL-6 and TNF-α levels after prolonged running. American Journal of Physiology. 2001;280(4):C769–C774. doi: 10.1152/ajpcell.2001.280.4.C769. [DOI] [PubMed] [Google Scholar]
- 30.Starkie RL, Angus DJ, Rolland J, Hargreaves M, Febbraio MA. Effect of prolonged, submaximal exercise and carbohydrate ingestion on monocyte intracellular cytokine production in humans. Journal of Physiology. 2000;528(3):647–655. doi: 10.1111/j.1469-7793.2000.t01-1-00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stienstra R, Duval C, Müller M, Kersten S. PPARs, obesity, and inflammation. PPAR Research. 2007;2007:10 pages. doi: 10.1155/2007/95974. Article ID 95974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ellacott KLJ, Murphy JG, Marks DL, Cone RD. Obesity-induced inflammation in white adipose tissue is attenuated by loss of melanocortin-3 receptor signaling. Endocrinology. 2007;148(12):6186–6194. doi: 10.1210/en.2007-0699. [DOI] [PubMed] [Google Scholar]
- 33.Trevaskis JL, Gawronska-Kozak B, Sutton GM, et al. Role of adiponectin and inflammation in insulin resistance of Mc3r and Mc4r knockout mice. Obesity. 2007;15(11):2664–2672. doi: 10.1038/oby.2007.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leoni G, Patel HB, Sampaio ALF, et al. Inflamed phenotype of the mesenteric microcirculation of melanocortin type 3 receptor-null mice after ischemia-reperfusion. FASEB Journal. 2008;22(12):4228–4238. doi: 10.1096/fj.08-113886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel HB, Bombardieri M, Sampaio ALF, et al. Anti-inflammatory and antiosteoclastogenesis properties of endogenous melanocortin receptor type 3 in experimental arthritis. FASEB Journal. 2010;24(12):4835–4843. doi: 10.1096/fj.10-167759. [DOI] [PubMed] [Google Scholar]
- 36.Maury E, Brichard SM. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Molecular and Cellular Endocrinology. 2010;314(1):1–16. doi: 10.1016/j.mce.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 37.Phillips MD, Flynn MG, McFarlin BK, Stewart LK, Timmerman KL, Ji H. Resistive exercise blunts LPS-stimulated TNF-α and Il-1β. International Journal of Sports Medicine. 2008;29(2):102–109. doi: 10.1055/s-2007-965115. [DOI] [PubMed] [Google Scholar]
- 38.Yanagita S, Amemiya S, Suzuki S, Kita I. Effects of spontaneous and forced running on activation of hypothalamic corticotropin-releasing hormone neurons in rats. Life Sciences. 2007;80(4):356–363. doi: 10.1016/j.lfs.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 39.Radosevich PM, Nash JA, Lacy DB, O’Donovan C, Williams PE, Abumrad NN. Effects of low- and high-intensity exercise on plasma and cerebrospinal fluid levels of ir-β-endorphin, ACTH, cortisol, norepinephrine and glucose in the conscious dog. Brain Research. 1989;498(1):89–98. doi: 10.1016/0006-8993(89)90402-2. [DOI] [PubMed] [Google Scholar]
- 40.Parsadaniantz SM, Levin N, Lenoir V, Roberts JL, Kerdelhue B. Human interleukin 1β: corticotropin releasing factor and ACTH release and gene expression in the male rat: in vivo and in vitro studies. Journal of Neuroscience Research. 1994;37(6):675–682. doi: 10.1002/jnr.490370602. [DOI] [PubMed] [Google Scholar]
- 41.Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. Journal of Leukocyte Biology. 2007;81(3):584–592. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]