Abstract
Background
Rhesus macaques (RM) co-infected with simian immunodeficiency virus (SIV) and rhesus macaque rhadinovirus (RRV) develop abnormal cellular proliferations characterized as extra-nodal lymphoma and retroperitoneal fibromatosis (RF). RRV encodes a viral interleukin-6 (vIL-6), much like Kaposi’s sarcoma-associated herpesvirus, and involvement of the viral cytokine was examined in proliferative lesions.
Methods
Formalin fixed tissue from RM co-infected with SIV and RRV were analyzed for RRV genomes by in situ hybridization and RRV vIL-6 expression by immunofluorescence analysis.
Results
In situ hybridization analysis indicated that RRV is present in both types of lesions. Immunofluorescence analysis of different lymphomas and RF revealed positive staining for vIL-6. Similarly to KS, RF lesion is positive for vimentin, CD117 (c-kit), and smooth muscle actin (SMA) and contains T cell, B cell and monocytes/macrophage infiltrates.
Conclusions
Our data support the idea that vIL-6 may be critical to the development and progression of lymphoproliferative disorder in RRV/SIV-infected RM.
Keywords: multi-centric Castleman’s disease, neoplasia, RRV and SIV
Introduction
Rhesus rhadinovirus (RRV) is a gamma-2 herpesvirus and is a natural pathogen of rhesus macaques (RM). The genome of RRV strain 17577 (RRV17577) has been determined and is essentially colinear with human herpesvirus 8 (HHV8), also known as Kaposi’s sarcoma-associated herpesvirus (KSHV) [6, 22]. KSHV is widely accepted to be the etiological agent responsible for Kaposi’s sarcoma (KS), primary effusion lymphoma, and multicentric Castleman’s disease (MCD), disease manifestations observed in human immunodeficiency virus-infected individuals [8]. RM experimentally inoculated with simian immunodeficiency virus (SIVmac239) and RRV17577 develop a hyperplastic B cell lymphoproliferative disorder (LPD) resembling MCD in humans. Hyperplastic LPD in RM is characterized by B cell hyperplasia, persistent lymphadenopathy, splenomegaly, elevated serum IgG, and persistent viremia. Approximately 25% of SIV/RRV-infected RM develop abnormal cellular proliferations that manifest as extranodal lymphoma and, in at least one instance, retroperitoneal fibromatosis (RF) [16, 22].
Kaposi’s sarcoma-associated herpesvirus encodes a number of homologs to cellular genes that are widely considered to be associated with KSHV pathogenesis. One viral gene of interest is K2, which encodes a structural and functional homolog of human interleukin 6 (IL-6) called viral IL-6 (vIL-6), which can stimulate all IL-6-induced signaling pathways [17]. RRV, like KSHV, encodes a vIL-6 that shares 17.8% amino acid identity with rhesus IL-6. RRV-encoded vIL-6 is biologically functional and elicits responses similar to cellular IL-6 [10, 16]. In this study, we evaluated the expression of vIL-6 in LPD in RM by immunofluorescence analysis. Our findings indicate that vIL-6 is expressed and that the viral-encoded cytokine could play a significant role in the genesis and maintenance of the abnormal cellular proliferations observed in our SIV/RRV-infected animals.
Materials and methods
Experimental animal infections
All aspects of the animal studies were conducted in accordance with institutional guidelines for animal care and use at the Oregon National Primate Research Center, and were performed as described [22]. H&E staining was performed according to standard procedures.
