Abstract
Escherichia coli O157:H7 has been responsible for multiple food- and waterborne outbreaks of diarrhea and/or hemorrhagic colitis (HC) worldwide. More importantly, a portion of E. coli O157:H7-infected individuals, particularly young children, develop a life-threatening sequela of infection called hemolytic uremic syndrome (HUS). Shiga toxin (Stx), a potent cytotoxin, is the major virulence factor linked to the presentation of both HC and HUS. Currently, treatment of E. coli O157:H7 and other Stx-producing E. coli (STEC) infections is limited to supportive care. To facilitate development of therapeutic strategies and vaccines for humans against these agents, animal models that mimic one or more aspect of STEC infection and disease are needed. In this paper, we focus on the characteristics of various mouse models that have been developed and that can be used to monitor STEC colonization, disease, pathology, or combinations of these features as well as the impact of Stx alone.
1. Introduction
Escherichia coli O157:H7 is a member of a group of pathogenic E. coli (known as enterohemorrhaghic E. coli or EHEC) that colonize the gastrointestinal tract and cause a condition known as hemorrhagic colitis (HC) or bloody diarrhea. E. coli O157:H7 is also a member of the larger category of Shiga toxin-producing E. coli (STEC). This group of E. coli is solely defined by its capacity to produce Shiga toxin type 1 (Stx1), Shiga toxin type 2 (Stx2), or both toxins (as well as variants of these). The capacity of STEC in general and E. coli O157:H7 and other EHEC in particular to produce Stxs makes them of particular concern because Stx has been linked to the development of hemolytic uremic syndrome (HUS) that can lead to kidney failure, particularly in children [1, 2].
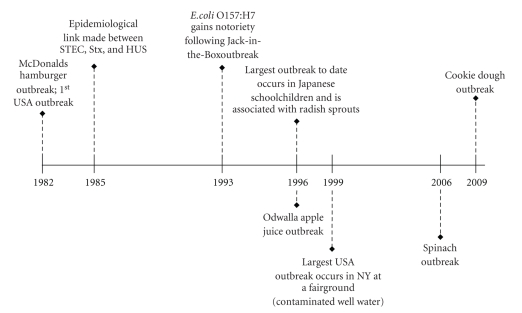
While a number of STEC serotypes are known to cause disease worldwide, E. coli O157:H7 infection generally represents the largest of the STEC disease burden, both in the United States (USA) and worldwide. Although the first recorded outbreak due to organisms of this serotype in the United States occurred in 1982, E. coli O157:H7 infection in both people and animals can be traced back as early as the 1970's. The prevalence of this pathogen has grown since its first description and, despite our best control measures, E. coli O157:H7 remains a serious health concern (Figure 1) [1, 3–8].
Figure 1.
Major outbreaks of E. coli O157:H7 infection. Outbreaks are listed by year and in the context of key discoveries that linked Stx with development of HUS.
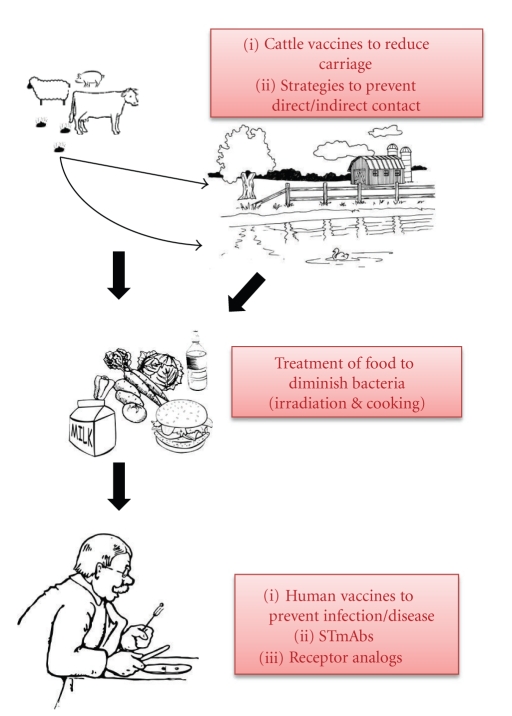
E. coli O157:H7 is responsible for an estimated 73,480 cases of illness, 2,168 hospitalizations, and 61 deaths annually in USA, according to data published by Mead et al. in 1999 [9]. The majority of such E. coli O157:H7 outbreaks in the USA are associated with foodborne transmission [4]. Cattle as well as other ruminants serve as a reservoir for E. coli O157:H7. In particular, surveys of beef and dairy cattle have demonstrated carriage rates less than 0.5 to greater than 2.0% [10]. As a result of E. coli O157:H7 carriage in cattle, beef, and dairy products often become contaminated and serve as the source of infection in outbreaks of E. coli O157:H7. Many vehicles for foodborne transmission of E. coli O157:H7 have been described including beef (ground beef, roast beef, steak, salami, etc.), produce (unpasteurized apple cider or juice, melons, grapes, lettuce, bean sprouts, spinach, etc.), and dairy products (raw milk, cheese, butter, etc.) [4]. The spread of E. coli O157:H7 by these various food matrices is facilitated not only by the pathogen's low infectious dose [11], but additionally by the pathogen's capacity to grow over a broad temperature range and to survive both freezing and acidic conditions [12]. In addition to transmission from contaminated food (or drink), person-to-person and waterborne transmission (both likely facilitated by the low infectious dose of E. coli O157:H7 [11, 13]) have also been reported (Figure 2).
Figure 2.
Modes of E. coli O157:H7 transmission to humans with emphasis on strategies for prevention/intervention. Other modes of transmission of E. coli O157:H7 have been reported and are described elsewhere.
E. coli O157:H7 infection can manifest in a variety of ways. Some individuals who are infected with the microbe remain asymptomatic, others experience diarrhea, but most develop hemorrhagic colitis, the hallmark of E. coli O157:H7 infection. Furthermore, children and the elderly appear to be especially susceptible to E. coli O157:H7-mediated disease and, for reasons that are unclear, may develop HUS (a triad of clinical manifestations including hemolytic anemia, thrombocytopenia, and renal failure [14]) and other systemic problems that include central nervous system (CNS) impairment.
A rise in both the hospitalization and HUS rates has been reported in association with more recent outbreaks of E. coli O157:H7. In data collected from outbreaks that occurred between 1982 and 2002, the average hospitalization rate was just over 17% and the average rate of HUS was ∼4% [4]. However, in the spinach outbreak in 2006 and the cookie dough outbreak in 2009, rates of hospitalization and HUS were even higher (approximately 51% and 16%, respectively, in the spinach outbreak and approximately 44% and 13%, respectively, in the cookie dough outbreak [7, 8]). This increase in disease severity among E. coli O157:H7 infected persons has led to speculation that more virulent strains of the pathogen have emerged [15].
Although it has been nearly 30 years since the discovery of E. coli O157:H7 as an enteric pathogen and despite the recent increase in the rate of severe disease associated with infection by the organism, no treatment yet exists. In general, antibiotic therapy is contraindicated as it may promote toxin expression from the lysogenized phage that typically carries Stx genes. Additionally, antimotility agents are not recommended as they can promote the sustained presence, and consequent toxin expression, of EHEC in the gastrointestinal tract. In instances where HUS develops, supportive care is provided. A variety of treatment and prevention strategies to protect against E. coli O157:H7 are currently in development; these include toxin receptor analogs, passive antibody therapy, and vaccines to protect humans against the systemic effects of the toxin. Since an E. coli O157:H7 vaccine has not been developed and licensed for immunization of humans (two vaccines are currently in use in cattle [16–19]), the most promising prevention strategies for E. coli O157:H7 focus on minimizing exposure to this pathogen (Figure 2).
2. Pathogenesis of E. coli O157:H7
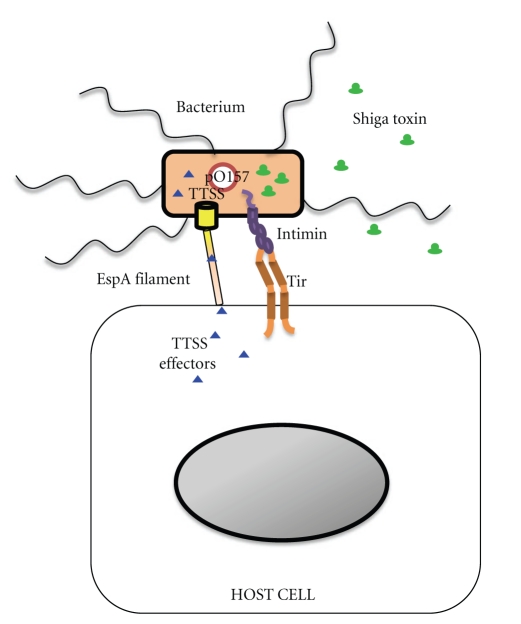
E. coli O157:H7 is well adapted to cause disease in humans. The organism has a number of virulence factors that contribute to its pathogenicity, and Shiga toxin (Stx) is certainly among them. Indeed, Stx is considered to be responsible for the severe complications of E. coli O157:H7 infection, including HUS [1, 2]. In addition to Shiga toxin, E. coli O157:H7 expresses several other important virulence factors that include intimin, translocated intimin receptor (or Tir), a type three secretion system (TTSS), and enterohemolysin (located on the pO157 plasmid) (see Figure 3). The genes for many of these factors are located on a 44 kb pathogenicity island known as the locus of enterocyte effacement, or the LEE locus.
Figure 3.
E. coli O157:H7 virulence factor expression and interaction with host cells. E. coli O157:H7 possesses a large plasmid (pO157), carries the LEE PAI (and thus is intimin positive), and expresses Shiga toxins. The LEE locus encodes a TTSS and TTSS effector proteins. One of the TSSS proteins, E. coli Secreted Protein (Esp) A, forms a filament that serves to translocate TTSS effector proteins from the bacterium into the host cell by way of a pore created by EspB and EspD. One of these effectors, Tir, serves as the receptor for the major adhesin, intimin, and thus allows adherence of the bacterium to the host cell.
The Stx family of AB5 toxins (composed of a single A or active subunit noncovalently associated with a pentameric ring of B or binding subunits) contains two subgroups, Stx1 and Stx2. In addition to these two Shiga toxin serotypes, variants of each have been described: Stx1c, Stx1d, Stx2c, Stx2d, Stx2d-activatable, Stx2e, and Stx2f (reviewed in [20]). While Stx1 and Stx2 have the same overall structure [21], these toxins are antigenically distinct such that antibody against Stx1 will not neutralize Stx2, and vice versa [22]. Stx1 and Stx2 also have the same mode of action; however, they differ in specific activities both in vitro and in mice. The 50% cytotoxic dose (CD50) of Stx1 for Vero cells is lower than that for Stx2 [23], while the 50% lethal dose (LD50) of purified Stx1 for systemically inoculated adult CD-1 mice is ∼125 ng compared to ∼1 ng for Stx2 [24]. Moreover differences in toxicity are also evident when human renal endothelial cells are treated with purified Stx1 or Stx2; Stx2 is about 1,000-fold more toxic [25]. Finally, epidemiological data suggest a difference in Stx1 and Stx2 toxicity in people; Stx2-producing E. coli O157:H7 strains are more frequently associated with HUS than are strains that produce Stx1 [26–28].
The B subunit of Stxs forms a homopentameric ring structure as it binds the cellular toxin receptor, globotriaosylceramide (also known as Gb3 or CD77), a Pk blood group antigen found on a variety of cells [29, 30]. The A1 fragment of the A subunit is translocated into the host cell cytoplasm [31], where it acts as an N-glycosidase to remove a single adenosine residue from the 28S ribosomal RNA of the 60S ribosome [32, 33]. Alteration of the 28S rRNA prevents binding of elongation factor to the ribosome so as to inhibit protein synthesis [34]. Stx-mediated inhibition of cellular protein synthesis generally results in death of the intoxicated cell by apoptosis.
Plasmid pO157 is a 93 kb plasmid (∼60 MDa) found in most E. coli O157:H7 isolates as well as other EHEC strains [35, 36]. The plasmid encodes an enterohemolysin, an adhesin known as ToxB, and a type two secretion system [37–39]. While the plasmid is thought to be important for virulence (studies have correlated pO157 with hemolytic activity [39] and intestinal adherence [40]), its exact role in pathogenesis remains unclear (reviewed in [35]).
The locus of enterocyte effacement, or LEE, is a chromosomally encoded pathogenicity island (PAI) that encodes the pathogenic determinants intimin (an adhesin), a type three secretion system (TTSS), and TTSS effector proteins. The TTSS of E. coli O157:H7 is a contact-dependent system for transport of bacterial proteins, or effectors, into host cells (such as intimin and Tir). Intimin is a 97 kDa outer membrane protein that facilitates intimate adherence of E. coli O157:H7 to the epithelial surface [41]. Although STEC strains that lack intimin can cause human disease [42, 43], E. coli O157:H7 appears to require intimin to establish colonization [44–50]. Several investigators have speculated that E. coli O157:H7 colonization initially proceeds via the interaction of the outer membrane protein intimin with cell-surface-expressed factors such as β1 integrins [51] and nucleolin [52, 53], before binding to the translocated intimin receptor. E. coli O157:H7 is unique among bacterial pathogens (with the exception of other LEE-encoding bacteria) in that it encodes its own receptor, Tir, which is injected into target host cells by way of the TTSS.
