Summary
Background and objectives
Calcineurin inhibitors, while representing advances for solid organ transplantation, have nephrotoxic potential that reduces their net benefit. Tacrolimus has been considered less nephrotoxic than cyclosporine, but direct quantitative comparisons of the changes in renal structure from baseline to follow-up biopsies have not been done. To avoid the pitfalls of renal allograft studies, including rejection and disease recurrence, we compared the development of calcineurin lesions in the native kidneys of 14 tacrolimus– and 12 calcineurin–treated pancreas transplant alone recipients cured of type 1 diabetes.
Design, setting, participants, & measurements
Research renal biopsies obtained before and at 5 years after transplantation were studied using established morphometric methods.
Results
The cyclosporine and tacrolimus groups had, respectively, on average, 33% versus 44% decline in GFR (ns), 27% versus 29% increase in cortical interstitial fractional volume (ns), 245% versus 347% increase in the fractional volume of cortical tubules that were atrophic (ns), and 291% versus 392% increase in the percent of globally sclerotic glomeruli (ns). Arteriolar hyalinosis did not change significantly in either group.
Conclusions
These studies indicate that the nephrotoxic potential of tacrolimus and cyclosporine are equivalent and support the development of strategies to reduce these negative effects.
Introduction
Calcineurin inhibitors (CNI) improved early renal allograft survival and revolutionized transplantation of nonrenal organs, including heart, liver, lung, and pancreas. Nevertheless, cyclosporine (CSA) and tacrolimus (TAC) have important adverse effects, especially nephrotoxicity (1). Thus, 5 years after liver transplantation, renal failure prevalence is 18% (2) and progressive renal damage in nonrenal organ transplant recipients is a significant contributor to ESRD (2). Both CSA and TAC have renal hemodynamic effects leading to rapid reductions in GFR which, early on, are reversible upon dose reduction or cessation (1). Eventually, GFR loss may become irreversible, reflecting structural changes, including tubular atrophy, interstitial fibrosis, glomerulosclerosis, and arteriolopathy (3). The suggestion that TAC may be less nephrotoxic than CSA (2) remains controversial (4), as recently outlined (5). Most studies comparing renal injury associated with these agents were in renal allografts where it is often impossible to discriminate between structural changes from drug toxicity versus other causes of cortical scarring (1). Whereas there are several biopsy studies in native kidneys of CSA patients (1), there is little information on TAC lesions in native kidneys. Studies in liver transplantation described similar renal pathologic findings in patients treated with CSA and TAC (6); however, kidney biopsies were performed for clinical indications and cannot be considered as representative of the liver transplant population. Finally, nearly all studies have been limited by the lack of baseline kidney biopsies. In liver transplant patients, for example, there may be pre-existing renal lesions from diabetes, hypertension, hepatitis C, and other disorders (7).
We reported that CSA-associated renal lesions develop in recipients with type 1 diabetes (T1DM) of successful pancreas transplant alone (PTA) who had protocol renal biopsies before and 5 years after PTA (8). Here, we evaluated renal structure in TAC-treated T1DM patients with successful PTA who underwent protocol renal biopsies before and 5 years after PTA, thus, allowing direct comparison between TAC- and CSA-related lesions.
Materials and Methods
Patients and Study Design
TAC group.
Fourteen patients met the entry criteria: T1DM recipients of successful PTA (normoglycemia and insulin independence for 5 years); on TAC as part of their immunosuppression; protocol kidney biopsies before and 5 years after PTA.
CSA group.
The study design and entry criteria were the same, but patients received CSA. These CSA studies have been previously published (8) and 12 of the original 13 patients (8) are included here: one patient, on CSA for only 1 year, was excluded so that this cohort was more comparable with the TAC group. Eleven of the 14 patients received TAC for all 5 years, and two discontinued during their fifth posttransplant year because of elevated serum creatinine levels and one because of migraine headaches. Patients in neither group were receiving other potentially nephrotoxic drugs at the time of the baseline or 5-year biopsies. One patient in each group required retransplantation because of rejection.