Immunohistochemistry and in situ hybridization for viral DNA in tissue sections
Immunohistochemistry and in situ hybridization was performed as previously described [16]. Briefly, sections of formalin-fixed paraffin-embedded tissues were deparaffinized and hydrated. After appropriate blocking and quenching, sections were incubated with panels of antibodies: goat antihuman IgM antibody (Southern Biotech, Birmingham, AL, USA), mouse monoclonal antibodies to cytokeratin, desmin, smooth muscle actin, CD20, CD68 (DakoCytomation, Carpinteria, CA, USA), ki-67 (BD Biosciences, San Jose, CA, USA), CD34 (Biomeda, Foster City, CA, USA), rabbit polyclonal antibodies to CD3, CD117 (DakoCytomation) or anti-vWF (Novocastra Reagents, Leica Biosystems Newcastle Ltd, Newcastle upon Tyne, UK). For detection of RRV by in situ hybridization, slides were treated with Proteinase K (100 μg/ml) (Promega, Madison, WI, USA). To detect the presence of RRV DNA in tissue, negative control SuperCos DNA and RRV cosmid clones 28b and 44 [20] were biotinylated and used for probes. Following hybridization, probes were identified within tissue using R. T. U. Vectastain Universal ABC kit, Elite (Vector Laboratories, Burlingame, CA), followed by peroxidase substrate DAB (3,3′-diaminobenzidine, DakoCytomation, Carpinteria, CA) or Vector SG-grey (Vector Laboratories, Burlingame, CA, USA). Stained slides were covered with DPX imaging medium (Molecular Probes, Carlsbad, CA, USA).
Immunofluorescence staining for viral IL-6
vIL-6-positive cells were detected with a mouse monoclonal α-vIL-6 (Clone No. 17D5 developed in The Vaccine and Gene Therapy Institute – Antibody Core facility) as described earlier [16]. Nuclei were visualized with Hoechst dye (Sigma-Aldrich, St Louis, MO, USA). Stained slides were covered with Slow Fade imaging medium (Molecular Probes).
Microscopic evaluation
A fluorescence microscope (Zeiss Axioscope 2 plus microscope, Carl Zeiss Microimaging Inc, Thorwood, NY, USA) equipped with digital camera (Zeiss Axio- Cam) and filter cubes was used to document bright field as well as specific Alexa 488, Alexa 594 and Hoechst fluorescence, which were edited in and overlaid by Axiovision Rel 4.6 software (Carl Zeiss Microimaging Inc, Thorwood, NY, USA) or Adobe Illustrator CS2 (Adobe Creative Suite 2 Premium Edition, Adobe System Incorporated, San Jose, CA, USA).
Results
We previously reported that the SIV/RRV-infected RM can serve as an excellent model to investigate KSHV-like pathogenesis and RRV is associated with non-Hodgkin’s lymphoma [16]. Histological examination of the kidney in animal 19455 that was experimentally infected with SIV and a plaque purified isolate of RRV17577 revealed the kidney contained numerous Castleman’s-like lesions that stained positively for viral IL-6 [16]. As this animal also developed lymphoproliferative lesions in the bone marrow, we analyzed this tissue for the presence of RRV. As shown in Fig. 1A, B, in situ hybridization reveals the presence of RRV genomes, suggesting the plaque-purified isolate is coincident with disease and is pathogenic. Moreover, the lesions contained numerous B cells that harbored RRV genomes (Fig. 1B), that also stained positively for vIL-6 (Fig. 1C).
Fig. 1.
Detection of Rhesus rhadinovirus (RRV) and vIL-6 in the multicentric Castleman’s disease (MCD) lesion of RRV-infected rhesus macaque. (A) In situ hybridization of MCD lesion isolated from the bone marrow of animal 19455 with RRV cosmid probe (brown), original magnification 630×. Arrows point to RRV-positive cells. (B) Combined in situ hybridization of the same lesion with RRV cosmid probe (gray dots) and immunocytochemistry with mouse anti-CD20 (brown). Arrows point to CD20+ cells positive for RRV (original magnification 630×). (C) Lesion from the same animal was stained for vIL-6 (green), CD20 (red), and nuclei (blue). Arrows point to the cells positive for both markers, original magnification 630×.
