Abstract
Obesity is an epidemic problem in the world and is associated with several health problems, including diabetes, cardiovascular disease, respiratory failure, muscle weakness, and cancer. The precise molecular mechanisms by which obesity induces these health problems are not yet clear. To better understand the pathomechanisms of human disease, good animal models are essential. In this paper, we will analyze animal models of obesity and their use in the research of obesity-associated human health conditions and diseases such as diabetes, cancer, and obstructive sleep apnea syndrome.
1. Introduction
Obesity, defined as a body mass index (BMI) >30 kg/m2, is a significant health problem [1]. Obesity has reached epidemic proportions globally, and the World Health Organization estimates that there are more than 1 billion overweight adults, of which at least 300 million are obese [2]. Societal changes and the worldwide nutrition transition have driven the obesity epidemic over recent decades. Economic growth as well as modernization, urbanization and globalization of food markets are some of the elements that have contributed to the obesity epidemic. Significant shifts toward less physically demanding work have been observed worldwide. Decreased physical activity has also been associated with increasing opportunities to use automated transport, have technology in the home, and engage in more passive leisure pursuits [2].
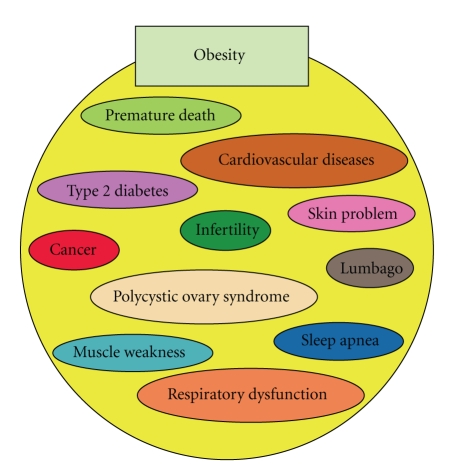
Obesity is associated with premature death through increasing the risk of many chronic diseases, including type 2 diabetes, cardiovascular disease, and certain cancers (Figure 1) [3, 4]. In addition, obesity is associated with respiratory difficulties, chronic musculoskeletal problems, lumbago, skin problems, and infertility (Figure 1) [4]. Most of the evidence proposing obesity-associated health problems has been obtained from epidemiological analyses of human subjects; the precise molecular mechanisms of obesity-associated health problems have not yet been determined. In this paper, we will summarize reports associated with obesity-related pathology using animal models and also propose further demand for animal research models to address the worldwide obesity epidemic.
Figure 1.
Obesity-associated complications. Obesity is associated with various health conditions in humans.
2. Animal Models of Obesity
There are many rodent and nonrodent models of obesity. We introduce several widely used important animal models of obesity in this section.
2.1. Rodent Models
2.1.1. Monogenic Mouse Obesity
Lethal Yellow Mutant Mouse (Ay) —
Among the several commonly existing obese mice currently used in research, the agouti mutation mouse was first reported more than century ago. In 1992, the agouti protein was cloned by Bultman et al., and agouti became the first obesity gene characterized at the molecular level [5]. Agouti is a pigment control gene transiently expressed in follicular melanocytes to induce the production of red/yellow pheomelanin pigment and inhibit black/brown pigment [6–8]. The lethal yellow mutant mouse (Ay) is one of five dominant agouti mutations and has been found to be an excellent mouse model of obesity [5]. The Ay mutation is characterized by the deletion of 120–170 kb genomic DNA, resulting in ubiquitous agouti expression due to loss of the tissue-specific control promoter element [9–11]. Ay mice exhibit several phenotypes such as a yellow coat color, mature-onset obesity, type-II diabetes, hyperleptinemia, increased linear growth, higher tumor susceptibility, and infertility [5]. Transgenic mice expressing ubiquitous agouti exhibited yellow coat color, obesity, hyperinsulinemia, and hyperglycemia similar to Ay mouse [12], revealing the molecular mechanism of agouti in mouse phenotype. Mice with adipose tissue-specific agouti overexpression exhibit an overgrowth of adipose tissue without alteration of food intake, suggesting that increased fat in this model is due to changes in energy metabolism [13]. The adipose tissue agouti overexpression model could be relevant to human obesity because agouti gene expression is found in human adipose tissue [14, 15] and is increased in the adipose tissue of type 2 diabetic subjects [16]. Likely the ectopic expression of agouti in mice pancreas stimulated the release of insulin by pancreatic β-cells, which may further enhance agouti-stimulated lipogenesis [17]. Transgenic Agouti expression in skin did not induce obesity, suggesting that the obesogenic role of agouti is tissue dependent [18].
Leptin Signaling Defects in Mice: ob/ob and db/db Mouse Models —
In 1949, researchers from the Jackson Laboratory discovered obese mice by chance [19]. Responsible mutation gene is named as obese (ob) gene. The ob/ob mutation is recessive, and neonatal mutant mice are normal when compared to unaffected control littermates. Mutant mice, however, gain weight rapidly throughout their lives, eventually reaching a weight that is three times that of control mice. The phenotype is clear, but identification of the gene responsible for the obese phenotype took nearly 50 years [20]. In 1994, Zhang et al. identified the mutation in the leptin gene as responsible for ob mutation by positional cloning [20]. Leptin is a gene expressed abundantly in the adipose tissue. Characterization of this mutation revealed a single base pair deletion in the leptin coding region that results in a frame shift and a premature stop codon [20]. The leptin protein plays an important role in appetite control. Therefore, ob/ob mice exhibit uncontrollable food intake, obesity, type 2 diabetes, and insulin resistance with hyperinsulinemia.
The db/db mouse was identified initially in 1966 by researchers in the Jackson Laboratory as an obese mouse [21]. The db (stands for “diabetes”) mutation is an autosomal recessive trait that encodes for a G-to-T point mutation in the leptin receptor gene, resulting in defective leptin signaling [22, 23]. Impaired leptin signaling in the hypothalamus leads to persistent hyperphagia and obesity, with consequent hyperleptinemia, insulin resistance, and increased insulin levels [22, 23]. At 1 month of age, db/db mice are larger/obese when compared to control (heterozygous) littermates, and db/db mice present increased fat deposition in the inguinal and axillary regions. db/db mice also develop frank hyperglycemia by 8 weeks of age. Consequently, these mice are widely used as a model for the study of type 2 diabetes [22, 23].
2.1.2. Polygenic Mouse Obesity
Although monogenic models provide important information on the biology of obesity, human obesity is most likely mediated by multiple genes. Therefore, polygenic models could be much more relevant to human obesity.
New Zealand Obese (NZO) Mouse —
The NZO strain is a polygenic mouse model of obesity that exhibits type 2 diabetes only in males. NZO mice increase their body weight rapidly during the first 2 months of life because of hyperphagia that may be associated with leptin resistance, although they have genetically normal leptin and leptin receptors. Among polygenic mouse models of obesity, NZO mice exhibit the most severe phenotype, with fat depots accounting for more than 40% of total body weight at 6 months of age [24]. Additionally, NZO mice exhibit decreased exercise activity when compared to control or even ob/ob mice [25]. This information suggests that like human obesity, obesity in NZO mice is due to a combination of hyperphagia, reduced energy expenditure, and insufficient physical activity.
Tsumura Suzuki Obese Diabetes (TSOD) Mouse —
Through the selection of obese and urine sugar positive colonies from the ddY strain of mice, Tsumura and Suzuki established two inbred strains. The TSOD strain develops obesity with diabetes, whereas the Tsumara Suzuki nonobese (TSNO) strain does not become obese [26]. Male TSOD mice exhibit polygenic obesity with hyperglycemia and hyperinsulinemia [26, 27]. Although the mean values of fed blood glucose concentrations in TSOD mice are increased with age (232 mg/dl at 13 weeks, 269 mg/dl at 16 weeks, and 346 mg/dl at 24 weeks), severe diabetes does not develop because TSOD mice display increased β-cell mass and maintain insulin secretion to control blood glucose [26]. Older TSOD mice display similar lesion as diabetic nephropathy and neuropathy [28].