These studies were approved by the Committee for the Use of Human Subjects in Research of the University of Minnesota, and all patients gave written informed consent before each evaluation.
Procedures
Patients were admitted to the General Clinical Research Center (GCRC) at the University of Minnesota for pre-PTA and follow-up evaluations. Patients underwent three 24-hour urine collections for measurements of creatinine clearance (CrCl) by the Jaffé reaction (normal range: 90 to 130 ml/min per 1.73 m2) and urinary albumin excretion rate (AER) by nephelometry (normal values <22 mg/24 h). GFR by plasma iohexol clearance was measured in most of the follow-up evaluations but only in some of the baseline evaluations; thus, only CrCl data are presented. However, CrCls obtained in the GCRC were highly correlated with iohexol GFR in 21 measurements in these patients (r = 0.88, P < 0.001). BP was measured repeatedly by the GCRC nursing staff. Glycated hemoglobin (A1c) was measured by HPLC (BioRad Diamat, BioRad Laboratories, Hercules, CA) (normal range: 4.0% to 6.1%). CSA and TAC trough blood levels were measured by HPLC.
Renal Biopsy Studies
Light microscopy tissue was fixed in Zenker solution, embedded in paraffin, cut in 2- to 3-μm sections, and stained with periodic acid–Schiff stain. Morphometric measurements were performed by a single masked observer (P.F.), unaware of the patient's identity.
Interstitial volume fraction per cortex [Vv(Int/cortex)] was estimated by point counting at 300×. Points falling on the interstitium, defined as the space outside Bowman's capsule, tubular basement membrane (TBM) and vessels larger than one tubular diameter, and total number of points overlying the cortical tissue were counted using a 1:4 grid with a distance between fine points of 13 mm (normal value: 0.15 ± 0.02). The presence of striped fibrosis, defined by bandlike interstitial fibrosis and tubular atrophy along the medullary rays, and the presence of isometric vacuolization of tubules were recorded as dichotomous variables (present or absent) by a masked observer (B.N.).
Percentage of global glomerulosclerosis (%GS) was determined when at least 15 glomeruli [26 (15 to 78); median (range)] on multiple sections were available (9) (normal value: <10%). Percentage of glomeruli with focal segmental glomerulosclerosis was similarly determined.
Fractional volume of atrophic tubules per total control tubules [Vv(AT/TT)] was estimated by point counting on all available cortical tissues. A 1:9 grid with a distance between fine points of 10 mm was used (normal value: 0). Tubular atrophy was defined by the presence of thickened or reduplicated TBM surrounding tubules of reduced diameters containing shortened tubular epithelial cells; in the absence of thickened TBM, atrophy was defined as tubular diameter reduced by more than 50% as compared with that of adjacent tubules (10).
The index of arteriolar hyalinosis (IAH) is an estimate of the severity of arteriolar wall infiltration by hyaline material. Arteries smaller than the average tubular diameter [24 (6 to 76) median (range) were scored by two masked observers (B.N. and M.M.) for estimation of the fraction of the vascular profile occupied by hyaline (11). Calculation of the index also includes weighting for higher fractions (11), based on earlier studies linking global glomerulosclerosis in T1DM only with more severe hyalinosis lesions (9).
Tissue for electron microscopy was processed and measured as previously detailed (12) to determine mesangial volume fraction per glomerulus [Vv(Mes/glom)] and glomerular basement membrane width.
Statistical Analyses
Results are expressed as mean ± SD, except for AER, which not normally distributed, is expressed as median and range. AER values were logarithmically transformed before analyses. Comparisons of baseline with 5-year follow-up data used the paired two-sided t test.
The changes from baseline to 5-year follow-up in the TAC and CSA groups were compared by unpaired two-sided t test. Linear regression analyses were used to evaluate the relationships between drug dose and plasma levels at different times, and between dose and levels and changes in the structural parameters over 5 years. Spearman rank order correlation was used to study relationships between nonparametric variables. Striped fibrosis at baseline and follow-up biopsies was compared using χ2 test or Fisher exact test where applicable. Statistical significance was set at P < 0.05; however, all P values <0.10 are provided.