2.1. Models of Pathogenesis
Knowledge of the pathogenesis of E. coli O157:H7-mediated disease in humans is quite limited. The use of human subjects to investigate the steps required for E. coli O157:H7 to evoke intestinal pathology is considered unethical because of the possibility that a volunteer could develop HUS. Thus, numerous in vitro assays and animal models have been developed in an attempt to mimic various aspects of E. coli O157:H7 disease in humans.
In vitro systems such as cell monolayers, transwells, organoids, and in vitro organ culture (referred to as IVOC) as well as ex vivo cultures of biopsies are useful for the study of several aspects of E. coli O157:H7 pathogenesis, such as adherence of the microbe to eukaryotic cells and the impact of Stx on those cells. In addition, the type of cells used often depends on the specific aspect of infection and/or pathogenesis under investigation. For example, epithelial cells (derived from a variety of different sources) are commonly used to evaluate E. coli O157:H7 adherence mechanisms [47, 54], while endothelial cells (human umbilical vein endothelial cells (HUVECs) or human glomerular microvascular endothelial cells (GMVECs)) are often used to examine the effect of Stx on vascular cell integrity and cytokine response [55]. In an attempt to model not only adherence but also the early steps in E. coli O157:H7 pathogenesis, polarized monolayers were generated with transwell systems. A key feature of the transwell approach is that it allows for the development of polarized cell monolayers that express features more characteristic of differentiated cells, such as the formation of tight junctions and the expression of unique cellular factors [56]. Thus, researchers have explored the mechanisms of both E. coli O157:H7 binding to and Stx transit across the epithelium [57, 58]. In this manner, Stx1 and Stx2 have been shown to translocate, at varying rates, across CaCo-2A, T84, and HCT-8 cells, all of which are of intestinal origin [59]. Another tissue culture model used to explore E. coli O157:H7 adherence and subsequent host cell damage is the organoid system in which cells grown on a scaffold under microgravity conditions form pieces of tissue-like material [60–62]. When an organoid derived from HCT-8 intestinal epithelial cells was infected with E. coli O157:H7, attaching and effacing (A/E) lesion formation and slight tissue damage were evident; the tissue damage was attributed to the Stx2 produced by the bacteria [63]. Finally, in vivo grown organ cultures of intestinal samples have been used to study adherence of E. coli O157:H7 to gut mucosal cells and subsequent damage to the intestinal cells [64–68].
Many animal models have been developed to facilitate study of EHEC pathogenesis in vivo. In general, these models exist in two varieties: those solely focused on the effects of Stx (in the absence of bacteria) and those that explore E. coli O157:H7 infection. Models that evaluate toxicity rely on injection of Stx (with or without LPS) and often measure mortality as the endpoint of the investigation. Such in vivo assays have been used to explore differences in relative toxicity among Stx toxin types [69], to assess the protective capacity of some factor [70–72], or to model the pathogenesis of HUS [73]. While each applicable model can be used to study one or more components of the steps in the pathogenesis of E. coli O157:H7- or other STEC-mediated disease (from initial colonization to mortality), no one animal model system that mimics the full spectrum of STEC-evoked illness in humans (to include the development of HC and HUS) has been described to date (reviewed in [74]).
Small animals that have served as models for EHEC infection and disease include mice [75–80], rats [81], and rabbits [82]. Larger animals that have been so used, albeit less frequently, include chickens [83, 84], pigs [85], cows [86], dogs [87], baboons [88], and macaques [89]. The presence of characteristic A/E lesions in the gastrointestinal tract of E. coli O157:H7-infected animals has been reported in gnotobiotic piglets, infant rabbits, calves, chickens, and macaques (reviewed in [90, 91]). Additionally, naturally occurring HUS-like diseases have been described in greyhounds (known as idiopathic cutaneous and renal glomerular vasculopathy of greyhounds (CRVGs) or “Alabama rot” [87, 92]) and rabbits [93].
3. Mouse Models of Infection
While large animal models, such as the gnotobiotic piglet, exhibit a number of features of E. coli O157:H7 pathogenesis, their breeding and maintenance require considerable veterinary skill, space, and financial support. Thus, small animal model systems are preferable for general use. Mouse models in particular offer a number of benefits that include the following: low relative costs for purchase and maintenance, ease of care and handling, ready availability of numerous immunological reagents, variations in genetic backgrounds among inbred mouse strains as well as access to transgenic and recombinant inbred animals, and, very importantly, the feasibility of using sufficient numbers of animals in a single study to perform meaningful statistical analyses on the resultant data. Popular mouse models of E. coli O157:H7 oral infection include axenic mice (no indigenous intestinal flora) or streptomycin-treated mice (reduced normal flora) because these animals have proven amenable to EHEC colonization. A summary of the mouse models that have been used for E. coli O157:H7 oral infection studies is presented in Table 1.
Table 1.
Commonly used mouse models of STEC colonization and/or disease.
| Model | Inoculum (CFU) | Inoculation Method | Features | Histopathology | Source |
|---|---|---|---|---|---|
| Str-treated | 1010 | Feeding | Colonization, morbidity, mortality with 933cu-rev | Kidney | [79] |
| Str-treated (O91:H21) | LD50 < 10 | Feeding | Colonization, morbidity, mortality | Kidney | [94] |
| MMC & str (O157:H-) | >109 (M)a | IG | Colonization, morbidity, mortality | Kidney, brain | [95] |
| Conventional | 107, 108 | IG | Morbidity, mortality | Kidney, intestines | [76] |
| Germfree | 2 × 102 (C)a, 2 × 109 (D, M)a |
IG | Colonization, morbidity, mortality | Kidney, intestines, brain | [96] |
| PCM | 2 × 105–2 × 107 | IG | Colonization, morbidity, mortality | Kidney, intestines, brain | [77] |
| Conventional | ~2 × 1010 | IG | Colonization | [97] | |
| Germfree | 5 × 107 | IG | Colonization, morbidity, Mortality with hypertoxigenic strain | Kidney, intestines, brain | [98] |
| Conventional | 1011/kg | IG | Colonization | [99] | |
| MMC & str | 5 × 103 | IG | Colonization, morbidity, mortality | Kidney, other | [78] |
| Germfree | 102–106 | IG | Colonization, morbidity, mortality | Kidney | [75] |
| Conventional (weaned) | 6 × 109/kg | IG | Morbidity and mortality | Kidney, intestines | [100] |
aC: colonization, D: disease, M: mortality.
3.1. Development of the Streptomycin-Treated Mouse Model for E. coli O157:H7 Infection
The first mouse system described for study of the pathogenesis of E. coli O157:H7 was the streptomycin-treated murine model developed by Wadolkowski et al. [79]. This E. coli O157:H7 mouse model incorporates streptomycin (str) treatment of animals via their drinking water as a means of reducing the animals' normal intestinal facultative flora so as to decrease bacterial competition for the infecting EHEC strain. This methodology was based on the work of Myhal et al. as described in 1982 [101]. The goal of the original study by Myhal and colleagues was to assess the relative colonizing capacities of different E. coli isolates. Prior to the report by Myhal et al., E. coli and several other bacteria (to include Salmonella and Vibrio) were shown to have the capacity to colonize mice if the animals had been antibiotic-treated or were axenic [102, 103]. However, Myhal et al. demonstrated that even a laboratory-adapted E. coli K12 given orally as a single strain challenge was capable of colonizing str-treated mice to levels equivalent to those observed for human fecal E. coli isolates. Thus, as the authors suggested, str-treated mice are best used to evaluate the relative colonization capacity of an E. coli strain if given with another isolate in a competitive infection study [101].
Myhal et al. used 5-6-week-old, male CD-1 (outbred mice, also known as ICR) mice [101]. Myhal et al. demonstrated that addition of 5 g/L of streptomycin sulfate to the animals' drinking water, for as little as one day, reduced the number of facultative anaerobes shed (from 108 CFU/g feces prior to streptomycin treatment down to <102 CFU/g feces) but had little or no effect on the number of obligate anaerobic bacteria present within the gastrointestinal tract (109 CFU shed/g feces). The authors selected streptomycin as the antibiotic treatment of choice because they reasoned that a mutation that rendered the bacteria resistant to streptomycin (a presumed alteration to the ribosomes) should have little effect on the bacterial surface and thereby on colonization. In their report, str-treated mice deprived of food/water were infected with 1010 CFU of str-resistant E. coli in a solution of 20% sucrose. Colony counts of feces collected daily indicated that high levels of colonization were achieved by all of the challenge strains when given alone (∼108 CFU/g feces), whereas in cofeeding or competition experiments varying colonizing capacities of the strains were observed. Furthermore, the authors found that human fecal isolates heavily colonized both the cecum and the large intestines. They concluded that the large intestines, which had slightly more adherent bacteria than were observed in the cecum, were the main site of E. coli colonization in their str-treated model [101]. This primary colonization site was consistent with what was previously reported for E. coli colonization in the untreated mouse model [104].
Wadolkowski et al. followed a very similar methodology to that described by Myhal et al. for their E. coli O157:H7 infection studies in str-treated mice [79, 101]. Male CD-1 mice were provided 5 g/L streptomycin sulfate in their drinking water to decrease the normal flora prior to an overnight fast and inoculation with 1010 CFU of the strain/s of interest in 20% sucrose (w/v). Food was returned after infection and animals were housed individually. Colonization levels of the infecting E. coli O157:H7 strain were determined by enumeration of str-resistant E. coli O157:H7 in shed feces.
Wadolkowski et al. tested three E. coli O157:H7 strains (n = 3 per strain; experiments done in triplicate (at least)) in their initial experiments: WT strain (933), a pO157-cured mutant (933cu), and an additional pO157-cured mutant recovered from co-infection studies (933cu-rev) [79]. Strains 933 and 933cu colonized to similar levels (107 CFU/g feces for 25 days) in single infection experiments with no observed disease manifestation. When co-infections were conducted, 933 outcompeted 933cu in 2/3 of the mice. In the remaining mouse, after a decline in the load of 933cu, there was a steady increase in levels of 933cu compared to 933. An isolate of 933cu recovered from the co-infected mouse was labeled 933cu-rev (“rev” meaning “revertant” to wild-type or virulent colonization levels). Although no signs of illness were apparent in the co-infected mouse with the high levels of 933-cu rev, disease manifestations were evident in mice subsequently infected with 933-cu-rev alone. The 933cu-rev-infected mice shed loose stools, were anorexic and lethargic, and died within a few days of disease presentation. Extensive necropsies (included the liver, brain, heart, stomach, small intestine, cecum, large intestines, spleen, and kidneys) of singly infected animals revealed that only the kidneys from animals infected with strain 933cu-rev demonstrated pathology of any kind. Histopathologic analysis of kidneys from 933cu-rev-infected animals indicated widespread bilateral acute renal cortical tubular necrosis despite apparently normal glomeruli with no evidence of fibrin deposits, elastic fibers, or bacteria. The authors concluded that this pathology was more indicative of insult from a toxin rather than a result of dehydration that might be expected in an anorexic animal with loose stools [79].
In this same study, Wadolkowski et al. recovered strains 933, 933cu, and 933cu-rev from epithelial cells of the small intestine, cecum, and large bowel. However, 933cu-rev demonstrated an increased capacity to colonize the small intestines and the mid and distal sections of the large intestines compared to the other two strains. This finding was consistent with the increased capacity of strain 933cu-rev to multiply within mucus obtained from the different intestinal segments (note: Wadolkowski et al. had previously shown that multiplication in cecal mucus was required for a human fecal isolate of E. coli to colonize the large intestine [105]). Wadolkowski et al. went on to speculate that the increased virulence of 933cu-rev may have been attributable in part to its broader range of colonization locales and, more specifically to the increased capacity of the distal small intestines (versus the cecum or large intestines) to absorb Stxs produced at that site [79]. Furthermore, the link between site of colonization and extent of disease was also suggested by Tzipori et al. in relation to EPEC colonization [106]. Strains that caused more severe disease colonized the proximal small intestines of piglets, whereas strains that caused milder pathogenesis tended to colonize distal regions of the small intestines as well as distal portions of the large intestines.