M16 Mouse —
The M16 mouse, an outbred mouse model of early-onset polygenic obesity, was developed through long-term selection for 3- to 6-week weight gain in an ICR background [29]. M16 mice exhibit hyperphagia, hyperinsulinemia, and hyperleptinemia compared to ICR controls. M16 males and females were found to be moderately hyperglycemic compared to ICR controls, with 56% and 22% higher fasted plasma glucose levels, respectively, at 8 weeks of age [29].
Kuo Kondo (KK) Mouse —
The KK mouse is a polygenic model of obesity that also exhibits type 2 diabetes. The KK mouse was developed in Japan with selective inbreeding for large body size [30]. KK mice display hyperphagia, hyperinsulinemia, and insulin resistance and show moderate obesity by 2 months of age [31, 32]. Insulin resistance in KK mice precedes the onset of obesity [33]. The KK mouse strain was modified to develop the KKAy mouse by transferring the lethal yellow obese gene (Ay); the KKAy mouse is widely used for obesity and diabetes research in the testing of experimental therapies [34].
2.1.3. Rat Models of Obesity
Zucker Fatty Rat (ZFR) —
In 1961, L. M. Zucker and T. F. Zucker reported a seminal finding in obesity research [35]: an autosomal recessive mutation in the fatty (fa) gene on chromosome 5. These rats are characterized by hyperphagia and early-onset obesity, which appears at 5 weeks of age as an accumulation of subcutaneous fat. Although ZFR also exhibits marked insulin resistance [36], their blood sugar levels remain normal [37]. Later, the fa gene was shown to be the leptin receptor gene [38]. A ZFR does not develop diabetes [37]. However, a substrain of the ZFR that exhibits frank diabetes was discovered and was designated the Zucker diabetic fatty (ZDF) rats [39].
Wistar Fatty Rat —
In 1981, Ikeda et al. reported another obesity rat model, the Wistar fatty rat (WFR) [40]. The WFR strain was derived by transferring the fa gene from ZFR (13 M strain) to Wistar Kyoto rats, which exhibit poor glucose tolerance [40]. WFR displays obesity from 3 weeks after birth and develops obesity-related diseases such as type 2 diabetes, hyperinsulinemia, and hyperlipidemia. Metabolic abnormalities are prominent in WFR males, but not in WFR females, which display only mild insulin resistance and some glucose intolerance [40]. The appearance of diabetes in WFR but not in ZFR, despite the presence of the fa (leptin receptor) mutation in both strains, could be explained by the presence of other genetic factors in WFR. The WFR strain is widely used for research in type 2 diabetes because aged WFR displays diabetic complications such as nephropathy and neuropathy [41–44].
Otsuka Long Evans Tokushima Fatty (OLETF) Rat —
OLETF rats, established by Otsuka Pharmaceuticals in Tokushima, Japan, were developed by the selection of spontaneously type 2 diabetic rats from the outbreeding of Long Evans rats in a closed colony of Charles River [45]. OLETF rats are hyperphagic beginning several weeks after birth, with increasing body weight eventually progressing to frank obesity [46]. At approximately 25 weeks after birth, all male OLETF rats display diabetes, as determined by oral glucose tolerance test, whereas only 30% of female OLETF rats develop diabetes even after 60 weeks of age. Hyperinsulinemia is observed beginning at 8 weeks of age, and insulin resistance is observed beginning at 12 weeks of age. At 25 weeks of age, male OLETF rats display hyperplasia of pancreatic islets, but islets become atrophic by 60 weeks of age [46, 47]. In contrast to ZDF rats, hyper-free fatty acidemia has not been observed in OLETF rats. Instead, hypertriglyceridemia is found to precede the onset of hyperglycemia and insulin resistance [47]. OLETF rats are widely used in obesity and diabetes research.
2.1.4. Diet-Induced Obesity
High-Fat Diet —
A high-fat diet (HFD) is often utilized in obesity research as a non-leptin-deficient model. There are mouse strain-specific differences in responses to the HFD (Table 1) [48]. Among the various strains, C57BL/6J mice are the most widely used for HFD-induced obesity because they exhibit abnormalities similar to human metabolic syndrome when fed the HFD [49]. Interestingly, within the C57 mouse strain, there are significant differences among substrains in response to the HFD. For instance, whereas C57BL/6J mice exhibit HFD-induced obesity, hyperinsulinemia, and insulin resistance that closely parallel the progression of human disease, C57BL/KsJ mice display a weak phenotype [49]. Beside C57BL/J6 mice, sand mice and spiny mice are also used in obesity/type 2 diabetes research. Sprague Dawley or Long Evans rats are also used for nonmouse rodent models of HFD-induced obesity [50].
Using such high-fat diet-induced obesity mice models, some clues to fight against human obesity have been reported; manipulation of diet may rescue the obesity phenotype even in high-fat-fed condition.
Watanabe et al. reported seminal finding about the antiobesitogenic role of bile acids (BAs) in mice. BAs have been long recognized as simple lipid solubilizers, however, during the last decades researchers revealed that BAs play pivotal roles in the complex metabolic regulations. Watanabe et al. found that high-fat diet supplemented with 0.5% cholic acid, the BA found in the largest amount, prevented weight gain and adiposity without alteration in the amount of food intake [51]. They found that BAs activate G-protein-coupledTGR5 receptor and induce type 2 deiodinase activity. Such activation of type 2 deiodinase results in the conversion of thyroxine (T4) to triiodothyronine (T3), which enhances energy expenditure [51]. Furthermore, agonistic compound for TGR5, INT-777, mimics such metabolic effect of BAs and inhibited the onset of steatosis in high-fat-fed mice [52]. Furthermore, INT-777 induces incretin effects via the secretion of glucagon-like peptide (GLP)-1 and therefore ameliorated glucose tolerance [52]. These researches revealed the potential importance of BAs for the prevention of diet-induced obesity and associated health problem. Interestingly postcholecystectomized patients have been shown to have high prevalence of type 2 diabetes [53].
Another nutritional intervention method, which could prevent metabolic abnormality, is the diet with high ketogenic essential amino acid (KAA) such as leucine, isoleucine, valine, lysine, and threonine. Zhang et al. have reported that high-leucine feeding in mice prevented high-fat diet-induced obesity [54]. Such enhanced administration of high-KAA mixture diet modulated lipid synthetic pathway and prevented hepatic steatosis and insulin resistance with the reduction of body weight under the high-fat diet [55]. Interestingly such high-KAA mixture has been shown to improve insulin sensitivity in elderly type 2 diabetic subjects [56]. These reports indicated that the high-fat diet-induced obesity animals could be the good model for the experimental therapy and the translational research to discover a novel therapeutic strategy for obesity epidemic.
Table 1.
Different phenotypes of inbred mouse strains with diet- or genetically induced obesity (summary of references [50, 74, 75]).