Results
TAC Group
These patients (four men and ten women) were 39.2 ± 8.3 years old, with T1DM for 22.1 ± 10.0 years (Table 1). Follow-up studies were at 4.6 ± 0.8 years after PTA. All were insulin-independent and normoglycemic during the study. A1c, 8.9 ± 1.9% before PTA, was normal 5 years after PTA (5.24 ± 0.7; P < 0.0001 versus baseline). Serum creatinine (SCr) increased from 0.86 ± 0.21 to 1.55 ± 0.66 mg/dl (P < 0.0001) and GFR decreased from 98 ± 24 to 52 ± 26 ml/min per 1.73 m2 from baseline to 5 years (P < 0.001).
Table 1.
Baseline clinical features and renal structural parameters in TAC- and CSA-treated PTA recipients
| TAC Group | CSA Group | P | |
|---|---|---|---|
| Gender (men/women) | 4/10 | 5/7 | NS |
| Age (years) | 39.2 ± 8.3 | 32.3 ± 7.3 | 0.023 |
| Diabetes duration (years) | 22.1 ± 10.0 | 18.8 ± 6.3 | NS |
| Serum creatinine (ml/dl) | 0.86 ± 0.21 | 0.89 ± 0.26 | NS |
| GFR (ml/min per 1.73 m2) | 98 ± 24 | 106 ± 19 | NS |
| AER (μg/min) | 25 (6 to 815) | 71 (5 to 886) | NS |
| Vv(Int/cortex) | 0.223 ± 0.042 | 0.248 ± 0.050 | NS |
| Vv(AT/TT) | 0.044 ± 0.043 | 0.042 ± 0.042 | NS |
| % Global glomerulosclerosis | 6.1 ± 7.5 | 9.1 ± 10.6 | NS |
| % Segmental glomerulosclerosis | 3.4 ± 7.1 | 5.6 ± 12.7 | NS |
| Index of arteriolar hyalinosis | 0.815 ± 0.56 | 0.826 ± 0.55 | NS |
| GBM width (nm) | 541 ± 107 | 585 ± 125 | NS |
| Vv(Mes/glom) | 0.33 ± 0.09 | 0.32 ± 0.07 | NS |
GBM, glomerular basement membrane.
AER was 25 (6 to 815) μg/min before and 9 (3 to 3012) μg/min at 5 years (ns). BP was 125 ± 15/70 ± 9 mmHg before and 129 ± 13/72 ± 11 at 5 years (ns). The number of patients receiving antihypertensive therapy increased from 6 before PTA to 10 at 5 years.
TAC dose was 6.2 ± 2.7 mg/d (0.074 ± 0.034 mg/kg per d) in the first year, 5.1 ± 2.01 (0.063 ± 0.028) in the fourth year, and 5.0 ± 2.4 (0.062 ± 0.029) in the fifth year (ns). TAC blood levels decreased from 9.9 ± 1.8 μg/L in the first year to 7.6 ± 1.2 μg/L at 4 years (P < 0.001 versus 1 year) and 6.9 ± 1.9 at 5 years (P < 0.005 versus 1 year).
CSA group.
These 12 patients (five men and seven women) were 32 ± 7 years old, with T1DM for 19 ± 6 years (Table 1). All were insulin-independent and normoglycemic during the study. SCr increased from 0.89 ± 0.28 to 1.24 ± 0.36 mg/dl (P < 0.001) and GFR decreased from 106 ± 19 to 70 ± 21 ml/min per 1.73 m2 at 5 years after PTA (P < 0.001). Neither AER [71 (5 to 886) μg/min at baseline and 21 (1.4 to 1986) μg/min at 5 years] nor BP changed significantly. Patients receiving antihypertensive therapy increased from 2 before PTA to 11 at 5 years.
CSA dose decreased from 8.4 ± 2.7 mg/kg per d during the first year to 4.6 ± 1.6 during the fifth year after PTA (P < 0.001). CSA blood levels decreased from 191 ± 65 μg/L in the first year to 92 ± 38 μg/L at 5 years (P < 0.005).