3.2. Extension of the Streptomycin-Treated Mouse Model for STEC Infection
The utility of the str-treated E. coli O157:H7 colonization model was extended from the original report of E. coli O157:H7 infection and colonization to include the evaluation of non-O157 STEC strains [94]. Moreover, in 1994 Fujii et al. used the str-treated model to demonstrate the development of neurological manifestations of disease by incorporation of mitomycin C (MMC) into the treatment regimen [95]. MMC treatment results in induction of phage expression by the bacterium, which concurrently leads to increases in toxin production. Agents that induce toxin expression, to include MMC or ciprofloxacin treatment, are frequently used in mouse models of STEC infection [78, 95, 107, 108]. Alterations to the str-treated model include a reduction in the amount of bacteria fed [78, 109]; an increase in the time of streptomycin treatment prior to infection [110, 111]; the application of intragastric inoculation of the organism [112]; finally, changes in the sex, age, or strain of mouse used [110, 112–115].
3.3. Protein-Calorie Malnutrition Mouse Model
While the str-treated mouse model has proven particularly useful for reproducibly attaining high levels of STEC colonization, this animal system has limitations. For example, very high inocula of E. coli O157:H7 are required to cause morbidity or mortality in a portion of str-treated animals, even though low to moderate doses of STEC strain B2F1 (E. coli O91:H21) have been used to achieve colonization of str-treated mice and induce disease [94]. In an effort to reduce the inoculum of E. coli O157:H7 required to evoke disease in mice and to bring the mouse inoculum closer to the predicted infectious dose for humans (thought to be around 50 organisms [116]), other mouse model systems have been developed. One such system described by Kurioka et al. was based on the observation that some children who contract STEC infections subsisted on an unbalanced diet prior to infection [77]. Therefore, the authors theorized that protein calorie malnourished (PCM) mice would be more readily infected by low doses of E. coli O157:H7 than would conventional mice. The authors' hypothesis proved correct in that the minimal infectious dose of E. coli O157:H7 in PCM mice was over 3 logs lower than that of control mice. The authors reported pathological changes in the intestinal tract of PCM mice infected with E. coli O157:H7 (underdevelopment of the intestinal epithelium in response to PCM, likely resulting in the animals' predisposition to E. coli O157:H7 colonization) and a slight increase in TNF-α in the blood of PCM mice compared to controls. However, they did not observe significant renal pathology (as was seen by Isogai et al. [117]) but did note minimal degeneration of renal tubules and weak staining of the cortical tubular epithelium for Stx [77]. Kurioka et al. also postulated that retardation of intestinal development by PCM might facilitate Stx and LPS transit across the intestinal barrier. That Stx likely did cross the mucosal barrier more readily in PCM-infected animals was strongly suggested by the CNS findings in the PCM mice; cerebral hemorrhage was evident and toxin was detected in the hippocampus. Thus, this report confirmed that Stx can affect the CNS of mice, and can cause death of the infected PCM mice within 10 days. These CNS findings in E. coli O157:H7-infected PCM mice are consistent with the observation noted by Kurioka et al. [77] that up to 30% of STEC-infected children display neurological manifestations of disease [118].
3.4. Germ-Free Mouse Models
In an attempt to extend the renal pathology evident in str-treated, STEC-infected mice to include evidence of glomerular lesions, Isogai et al. infected germ-free mice with E. coli O157:H7 [117]. Although these animals became colonized with E. coli O157:H7 following a low-dose challenge, the animals did not display signs of disease. High inocula (comparable to those reported for the str-treated model) were necessary to cause pathology (colon, kidneys, and brain), disease (lethargy, paralysis, anorexia, dehydration), and death within 7 days. However, when these mice were treated with TNF-α and then infected with a low dose of E. coli O157:H7, systemic disease, neurological manifestations, and glomerular lesions were observed [117].
Since the initial report by Isogai et al., other groups have used germ-free animals to evaluate E. coli O157:H7 pathogenesis. Sawamura et al. explored the effects of antibiotic treatment on E. coli O157:H7 infection in a germ-free mouse model they developed [119] and later used to investigate the role of bacterial internalization by epithelial cells in STEC pathogenesis [120]. Isogai and colleagues subsequently used germ-free mice to explore the effects of antibiotic and green tea extract treatment on E. coli O157:H7 disease [96, 121–123]. Taguchi et al. developed a variation of the germ-free mouse model in which inoculation with a hyper-toxigenic strain of E. coli O157:H7 caused 100% mortality among infected animals [98]. Takahashi et al. went on to use this latter model to investigate the effect of probiotics on E. coli O157:H7 [124]. In 2007, Jeon et al. used a germ-free mouse model to assess the virulence of a mutant strain of E. coli O157:H7 [125]. Furthermore, Eaton and colleagues exploited germ-free Swiss Webster mice to explore the roles of different E. coli O157:H7 strains and Stx types, as well as host factors such as age and gender of the mouse [75]. Eaton et al. reported that gut-adherent E. coli O157:H7 organisms were seen in the ileum and cecum but not the colon. The absence of detectable mucosally-adherent E. coli O157:H7 in the colon of germ-free mice is in contrast to the observations of Wadolkowski et al. in the str-treated model [79].
3.5. Conventional Mouse Models
While str-treated or axenic animals are useful as models for assessment of disease outcome, they rely on the absence of colonization resistance. The term “colonization resistance” was originally coined by van der Waaij in 1971 to explain a phenomenon whereby “a complex intestinal microflora provides protection against colonization by many pathogenic infectious agents” [107]. As a result of the partial or complete absence of a competing microbiota in antibiotic-treated or axenic animals, the inoculated microorganism has a colonization advantage. Thus, studies that apply these models are of limited utility for the assessment of the capacity of an STEC strain to colonize in the face of the physiologically more relevant situation where normal bowel flora are present.
An alternative model that did not require the alteration of the indigenous flora of mice was described in 1997 by Karpman and colleagues [76]. These investigators administered high doses of E. coli O157:H7 intragastrically to C3H/HeN and C3H/HeJ mice and reported significant morbidity and mortality in the infected animals. Mice in this model developed gastrointestinal, neurological, and systemic disease manifestations. Renal pathology included both glomerular mesangial changes and tubular necrosis. Focal areas of colonic necrosis were also evident. Of note, administration of anti-Stx2 antibodies protected animals from disease symptoms and pathology [76].
Like Karpman et al., Conlan and Perry investigated conventional mice as a model for E. coli O157:H7 infection [97]. In their report, Conlan and Perry considered three strains of female mice: CD-1 (outbred), BALB/c (inbred) and C57BL/6 (inbred) to screen potential vaccine candidates. Following intragastric administration of ∼1010 CFU E. coli O157:H7 to one of the three mouse strains, fecal shedding of the organism was monitored as a surrogate for colonization. Although all mouse strains were colonized after infection (generally for 1-2 weeks), no morbidity or mortality was observed. Interestingly, only BALB/c mice seemed to be relatively resistant to re-infection; they shed E. coli O157:H7 for a shorter duration than did other mouse strains that had also received a second challenge with the microbe. BALB/c mice produced more O157-specific IgA (both serum and fecal) in response to both primary and secondary infection despite significantly lower serum anti-O157 IgG titers following secondary infection, when compared to C57BL/6 mice. Thus, BALB/c mice can serve as models for E. coli O157:H7 vaccine studies, despite their reported lack of mortality and/or morbidity in the study by Conlan and Perry [97].
The reports by Conlan and Perry and Karpman et al. indicated that E. coli O157:H7 could colonize conventional mice [76, 97]. To extend these findings, Nagano and colleagues determined the functionality of specific pathogen free (SPF) mice as models for E. coli O157:H7 colonization through exploration of the susceptibility of various mouse strains to colonization after infection with E. coli O157:H7 [99]. The mouse strains they examined included ICR (also known as CD-1), BALB/c, C3H/HeN, C3H/HeJ, and A/J (all female SPF mice). At one week after infection, even with a high inoculum of E. coli O157:H7, only the ICR animals remained colonized as a group. In fact, the majority of ICR mice stayed colonized and shed E. coli O157:H7 in their feces for the duration of the study (28 days). Moreover, E. coli O157:H7 were detected in both the cecum and the colon later in infection. However, Nagano et al. concluded that the cecum was the primary site of colonization because only at that location were E. coli O157:H7 colonies observed adherent to epithelial cell surfaces. In spite of this persistent colonization of ICR mice by E. coli O157:H7, no morbidity or mortality was observed after E. coli O157:H7 infection of these animals [99].
Recently, Brando et al. used weaned BALB/c mice to investigate E. coli O157:H7 pathogenesis [100]. Weaned mice were selected over adult mice in an attempt to render the animals more susceptible to E. coli O157:H7 infection; age has been described to be an important factor in susceptibility of animals to EHEC disease [75]. In their model, Brando et al. showed that only mice <21 days of age demonstrated systemic manifestations of disease, which included mortality within 96 hours and increased plasma urea levels [100]. Occult blood was observed in the stools of mice that succumbed to infection. Histologic analysis revealed tubular necrosis (consistent with other STEC models) and glomerular alterations. Only a portion of infected mice had detectable bacteria by 72 hours after infection, and all survivors cleared the infection by day 7. Furthermore, only transient colonization of the small and large intestine (the cecum was not analyzed) was observed. However, histological analysis revealed damage to and inflammatory infiltrates in the intestinal epithelium. The incompletely developed intestinal epithelium in weanling mice likely contributed to the pathologic features in the intestine and the systemic absorption of toxin, as was seen in PCM-infected mice [77, 100].
3.6. Intact Commensal Flora Model
Other studies that used conventional mice to study STEC pathogenesis can be found in the literature, although they are less thoroughly described [111, 126–129]. However, a common feature among many of these conventional mouse model investigations of E. coli O157:H7 infection was that they either assessed colonization or monitored the development of disease. For example, in the studies by Nagano et al. and Conlan and Perry, colonization was followed but morbidity of infected mice was not observed [97, 99]. Conversely, in the study by Karpman and colleagues, morbidity and mortality were observed but colonization was not monitored [76]. Thus, until our report of a new conventional BALB/c mouse model of E. coli O157:H7 infection [80], no adult mouse studies with conventional animals that explored in detail both colonization and disease after E. coli O157:H7 oral infection had been reported.
We described the use of conventional BALB/c mice to model E. coli O157:H7 oral infection [80]. Female animals with an intact commensal flora (ICF) were inoculated with high doses (109 CFU or greater) of E. coli O157:H7 strain 86-24. Thus, E. coli O157:H7 introduced orally by either pipette feeding or intragastric administration (gavage) were forced to compete with commensal flora to become established within the gastrointestinal tract. The use of an ICF animal model for studies of E. coli O157:H7 infection and pathogenesis has the advantage of reflecting a complex microbiological environment in the gut such as that typically found in an individual who ingests E. coli O157:H7 in contaminated food or water or through person-to-person contact. Upon infection, mice were colonized with E. coli O157:H7 for the seven day course of the experiments; however, high doses of E. coli O157:H7 were required to achieve consistent, persistent colonization in the face of the ICF of the mouse. The primary site of E. coli O157:H7 colonization was demonstrated to be in the cecum of these animals. We surmised that the bacteria were shed into the cecal content where they transiently colonized or directly passed through the large intestine in the luminal contents prior to becoming encased in fecal pellets that were subsequently expelled [80].
The ICF model of E. coli O157:H7 infection is unique in that we were able to monitor both colonization and disease, which included ruffled fur, lethargy, weight loss, and, on average, ∼30% mortality [80]. In addition, ICF mice infected with E. coli O157:H7 displayed evidence of renal tubular damage with increased blood urea nitrogen (BUN) and slightly increased levels of creatinine, both of which can indicate renal impairment. These blood chemistry findings are seen in patients with HUS that sometimes follows E. coli O157:H7 infection. Therefore, through these data and others, we inferred that Stx2 produced by E. coli O157:H7 at the site of infection within the gastrointestinal tract could enter the bloodstream and harm the kidneys of the mice in a manner analogous to that presumed to occur in some E. coli O157:H7-infected humans [80].
3.7. Citrobacter Rodentium as a Surrogate for E. coli O157:H7
Another mouse model system used to evaluate the virulence mechanisms of EHEC employs the natural mouse pathogen Citrobacter rodentium as a surrogate for E. coli O157:H7. C. rodentium is similar to both EPEC and EHEC in that it carries a homolog of the LEE pathogenicity island of EPEC and EHEC and has the capacity to evoke A/E lesions. Thus, Citrobacter has been used to study the molecular basis for A/E lesion formation in mice because it is the only known LEE-positive organism that is naturally pathogenic for rodents [130]. C. rodentium causes transmissible murine colonic hyperplasia (TMCH) [130]. While the organism is useful for assessment of the contribution of various genes/proteins to virulence, this model fails to recapitulate the pathogenesis caused by EHEC as a whole because it does not make Shiga toxin (although there was one report of a strain of Citrobacter freundii that produces Stx2 [131]).