| Strain | Characteristics | Crossed with obese mice, and so forth |
|---|---|---|
| C57BL/6J | High-fat diet-induced diabetes and obesity [119] | Lepob/ob mice exhibit obesity but not diabetes [120] |
| C57BLKS/J | High-fat diet-induced diabetes and obesity weaker than C57BL/6J [49] | Lepob/ob mice exhibit obesity with severe diabetes [120] |
| DBA/2 | More glucose tolerance than C57BL/6 on a high-fat diet [121] | Lepob/ob mice exhibit obesity with severe diabetes [122] |
| 129sv | Low insulin; more glucose tolerance than other strains on a high-fat diet [123] | Homozygous for db allele db3J mice display mild/transient hyperglycemia with marked hyperinsulinemia and develop hypoglycemia leading to sudden death [124] |
| BTBR | Abdominal obesity with peripheral, but not hepatic insulin resistance [125] | Lepob/ob mice exhibit obesity with severe diabetes [126] |
| A/J | Low glucose level even on a high-fat diet; obesity and diabetes-resistant [119, 127, 128] | Not reported |
| BALB/c | Similar to A/J; glucose tolerance [121] | Lepob/ob mice exhibit reduced adiposity and increased thermogenesis and are fertile [129] |
| C3H | High glucose tolerance with robust insulin secretion [121] | Not reported |
| AKR | Sensitive to diet-induced obesity with hyperinsulinemia and insulin resistance [48] | Not reported |
| CAST/Ei | Lean at 12 weeks on a high-fat diet [130] | Not reported |
| Nonobese Diabetic | 45% fat diet-induced transient hyperglycemia with severe obesity [75] | Not reported |
| New Zealand Obese | Resemble metabolic syndrome in humans; high-fat diet-induced obesity and hyperglycemia [24, 25] | Not reported |
| FVB | High glucose levels with lower levels of insulin on normal chow [75] | Leprdb/db mice display insulin resistance, severe hyperglycemia, and marked hyperinsulinemia compared to C57BL/6 [131] |
| Kuo Kondo | Obesity; hyperleptinemia; increased glucose and HbA1c; hyperinsulinemia similar to C57BLKS/J Leprdb/db mice [30] | Ay mutation in agouti results in frank diabetes with nephropathy [34] |
| TallyHo | Natural model of obesity with type 2 diabetes [132] | Not reported |
| Nagoya-Shibata-Yasuda | Exhibit obesity; all males and 1/3 of females exhibit glucose intolerance [133] | Not reported |
| ALS/Lt | Sensitive to alloxan-induced diabetes [134] | Introduction of Ay mutation induces diabetes in 100% of males and 60% of females at 24 weeks of age [135] |
| M16 | Increased body weight, fat and food intake; both males and females develop hyperinsulinemia; only males develop moderate hyperglycemia [29] | Not reported |
| LG and SM | High-fat diet-stimulated body weight gain and increased plasma glucose more in SM than LG mice [136] | Not reported |
| Tsumura, Suzuki, Obese Diabetes | Obesity and moderate diabetes [26, 27] | Not reported |
| Akita | Mutation in insulin 2 gene; nonobese [137] | Not reported |
2.2. Nonrodent Models of Obesity
Obese Monkeys —
During evolution, primates diverged from rodent lineages about 65–85 million years ago [57]. In comparison, humans and other great apes (Hominoidea) diverged from Old World monkeys (Cercopithecoidea) a relatively recent 25 million years ago [58]. Obesity models in Old World monkeys, such as macaques, rhesus monkey, and baboons, would therefore provide information relevant to human obesity. When raised in indoor cages, Rhesus monkeys exhibit increased rates of obesity, with some of them developing obesity-associated diseases [59–61]. Captive macaques display obesity in an age-dependent manner when given food ad libitum [62]. Like humans, these monkeys develop type 2 diabetes and diabetic complications. It is likely that reduced exercise increases the risk of obesity in these monkeys [62–64]. Spontaneous obesity is also found in wild baboons and in a pedigreed colony [65–67] and occurs in free-ranging rhesus monkeys [68]. Furthermore, a species of Japanese monkey, Macaca fuscata, develops obesity without frank diabetes [69].
3. Human Disease and Obesity Animal Models
Genetic models provide useful information about the biology of obesity in humans. This does not mean, however, that these models can provide information on how obesity can cause other health problems. In this section, we introduce several animal models for analyzing human obesity-associated disease pathology.
3.1. Diabetes and Obesity
Type 2 diabetes is associated with insulin resistance and is one of the most common metabolic diseases. The incidence of type 2 diabetes has dramatically increased in the past two decades, coinciding with the epidemic of obesity. The pathogenesis of insulin resistance and diabetes-associated complications remains unclear. Research on type 2 diabetes using animal models of obesity is therefore quite significant.
Models of obesity with type 2 diabetes are classified into two categories: (1) those containing a mutation in the leptin or leptin receptor gene and (2) polygenic models. Obese rodents, such as Zucker rats, ob/ob mice, and db/db mice, are used as models for type 2 diabetes. Obesity in these models is due to leptin signaling deficiency. These rodent models exhibit microvascular complications similar to humans, such as diabetic retinopathy and nephropathy, and provide important models for testing experimental therapeutics. However, leptin abnormalities only comprise a minority of obesity/diabetes cases in humans [70–72] and are not the same condition of type 2 diabetes that is a worldwide epidemic.
Polygenic models of obesity with diabetes may provide more insight to the human condition. Certain inbred strains of mice exhibit remarkable obesity when fed on HFD, whereas others remain lean [48, 49, 73], suggesting gene-diet interactions. Furthermore, some of the strains exhibit obesity with severe insulin resistance and glucose intolerance, whereas others are highly sensitive to insulin-mediated glucose uptake and are resistant to the onset of diabetes (Table 1) [50, 74, 75]. In contrast, some strains are very prone to type 2 diabetes but not severely obese. Those polygenic models allow for analysis of diabetic phenotypes alone, or the mice can be fed on HFD or crossed with another obesity mouse model, such as ob/ob, db/db, or Ay (Table 1) [50, 74, 75].
3.2. Cancer and Obesity
Obesity in humans is associated with the incidence of several cancers. Likewise, type 2 diabetes has been associated with an increased risk of cancer. Several mechanisms have been proposed to explain the interaction between obesity and cancer development, including the prevalence of type 2 diabetes, increased insulin resistance, elevated levels of insulin-like growth factor 1 (IGF-1), and increased production of sex steroid hormones and adipocytokines [76–80]. However, clear molecular mechanisms that explain obesity-associated cancer have yet to be determined. Recently, Park et al. reported a breakthrough observation in carcinogenesis in obesity [81]. They found that diethylnitrosamine-induced HCC is significantly higher in both genetically (ob/ob) and HFD-induced (59% fat, 15% protein, 26% carbohydrates) obese mice [81]. Furthermore, HFD induced the growth of subcutaneously injected HCC, suggesting that obesity has a systemic effect on tumorigenesis [81]. With regard to the mechanisms of tumorigenesis in obesity, they found that obesity is associated with increased intracellular transcriptional factor STAT signaling and liver inflammation [81]. This inflammation was demonstrated to be essential for the tumor promoting effects of obesity because the depletion of signaling by inflammatory cytokines IL-6 and TNF-α abolished the tumor promoting effects of obesity [81].
Metformin belongs to the biguanide class of antitype 2 diabetic drugs. Since the middle ages, the biguanide Galega officinalis (goat's rue or French lilac) has been used to treat diabetic patients. Accumulating evidence suggests that metformin reduces cancer incidence in type 2 diabetic patients. Metformin activates AMPK and inhibits the mTOR signaling pathway via various mechanisms [82–86]. Metformin treatment has been shown to result in a gene expression profile similar to that of long-term caloric restriction [87], which can reduce the incidence of many age-related diseases, including cancer [88, 89]. Metformin treatment inhibits high-energy diet-stimulated colon cancer cell growth [90] and breast tumor growth in HFD-fed mice but did not inhibit tumor growth in mice fed normal chow [91]. Although the effects of metformin in obesity-related cancer biology are not clear, these reports suggest that the tumor suppressive effect of metformin may involve the amelioration of a systemic metabolic profile associated with a high-energy diet and obesity.
Elevated leptin levels, often found in obesity, may affect cancer cell growth. Recently, Ribeiro et al. injected androgen-insensitive murine prostate carcinoma RM1 cells into control male C57BL6 and genetic (ob/ob mice and db/db mice) and HFD mouse models of obesity [92]. They found that low-leptin models (ob/ob and HFD mice) exhibited large tumors, whereas high-leptin (db/db) mice exhibited small tumors, suggesting that leptin may inhibit RM1 tumor growth [92]. In contrast, Gonzalez et al. reported that leptin may accelerate the growth of breast tumors in mice via induction of VEGF-VEGFR2-mediated angiogenesis [93], although their study used immunodeficient SCID mice and not a mouse model of obesity [94]. Similar leptin-induced proliferation and invasiveness has been shown in endometrial cancer [95]. Bartucci et al. reported that colorectal cancer stem cells express leptin receptors, and therefore leptin may induce tumor growth and interfere with the cytotoxic effects of the anticancer drug 5-FU [96]. The reports regarding the role of leptin in carcinogenesis are still very controversial and require further followup studies.