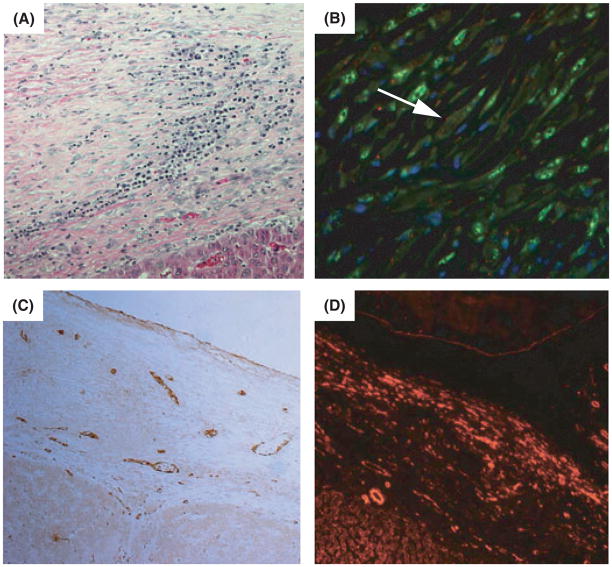
We further characterized the single case of simian RF as this is the only case of RF in an animal experimentally inoculated with SIV and RRV. RF has been reported in other non-human primate colonies, but these cases were observed in animals that were simian Type D retrovirus (SRV-2)-positive [11, 21]. As such, additional analysis of the RF is required. Animal 18483 developed hepatomegaly at 517 days post-RRV infection, whereas control animals inoculated with SIV alone did not. Two weeks later the animal was diagnosed with marked abdominal distention associated with ascites and dyspnea. The animal’s health deteriorated rapidly and it was humanely euthanized on 531 day p.i. Necropsy revealed that the abdominal distention was due to accumulation of ascites and generalized RF. Histological examination revealed the lesions were composed of thick, laminar, subserosal proliferation of plump or spindle-shaped cells with vesicular nuclei set in a collagen-rich, often edematous stroma (Fig. 2A). RF has been characterized as a vascular fibroproliferative neoplasm strongly resembling KS in humans [3]. In our previous article we showed that similar to KS lesions, the neoplastic spindeloid cells comprising the RF lesion were diffusely positive for vimentin suggesting its mesenchymal origin [1, 12, 16]. We further confirmed that RF cells were negative for cytokeratin and desmin (data not shown) and stained positively for CD117 (c-kit) (Fig. 2B). Staining for von Willebrand factor (vWF) was mostly localized within vascular smooth muscle cells; SMA antigen was present in both, vascular smooth muscle cells and in the neoplastic cells (Fig. 2C, D, respectively). The antibodies to CD34 did not cross react with macaque fixed tissues and the results are summarized in Table 1.
Fig. 2.
Retroperitoneal fibromatosis (RF) in Rhesus rhadinovirus-infected rhesus macaque. (A) Hematoxylin and eosin stain of RF isolated from the liver of animal 18483, original magnification 200×. (B) Positive staining for CD117 (dotted pattern-red, white arrow), vIL-6 (green) and nuclei (blue), original magnification 630×. (C) Staining of the same lesion with vWF antibody. Brown staining is associated with vascular smooth muscle cells, original magnification 200×. (D) Positive labeling for smooth muscle actin (red), original magnification 200×.
Table 1.
Immunophenotypic characteristic of retroperitoneal fibromatosis lesion vs. KS
| CD117 | CD34 | vWF | SMA | Desmin | CKs | Vimentin | Ki-67 | vIL-6 | |
|---|---|---|---|---|---|---|---|---|---|
| KS lesions | + | + | ± | + | − | − | + | + | + |
| RF | + | ? | ± | + | − | − | + | + | + |
(?) no results due to lack of working antibody.
Other cell types were also found to be present in the lesion. Specifically, immunohistochemical analysis revealed numerous CD3+, CD20+, and CD68+ cells dispersed throughout (data not shown). Proliferating Ki67+ cells were also detected within the RF lesion; however, the phenotype of the cells should be further defined.