Baseline Comparisons
TAC and CSA groups were comparable for all baseline clinical characteristics, except age (P < 0.03; Table 1). Baseline Vv(Int/cortex), Vv(AT/TT), %GS, % segmental glomerulosclerosis, and the index of IAH were superimposable in the two groups, as was the severity of diabetic glomerulopathy (Table 1).
Comparison of Renal Functional and Structural Changes in the TAC and CSA Groups
The decline in GFR was similar in the TAC and CSA groups (Table 2); AER did not change significantly in either group (Table 2).
Table 2.
Changes in renal function and structure from baseline to 5-year post-PTA in the TAC and CSA groups
| Change in 5 years | TAC Group | CSA Group | P |
|---|---|---|---|
| Serum creatinine (ml/dl) | 0.65 ± 0.38 | 0.35 ± 0.08 | NS |
| GFR (ml/min per 1.73 m2) | −43 ± 19 | −35 ± 16 | NS |
| AER (μg/min) | −3 (−755 to 2987) | −2 (−858 to 1722) | NS |
| Vv(Int/cortex) | 0.057 ± 0.062 | 0.067 ± 0.07 | NS |
| Vv(AT/TT) | 0.143 ± 0.149 | 0.102 ± 0.09 | NS |
| % Global glomerulosclerosis | 22.9 ± 14.3 | 23.3 ± 20.4 | NS |
| % Segmental glomerulosclerosis | 4.7 ± 7.9 | 9.9 ± 8.3 | NS |
| Index of arteriolar hyalinosis | 0.053 ± 0.048 | −0.187 ± 0.34 | NS |
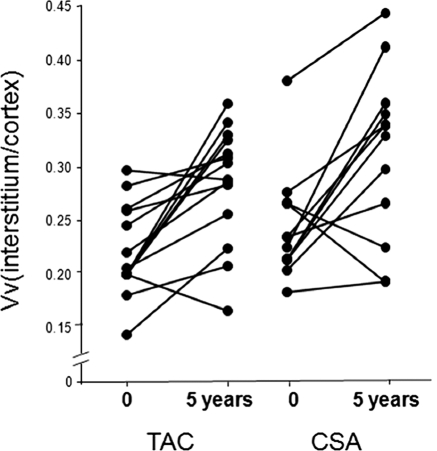
Vv(Int/cortex), elevated at baseline in the TAC group (0.223 ± 0.042), increased significantly at 5 years (0.285 ± 0.054, P < 0.001). This 29% increase was similar to the 27% increase in the CSA group (from 0.248 ± 0.050 to 0.314 ± 0.077, P < 0.005) (Figure 1, Table 2). Whereas there was no striped fibrosis in either group at baseline, 29% of TAC (χ2 = 4.67, P < 0.05) and 27% of CSA biopsies (χ2 = 3.47, P = ns) showed striped fibrosis at 5 years, with no difference between the groups.
Figure 1.
Interstitial volume fraction [Vv(Int/cortex)] at baseline and 5 years after PTA in TAC- and CSA-treated groups. Data for individual patients are connected by lines.
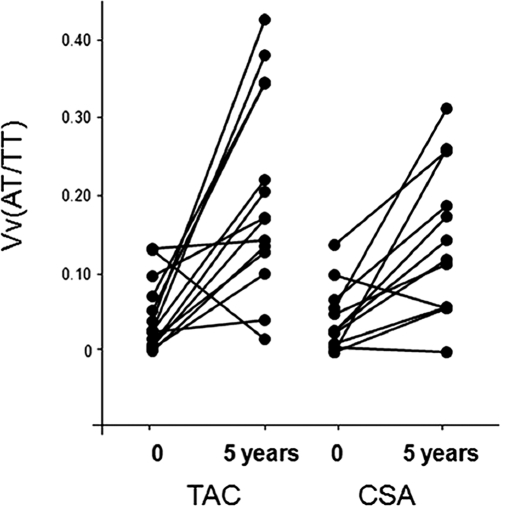
Vv(AT/TT) increased by 346% in the TAC group (from 0.044 ± 0.043 to 0.201 ± 0.127, P = 0.001) and by 245% in the CSA group (from 0.042 ± 0.042 to 0.145 ± 0.096, P < 0.005) (Figure 2, Table 2). There was no isometric vacuolization of tubules at baseline or 5 years in either group.