3.3. Obstructive Sleep Apnea and Obesity
Obstructive sleep apnea (OSA) is an important obesity-associated health problem that is characterized by obstruction of the airway and depletion of oxygen tone in the blood. OSA may be associated with the onset of hypertension, diabetes, and coronary heart disease. Although OSA is of clinical importance, the etiology of OSA is not yet clear, perhaps due to the lack of appropriate animal models. The first animal model reported to exhibit sleep apnea was the English bulldog [97]. These animals exhibit respiration disorders and decreased O2 saturation that worsen during rapid-eye-movement sleep. Most bulldogs experience less than 90% O2 saturation for prolonged durations [97]. Two varieties of obese pigs were also found to be good models of OSA [98, 99]. Although these models provide important information about the pathomechanisms of OSA, large-animal-based research is technically difficult. Therefore, for the development of experimental therapies and drugs, rodent models are superior. In 1996, Van Lunteren et al. reported altered respiratory-associated muscle contraction in genetically obese ZFR [100]. Later, this model was found to exhibit sleep apnea syndrome [101]. ZFRs have since been used for various experimental therapies and have provided important information about OSA [102–104].
Although these dog, pig, and rat models help improve our understanding of the pathophysiology of OSA, mouse models are critical in identifying the genes conferring disease risk [105]. Tagaito et al. developed a gas-delivery system that alters O2 levels depending on the sleep-wake status of C57BL/6 male mice. However, this model is not a natural OSA model and is not a good representation of OSA [106]. Recently, NZO mice were used as a model mouse for sleep apnea syndrome [107]. NZO mice exhibit polygenic obesity and metabolic syndromes, such as insulin resistance, diabetes, hyperlipidemia, and hypertension (Table 1), much like a human sleep apnea patient. This report suggests that the NZO mouse may be a useful model for testing new drugs and experimental therapies for OSA.
4. Why Do Humans Gain Weight? Why Do Humans Like to Eat Fat?
All the data above might be focused mainly on the pathogenesis of obesity/obesity-related complications. Most publications may shed light on the pathology of obesity by forcing HFD or genetic mutations in rodents; however, the conditions are very different from the real problems that humans are facing. For example, although leptin-deficient rodents have been used in many obesity-associated studies, leptin/leptin receptor mutations are rare in humans [70–72]. The main difference between experimental animal models and human obesity is that humans do not have induced gene mutations and are not forced to eat HFDs. Instead, humans tend to enjoy eating such diets. If we can answer the question of why some individuals prefer to eat high-fat food and others do not, we would have a direct solution for obesity. Little evidence is currently available on this topic, but some seminal results have been shown [108, 109]. It has also been reported that the variation in fat consumption (ranging from 26 to 83% of total energy) is dependent on the response of inbred mouse strains to the macronutrient diet selection paradigm [110]. This theory suggests that there are strain-specific differences in food selection behavior, which could potentially be mediated by differences in brain neuropeptides [111–116]. These reports indicate that further investigation on this topic in conjunction with human epidemiological and genetic studies is required.
5. Conclusion
We have summarized many current animal models of obesity and obesity-associated human diseases. However, animal models have not yet been established for some devastating obesity-associated human diseases, including polycystic ovary syndrome [117, 118], which is extremely prevalent and constitutes one of the most common endocrinopathy in women of reproductive age. Suitable animal models are fundamental to testing novel therapeutic strategies against disease. Therefore, intensive and continuous efforts should be made to establish novel obesity-associated animal models that mimic human health problems.
Acknowledgments
The authors declare no conflict of interest. Author's laboratory is supported by the grant from Japan Society for the Promotion of Science to D. Koya and a grant from the Uehara Memorial Foundation to D. Koya. K. Kanasaki is currently supported by a grant from Kanae Foundation for the Promotion of Medical Science.
References
- 1.Kopelman P. Health risks associated with overweight and obesity. Obesity Reviews. 2007;8(1):13–17. doi: 10.1111/j.1467-789X.2007.00311.x. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Fact sheet: Obesity and overweight. http://www.who.int/hpr/gs.fs.obesity.shtml.
- 3.Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9, article 88 doi: 10.1186/1471-2458-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown WV, Fujioka K, Wilson PW, Woodworth KA. Obesity: why be concerned? The American Journal of Medicine. 2009;122(4):S4–S11. doi: 10.1016/j.amjmed.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Bultman SJ, Michaud EJ, Woychik RP. Molecular characterization of the mouse agouti locus. Cell. 1992;71(7):1195–1204. doi: 10.1016/s0092-8674(05)80067-4. [DOI] [PubMed] [Google Scholar]
- 6.Lu D, Willard D, Patel IR, et al. Agouti protein is an antagonist of the melanocyte-stimulating-hormone receptor. Nature. 1994;371(6500):799–802. doi: 10.1038/371799a0. [DOI] [PubMed] [Google Scholar]
- 7.Millar SE, Miller MW, Stevens ME, Barsh GS. Expression and transgenic studies of the mouse agouti gene provide insight into the mechanisms by which mammalian coat color patterns are generated. Development. 1995;121(10):3223–3232. doi: 10.1242/dev.121.10.3223. [DOI] [PubMed] [Google Scholar]
- 8.Matsunaga N, Virador V, Santis C, et al. In situ localization of agouti signal protein in murine skin using immunohistochemistry with an ASP-specific antibody. Biochemical and Biophysical Research Communications. 2000;270(1):176–182. doi: 10.1006/bbrc.2000.2409. [DOI] [PubMed] [Google Scholar]
- 9.Michaud EJ, Bultman SJ, Stubbs LJ, Woychik RP. The embryonic lethality of homozygous lethal yellow mice (A(y)/A(y)) is associated with the disruption of a novel RNA-binding protein. Genes and Development. 1993;7(7 A):1203–1213. doi: 10.1101/gad.7.7a.1203. [DOI] [PubMed] [Google Scholar]
- 10.Duhl DMJ, Stevens ME, Vrieling H, et al. Pleiotropic effects of the mouse lethal yellow (A(y)) mutation explained by deletion of a maternally expressed gene and the simultaneous production of agouti fusion RNAs. Development. 1994;120(6):1695–1708. doi: 10.1242/dev.120.6.1695. [DOI] [PubMed] [Google Scholar]