3.8. Alternative Models to Oral Infection
In studies of mice infected with E. coli O157:H7, the oral route of challenge is used most frequently. Nevertheless, alternative means of inoculation with STEC, although infrequent, have been described. For instance, one of the earliest published murine models of STEC infection relied on the subcutaneous injection of large quantities (108 CFU) of E. coli O157:H7 into SPF mice [132]. Intravenous injection of mice with spontaneously-derived motility mutants of E. coli O157:H7 was explored as a system to assess potential differences in virulence of the strains [133]. Most recently, Gao et al. evaluated the protective impact of an Stx fusion protein vaccine on intraperitoneal challenge with E. coli O157:H7 [134].
4. Mouse Models of Intoxication
HC and HUS, which are the most severe manifestations of disease observed after infection with E. coli O157:H7 in particular but with other STEC isolates as well, have been attributed to the production of Stx by the organisms. As such, mouse models are often used to explore the effects of Shiga toxin intoxication on an animal. While this can be achieved by inoculation with toxigenic strains [135–138], toxin is more commonly administered to mice by means of parenteral injection [24, 69, 135, 139–142]. Injection of purified Stx1 or Stx2 alone causes paralysis, damage to renal cortical tubule epithelial cells, and ultimately kills mice [69]. The LD50 for Stx1 is ∼125 ng and for Stx2 is ∼1 ng [24]; these LD50 data conflict with in vitro analyses of Stx that show that the CD50 of Stx1 for Vero cells is lower than that of Stx2 [23]. However, the mouse LD50s support data that show that purified Stx2 is about 1,000-fold more toxic to human renal endothelial cells [25] as well as epidemiological findings that suggest a difference in Stx1 and Stx2 toxicity for people; Stx2-producing E. coli O157:H7 strains are more frequently associated with HUS than are strains that produce Stx1 [26–28]. Mice fed Stx2-producing DH5α experienced mortality and exhibited renal lesions similar to those observed in STEC (E. coli O91:H21)-infected mice [135].
4.1. Administration of Stx and Endotoxin
Because injection of Stx alone initially failed to recapitulate all features of HUS, a mouse model that mimics the course of human E. coli O157:H7 disease was developed. In this model, mice were injected with a combination of Stx and LPS [139–141, 143, 144]. Systemic exposure to both virulence factors caused characteristic features of HUS that included neutrophilia, thrombocytopenia, red cell hemolysis, and increased BUN and creatinine levels [139]. In addition, evidence of fibrin deposition and red blood cell infiltration were observed in the glomeruli, capillaries, and intertubular spaces [139]. The renal damage was caused by Stx-induced apoptosis of the Gb3-expressing renal cortical and medullary tubular cells, which led to loss of function in the renal collecting ducts and subsequent dehydration [140]. The role of LPS in the mouse model of HUS, however, is controversial. Palermo et al. determined that mortality caused by Stx and LPS was dependent on the timing of the LPS administration relative to Stx intoxication [145]. In contrast, Suzuki et al. observed no synergistic effect of coadministration of LPS and Stx based on analysis of cytokine induction and mortality [146]. Recent studies demonstrated that repeated exposure to sublethal doses of Stx2 in the absence of LPS resulted in the development of features of HUS [73]. Extended exposure to Stx would be expected following infection with E. coli O157:H7 or another STEC.
4.2. Feeding of Stx
It has been postulated that toxin produced by E. coli O157:H7 and liberated into consumables may result in intoxication in the absence of infection [147]. To test this hypothesis in a mouse model, toxin was delivered to mice orally in an attempt to cause systemic intoxication. Our lab found that mice fed large amounts of Stx2 succumbed to the effects of toxin (unpublished data). Furthermore, Rasooly and colleagues showed that mice fed Stx also died within a few days of oral intoxication [148]. In their study, toxin-mediated damage to distal organs was observed, which indicated that Stx was able to migrate out of the digestive tract in the absence of E. coli O157:H7 [148].
5. Applications of Mouse Models
5.1. The Role of Stx
In 2006, Robinson et al. demonstrated that Stx2 facilitated adherence of E. coli O157:H7 to epithelial cells in vitro and colonization of the intestine in vivo [149]. They showed that an isogenic E. coli O157:H7 stx2 mutant adhered to HEp-2 cells to a lesser extent than did the wild-type E. coli O157:H7 strain; furthermore, this adherence deficiency could be overcome by treatment of the HEp-2 cells with purified Stx2. In single infections of conventional mice, the Stx2-producing wild-type E. coli O157:H7 strain colonized the mouse intestine at significantly higher levels than did its isogenic stx2 mutant. However, in a co-infection, the E. coli O157:H7 wild-type and stx2 mutant strains colonized mice similarly. These data led Robinson et al. to conclude that Stx2 produced by the wild-type complemented the stx2 defect in the mutant in vivo [149]. Stx was also shown to facilitate colonization of C57BL/6 mice with E. coli O157:H7 [150]. Calderon-Toledo et al. reported higher E. coli O157:H7 counts in the feces of str-treated mice infected with a Stx2-producing strain when compared to a Stx2-nonproducing strain [150].
More recently, we confirmed the capacity of Stx2 to promote E. coli O157:H7 colonization in the ICF mouse model [151]. In our study, we exogenously supplied Stx2 to an stx2 mutant E. coli O157:H7. This addition of Stx2 in trans to the stx2 mutant restored the capacity of the mutant to colonize the intestines of mice with an ICF. Furthermore, we discovered that anti-Stx2 neutralizing antibodies administered prior to infection significantly decreased the likelihood that a mouse would become highly colonized with E. coli O157:H7. We also confirmed that these Stx2-neutralizing antibodies could protect E. coli O157:H7-infected mice against manifestations of Stx2-mediated disease such as weight loss and death. Moreover, mice repeatedly immunized with a Stx2 toxoid developed a Stx2-neutralizing fecal antibody response; when challenged with E. coli O157:H7, immunized mice shed fewer organisms in their feces for a shorter duration than did sham-vaccinated control mice [151].
5.2. The Role of Gb3
For Stx to intoxicate a host, either directly via injection or by infection with STEC, the toxin must gain entry into host cells by binding to the Stx receptor Gb3. Thus, for an animal model of Stx intoxication to be relevant, surface-expressed Gb3 must be present in the host. In addition, the degree of receptor expression by particular cells dictates the focus of toxin damage. In humans, the gene that encodes the Gb3 synthase is expressed to varying levels in the heart, kidney, spleen, liver, lung, stomach, small intestine, and colon [152, 153]. Furthermore, Gb3-expressing cells have been identified in the colon, endothelium, kidney, and CNS [153–160].
In mice, Okuda and colleagues used Gb3 knockout mice to demonstrate that the effects of Stx injection are indeed dependent on the expression of Gb3 [161]. The concept that Gb3 may be differentially distributed in murine tissues in comparison to human tissue was supported by the work of Fujii et al., who reported that the Gb3 synthase gene is expressed most highly in the kidney and lungs, with lower expression levels in the brain, heart, gastrointestinal tract, and spleen [162]. They and others have shown that the actual glycolipid Gb3 is present in the kidney (tubules but not glomeruli, despite reports of toxin-associated glomerular damage [73, 76, 139, 144, 161]), as well as the lung, brain, and spleen [69, 161, 163, 164]. In summation, even though the relative Gb3 expression levels between humans and mice are not known, the overall tissue distribution of Gb3 between adult humans and mice appears to be similar.
5.3. Stx and Pathogenesis
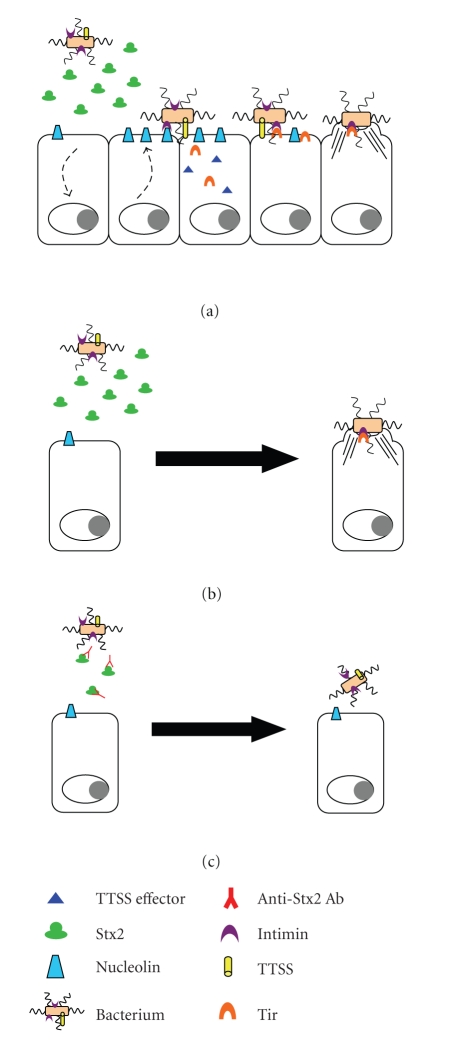
The use of mouse models to explore the role of Stx, arguably the most important virulence factor of E. coli O157:H7, in colonization and disease has served to provide a better understanding of E. coli O157:H7 pathogenesis. A model has been postulated whereby the bacteria enter the gastrointestinal tract and then transit to the primary site of colonization (the cecum in mice [80, 99]) where they then elaborate Stx2. Data generated in vitro with HEp-2 cells indicate that the action of the toxin causes an increase in cell surface-localized nucleolin [149]. Nucleolin is a eukaryotic-binding partner for intimin and thus a eukaryotic receptor for intimin-expressing E. coli O157:H7 [48, 52]. Intimin from the E. coli O157:H7 and cell-surface nucleolin interact to provide an initial point of attachment for the bacterium. Once the organism associates with the host cell via the intimin/nucleolin interaction, intimin binds to Tir, the bacterially encoded intimin receptor that is translocated by the TTSS into the host cell. The intimin/Tir interaction, coupled with the effects of other TTSS effector proteins, culminates in the formation of the characteristic A/E lesion/pedestal (Figure 4(a)).
Figure 4.
Model of the role of Stx2 in E. coli O157:H7 adherence and colonization. (a) E. coli O157:H7 elaborates Shiga toxin early during the colonization/adherence process. Stx2 exerts an effect on the host cell epithelium that leads to increased levels of cell surface-localized nucleolin. Nucleolin acts as an initial receptor for intimin, an interaction that allows E. coli O157:H7 to bind to the host epithelium and inject Tir and other TTSS effectors into the host cell. Intimin then engages Tir which, coupled with the cellular effects of other TTSS effectors, leads to host cell cytoskeletal rearrangement and formation of the characteristic A/E lesion. (b) Stx2, produced by the wild-type organism or provided to a stx2 mutant, facilitates colonization of the gastrointestinal tract. (c) Neutralizing anti-Stx2 antibody present prior to and during E. coli O157:H7 infection results in reduced levels of E. coli O157:H7 colonization of the gastrointestinal tract.
Stx2 production may occur during transit of the organism through the intestines. Stx2 was found in the cecum and at other sites where E. coli O157:H7 were abundant early in infection [80]. Furthermore, in our study, Stx2 was more abundant in the cecal and large intestinal luminal contents than in the corresponding tissue; these observations may indicate that toxin is absorbed into the circulation from both the cecum and the large intestine [80]. At least a portion of the toxin produced within the gastrointestinal tract enters the bloodstream because (1) renal tubular damage was evident in infected mice, and toxin was detected in the kidney of at least one infected animal [80], and (2) parenteral inoculation of antitoxin antibody protected mice from the systemic manifestations of disease [151].
Given that Stx not only acts systemically but also facilitates E. coli O157:H7 colonization [151], a therapeutic strategy directed against toxin has potential to be quite beneficial. To this end, we demonstrated that toxin provided exogenously to a toxin-null mutant resulted in increased E. coli O157:H7 colonization [151]. Thus, toxin, produced by the wild-type or provided exogenously to the mutant, had the capacity to increase the colonization levels of E. coli O157:H7 in mice (Figure 4(b)). Additionally, anti-toxin antibody, either administered to animals or actively generated following vaccination, reduced the overall levels of E. coli O157:H7 shed by mice and protected animals from systemic manifestations of disease (Figure 4(c)).
6. Conclusions
As a result of the increased rate of HUS over the last several years and the lack of therapies for treatment of HUS, further research is necessary to define mechanisms involved in the pathogenesis of E. coli O157:H7 and to identify potential disease prevention strategies and therapeutics. The application of animal model systems is vital to achieve these goals. While no one model recapitulates all features of E. coli O157:H7 infection, many valuable mouse models have been developed that permit exploration of E. coli O157:H7 pathogenesis and that can help pinpoint the means by which E. coli O157:H7 infection and/or disease can be controlled or prevented.
Acknowledgments
The authors would like to thank Dr. Christy Ventura for assistance in the proofreading and editing of this paper. This work was supported by R37 AI20148 from the National Institute of Allergy and Infectious Diseases (ADO), and by intramural awards R073NQ (ADO) and T073MR (KLM) from the Uniformed Services University of the Health Sciences.