Molecular genetic approaches to investigate how viruses induce disease are important to identify viral determinants associated with pathogenesis. These types of approaches benefit tremendously with an infectious molecular clone that is pathogenic. For RRV, earlier we reported the successful creation of a bacterial artificial chromosome (BAC) harboring the RRV genome, based upon the pathogenic plaque-purified isolate that induced disease in animal 19455. This RRV-BAC is capable of producing virus that is infectious in RM [7]. Further analysis of animals infected with SIV and the BAC-derived virus revealed an additional case of LPD. In particular, animal 23545 developed paralyses in both legs 525 days post-RRV infection and was humanely euthanized. Pathological examination of animal 23545 detected a lymphoproliferative lesion adjacent to and infiltrating the spinal cord, which we believe led to bi-lateral paralysis (Fig. 3A). In situ hybridization of this lesion demonstrated numerous RRV- and vIL-6-positive cells (Fig. 3B, C, respectively). These data imply that the BAC-derived RRV generated from a molecular clone is not only infectious but also pathogenic.
Fig. 3.

Detection of Rhesus rhadinovirus (RRV) and vIL-6 in the lesion of an RRV-BAC- infected rhesus macaque. (A) Hematoxylin and eosin stain of the lesion isolated from the spinal cord of animal 23545, original magnification 200×. (B) In situ hybridization of the same lesion with RRV cosmid probe (brown). Arrows point to RRV-positive cells, original magnification 630×. (C) Lesion was stained for vIL-6 (red), and nuclei (blue), original magnification 630×. Yellow spots are red blood cells.
Discussion
Kaposi’s sarcoma-associated herpesvirus-encoded vIL-6 has been shown to be expressed in spindle cells in KS, in MCD, in lymphoid cells and in follicular dendritic cell network, and is considered to be an important viral determinant associated with disease [4]. The data presented here with the closely related RRV/RM model provides further evidence of the potential impact vIL-6 has on development of lympho- and mesenchymal-proliferative disorders. Here we confirmed that RRV is present in the LPD lesions observed in the bone marrow of an animal, which was described earlier with vIL-6+/CD20+ LPD in the kidney [16]. In addition, we further analyzed an RF lesion found in one of our RM experimentally infected with SIV and RRV. RF is a rare tumor that can appear in a localized and a progressive form growing rapidly, filling the abdominal cavity, encasing intestines and internal organs [3]. Previous publications have reported the presence of RF co-incident with SRV-2 infection [11, 21]; however, the KSHV-related macaque rhadinoviruses 1 (RV1) and 2 (RV2) may play a role in the etiology of the macaque tumors [5, 19]. Interestingly, presence of retroperitoneal fibromatosis herpesvirus (RFHV) was detected in an intestinal stromal tumor of an SIV-infected, SRV-2- negative RM [2] and we revealed RRV in RF in the absence of RFHV [6, 16], implying one or both agents could be associated with RF.
As this was the first documented report of RF in an SIV/RRV-infected RM, we chose to better characterize the proliferative lesion to determine if there are morphological and histological similarities to human KS. We showed that neoplastic spindeloid cells of an RF lesion were positive for vimentin and SMA, negative for desmin and cytokeratin, which strongly resembles KS [12]. The neoplastic spindeloid cells were also negative for vWF factor. It has been shown that lymphatic endothelium express the vWF antigen, although staining is less intense than in blood vessel endothelia [15]. The RF lesion characterized here only exhibited vWF staining in the endothelial cells of blood vessel, which is consistent with vWF staining in KS lesions [9]. In addition, we verified that the transmembrane receptor tyrosine kinase c-kit, which is defined by the CD117 antigen and is the product of the c-kit proto-oncogene was expressed in RF cells. Similarly, upregulation of c-kit by KSHV has been shown in endothelial cells [14]. In our previous report we showed that neoplastic cells stained positively for vIL-6, which is in agreement with other reports that RF requires IL-6 for growth [13, 16, 18]. Here we now demonstrate that the RF contains numerous B and T cells, and monocyte infiltrates. Moreover, numerous cells in the RF were Ki-67+, a marker of cell proliferation in many invasive cancers; however, we need to further determine the nature of the proliferating cells.
Importantly, we also report that BAC-derived RRV is also associated with pathogenesis, as one animal experimentally inoculated with infectious virus derived from the molecular clone developed LPD. This is extremely relevant for studies involving viral pathogenesis, as it establishes the foundation for future molecular virology studies to identify viral determinants associated with viral induced disease. In summary, our observations indicate that RRV is associated with LPD in SIV-infected RM and that RRV-encoded vIL-6 may contribute in the progression of the disease.