- 11.Michaud EJ, Bultman SJ, Klebig ML, et al. A molecular model for the genetic and phenotypic characteristics of the mouse lethal yellow (A(y)) mutation. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(7):2562–2566. doi: 10.1073/pnas.91.7.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klebig ML, Wilkinson JE, Geisler JG, Woychik RP. Ectopic expression of the agouti gene in transgenic mice causes obesity, features of type II diabetes, and yellow fur. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(11):4728–4732. doi: 10.1073/pnas.92.11.4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mynatt RL, Miltenberger RJ, Klebig ML, et al. Combined effects of insulin treatment and adipose tissue-specific agouti expression on the development of obesity. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(3):919–922. doi: 10.1073/pnas.94.3.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwon HY, Bultman SJ, Löffler C, et al. Molecular structure and chromosomal mapping of the human homolog of the agouti gene. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(21):9760–9764. doi: 10.1073/pnas.91.21.9760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson BD, Ollmann MM, Kang L, Stoffel M, Bell GI, Barsh GS. Structure and function of ASP, the human homolog of the mouse agouti gene. Human Molecular Genetics. 1995;4(2):223–230. doi: 10.1093/hmg/4.2.223. [DOI] [PubMed] [Google Scholar]
- 16.Smith SR, Gawronska-Kozak B, Janderová L, et al. Agouti expression in human adipose tissue: functional consequences and increased expression in type 2 diabetes. Diabetes. 2003;52(12):2914–2922. doi: 10.2337/diabetes.52.12.2914. [DOI] [PubMed] [Google Scholar]
- 17.Xue BZ, Wilkison WO, Mynatt RL, Moustaid N, Goldman M, Zemel MB. The agouti gene product stimulates pancreatic β-cell Ca2+ signaling and insulin release. Physiological Genomics. 1999;1999(1):11–19. doi: 10.1152/physiolgenomics.1999.1.1.11. [DOI] [PubMed] [Google Scholar]
- 18.Kucera GT, Bortner DM, Rosenberg MP. Overexpression of an Agouti cDNA in the skin of transgenic mice recapitulates dominant coat color phenotypes of spontaneous mutants. Developmental Biology. 1996;173(1):162–173. doi: 10.1006/dbio.1996.0014. [DOI] [PubMed] [Google Scholar]
- 19.Ingalls AM, Dickie MM, Snell GD. Obese, a new mutation in the house mouse. The Journal of Heredity. 1950;41(12):317–318. doi: 10.1093/oxfordjournals.jhered.a106073. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 21.Hummel KP, Dickie MM, Coleman DL. Diabetes, a new mutation in the mouse. Science. 1966;153(3740):1127–1128. doi: 10.1126/science.153.3740.1127. [DOI] [PubMed] [Google Scholar]
- 22.Chen H, Charlat O, Tartaglia LA, et al. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84(3):491–495. doi: 10.1016/s0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- 23.Lee GH, Proenca R, Montez JM, et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379(6566):632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 24.Herberg L, Coleman DL. Laboratory animals exhibiting obesity and diabetes syndromes. Metabolism. 1977;26(1):59–99. doi: 10.1016/0026-0495(77)90128-7. [DOI] [PubMed] [Google Scholar]
- 25.Jürgens HS, Schürmann A, Kluge R, et al. Hyperphagia, lower body temperature, and reduced running wheel activity precede development of morbid obesity in New Zealand obese mice. Physiological Genomics. 2006;25(2):234–241. doi: 10.1152/physiolgenomics.00252.2005. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki W, Iizuka S, Tabuchi M, et al. A new mouse model of spontaneous diabetes derived from ddY strain. Experimental Animals. 1999;48(3):181–189. doi: 10.1538/expanim.48.181. [DOI] [PubMed] [Google Scholar]
- 27.Hirayama I, Yi Z, Izumi S, et al. Genetic analysis of obese diabetes in the TSOD mouse. Diabetes. 1999;48(5):1183–1191. doi: 10.2337/diabetes.48.5.1183. [DOI] [PubMed] [Google Scholar]
- 28.Iizuka S, Suzuki W, Tabuchi M, et al. Diabetic complications in a new animal model (TSOD mouse) of spontaneous NIDDM with obesity. Experimental Animals. 2005;54(1):71–83. doi: 10.1538/expanim.54.71. [DOI] [PubMed] [Google Scholar]
- 29.Allan MF, Eisen EJ, Pomp D. The M16 mouse: an outbred animal model of early onset polygenic obesity and diabesity. Obesity Research. 2004;12(9):1397–1407. doi: 10.1038/oby.2004.176. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura M, Yamada K. Studies on a diabetic (KK) strain of the mouse. Diabetologia. 1967;3(2):212–221. doi: 10.1007/BF01222198. [DOI] [PubMed] [Google Scholar]
- 31.Igel M, Taylor BA, Phillips SJ, Becker W, Herberg L, Joost HG. Hyperleptinemia and leptin receptor variant Asp600Asn in the obese, hyperinsulinemic KK mouse strain. Journal of Molecular Endocrinology. 1998;21(3):337–345. doi: 10.1677/jme.0.0210337. [DOI] [PubMed] [Google Scholar]
- 32.Shike T, Hirose S, Kobayashi M, Funabiki K, Shirai T, Tomino Y. Susceptibility and negative epistatic loci contributing to type 2 diabetes and related phenotypes in a KK/Ta mouse model. Diabetes. 2001;50(8):1943–1948. doi: 10.2337/diabetes.50.8.1943. [DOI] [PubMed] [Google Scholar]
- 33.Ikeda H. KK mouse. Diabetes Research and Clinical Practice. 1994;24(supplement):S313–S316. doi: 10.1016/0168-8227(94)90268-2. [DOI] [PubMed] [Google Scholar]
- 34.Okazaki M, Saito Y, Udaka Y, et al. Diabetic nephropathy in KK and KK-A mice. Experimental Animals. 2002;51(2):191–196. doi: 10.1538/expanim.51.191. [DOI] [PubMed] [Google Scholar]
- 35.Zucker LM, Zucker TF. Fatty, a new mutation in the rat. Journal of Heredity. 1961;52(6):275–278. [Google Scholar]
- 36.Zucker LM, Antoniades HN. Insulin and obesity in the Zucker genetically obese rat "fatty". Endocrinology. 1972;90(5):1320–1330. doi: 10.1210/endo-90-5-1320. [DOI] [PubMed] [Google Scholar]
- 37.Bray GA. The Zucker fatty rat: a review. Federation Proceedings. 1977;36(2):148–153. [PubMed] [Google Scholar]
- 38.Ogawa Y, Masuzaki H, Isse N, et al. Molecular cloning of rat obese cDNA and augmented gene expression in genetically obese Zucker fatty (fa/fa) rats. Journal of Clinical Investigation. 1995;96(3):1647–1652. doi: 10.1172/JCI118204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Friedman JE, De Vente JE, Peterson RG, Dohm GL. Altered expression of muscle glucose transporter GLUT-4 in diabetic fatty Zucker rats (ZDF/Drt-fa) American Journal of Physiology. 1991;261(6):E782–E788. doi: 10.1152/ajpendo.1991.261.6.E782. [DOI] [PubMed] [Google Scholar]
- 40.Ikeda H, Shino A, Matsuo T, Iwatsuka H, Suzuoki Z. A new genetically obese-hyperglycemic rat (Wistar fatty) Diabetes. 1981;30(12):1045–1050. doi: 10.2337/diab.30.12.1045. [DOI] [PubMed] [Google Scholar]
- 41.Matsui H, Suzuki M, Tsukuda R, Iida K, Miyasaka M, Ikeda H. Expression of ICAM-1 on glomeruli is associated with progression of diabetic nephropathy in a genetically obese diabetic rat, Wistar fatty. Diabetes Research and Clinical Practice. 1996;32(1-2):1–9. doi: 10.1016/0168-8227(96)01209-0. [DOI] [PubMed] [Google Scholar]
- 42.Imai G, Satoh T, Kumai T, et al. Hypertension accelerates diabetic nephropathy in Wistar fatty rats, a model of type 2 diabetes mellitus, via mitogen-activated protein kinase cascades and transforming growth factor-β1. Hypertension Research. 2003;26(4):339–347. doi: 10.1291/hypres.26.339. [DOI] [PubMed] [Google Scholar]
- 43.Imai K, Kudo N, Koyama M, Kawashima Y. Effects of dehydroepiandrosterone on oleic acid accumulation in rat liver. Biochemical Pharmacology. 2003;65(10):1583–1591. doi: 10.1016/s0006-2952(03)00146-1. [DOI] [PubMed] [Google Scholar]
- 44.Berti-Mattera LN, Lowery J, Day SF, Peterson RG, Eichberg J. Alteration of phosphoinositide metabolism, protein phosphorylation, and carbohydrate levels in sciatic nerve from Wistar fatty diabetic rats. Diabetes. 1989;38(3):373–378. doi: 10.2337/diab.38.3.373. [DOI] [PubMed] [Google Scholar]
- 45.Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications: Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes. 1992;41(11):1422–1428. doi: 10.2337/diab.41.11.1422. [DOI] [PubMed] [Google Scholar]