References
- 1.Karmali MA, Petric M, Lim C. The association between idiopathic hemolytic uremic syndrome and infection by verotoxin-producing Escherichia coli. Journal of Infectious Diseases. 1985;151(5):775–782. doi: 10.1093/infdis/151.5.775. [DOI] [PubMed] [Google Scholar]
- 2.Karmali MA, Steele BT, Petric M, Lim C. Sporadic cases of haemolytic-uraemic syndrome associated with faecal cytotoxin and cytotoxin-producing Escherichia coli in stools. The Lancet. 1983;1(8325):619–620. doi: 10.1016/s0140-6736(83)91795-6. [DOI] [PubMed] [Google Scholar]
- 3.Riley LW, Remis RS, Helgerson SD, et al. Hemmorhagic colitis associated with a rare Escherichia coli serotype. New England Journal of Medicine. 1983;308(12):681–685. doi: 10.1056/NEJM198303243081203. [DOI] [PubMed] [Google Scholar]
- 4.Rangel JM, Sparling PH, Crowe C, Griffin PM, Swerdlow DL. Epidemiology of Escherichia coli O157:H7 outbreaks, United States, 1982–2002. Emerging Infectious Diseases. 2005;11(4):603–609. doi: 10.3201/eid1104.040739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michino H, Araki K, Minami S, et al. Massive outbreak of Escherichia coli O157:H7 infection in schoolchildren in Sakai City, Japan, associated with consumption of white radish sprouts. American Journal of Epidemiology. 1999;150(8):787–796. doi: 10.1093/oxfordjournals.aje.a010082. [DOI] [PubMed] [Google Scholar]
- 6.Cody SH, Glynn MK, Farrar JA, et al. An outbreak of Escherichia coli O157:H7 infection from unpasteurized commercial apple juice. Annals of Internal Medicine. 1999;130(3):202–209. doi: 10.7326/0003-4819-130-3-199902020-00005. [DOI] [PubMed] [Google Scholar]
- 7.CDC. Update on Multi-State Outbreak of E. coli O157:H7 Infections From Fresh Spinach, October 6, 2006. October 2006, http://www.cdc.gov/foodborne/ecolispinach/100606.htm.
- 8.CDC. Multistate Outbreak of E. coli O157:H7 Infections Linked to Eating Raw Refrigerated Prepackaged Cookie Dough. August 2009, http://www.cdc.gov/ecoli/2009/0807.html.
- 9.Mead PS, Slutsker L, Dietz V, et al. Food-related illness and death in the United States. Emerging Infectious Diseases. 1999;5(5):607–625. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hancock DD, Besser TE, Rice DH. Ecology of Escherichia coli O157:H7 in cattle and impact of management practices. In: Kaper JB, O'Brien AD, editors. Escherichia coli O157:H7 and Other Shiga Toxin-Producing E. coli Strains. Washington, DC, USA: ASM Press; 1998. pp. 85–91. [Google Scholar]
- 11.Griffin PM, Tauxe RV. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiologic Reviews. 1991;13:60–98. doi: 10.1093/oxfordjournals.epirev.a036079. [DOI] [PubMed] [Google Scholar]
- 12.Meng J, Doyle MP. Microbiology of Shiga toxin-producing Escherichia coli in foods. In: Kaper JB, O'Brien AD, editors. Escherichia coli O157:H7 and Other Shiga Toxin-Producing E. coli Strains. Washington, DC, USA: ASM Press; 1998. pp. 92–108. [Google Scholar]
- 13.Griffin PM. Escherichia coli O157:H7 and other enterohemorrhagic Escherichia coli. In: Blaser MJ, Smith PD, Ravdin JI, Greenberg HB, Guerrant RL, editors. Infections of the Gastrointestinal Tract. New York, NY, USA: Raven Press; 1995. pp. 739–761. [Google Scholar]
- 14.Brandt ML, O’Regan S, Rousseau E, Yazbeck S. Surgical complications of the hemolytic-uremic syndrome. Journal of Pediatric Surgery. 1990;25(11):1109–1112. doi: 10.1016/0022-3468(90)90741-q. [DOI] [PubMed] [Google Scholar]
- 15.Manning SD, Motiwala AS, Springman AC, et al. Variation in virulence among clades of Escherichia coli O157:H7 associated with disease outbreaks. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(12):4868–4873. doi: 10.1073/pnas.0710834105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fox JT, Thomson DU, Drouillard JS, et al. Efficacy of Escherichia coli O157:H7 siderophore receptor/porin proteins-based vaccine in feedlot cattle naturally shedding E. coli O157. Foodborne Pathogens and Disease. 2009;6(7):893–899. doi: 10.1089/fpd.2009.0336. [DOI] [PubMed] [Google Scholar]
- 17.Moxley RA, Smith DR, Luebbe M, Erickson GE, Klopfenstein TJ, Rogan D. Escherichia coli O157:H7 vaccine dose-effect in feedlot cattle. Foodborne Pathogens and Disease. 2009;6(7):879–884. doi: 10.1089/fpd.2009.0297. [DOI] [PubMed] [Google Scholar]
- 18.Smith DR, Moxley RA, Klopfenstein TJ, Erickson GE. A randomized longitudinal trial to test the effect of regional vaccination within a cattle feedyard on Escherichia coli O157:H7 rectal colonization, fecal shedding, and hide contamination. Foodborne Pathogens and Disease. 2009;6(7):885–892. doi: 10.1089/fpd.2009.0299. [DOI] [PubMed] [Google Scholar]
- 19.Thomson DU, Loneragan GH, Thornton AB, et al. Use of a siderophore receptor and porin proteins-based vaccine to control the burden of Escherichia coli O157:H7 in feedlot cattle. Foodborne Pathogens and Disease. 2009;6(7):871–877. doi: 10.1089/fpd.2009.0290. [DOI] [PubMed] [Google Scholar]
- 20.Melton-Celsa AR, Smith MJ, O’Brien AD. Shiga toxins: potent poisons, pathogenicity determinants, and pharmacological agents. In: Bock A, Curtiss R III, Kaper JB, et al., editors. EcoSal—Escherichia coli and Salmonella: Cellular and Molecular Biology. Washington, DC, USA: ASM Press; 2005. [DOI] [PubMed] [Google Scholar]
- 21.O’Brien AD, Holmes RK. Shiga and Shiga-like toxins. Microbiological Reviews. 1987;51(2):206–220. doi: 10.1128/mr.51.2.206-220.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wen SX, Teel LD, Judge NA, O’Brien AD. Genetic toxoids of Shiga toxin types 1 and 2 protect mice against homologous but not heterologous toxin challenge. Vaccine. 2006;24(8):1142–1148. doi: 10.1016/j.vaccine.2005.08.094. [DOI] [PubMed] [Google Scholar]
- 23.Smith MJ, Teel LD, Carvalho HM, Melton-Celsa AR, O’Brien AD. Development of a hybrid Shiga holotoxoid vaccine to elicit heterologous protection against Shiga toxins types 1 and 2. Vaccine. 2006;24(19):4122–4129. doi: 10.1016/j.vaccine.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 24.Smith MJ, Carvalho HM, Melton-Celsa AR, O’Brien AD. The 13C4 monoclonal antibody that neutralizes Shiga toxin type 1 (Stx1) recognizes three regions on the Stx1 B subunit and prevents Stx1 from binding to its eukaryotic receptor globotriaosylceramide. Infection and Immunity. 2006;74(12):6992–6998. doi: 10.1128/IAI.01247-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Louise CB, Obrig TG. Specific interaction of Escherichia coli O157:H7-derived Shiga-like toxin II with human renal endothelial cells. Journal of Infectious Diseases. 1995;172(5):1397–1401. doi: 10.1093/infdis/172.5.1397. [DOI] [PubMed] [Google Scholar]
- 26.Boerlin P, McEwen SA, Boerlin-Petzold F, Wilson JB, Johnson RP, Gyles CL. Associations between virulence factors of Shiga toxin-producing Escherichia coli and disease in humans. Journal of Clinical Microbiology. 1999;37(3):497–503. doi: 10.1128/jcm.37.3.497-503.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ostroff SM, Tarr PI, Neill MA, Lewis JH, Hargret-Bean N, Kobayashi JM. Toxin genotypes and plasmid profiles as determinants of systemic sequelae in Escherichia coli O157:H7 infections. Journal of Infectious Diseases. 1989;160(6):994–998. doi: 10.1093/infdis/160.6.994. [DOI] [PubMed] [Google Scholar]
- 28.Scotland SM, Willshaw GA, Smith HR, Rowe B. Properties of strains of Escherichia coli belonging to serogroup O157 with special reference to production of Vero cytotoxins VT1 and VT2. Epidemiology and Infection. 1987;99(3):613–624. doi: 10.1017/s0950268800066462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lingwood CA. Role of verotoxin receptors in pathogenesis. Trends in Microbiology. 1996;4(4):147–153. doi: 10.1016/0966-842x(96)10017-2. [DOI] [PubMed] [Google Scholar]
- 30.Lingwood CA. Verotoxins and their glycolipid receptors. Advances in Lipid Research. 1993;25:189–211. [PubMed] [Google Scholar]
- 31.Johannes L, Römer W. Shiga toxins from cell biology to biomedical applications. Nature Reviews Microbiology. 2010;8(2):105–116. doi: 10.1038/nrmicro2279. [DOI] [PubMed] [Google Scholar]
- 32.Endo Y, Tsurugi K, Yutsudo T, Takeda Y, Ogasawara T, Igarashi K. Site of action of a Vero toxin (VT2) from Escherichia coli O157:H7 and of Shiga toxin on eukaryotic ribosomes. RNA N-glycosidase activity of the toxins. European Journal of Biochemistry. 1988;171(1-2):45–50. doi: 10.1111/j.1432-1033.1988.tb13756.x. [DOI] [PubMed] [Google Scholar]
- 33.Saxena SK, O’Brien AD, Ackerman EJ. Shiga toxin, Shiga-like toxin II variant, and ricin are all single-site RNA N-glycosidases of 28 S RNA when microinjected into Xenopus oocytes. Journal of Biological Chemistry. 1989;264(1):596–601. [PubMed] [Google Scholar]
- 34.Obrig TG. Shiga toxin mode of action in E. coli O157:H7 disease. Frontiers in Bioscience. 1997;2:d635–d642. doi: 10.2741/a219. [DOI] [PubMed] [Google Scholar]
- 35.Nataro JP, Kaper JB. Diarrheagenic Escherichia coli. Clinical Microbiology Reviews. 1998;11(1):142–201. doi: 10.1128/cmr.11.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levine MM, Xu J, Kaper JB, et al. A DNA probe to identify enterohemorrhagic Escherichia coli of O157:H7 and other serotypes that cause hemorrhagic colitis and hemolytic uremic syndrome. Journal of Infectious Diseases. 1987;156(1):175–182. doi: 10.1093/infdis/156.1.175. [DOI] [PubMed] [Google Scholar]
- 37.Burland V, Shao Y, Perna NT, Plunkett G, Sofia HJ, Blattner FR. The complete DNA sequence and analysis of the large virulence plasmid of Escherichia coli O157:H7. Nucleic Acids Research. 1998;26(18):4196–4204. doi: 10.1093/nar/26.18.4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tatsuno I, Horie M, Abe H, et al. toxB gene on pO157 of enterohemorrhagic Escherichia coli O157:H7 is required for full epithelial cell adherence phenotype. Infection and Immunity. 2001;69(11):6660–6669. doi: 10.1128/IAI.69.11.6660-6669.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmidt H, Karch H, Beutin L. The large-sized plasmids of enterohemorrhagic Escherichia coli O157 strains encode hemolysins which are presumably members of the E. coliα-hemolysin family. FEMS Microbiology Letters. 1994;117(2):189–196. doi: 10.1111/j.1574-6968.1994.tb06763.x. [DOI] [PubMed] [Google Scholar]
- 40.Karch H, Heeseman J, Laufs R, et al. A plasmid of enterohemorrhagic Escherichia coli O157:H7 is required for expression of a new fimbrial antigen and for adhesion to epithelial cells. Infection and Immunity. 1987;55(2):455–461. doi: 10.1128/iai.55.2.455-461.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frankel G, Phillips AD, Rosenshine I, Dougan G, Kaper JB, Knutton S. Enteropathogenic and enterohaemorrhagic Escherichia coli: more subversive elements. Molecular Microbiology. 1998;30(5):911–921. doi: 10.1046/j.1365-2958.1998.01144.x. [DOI] [PubMed] [Google Scholar]