Acknowledgments
This work was supported by Public Health Service grants RR00163 and CA 75922 (SWW). The authors thank Ms Lori Boshears for editorial assistance.
Footnotes
Conflicts of interest
The authors have declared no conflicts of interest.
References
- 1.Azumi N, Battifora H. The distribution of vimentin and keratin in epithelial and nonepithelial neoplasms. A comprehensive immunohistochemical study on formalin-and alcohol-fixed tumors. Am J Clin Pathol. 1987;88:286–96. doi: 10.1093/ajcp/88.3.286. [DOI] [PubMed] [Google Scholar]
- 2.Bielefeldt-Ohmann H, Barouch DH, Bakke AM, Bruce AG, Durning M, Grant R, Letvin NL, Ryan JT, Schmidt A, Thouless ME, Rose TM. Intestinal stromal tumors in a simian immunodeficiency virus-infected, simian retrovirus-2 negative rhesus macaque (Macaca mulatta) Vet Pathol. 2005;42:391–6. doi: 10.1354/vp.42-3-391. [DOI] [PubMed] [Google Scholar]
- 3.Bosch ML, Harper E, Schmidt A, Strand KB, Thormahlen S, Thouless ME, Wang Y. Activation in vivo of retroperitoneal fibromatosis-associated herpesvirus, a simian homologue of human herpesvirus-8. J Gen Virol. 1999;80:467–75. doi: 10.1099/0022-1317-80-2-467. [DOI] [PubMed] [Google Scholar]
- 4.Brousset P, Cesarman E, Meggetto F, Lamant L, Delsol G. Colocalization of the viral interleukin-6 with latent nuclear antigen-1 of human herpesvirus-8 in endothelial spindle cells of Kaposi’s sarcoma and lymphoid cells of multicentric Castleman’s disease. Hum Pathol. 2001;32:95–100. doi: 10.1053/hupa.2001.21131. [DOI] [PubMed] [Google Scholar]
- 5.Bruce AG, Bakke AM, Bielefeldt-Ohmann H, Ryan JT, Thouless ME, Tsai CC, Rose TM. High levels of retroperitoneal fibromatosis (RF)-associated herpesvirus in RF lesions in macaques are associated with ORF73 LANA expression in spindleoid tumour cells. J Gen Virol. 2006;87:3529–38. doi: 10.1099/vir.0.82339-0. [DOI] [PubMed] [Google Scholar]
- 6.Desrosiers RC, Sasseville VG, Czajak SC, Zhang X, Mansfield KG, Kaur A, Johnson RP, Lackner AA, Jung JU. A herpesvirus of rhesus monkeys related to the human Kaposi’s sarcoma-associated herpesvirus. J Virol. 1997;71:9764–9. doi: 10.1128/jvi.71.12.9764-9769.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Estep RD, Powers MF, Yen BK, Li H, Wong SW. Construction of an infectious rhesus rhadinovirus bacterial artificial chromosome for the analysis of Kaposi’s sarcoma- associated herpesvirus-related disease development. J Virol. 2007;81:2957–69. doi: 10.1128/JVI.01997-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ganem D. KSHV and Kaposi’s sarcoma: the end of the beginning? Cell. 1997;91:157–60. doi: 10.1016/s0092-8674(00)80398-0. [DOI] [PubMed] [Google Scholar]
- 9.Jussila L, Valtola R, Partanen TA, Salven P, Heikkila P, Matikainen MT, Renkonen R, Kaipainen A, Detmar M, Tschachler E, Alitalo R, Alitalo K. Lymphatic endothelium and Kaposi’s sarcoma spindle cells detected by antibodies against the vascular endothelial growth factor receptor-3. Cancer Res. 1998;58:1599–604. [PubMed] [Google Scholar]