- 46.Kawano K, Hirashima T, Mori S, Natori T. OLETF (Otsuka Long-Evans Tokushima fatty) rat: a new NIDDM rat strain. Diabetes Research and Clinical Practice. 1994;24:S317–S320. doi: 10.1016/0168-8227(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 47.Jia D, Taguchi M, Otsuki M. Synthetic protease inhibitor camostat prevents and reverses dyslipidemia, insulin secretory defects, and histological abnormalities of the pancreas in genetically obese and diabetic rats. Metabolism. 2005;54(5):619–627. doi: 10.1016/j.metabol.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 48.West DB, Boozer CN, Moody DL, Atkinson RL. Dietary obesity in nine inbred mouse strains. American Journal of Physiology. 1992;262(6):R1025–R1032. doi: 10.1152/ajpregu.1992.262.6.R1025. [DOI] [PubMed] [Google Scholar]
- 49.Collins S, Martin TL, Surwit RS, Robidoux J. Genetic vulnerability to diet-induced obesity in the C57BL/6J mouse: physiological and molecular characteristics. Physiology and Behavior. 2004;81(2):243–248. doi: 10.1016/j.physbeh.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 50.Srinivasan K, Ramarao P. Animal models in type 2 diabetes research: an overview. Indian Journal of Medical Research. 2007;125(3):451–472. [PubMed] [Google Scholar]
- 51.Watanabe M, Houten SM, Mataki C, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439(7075):484–489. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- 52.Thomas C, Gioiello A, Noriega L, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metabolism. 2009;10(3):167–177. doi: 10.1016/j.cmet.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Santis A, Attili AF, Corradini SG, et al. Gallstones and diabetes: a case-control study in a free-living population sample. Hepatology. 1997;25(4):787–790. doi: 10.1002/hep.510250401. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Y, Guo K, LeBlanc RE, Loh D, Schwartz GJ, Yu YH. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes. 2007;56(6):1647–1654. doi: 10.2337/db07-0123. [DOI] [PubMed] [Google Scholar]
- 55.Noguchi Y, Nishikata N, Shikata N, et al. Ketogenic essential amino acids modulate lipid synthetic pathways and prevent hepatic steatosis in mice. PLoS One. 2010;5(8) doi: 10.1371/journal.pone.0012057. Article ID e12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Solerte SB, Gazzaruso C, Schifino N, et al. Metabolic effects of orally administered amino acid mixture in elderly subjects with poorly controlled type 2 diabetes mellitus. American Journal of Cardiology. 2004;93(8):23A–29A. doi: 10.1016/j.amjcard.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 57.Eizirik E, Murphy WJ, O’Brien SJ. Molecular dating and biogeography of the early placental mammal radiation. Journal of Heredity. 2001;92(2):212–219. doi: 10.1093/jhered/92.2.212. [DOI] [PubMed] [Google Scholar]
- 58.Page SL, Goodman M. Catarrhine phylogeny: noncoding DNA evidence for a diphyletic origin of the mangabeys and for a human-chimpanzee clade. Molecular Phylogenetics and Evolution. 2001;18(1):14–25. doi: 10.1006/mpev.2000.0895. [DOI] [PubMed] [Google Scholar]
- 59.Hansen BC, Bodkin NL. Heterogeneity of insulin responses: phases leading to type 2 (non-insulin-dependent) diabetes mellitus in the rhesus monkey. Diabetologia. 1986;29(10):713–719. doi: 10.1007/BF00870281. [DOI] [PubMed] [Google Scholar]
- 60.Bodkin NL, Hannah JS, Ortmeyer HK, Hansen BC. Central obesity in rhesus monkeys: association with hyperinsulinemia, insulin resistance and hypertriglyceridemia? International Journal of Obesity. 1993;17(1):53–61. [PubMed] [Google Scholar]
- 61.Bodkin NL, Ortmeyer HK, Hansen BC. Diversity of insulin resistance in monkeys with normal glucose tolerance. Obesity Research. 1993;1(5):364–370. doi: 10.1002/j.1550-8528.1993.tb00014.x. [DOI] [PubMed] [Google Scholar]
- 62.Kemnitz JW. Obesity in macaques: spontaneous and induced. Advances in Veterinary Science and Comparative Medicine. 1984;28:81–114. doi: 10.1016/b978-0-12-039228-5.50009-7. [DOI] [PubMed] [Google Scholar]
- 63.Hansen BC, Bodkin NL. Primary prevention of diabetes mellitus by prevention of obesity in monkeys. Diabetes. 1993;42(12):1809–1814. doi: 10.2337/diab.42.12.1809. [DOI] [PubMed] [Google Scholar]
- 64.Hansen BC, Ortmeyer HK, Bodkin NL. Prevention of obesity in middle-aged monkeys: food intake during body weight clamp. Obesity Research. 1995;3(supplement 2):199s–204s. doi: 10.1002/j.1550-8528.1995.tb00464.x. [DOI] [PubMed] [Google Scholar]
- 65.Altmann J, Schoeller D, Altmann SA, Muruthi P, Sapolsky RM. Body size and fatness of free-living baboons reflect food availability and activity levels. American Journal of Primatology. 1993;30:149–161. doi: 10.1002/ajp.1350300207. [DOI] [PubMed] [Google Scholar]
- 66.Banks WA, Altmann J, Sapolsky RM, Phillips-Conroy JE, Morley JE. Serum leptin levels as a marker for a syndrome X-like condition in wild baboons. Journal of Clinical Endocrinology and Metabolism. 2003;88(3):1234–1240. doi: 10.1210/jc.2002-021695. [DOI] [PubMed] [Google Scholar]
- 67.Banks WA, Phillips-Conroy JE, Jolly CJ, Morley JE. Serum leptin levels in wild and captive populations of baboons (papio): implications for the ancestral role of leptin. Journal of Clinical Endocrinology and Metabolism. 2001;86(9):4315–4320. doi: 10.1210/jcem.86.9.7874. [DOI] [PubMed] [Google Scholar]
- 68.Schwartz SM, Kemnitz JW, Howard Jr. CF. Obesity in free-ranging rhesus macaques. International Journal of Obesity. 1993;17(1):1–9. [PubMed] [Google Scholar]
- 69.Takahashi T, Higashino A, Takagi K, et al. Characterization of obesity in Japanese monkeys (Macaca fuscata) in a pedigreed colony. Journal of Medical Primatology. 2006;35(1):30–37. doi: 10.1111/j.1600-0684.2005.00138.x. [DOI] [PubMed] [Google Scholar]
- 70.Matsuoka N, Ogawa Y, Hosoda K, et al. Human leptin receptor gene in obese Japanese subjects: evidence against either obesity-causing mutations or association of sequence variants with obesity. Diabetologia. 1997;40(10):1204–1210. doi: 10.1007/s001250050808. [DOI] [PubMed] [Google Scholar]
- 71.Clément K, Vaisse C, Lahlou N, et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392(6674):398–401. doi: 10.1038/32911. [DOI] [PubMed] [Google Scholar]
- 72.Rolland V. Leptin receptor gene in a large cohort of massively obese subjects: no indication of the fa/fa rat mutation. Detection of an intronic variant with no association with obesity. Obesity Research. 1998;6(2):122–127. doi: 10.1002/j.1550-8528.1998.tb00325.x. [DOI] [PubMed] [Google Scholar]
- 73.Surwit RS, Seldin MF, Kuhn CM, Cochrane C, Feinglos MN. Control of expression of insulin resistance and hyperglycemia by different genetic factors in diabetic C57BL/6J mice. Diabetes. 1991;40(1):82–87. doi: 10.2337/diab.40.1.82. [DOI] [PubMed] [Google Scholar]
- 74.Speakman J, Hambly C, Mitchell S, Król E. Animal models of obesity. Obesity Reviews. 2007;8(1):55–61. doi: 10.1111/j.1467-789X.2007.00319.x. [DOI] [PubMed] [Google Scholar]
- 75.Clee SM, Attie AD. The genetic landscape of type 2 diabetes in mice. Endocrine Reviews. 2007;28(1):48–83. doi: 10.1210/er.2006-0035. [DOI] [PubMed] [Google Scholar]
- 76.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nature Reviews Cancer. 2004;4(8):579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 77.Calle EE, Rodriguez C, Jacobs EJ, et al. The American Cancer Society Cancer Prevention Study II Nutrition Cohort: rationale, study design, and baseline characteristics. Cancer. 2002;94(9):2490–2501. doi: 10.1002/cncr.101970. [DOI] [PubMed] [Google Scholar]