- 42.Feng P, Weagant SD, Monday SR. Genetic analysis for virulence factors in Escherichia coli O104:H21 that was implicated in an outbreak of hemorrhagic colitis. Journal of Clinical Microbiology. 2001;39(1):24–28. doi: 10.1128/JCM.39.1.24-28.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paton AW, Woodrow MC, Doyle RM, Lanser JA, Paton JC. Molecular characterization of a Shiga toxigenic Escherichia coli O113:H21 strain lacking eae responsible for a cluster of cases of hemolytic- uremic syndrome. Journal of Clinical Microbiology. 1999;37(10):3357–3361. doi: 10.1128/jcm.37.10.3357-3361.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cornick NA, Booher SL, Moon HW. Intimin facilitates colonization by Escherichia coli O157:H7 in adult ruminants. Infection and Immunity. 2002;70(5):2704–2707. doi: 10.1128/IAI.70.5.2704-2707.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Donnenberg MS, Tzipori S, McKee ML, O’Brien AD, Alroy J, Kaper JB. The role of the eae gene of enterohemorrhagic Escherichia coli in intimate attachment in vitro and in a porcine model. Journal of Clinical Investigation. 1993;92(3):1418–1424. doi: 10.1172/JCI116718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Judge NA, Mason HS, O’Brien AD. Plant cell-based intimin vaccine given orally to mice primed with intimin reduces time of Escherichia coli O157:H7 shedding in feces. Infection and Immunity. 2004;72(1):168–175. doi: 10.1128/IAI.72.1.168-175.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McKee ML, Melton-Celsa AR, Moxley RA, Francis DH, O’Brien AD. Enterohemorrhagic Escherichia coli O157:H7 requires intimin to colonize the gnotobiotic pig intestine and to adhere to HEp-2 cells. Infection and Immunity. 1995;63(9):3739–3744. doi: 10.1128/iai.63.9.3739-3744.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sinclair JF, Dean-Nystrom EA, O’Brien AD. The established intimin receptor Tir and the putative eucaryotic intimin receptors nucleolin and β1 integrin localize at or near the site of enterohemorrhagic Escherichia coli O157:H7 adherence to enterocytes in vivo. Infection and Immunity. 2006;74(2):1255–1265. doi: 10.1128/IAI.74.2.1255-1265.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tzipori S, Gunzer F, Donnenberg MS, De Montigny L, Kaper JB, Donohue- Rolfe A. The role of the eaeA gene in diarrhea and neurological complications in a gnotobiotic piglet model of enterohemorrhagic Escherichia coli infection. Infection and Immunity. 1995;63(9):3621–3627. doi: 10.1128/iai.63.9.3621-3627.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woods JB, Schmitt CK, Darnell SC, Meysick KC, O’Brien AD. Ferrets as a model system for renal disease secondary to intestinal infection with Escherichia coli O157:H7 and other Shiga toxin-producing E. coli. Journal of Infectious Diseases. 2002;185(4):550–554. doi: 10.1086/338633. [DOI] [PubMed] [Google Scholar]
- 51.Frankel G, Lider O, Hershkoviz R, et al. The cell-binding domain of intimin from enteropathogenic Escherichia coli binds to β integrins. Journal of Biological Chemistry. 1996;271(34):20359–20364. doi: 10.1074/jbc.271.34.20359. [DOI] [PubMed] [Google Scholar]
- 52.Sinclair JF, O’Brien AD. Cell surface-localized nucleolin is a eukaryotic receptor for the adhesin intimin-γ of enterohemorrhagic Escherichia coli O157:H7. Journal of Biological Chemistry. 2002;277(4):2876–2885. doi: 10.1074/jbc.M110230200. [DOI] [PubMed] [Google Scholar]
- 53.Sinclair JF, O’Brien AD. Intimin types α, β, and γ bind to nucleolin with equivalent affinity but lower avidity than to the translocated intimin receptor. Journal of Biological Chemistry. 2004;279(32):33751–33758. doi: 10.1074/jbc.M401616200. [DOI] [PubMed] [Google Scholar]
- 54.Tarr PI, Bilge SS. Intimin-independent adherence mechanisms of Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. In: Kaper JB, O’Brien AD, editors. Escherichia coli O157:H7 and Other Shiga Toxin-Producing E. coli Strains. Washington, DC, USA: ASM Press; 1998. pp. 157–162. [Google Scholar]
- 55.Monnens L, Savage CO, Taylor CM. Pathophysiology of hemolytic-uremic syndrome. In: Kaper JB, O’Brien AD, editors. Escherichia coli O157:H7 and Other Shiga Toxin-Producing E. coli Strains. Washington, DC, USA: ASM Press; 1998. pp. 287–292. [Google Scholar]
- 56.Madara JL, Stafford J, Dharmsathaphorn K, Carlson S. Structural analysis of a human intestinal epithelial cell line. Gastroenterology. 1987;92(5, part 1):1133–1145. doi: 10.1016/s0016-5085(87)91069-9. [DOI] [PubMed] [Google Scholar]
- 57.Acheson DWK, Moore R, De Breucker S, et al. Translocation of shiga toxin across polarized intestinal cells in tissue culture. Infection and Immunity. 1996;64(8):3294–3300. doi: 10.1128/iai.64.8.3294-3300.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Izumikawa K, Hirakata Y, Yamaguchi T, et al. Escherichia coli O157 interactions with human intestinal Caco-2 cells and the influence of fosfomycin. Journal of Antimicrobial Chemotherapy. 1998;42(3):341–347. doi: 10.1093/jac/42.3.341. [DOI] [PubMed] [Google Scholar]
- 59.Acheson DWK, Lincicome LL, Jacewicz MS, et al. Shiga toxin interaction with intestinal epithelial cells. In: Kaper JB, O’Brien AD, editors. Escherichia coli O157:H7 and Other Shiga Toxin-Producing E. coli Strains. Washington, DC, USA: ASM Press; 1998. pp. 140–147. [Google Scholar]
- 60.Hammond TG, Hammond JM. Optimized suspension culture: the rotating-wall vessel. American Journal of Physiology. 2001;281(1):F12–F25. doi: 10.1152/ajprenal.2001.281.1.F12. [DOI] [PubMed] [Google Scholar]
- 61.Nickerson CA, Goodwin TJ, Terlonge J, et al. Three-dimensional tissue assemblies: novel models for the study of Salmonella enterica serovar typhimurium pathogenesis. Infection and Immunity. 2001;69(11):7106–7120. doi: 10.1128/IAI.69.11.7106-7120.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Unsworth BR, Lelkes PI. Growing tissues in microgravity. Nature Medicine. 1998;4(8):901–907. doi: 10.1038/nm0898-901. [DOI] [PubMed] [Google Scholar]
- 63.Carvalho HM, Teel LD, Goping G, O’Brien AD. A three-dimensional tissue culture model for the study of attach and efface lesion formation by enteropathogenic and enterohaemorrhagic Escherichia coli. Cellular Microbiology. 2005;7(12):1771–1781. doi: 10.1111/j.1462-5822.2004.00594.x. [DOI] [PubMed] [Google Scholar]
- 64.Baines D, Masson L, McAllister T. Escherichia coli O157:H7-secreted cytotoxins are toxic to enterocytes and increase Escherichia coli O157:H7 colonization of jejunum and descending colon in cattle. Canadian Journal of Animal Science. 2008;88(1):41–50. [Google Scholar]
- 65.Chong Y, Fitzhenry R, Heuschkel R, Torrente F, Frankel G, Phillips AD. Human intestinal tissue tropism in Escherichia coli O157:H7—initial colonization of terminal ileum and Peyer’s patches and minimal colonic adhesion ex vivo. Microbiology. 2007;153(3):794–802. doi: 10.1099/mic.0.2006/003178-0. [DOI] [PubMed] [Google Scholar]
- 66.Girard F, Batisson I, Frankel GM, Harel J, Fairbrother JM. Interaction of enteropathogenic and Shiga toxin-producing Escherichia coli and porcine intestinal mucosa: role of intimin and Tir in adherence. Infection and Immunity. 2005;73(9):6005–6016. doi: 10.1128/IAI.73.9.6005-6016.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Girard F, Dziva F, Van Diemen P, Phillips AD, Stevens MP, Frankel G. Adherence of enterohemorrhagic Escherichia coli O157, O26, and O111 strains to bovine intestinal explants ex vivo. Applied and Environmental Microbiology. 2007;73(9):3084–3090. doi: 10.1128/AEM.02893-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Phillips AD, Navabpour S, Hicks S, Dougan G, Wallis T, Frankel G. Enterohaemorrhagic Escherichia coli O157:H7 target Peyer’s patches in humans and cause attaching/effacing lesions in both human and bovine intestine. Gut. 2000;47(3):377–381. doi: 10.1136/gut.47.3.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tesh VL, Burris JA, Owens JW, et al. Comparison of the relative toxicities of Shiga-like toxins type I and type II for mice. Infection and Immunity. 1993;61(8):3392–3402. doi: 10.1128/iai.61.8.3392-3402.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Armstrong GD, Mulvey GL, Marcato P, et al. Human serum amyloid P component protects against Escherichia coli O157:H7 shiga toxin 2 in vivo: therapeutic implications for hemolytic-uremic syndrome. Journal of Infectious Diseases. 2006;193(8):1120–1124. doi: 10.1086/501472. [DOI] [PubMed] [Google Scholar]
- 71.Bentancor LV, Bilen M, Brando RJF, et al. A DNA vaccine encoding the enterohemorragic Escherichia coli shiga-like toxin 2 A and B subunits confers protective immunity to shiga toxin challenge in the murine model. Clinical and Vaccine Immunology. 2009;16(5):712–718. doi: 10.1128/CVI.00328-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sheoran AS, Chapman-Bonofiglio S, Harvey BR, et al. Human antibody against Shiga toxin 2 administered to piglets after the onset of diarrhea due to Escherichia coli O157:H7 prevents fatal systemic complications. Infection and Immunity. 2005;73(8):4607–4613. doi: 10.1128/IAI.73.8.4607-4613.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sauter KAD, Melton-Celsa AR, Larkin K, Troxell ML, O’Brien AD, Magun BE. Mouse model of hemolytic-uremic syndrome caused by endotoxin-free Shiga toxin 2 (Stx2) and protection from lethal outcome by anti-Stx2 antibody. Infection and Immunity. 2008;76(10):4469–4478. doi: 10.1128/IAI.00592-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Melton-Celsa AR, O’Brien AD. Animal models for STEC-mediated disease. Methods in Molecular Medicine. 2003;73:291–305. doi: 10.1385/1-59259-316-x:291. [DOI] [PubMed] [Google Scholar]
- 75.Eaton KA, Friedman DI, Francis GJ, et al. Pathogenesis of renal disease due to enterohemorrhagic Escherichia coli in germ-free mice. Infection and Immunity. 2008;76(7):3054–3063. doi: 10.1128/IAI.01626-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Karpman D, Council H, Svensson M, Scheutz F, Aim P, Svanborg C. The role of lipopolysaccharide and Shiga-like toxin in a mouse model of Escherichia coli O157:H7 infection. Journal of Infectious Diseases. 1997;175(3):611–620. doi: 10.1093/infdis/175.3.611. [DOI] [PubMed] [Google Scholar]
- 77.Kurioka T, Yunou Y, Kita E. Enhancement of susceptibility to Shiga toxin-producing Escherichia coli O157:H7 by protein calorie malnutrition in mice. Infection and Immunity. 1998;66(4):1726–1734. doi: 10.1128/iai.66.4.1726-1734.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shimizu K, Asahara T, Nomoto K, et al. Development of a lethal Shiga toxin-producing Escherichia coli-infection mousemodel using multiple mitomycin C treatment. Microbial Pathogenesis. 2003;35(1):1–9. doi: 10.1016/s0882-4010(03)00065-2. [DOI] [PubMed] [Google Scholar]
- 79.Wadolkowski EA, Burris JA, O’Brien AD. Mouse model for colonization and disease caused by enterohemorrhagic Escherichia coli O157:H7. Infection and Immunity. 1990;58(8):2438–2445. doi: 10.1128/iai.58.8.2438-2445.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mohawk KL, Melton-Celsa AR, Zangari T, Carroll EE, O’Brien AD. Pathogenesis of Escherichia coli O157:H7 strain 86-24 following oral infection of BALB/c mice with an intact commensal flora. Microbial Pathogenesis. 2010;48(3-4):131–142. doi: 10.1016/j.micpath.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zotta E, Lago N, Ochoa F, Repetto HA, Ibarra C. Development of an experimental hemolytic uremic syndrome in rats. Pediatric Nephrology. 2008;23(4):559–567. doi: 10.1007/s00467-007-0727-4. [DOI] [PubMed] [Google Scholar]
- 82.García A, Bosques CJ, Wishnok JS, et al. Renal injury is a consistent finding in Dutch Belted rabbits experimentally infected with enterohemorrhagic Escherichia coli. Journal of Infectious Diseases. 2006;193(8):1125–1134. doi: 10.1086/501364. [DOI] [PubMed] [Google Scholar]