- 10.Kaleeba JA, Bergquam EP, Wong SW. A rhesus macaque rhadinovirus related to Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8 encodes a functional homologue of interleukin-6. J Virol. 1999;73:6177–81. doi: 10.1128/jvi.73.7.6177-6181.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marx PA, Lowenstine LJ. Mesenchymal neoplasms associated with type D retroviruses in macaques. Cancer Surv. 1987;6:101–15. [PubMed] [Google Scholar]
- 12.Massarelli G, Scott CA, Ibba M, Tanda F, Cossu A. Immunocytochemical profile of Kaposi’s sarcoma cells: their reactivity to a panel of antibodies directed against different tissue cell markers. Appl Pathol. 1989;7:34–41. [PubMed] [Google Scholar]
- 13.Miles SA, Rezai AR, Salazar-Gonzalez JF, Vander Meyden M, Stevens RH, Logan DM, Mitsuyasu RT, Taga T, Hirano T, Kishimoto T. AIDS Kaposi sarcoma- derived cells produce and respond to interleukin 6. Proc Natl Acad Sci USA. 1990;87:4068–72. doi: 10.1073/pnas.87.11.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moses AV, Jarvis MA, Raggo C, Bell YC, Ruhl R, Luukkonen BG, Griffith DJ, Wait CL, Druker BJ, Heinrich MC, Nelson JA, Fruh K. A functional genomics approach to Kaposi’s sarcoma. Ann N Y Acad Sci. 2002;975:180–91. doi: 10.1111/j.1749-6632.2002.tb05951.x. [DOI] [PubMed] [Google Scholar]
- 15.Nagle RB, Witte MH, Martinez AP, Witte CL, Hendrix MJ, Way D, Reed K. Factor VIII-associated antigen in human lymphatic endothelium. Lymphology. 1987;20:20–4. [PubMed] [Google Scholar]
- 16.Orzechowska BU, Powers MF, Sprague J, Li H, Yen B, Searles RP, Axthelm MK, Wong SW. Rhesus macaque rhadinovirus-associated non-Hodgkin lymphoma: animal model for KSHV-associated malignancies. Blood. 2008;112:4227–34. doi: 10.1182/blood-2008-04-151498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osborne J, Moore PS, Chang Y. KSHV-encoded viral IL-6 activates multiple human IL-6 signaling pathways. Hum Immunol. 1999;60:921–7. doi: 10.1016/s0198-8859(99)00083-x. [DOI] [PubMed] [Google Scholar]
- 18.Roodman ST, Woon MD, Hoffmann JW, Theodorakis P, Tsai CC, Wu HN, Tsai CC. Interleukin-6 and retroperitoneal fibromatosis from SRV-2-infected macaques with simian AIDS. J Med Primatol. 1991;20:201–5. [PubMed] [Google Scholar]
- 19.Rose TM, Strand KB, Schultz ER, Schaefer G, Rankin GW, Jr, Thouless ME, Tsai CC, Bosch ML. Identification of two homologs of the Kaposi’s sarcoma-associated herpesvirus (human herpesvirus 8) in retroperitoneal fibromatosis of different macaque species. J Virol. 1997;71:4138–44. doi: 10.1128/jvi.71.5.4138-4144.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Searles RP, Bergquam EP, Axthelm MK, Wong SW. Sequence and genomic analysis of a Rhesus macaque rhadinovirus with similarity to Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8. J Virol. 1999;73:3040–53. doi: 10.1128/jvi.73.4.3040-3053.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsai CC, Tsai CC, Roodman ST, Woon MD. Mesenchymoproliferative disorders (MPD) in simian AIDS associated with SRV-2 infection. J Med Primatol. 1990;19:189–202. [PubMed] [Google Scholar]
- 22.Wong SW, Bergquam EP, Swanson RM, Lee FW, Shiigi SM, Avery NA, Fanton JW, Axthelm MK. Induction of B cell hyperplasia in simian immunodeficiency virus-infected rhesus macaques with the simian homologue of Kaposi’s sarcoma-associated herpesvirus. J Exp Med. 1999;190:827–40. doi: 10.1084/jem.190.6.827. [DOI] [PMC free article] [PubMed] [Google Scholar]