- 78.Calle EE, Rodriguez C, Jacobs EJ, et al. The American Cancer Society Cancer Prevention Study II Nutrition Cohort: rationale, study design, and baseline characteristics. Cancer. 2002;94(9):2490–2501. doi: 10.1002/cncr.101970. [DOI] [PubMed] [Google Scholar]
- 79.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. Adults. The New England Journal of Medicine. 2003;348(17):1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 80.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW., Jr. Body-mass index and mortality in a prospective cohort of U.S. adults. The New England Journal of Medicine. 1999;341(15):1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 81.Park EJ, Lee JH, Yu GY, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140(2):197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shaw RJ, Lamia KA, Vasquez D, et al. Medicine: the kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310(5754):1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dowling RJO, Zakikhani M, Fantus IG, Pollak M, Sonenberg N. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Research. 2007;67(22):10804–10812. doi: 10.1158/0008-5472.CAN-07-2310. [DOI] [PubMed] [Google Scholar]
- 84.Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nature Reviews Cancer. 2009;9(8):563–575. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kalender A, Selvaraj A, Kim SY, et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metabolism. 2010;11(5):390–401. doi: 10.1016/j.cmet.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Saeedi R, Parsons HL, Wambolt RB, et al. Metabolic actions of metformin in the heart can occur by AMPK-independent mechanisms. American Journal of Physiology. 2008;294(6):H2497–H2506. doi: 10.1152/ajpheart.00873.2007. [DOI] [PubMed] [Google Scholar]
- 87.Dhahbi JM, Mote PL, Fahy GM, Spindler SR. Identification of potential caloric restriction mimetics by microarray profiling. Physiological Genomics. 2005;23(3):343–350. doi: 10.1152/physiolgenomics.00069.2005. [DOI] [PubMed] [Google Scholar]
- 88.Merry BJ. Molecular mechanisms linking calorie restriction and longevity. International Journal of Biochemistry and Cell Biology. 2002;34(11):1340–1354. doi: 10.1016/s1357-2725(02)00038-9. [DOI] [PubMed] [Google Scholar]
- 89.Dhahbi JM, Kim HJ, Mote PL, Beaver RJ, Spindler SR. Temporal linkage between the phenotypic and genomic responses to caloric restriction. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(15):5524–5529. doi: 10.1073/pnas.0305300101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Algire C, Amrein L, Zakikhani M, Panasci L, Pollak M. Metformin blocks the stimulative effect of a high-energy diet on colon carcinoma growth in vivo and is associated with reduced expression of fatty acid synthase. Endocrine-Related Cancer. 2010;17(2):351–360. doi: 10.1677/ERC-09-0252. [DOI] [PubMed] [Google Scholar]
- 91.Phoenix KN, Vumbaca F, Fox MM, Evans R, Claffey KP. Dietary energy availability affects primary and metastatic breast cancer and metformin efficacy. Breast Cancer Research and Treatment. 2010;123(2):333–344. doi: 10.1007/s10549-009-0647-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ribeiro AM, Andrade S, Pinho F, et al. Prostate cancer cell proliferation and angiogenesis in different obese mice models. International Journal of Experimental Pathology. 2010;91(4):374–386. doi: 10.1111/j.1365-2613.2010.00726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gonzalez RR, Cherfils S, Escobar M, et al. Leptin signaling promotes the growth of mammary tumors and increases the expression of vascular endothelial growth factor (VEGF) and its receptor type two (VEGF-R2) Journal of Biological Chemistry. 2006;281(36):26320–26328. doi: 10.1074/jbc.M601991200. [DOI] [PubMed] [Google Scholar]
- 94.Rene Gonzalez R, Watters A, Xu Y, et al. Leptin-signaling inhibition results in efficient anti-tumor activity in estrogen receptor positive or negative breast cancer. Breast Cancer Research. 2009;11(3):p. R36. doi: 10.1186/bcr2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sharma D, Saxena NK, Vertino PM, Anania FA. Leptin promotes the proliferative response and invasiveness in human endometrial cancer cells by activating multiple signal-transduction pathways. Endocrine-Related Cancer. 2006;13(2):629–640. doi: 10.1677/erc.1.01169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bartucci M, Svensson S, Ricci-Vitiani L, et al. Obesity hormone leptin induces growth and interferes with the cytotoxic effects of 5-fluorouracil in colorectal tumor stem cells. Endocrine-Related Cancer. 2010;17(3):823–833. doi: 10.1677/ERC-10-0083. [DOI] [PubMed] [Google Scholar]
- 97.Hendricks JC, Kline LR, Kovalski RJ, O’Brien JA, Morrison AR, Pack AI. The English bulldog: a natural model of sleep-disordered breathing. Journal of Applied Physiology. 1987;63(4):1344–1350. doi: 10.1152/jappl.1987.63.4.1344. [DOI] [PubMed] [Google Scholar]
- 98.Lonergan RP, Ware JC, Atkinson RL, Winter WC, Suratt PM. Sleep apnea in obese miniature pigs. Journal of Applied Physiology. 1998;84(2):531–536. doi: 10.1152/jappl.1998.84.2.531. [DOI] [PubMed] [Google Scholar]
- 99.Tuck SA, Dort JC, Olson ME, Remmers JE. Monitoring respiratory function and sleep in the obese Vietnamese pot-bellied pig. Journal of Applied Physiology. 1999;87(1):444–451. doi: 10.1152/jappl.1999.87.1.444. [DOI] [PubMed] [Google Scholar]
- 100.van Lunteren E. Effects of genetic obesity on rat upper airway muscle and diaphragm contractile properties. European Respiratory Journal. 1996;9(10):2139–2144. [PubMed] [Google Scholar]
- 101.Radulovacki M, Trbovic S, Carley DW. Hypotension reduces sleep apneas in Zucker lean and Zucker obese rats. Sleep. 1996;19(10):767–773. doi: 10.1093/sleep/19.10.767. [DOI] [PubMed] [Google Scholar]
- 102.Megirian D, Dmochowski J, Farkas GA. Mechanism controlling sleep organization of the obese Zucker rats. Journal of Applied Physiology. 1998;84(1):253–256. doi: 10.1152/jappl.1998.84.1.253. [DOI] [PubMed] [Google Scholar]
- 103.Brennick MJ, Pickup S, Cater JR, Kuna ST. Phasic respiratory pharyngeal mechanics by magnetic resonance imaging in lean and obese Zucker rats. American Journal of Respiratory and Critical Care Medicine. 2006;173(9):1031–1037. doi: 10.1164/rccm.200505-705OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nakano H, Magalang UJ, Lee SD, Krasney JA, Farkas GA. Serotonergic modulation of ventilation and upper airway stability in obese Zucker rats. American Journal of Respiratory and Critical Care Medicine. 2001;163(5):1191–1197. doi: 10.1164/ajrccm.163.5.2004230. [DOI] [PubMed] [Google Scholar]
- 105.Peters LL, Robledo RF, Bult CJ, Churchill GA, Paigen BJ, Svenson KL. The mouse as a model for human biology: a resource guide for complex trait analysis. Nature Reviews Genetics. 2007;8(1):58–69. doi: 10.1038/nrg2025. [DOI] [PubMed] [Google Scholar]
- 106.Tagaito Y, Polotsky VY, Campen MJ, et al. A model of sleep-disordered breathing in the C57BL/6J mouse. Journal of Applied Physiology. 2001;91(6):2758–2766. doi: 10.1152/jappl.2001.91.6.2758. [DOI] [PubMed] [Google Scholar]