- 83.Beery JT, Doyle MP, Schoeni JL. Colonization of chicken cecae by Escherichia coli associated with haemorrhagic colitis. Applied and Environmental Microbiology. 1985;49(2):310–315. doi: 10.1128/aem.49.2.310-315.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sueyoshi M, Nakazawa M. Experimental infection of young chicks with attaching and effacing Escherichia coli. Infection and Immunity. 1994;62(9):4066–4071. doi: 10.1128/iai.62.9.4066-4071.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tzipori S, Wachsmuth IK, Chapman C. The pathogenesis of hemorrhagic colitis caused by Escherichia coli O157:H7 in gnotobiotic piglets. Journal of Infectious Diseases. 1986;154(4):712–716. doi: 10.1093/infdis/154.4.712. [DOI] [PubMed] [Google Scholar]
- 86.Dean-Nystrom EA, Stoffregen WC, Bosworth BT, Moon HW, Pohlenz JF. Early attachment sites for shiga-toxigenic Escherichia coli O157:H7 in experimentally inoculated weaned calves. Applied and Environmental Microbiology. 2008;74(20):6378–6384. doi: 10.1128/AEM.00636-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fenwick BW, Cowan LA. Canine model of hemolytic-uremic syndrome. In: Kaper JB, O’Brien AD, editors. Escherichia coli O157:H7 and Other Shiga Toxin-Producing E. coli Strains. Washington, DC, USA: ASM Press; 1998. pp. 268–277. [Google Scholar]
- 88.Taylor FB, Tesh VL, DeBault L, et al. Characterization of the baboon responses to Shiga-like toxin. Descriptive study of a new primate model of toxic responses to Stx-1. American Journal of Pathology. 1999;154(4):1285–1299. doi: 10.1016/S0002-9440(10)65380-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kang G, Pulimood AB, Koshi R, et al. A monkey model for enterohemorrhagic Escherichia coli infection. Journal of Infectious Diseases. 2001;184(2):206–210. doi: 10.1086/322011. [DOI] [PubMed] [Google Scholar]
- 90.Moxley RA, Francis DH. Overview of animal models. In: Kaper JB, O’Brien AD, editors. Escherichia coli O157:H7 and Other Shiga Toxin-Producing E. coli Strains. Washington, DC, USA: ASM Press; 1998. pp. 249–260. [Google Scholar]
- 91.Dean-Nystrom EA, Bosworth BT, Moon HW, et al. Bovine infection with Shiga toxin-producing Escherichia coli. In: Kaper JB, O’Brien AD, editors. Escherichia coli O157:H7 and Other Shiga Toxin-Producing E. coli Strains. Washington, DC, USA: ASM Press; 1998. pp. 261–267. [Google Scholar]
- 92.Cowan LA, Hertzke DM, Fenwick BW, Andreasen CB. Clinical and clinicopathologic abnormalities in Greyhounds with cutaneous and renal glomerular vasculopathy: 18 cases (1992–1994) Journal of the American Veterinary Medical Association. 1997;210(6):789–793. [PubMed] [Google Scholar]
- 93.García A, Marini RP, Feng Y, et al. A naturally occurring rabbit model of enterohemorrhagic Escherichia coli-induced disease. Journal of Infectious Diseases. 2002;186(11):1682–1686. doi: 10.1086/345371. [DOI] [PubMed] [Google Scholar]
- 94.Lindgren SW, Melton AR, O’Brien AD. Virulence of enterohemorrhagic Escherichia coli O91:H21 clinical isolates in an orally infected mouse model. Infection and Immunity. 1993;61(9):3832–3842. doi: 10.1128/iai.61.9.3832-3842.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fujii J, Kita T, Yoshida SI, et al. Direct evidence of neuron impairment by oral infection with verotoxin-producing Escherichia coli O157:H- in mitomycin-treated mice. Infection and Immunity. 1994;62(8):3447–3453. doi: 10.1128/iai.62.8.3447-3453.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Isogai E, Isogai H, Takeshi K, Nishikawa T. Protective effect of Japanese green tea extract on gnotobiotic mice infected with an Escherichia coli O157:H7 strain. Microbiology and Immunology. 1998;42(2):125–128. doi: 10.1111/j.1348-0421.1998.tb02260.x. [DOI] [PubMed] [Google Scholar]
- 97.Conlan JW, Perry MB. Susceptibility of three strains of conventional adult mice to intestinal colonization by an isolate of Escherichia coli O157:H7. Canadian Journal of Microbiology. 1998;44(8):800–805. doi: 10.1139/cjm-44-8-800. [DOI] [PubMed] [Google Scholar]
- 98.Taguchi H, Takahashi M, Yamaguchi H, et al. Experimental infection of germ-free mice with hyper-toxigenic enterohaemorrhagic Escherichia coli O157:H7, strain 6. Journal of Medical Microbiology. 2002;51(4):336–343. doi: 10.1099/0022-1317-51-4-336. [DOI] [PubMed] [Google Scholar]
- 99.Nagano K, Taguchi K, Hara T, Yokoyama SI, Kawada K, Mori H. Adhesion and colonization of enterohemorrhagic Escherichia coli O157:H7 in cecum of mice. Microbiology and Immunology. 2003;47(2):125–132. doi: 10.1111/j.1348-0421.2003.tb02795.x. [DOI] [PubMed] [Google Scholar]
- 100.Brando RJF, Miliwebsky E, Bentancor L, et al. Renal damage and death in weaned mice after oral infection with Shiga toxin 2-producing Escherichia coli strains. Clinical and Experimental Immunology. 2008;153(2):297–306. doi: 10.1111/j.1365-2249.2008.03698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Myhal ML, Laux DC, Cohen PS. Relative colonizing abilities of human fecal and K 12 strains of Escherichia coli in the large intestines of streptomycin-treated mice. European Journal of Clinical Microbiology. 1982;1(3):186–192. doi: 10.1007/BF02019621. [DOI] [PubMed] [Google Scholar]
- 102.Meynell GG, Subbaiah TV. Antibacterial mechanisms of the mouse gut. I. Kinetics of infection by Salmonella typhimurium in normal and streptomycin-treated mice studied with abortive transductants. British Journal of Experimental Pathology. 1963;44:197–208. [PMC free article] [PubMed] [Google Scholar]
- 103.Miller CE, Wong KH, Feeley JC, Forlines ME. Immunological conversion of Vibrio chorlerae in gnotobiotic mice. Infection and Immunity. 1972;6(5):739–742. doi: 10.1128/iai.6.5.739-742.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Savage DC. Microbial ecology of the gastrointestinal tract. Annual Review of Microbiology. 1977;31:107–133. doi: 10.1146/annurev.mi.31.100177.000543. [DOI] [PubMed] [Google Scholar]
- 105.Wadolkowski EA, Laux DC, Cohen PS. Colonization of the streptomycin-treated mouse large intestine by a human fecal Escherichia coli strain: role of growth in mucus. Infection and Immunity. 1988;56(5):1030–1035. doi: 10.1128/iai.56.5.1030-1035.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tzipori S, Gibson R, Montanaro J. Nature and distribution of mucosal lesions associated with enteropathogenic and enterohemorrhagic Escherichia coli in piglets and the role of plasmid-mediated factors. Infection and Immunity. 1989;57(4):1142–1150. doi: 10.1128/iai.57.4.1142-1150.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Asahara T, Shimizu K, Nomoto K, Hamabata T, Ozawa A, Takeda Y. Probiotic bifidobacteria protect mice from lethal infection with Shiga toxin-producing Escherichia coli O157:H7. Infection and Immunity. 2004;72(4):2240–2247. doi: 10.1128/IAI.72.4.2240-2247.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Teel LD, Melton-Celsa AR, Schmitt CK, O’Brien AD. One of two copies of the gene for the activatable Shiga toxin type 2d in Escherichia coli O91:H21 strain B2F1 is associated with an inducible bacteriophage. Infection and Immunity. 2002;70(8):4282–4291. doi: 10.1128/IAI.70.8.4282-4291.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Melton-Celsa AR, Darnell SC, O’Brien AD. Activation of Shiga-like toxins by mouse and human intestinal mucus correlates with virulence of enterohemorrhagic Escherichia coli O91:H21 isolates in orally infected, streptomycin-treated mice. Infection and Immunity. 1996;64(5):1569–1576. doi: 10.1128/iai.64.5.1569-1576.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Funatogawa K, Ide T, Kirikae F, Saruta K, Nakano M, Kirikae T. Use of immunoglobulin enriched bovine colostrum against oral challenge with enterohaemorrhagic Escherichia coli O157:H7 in mice. Microbiology and Immunology. 2002;46(11):761–766. doi: 10.1111/j.1348-0421.2002.tb02761.x. [DOI] [PubMed] [Google Scholar]
- 111.Gagnon M, Kheadr EE, Dabour N, Richard D, Fliss I. Effect of Bifidobacterium thermacidophilum probiotic feeding on enterohemorrhagic Escherichia coli O157:H7 infection in BALB/c mice. International Journal of Food Microbiology. 2006;111(1):26–33. doi: 10.1016/j.ijfoodmicro.2006.04.041. [DOI] [PubMed] [Google Scholar]
- 112.Gamage SD, Strasser JE, Chalk CL, Weiss AA. Nonpathogenic Escherichia coli can contribute to the production of Shiga toxin. Infection and Immunity. 2003;71(6):3107–3115. doi: 10.1128/IAI.71.6.3107-3115.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Babiuk S, Asper DJ, Rogan D, Mutwiri GK, Potter AA. Subcutaneous and intranasal immunization with type III secreted proteins can prevent colonization and shedding of Escherichia coli O157:H7 in mice. Microbial Pathogenesis. 2008;45(1):7–11. doi: 10.1016/j.micpath.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 114.Gu J, Liu Y, Yu S, et al. Enterohemorrhagic Escherichia coli trivalent recombinant vaccine containing EspA, intimin and Stx2 induces strong humoral immune response and confers protection in mice. Microbes and Infection. 2009;11(10-11):835–841. doi: 10.1016/j.micinf.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 115.Uhlich GA, Keen JE, Elder RO. Variations in the csgD promoter of Escherichia coli O157:H7 associated with increased virulence in mice and increased invasion of HEp-2 cells. Infection and Immunity. 2002;70(1):395–399. doi: 10.1128/IAI.70.1.395-399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tilden J, Jr., Young W, McNamara AM, et al. A new route of transmission for Escherichia coli: infection from dry fermented salami. American Journal of Public Health. 1996;86(8):1142–1145. doi: 10.2105/ajph.86.8_pt_1.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Isogai E, Isogai H, Kimura K, et al. Role of tumor necrosis factor alpha in gnotobiotic mice infected with an Escherichia coli O157:H7 Strain. Infection and Immunity. 1998;66(1):197–202. doi: 10.1128/iai.66.1.197-202.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cimolai N, Morrison BJ, Carter JE. Risk factors for the central nervous system manifestations of gastroenteritis-associated hemolytic-uremic syndrome. Pediatrics. 1992;90(4):616–621. [PubMed] [Google Scholar]
- 119.Sawamura S, Tanaka K, Koga Y. Therapeutic effects of antibiotics against enterohemorrhagic Escherichia coli (EHEC) O157:H7 (O157) infection: in vivo analysis using germfree mice. The Journal of the Japanese Association for Infectious Diseases. 1999;73(10):1054–1063. doi: 10.11150/kansenshogakuzasshi1970.73.1054. [DOI] [PubMed] [Google Scholar]
- 120.Aiba Y, Ishikawa H, Shimizu K, et al. Role of internalization in the pathogenicity of shiga toxin-producing Escherichia coli infection in a gnotobiotic murine model. Microbiology and Immunology. 2002;46(11):723–731. doi: 10.1111/j.1348-0421.2002.tb02757.x. [DOI] [PubMed] [Google Scholar]
- 121.Isogai E, Isogai H, Hayashi S, et al. Effect of antibiotics, levofloxacin and fosfomycin, on a mouse model with Escherichia coli O157 infection. Microbiology and Immunology. 2000;44(2):89–95. doi: 10.1111/j.1348-0421.2000.tb01251.x. [DOI] [PubMed] [Google Scholar]
- 122.Isogai E, Isogai H, Hirose K, Hayashi S, Oguma K. In vivo synergy between green tea extract and levofloxacin against enterohemorrhagic Escherichia coli O157 infection. Current Microbiology. 2001;42(4):248–251. doi: 10.1007/s0028403357. [DOI] [PubMed] [Google Scholar]
- 123.Isogai E, Isogai H, Hirose K, et al. Therapeutic effect of anti-TNF-α antibody and levofloxacin (LVFX) in a mouse model of enterohemorrhagic Escherichia coli 0157 infection. Comparative Immunology, Microbiology and Infectious Diseases. 2001;24(4):217–231. doi: 10.1016/s0147-9571(01)00009-1. [DOI] [PubMed] [Google Scholar]