- 107.Brennick MJ, Pack AI, Ko K, et al. Altered upper airway and soft tissue structures in the New Zealand obese mouse. American Journal of Respiratory and Critical Care Medicine. 2009;179(2):158–169. doi: 10.1164/rccm.200809-1435OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Smith BK, West DB, York DA. Carbohydrate versus fat intake: differing patterns of macronutrient selection in two inbred mouse strains. American Journal of Physiology. 1997;272(1):R357–R362. doi: 10.1152/ajpregu.1997.272.1.R357. [DOI] [PubMed] [Google Scholar]
- 109.Smith BK, Kelly LA, Piña R, York DA, Bray GA. Preferential fat intake increases adiposity but not body weight in Sprague-Dawley rats. Appetite. 1998;31(2):127–139. doi: 10.1006/appe.1998.0162. [DOI] [PubMed] [Google Scholar]
- 110.Smith BK, Andrews PK, West DB. Macronutrient diet selection in thirteen mouse strains. American Journal of Physiology. 2000;278(4):R797–R805. doi: 10.1152/ajpregu.2000.278.4.R797. [DOI] [PubMed] [Google Scholar]
- 111.Schwartz MW, Woods SC, Porte D, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404(6778):661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 112.Crawley JN. The role of galanin in feeding behavior. Neuropeptides. 1999;33(5):369–375. doi: 10.1054/npep.1999.0049. [DOI] [PubMed] [Google Scholar]
- 113.Odorizzi M, Fernette B, Angel E, Burlet C, Tankosic P, Burlet A. Galanin receptor antagonists decrease fat preference in Brattleboro rat. Neuropharmacology. 2002;42(1):134–141. doi: 10.1016/s0028-3908(01)00115-0. [DOI] [PubMed] [Google Scholar]
- 114.Smith BK, York DA, Bray GA. Chronic cerebroventricular galanin does not induce sustained hyperphagia or obesity. Peptides. 1994;15(7):1267–1272. doi: 10.1016/0196-9781(94)90152-x. [DOI] [PubMed] [Google Scholar]
- 115.Smith BK, Berthoud HR, York DA, Bray GA. Differential effects of baseline macronutrient preferences on macronutrient selection after galanin, NPY, and an overnight fast. Peptides. 1997;18(2):207–211. doi: 10.1016/s0196-9781(96)00318-x. [DOI] [PubMed] [Google Scholar]
- 116.Jhanwar-Uniyal M, Beck B, Jhanwar YS, Burlet C, Leibowitz SF. Neuropeptide Y projection from arcuate nucleus to parvocellular division of paraventricular nucleus: specificfd relation to the ingestion of carbohydrate. Brain Research. 1993;631(1):97–106. doi: 10.1016/0006-8993(93)91192-u. [DOI] [PubMed] [Google Scholar]
- 117.Carmina E, Lobo RA. Polycystic ovary syndrome (PCOS): arguably the most common endocrinopathy is associated with significant morbidity in women. Journal of Clinical Endocrinology and Metabolism. 1999;84(6):1897–1899. doi: 10.1210/jcem.84.6.5803. [DOI] [PubMed] [Google Scholar]
- 118.Glintborg D, Andersen M. An update on the pathogenesis, inflammation, and metabolism in hirsutism and polycystic ovary syndrome. Gynecological Endocrinology. 2010;26(4):281–296. doi: 10.3109/09513590903247873. [DOI] [PubMed] [Google Scholar]
- 119.Rebuffe-Scrive M, Surwit R, Feinglos M, Kuhn C, Rodin J. Regional fat distribution and metabolism in a new mouse model (C57BL/6J) of non-insulin-dependent diabetes mellitus. Metabolism. 1993;42(11):1405–1409. doi: 10.1016/0026-0495(93)90190-y. [DOI] [PubMed] [Google Scholar]
- 120.Coleman DL, Hummel KP. The influence of genetic background on the expression of the obese (Ob) gene in the mouse. Diabetologia. 1973;9(4):287–293. doi: 10.1007/BF01221856. [DOI] [PubMed] [Google Scholar]
- 121.Toye AA, Lippiat JD, Proks P, et al. A genetic and physiological study of impaired glucose homeostasis control in C57BL/6J mice. Diabetologia. 2005;48(4):675–686. doi: 10.1007/s00125-005-1680-z. [DOI] [PubMed] [Google Scholar]
- 122.Coleman DL. The influence of genetic background on the expression of mutations at the diabetes (db) locus in the mouse VI: hepatic malic enzyme activity is associated with diabetes severity. Metabolism. 1992;41(10):1134–1136. doi: 10.1016/0026-0495(92)90299-p. [DOI] [PubMed] [Google Scholar]
- 123.Almind K, Kahn CR. Genetic determinants of energy expenditure and insulin resistance in diet-induced obesity in mice. Diabetes. 2004;53(12):3274–3285. doi: 10.2337/diabetes.53.12.3274. [DOI] [PubMed] [Google Scholar]
- 124.Leiter EH, Coleman DL, Eisenstein AB, Strack I. A new mutation (db3J) at the diabetes locus in strain 129/J mice. I. Physiological and histological characterization. Diabetologia. 1980;19:58–65. doi: 10.1007/BF00258313. [DOI] [PubMed] [Google Scholar]
- 125.Flowers JB, Oler AT, Nadler ST, et al. Abdominal obesity in BTBR male mice is associated with peripheral but not hepatic insulin resistance. American Journal of Physiology. 2007;292(3):E936–E945. doi: 10.1152/ajpendo.00370.2006. [DOI] [PubMed] [Google Scholar]
- 126.Clee SM, Nadler ST, Attie AD. Genetic and genomic studies of the BTBR ob/ob mouse model of type 2 diabetes. American Journal of Therapeutics. 2005;12(6):491–498. doi: 10.1097/01.mjt.0000178781.89789.25. [DOI] [PubMed] [Google Scholar]
- 127.Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Diet-induced type II diabetes in C57BL/6J mice. Diabetes. 1988;37(9):1163–1167. doi: 10.2337/diab.37.9.1163. [DOI] [PubMed] [Google Scholar]
- 128.Surwit RS, Feinglos MN, Rodin J, et al. Differential effects of fat and sucrose on the development of obesity and diabetes in C57BL/6J and A/J mice. Metabolism. 1995;44(5):645–651. doi: 10.1016/0026-0495(95)90123-x. [DOI] [PubMed] [Google Scholar]
- 129.Qiu J, Ogus S, Mounzih K, Ewart-Toland A, Chehab FF. Leptin-deficient mice backcrossed to the BALB/cJ genetic background have reduced adiposity, enhanced fertility, normal body temperature, and severe diabetes. Endocrinology. 2001;142(8):3421–3425. doi: 10.1210/endo.142.8.8323. [DOI] [PubMed] [Google Scholar]
- 130.York B, Lei K, West DB. Sensitivity to dietary obesity linked to a locus on chromosome 15 in a CAST/Ei × C57BL/6J F intercross. Mammalian Genome. 1996;7(9):677–681. doi: 10.1007/s003359900204. [DOI] [PubMed] [Google Scholar]
- 131.Chua S, Liu SM, Li Q, Yang L, Thassanapaff V, Fisher P. Differential beta cell responses to hyperglycaemia and insulin resistance in two novel congenic strains of diabetes (FVB-Lepr) and obese (DBA-Lep) mice. Diabetologia. 2002;45(7):976–990. doi: 10.1007/s00125-002-0880-z. [DOI] [PubMed] [Google Scholar]
- 132.Kim JH, Sen A, Avery CS, et al. Genetic analysis of a new mouse model for non-insulin-dependent diabetes. Genomics. 2001;74(3):273–286. doi: 10.1006/geno.2001.6569. [DOI] [PubMed] [Google Scholar]
- 133.Ueda H, Ikegami H, Yamato E, et al. The NSY mouse: a new animal model of spontaneous NIDDM with moderate obesity. Diabetologia. 1995;38(5):503–508. doi: 10.1007/BF00400717. [DOI] [PubMed] [Google Scholar]
- 134.Mathews CE, Leiter EH. Resistance of ALR/Lt islets to free radical-mediated diabetogenic stress is inherited as a dominant trait. Diabetes. 1999;48(11):2189–2196. doi: 10.2337/diabetes.48.11.2189. [DOI] [PubMed] [Google Scholar]
- 135.Sekiguchi F, Ishibashi K, Kawamoto Y, Ino T. Diabetic peculiarity of the ALS-Ay and ALR-Ay strains. Jikken Dobutsu. 1991;40(3):323–329. doi: 10.1538/expanim1978.40.3_323. [DOI] [PubMed] [Google Scholar]
- 136.Ehrich TH, Kenney JP, Vaughn TT, Pletscher LS, Cheverud JM. Diet, obesity, and hyperglycemia in LG/J and SM/J mice. Obesity Research. 2003;11(11):1400–1410. doi: 10.1038/oby.2003.189. [DOI] [PubMed] [Google Scholar]
- 137.Yoshioka M, Kayo T, Ikeda T, Koizumi A. A novel locus, Mody4, distal to D7Mit189 on chromosome 7 determines early-onset NIDDM in nonobese C57BL/6 (Akita) mutant mice. Diabetes. 1997;46(5):887–894. doi: 10.2337/diab.46.5.887. [DOI] [PubMed] [Google Scholar]