- 124.Takahashi M, Taguchi H, Yamaguchi H, Osaki T, Komatsu A, Kamiya S. The effect of probiotic treatment with Clostridium butyricum on enterohemorrhagic Escherichia coli O157:H7 infection in mice. FEMS Immunology and Medical Microbiology. 2004;41(3):219–226. doi: 10.1016/j.femsim.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 125.Jeon B, Itoh K. Production of shiga toxin by a luxS mutant of Escherichia coli O157:H7 in vivo and in vitro. Microbiology and Immunology. 2007;51(4):391–396. doi: 10.1111/j.1348-0421.2007.tb03926.x. [DOI] [PubMed] [Google Scholar]
- 126.Kolling GL, Matthews KR. Examination of recovery in vitro and in vivo of nonculturable Escherichia coli O157:H7. Applied and Environmental Microbiology. 2001;67(9):3928–3933. doi: 10.1128/AEM.67.9.3928-3933.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.LeBlanc J, Fliss I, Matar C. Induction of a humoral immune response following an Escherichia coli O157:H7 infection with an immunomodulatory peptidic fraction derived from Lactobacillus helveticus-fermented milk. Clinical and Diagnostic Laboratory Immunology. 2004;11(6):1171–1181. doi: 10.1128/CDLI.11.6.1171-1181.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mundy R, Girard F, FitzGerald AJ, Frankel G. Comparison of colonization dynamics and pathology of mice infected with enteropathogenic Escherichia coli, enterohaemorrhagic E. coli and Citrobacter rodentium. FEMS Microbiology Letters. 2006;265(1):126–132. doi: 10.1111/j.1574-6968.2006.00481.x. [DOI] [PubMed] [Google Scholar]
- 129.Sheng H, Knecht HJ, Kudva IT, Hovde CJ. Application of bacteriophages to control intestinal Escherichia coli O157:H7 levels in ruminants. Applied and Environmental Microbiology. 2006;72(8):5359–5366. doi: 10.1128/AEM.00099-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Luperchio SA, Schauer DB. Molecular pathogenesis of Citrobacter rodentium and transmissible murine colonic hyperplasia. Microbes and Infection. 2001;3(4):333–340. doi: 10.1016/s1286-4579(01)01387-9. [DOI] [PubMed] [Google Scholar]
- 131.Tschape H, Prager R, Streckel W, Fruth A, Tietze E, Bohme G. Verotoxinogenic Citrobacter freundii associated with severe gastroenteritis and cases of haemolytic uraemic syndrome in a nursery school: green butter as the infection source. Epidemiology and Infection. 1995;114(3):441–450. doi: 10.1017/s0950268800052158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lai XH, Xu JG, Liu BY. Experimental infection of specific-pathogen-free mice with enterohemorrhagic Escherichia coli 0157:H7. Microbiology and Immunology. 1991;35(7):515–524. doi: 10.1111/j.1348-0421.1991.tb01582.x. [DOI] [PubMed] [Google Scholar]
- 133.Tóth I, Csík M, Emody L. Spontaneous antibiotic resistance mutation associated pleiotropic changes in Escherichia coli O157:H7. Acta Veterinaria Hungarica. 2003;51(1):29–44. doi: 10.1556/AVet.51.2003.1.3. [DOI] [PubMed] [Google Scholar]
- 134.Gao X, Cai K, Shi J, et al. Immunogenicity of a novel Stx2B-Stx1B fusion protein in a mice model of Enterohemorrhagic Escherichia coli O157:H7 infection. Vaccine. 2009;27(14):2070–2076. doi: 10.1016/j.vaccine.2009.01.115. [DOI] [PubMed] [Google Scholar]
- 135.Lindgren SW, Samuel JE, Schmitt CK, O’Brien AD. The specific activities of Shiga-like toxin type II (SLT-II) and SLT-II- related toxins of enterohemorrhagic Escherichia coli differ when measured by Vero cell cytotoxicity but not by mouse lethality. Infection and Immunity. 1994;62(2):623–631. doi: 10.1128/iai.62.2.623-631.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Paton AW, Bourne AJ, Manning PA, Paton JC. Comparative toxicity and virulence of Escherichia coli clones expressing variant and chimeric shiga-like toxin type II operons. Infection and Immunity. 1995;63(7):2450–2458. doi: 10.1128/iai.63.7.2450-2458.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Paton JC, Paton AW. Survival rate of mice after transient colonization with Escherichia coli clones carrying variant Shiga-like toxin type II operons. Microbial Pathogenesis. 1996;20(6):377–383. doi: 10.1006/mpat.1996.0035. [DOI] [PubMed] [Google Scholar]
- 138.Nakagawa I, Nakata M, Yamamura T, Wakisaka S, Kawabata S, Hamada S. Infection and pathogenesis of a murine strain of Escherichia coli with genetically introduced Shiga toxin type I operon in conventional mice. Microbial Pathogenesis. 2002;33(2):63–72. doi: 10.1006/mpat.2002.0518. [DOI] [PubMed] [Google Scholar]
- 139.Keepers TR, Psotka MA, Gross LK, Obrig TG. A murine model of HUS: Shiga toxin with lipopolysaccharide mimics the renal damage and physiologic response of human disease. Journal of the American Society of Nephrology. 2006;17(12):3404–3414. doi: 10.1681/ASN.2006050419. [DOI] [PubMed] [Google Scholar]
- 140.Psotka MA, Obata F, Rolling GL, et al. Shiga toxin 2 targets the murine renal collecting duct epithelium. Infection and Immunity. 2009;77(3):959–969. doi: 10.1128/IAI.00679-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Barrett TJ, Potter ME, Wachsmuth IK. Bacterial endotoxin both enhances and inhibits the toxicity of Shiga-like toxin II in rabbits and mice. Infection and Immunity. 1989;57(11):3434–3437. doi: 10.1128/iai.57.11.3434-3437.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.O’Brien AD, LaVeck GD. Purification and characterization of a Shigella dysenteriae 1-like toxin produced by Escherichia coli. Infection and Immunity. 1983;40(2):675–683. doi: 10.1128/iai.40.2.675-683.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yuhas Y, Weizman A, Dinari G, Ashkenazi S. An animal model for the study of neurotoxicity of bacterial products and application of the model to demonstrate that Shiga toxin and lipopolysaccharide cooperate in inducing neurologic disorders. Journal of Infectious Diseases. 1995;171(5):1244–1249. doi: 10.1093/infdis/171.5.1244. [DOI] [PubMed] [Google Scholar]
- 144.Ikeda M, Ito S, Honda M. Hemolytic uremic syndrome induced by lipopolysaccharide and Shiga-like toxin. Pediatric Nephrology. 2004;19(5):485–489. doi: 10.1007/s00467-003-1395-7. [DOI] [PubMed] [Google Scholar]
- 145.Palermo M, Alves-Rosa F, Rubel C, et al. Pretreatment of mice with lipopolysaccharide (LPS) or IL-1β exerts dose-dependent opposite effects on Shiga toxin-2 lethality. Clinical and Experimental Immunology. 2000;119(1):77–83. doi: 10.1046/j.1365-2249.2000.01103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Suzuki K, Tateda K, Matsumoto T, Gondaira F, Tsujimoto S, Yamaguchi K. Effects of interaction between Escherichia coli verotoxin and lipopolysaccharide on cytokine induction and lethality in mice. Journal of Medical Microbiology. 2000;49(10):905–910. doi: 10.1099/0022-1317-49-10-905. [DOI] [PubMed] [Google Scholar]
- 147.Rasooly R, Do PM. Shiga toxin Stx2 is heat-stable and not inactivated by pasteurization. International Journal of Food Microbiology. 2010;136(3):290–294. doi: 10.1016/j.ijfoodmicro.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 148.Rasooly R, Do PM, Griffey SM, Vilches-Moure JG, Friedman M. Ingested shiga toxin 2 (stx2) causes histopathological changes in kidney, spleen, and thymus tissues and mortality in mice. Journal of Agricultural and Food Chemistry. 2010;58(16):9281–9286. doi: 10.1021/jf101744z. [DOI] [PubMed] [Google Scholar]
- 149.Robinson CM, Sinclair JF, Smith MJ, O’Brien AD. Shiga toxin of enterohemorrhagic Escherichia coli type 0157:H7 promotes intestinal colonization. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(25):9667–9672. doi: 10.1073/pnas.0602359103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Toledo CC, Rogers TJ, Svensson M, et al. Shiga toxin-mediated disease in MyD88-deficient mice infected with Escherichia coli O157:H7. American Journal of Pathology. 2008;173(5):1428–1439. doi: 10.2353/ajpath.2008.071218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mohawk KL, Melton-Celsa AR, Robinson CM, O’Brien AD. Neutralizing antibodies to Shiga toxin type 2 (Stx2) reduce colonization of mice by Stx2-expressing Escherichia coli O157:H7. Vaccine. 2010;28(30):4777–4785. doi: 10.1016/j.vaccine.2010.04.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kojima Y, Fukumoto S, Furukawa K, et al. Molecular cloning of globotriaosylceramide/CD77 synthase, a glycosyltransferase that initiates the synthesis of globo series glycosphingolipids. Journal of Biological Chemistry. 2000;275(20):15152–15156. doi: 10.1074/jbc.M909620199. [DOI] [PubMed] [Google Scholar]
- 153.Zumbrun SD, Hanson L, Sinclair JF, et al. Human intestinal tissue and cultured colonic cells contain globotriaosylceramide synthase mRNA and the alternate shiga toxin receptor globotetraosylceramide. Infection and Immunity. 2010;78(11):4488–4499. doi: 10.1128/IAI.00620-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Boyd B, Lingwood C. Verotoxin receptor glycolipid in human renal tissue. Nephron. 1989;51(2):207–210. doi: 10.1159/000185286. [DOI] [PubMed] [Google Scholar]
- 155.Eisenhauer PB, Chaturvedi P, Fine RE, et al. Tumor necrosis factor alpha increases human cerebral endothelial cell Gb and sensitivity to Shiga toxin. Infection and Immunity. 2001;69(3):1889–1894. doi: 10.1128/IAI.69.3.1889-1894.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kasai K, Galton J, Terasaki PI. Tissue distribution of the P(k) antigen as determined by a monoclonal antibody. Journal of Immunogenetics. 1985;12(4-5):213–220. doi: 10.1111/j.1744-313x.1985.tb00848.x. [DOI] [PubMed] [Google Scholar]
- 157.Lingwood CA. Verotoxin-binding in human renal sections. Nephron. 1994;66(1):21–28. doi: 10.1159/000187761. [DOI] [PubMed] [Google Scholar]
- 158.Obata F, Tohyama K, Bonev AD, et al. Shiga toxin 2 affects the central nervous system through receptor globotriaosylceramide localized to neurons. Journal of Infectious Diseases. 2008;198(9):1398–1406. doi: 10.1086/591911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Oosterwijk E, Kalisiak A, Wakka JC, Scheinberg DA, Old LJ. Monoclonal antibodies against Galα1-4Galβ1-4Glc (P(k), CD77) produced with a synthetic glycoconjugate as immunogen: reactivity with carbohydrates, with fresh frozen human tissues and hematopoietic tumors. International Journal of Cancer. 1991;48(6):848–854. doi: 10.1002/ijc.2910480610. [DOI] [PubMed] [Google Scholar]
- 160.Uchida H, Kiyokawa N, Taguchi T, Horie H, Fujimoto J, Takeda T. Shiga toxins induce apoptosis in pulmonary epithelium-derived cells. Journal of Infectious Diseases. 1999;180(6):1902–1911. doi: 10.1086/315131. [DOI] [PubMed] [Google Scholar]
- 161.Okuda T, Tokuda N, Numata SI, et al. Targeted disruption of Gb3/CD77 synthase gene resulted in the complete deletion of globo-series glycosphingolipids and loss of sensitivity to verotoxins. Journal of Biological Chemistry. 2006;281(15):10230–10235. doi: 10.1074/jbc.M600057200. [DOI] [PubMed] [Google Scholar]
- 162.Fujii Y, Numata SI, Nakamura Y, et al. Murine glycosyltransferases responsible for the expression of globo-series glycolipids: CDNA structures, mRNA expression, and distribution of their products. Glycobiology. 2005;15(12):1257–1267. doi: 10.1093/glycob/cwj015. [DOI] [PubMed] [Google Scholar]
- 163.Fujii J, Iuchi Y, Okada F. Fundamental roles of reactive oxygen species and protective mechanisms in the female reproductive system. Reproductive Biology and Endocrinology. 2005;3 doi: 10.1186/1477-7827-3-43. Article ID 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rutjes NWP, Binnington BA, Smith CR, Maloney MD, Lingwood CA. Differential tissue targeting and pathogenesis of verotoxins 1 and 2 in the mouse animal model. Kidney International. 2002;62(3):832–845. doi: 10.1046/j.1523-1755.2002.00502.x. [DOI] [PubMed] [Google Scholar]