Figure 2.
Fractional volume of atrophic tubules [Vv(AT/TT)] at baseline and 5 years after PTA in TAC- and CSA-treated groups. Data for individual patients are connected by lines.
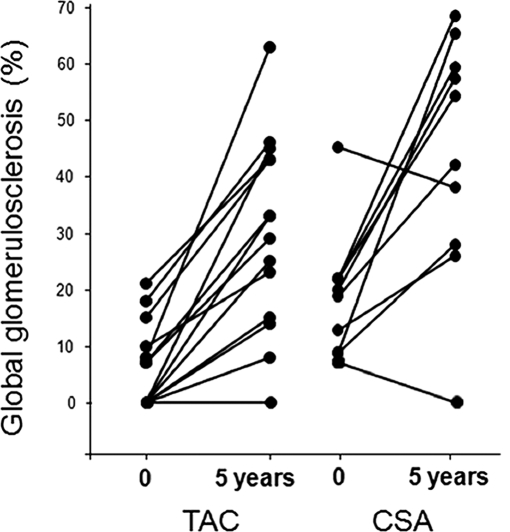
The increase in %GS from 6.1% ± 7.5% to 30% ± 17.2% (392%, P < 0.0001) in the TAC group was similar to that in the CSA group (from 9.1% ± 10.6% to 35.6% ± 19.3%; 291%, P < 0.002; Figure 3, Table 2). The change in % segmental glomerulosclerosis between the baseline and 5-year biopsies was not statistically significant in either the TAC or the CSA groups (Tables 1 and 2).
Figure 3.
Percentage of globally sclerosed glomeruli at baseline and 5 years after PTA in TAC- and CSA-treated groups. Data for individual patients are connected by lines.
IAH did not change in the TAC group (0.815 ± 0.55 at baseline and 0.867 ± 0.60 at 5 years, ns) or the CSA group (0.827 ± 0.55 and 0.639 ± 0.37, P = 0.08), and these changes were not significantly different between the two groups (Table 2). There were no significant correlations between the changes in IAH and in Vv(Int/cortex), Vv(AT/TT), or %GS in either group (highest r = 0.27, smallest P = 0.40).
Changes in Vv(Int/cortex) in the TAC group correlated with the changes in Vv(AT/TT) (r = 0.70, P < 0.004) and tended to correlate with the changes in %GS (r = 0.47, P = 0.07). There were significant inverse correlations between the GFR decrease and the increase in Vv(Int/cortex) (r = 0.68, P = 0.005) and in Vv(AT/TT) (r = 0.55, P = 0.035). There were no significant correlations between the increase in %GS and renal function.
There was no correlation between TAC dose and blood levels and changes in renal structural and functional parameters whereas, as reported elsewhere (8), CSA dose and blood levels in the first post-PTA year correlated with the changes in GFR from baseline to 1 year and with the changes in Vv(Int/cortex) from baseline to 5 years.
Discussion
CNI nephrotoxicity is a potentially serious side effect that may contribute to the high incidence of chronic kidney disease and ESRD in nonrenal organ transplant patients and to long-term renal allograft loss (1–3,13). Chronic CNI nephrotoxicity lesions of interstitial fibrosis, tubular atrophy, glomerulosclerosis, and arteriolar hyalinosis (14–17) developed in nearly all PTA patients after 5 years of CSA treatment, albeit with variable severity (8). TAC has been suggested to be more effective than CSA in increasing renal allograft survival and in preventing rejection in the short term (18); unfortunately, TAC is also nephrotoxic. Thus, renal insufficiency has been reported in 29% of liver transplantation patients receiving TAC versus 23% of those receiving CSA (19).
Although some have suggested that TAC is less nephrotoxic than CSA (4,20), these data are controversial and often based on short-term studies and on renal function (21). Most studies comparing TAC and CSA nephrotoxicity are in renal allografts where chronic rejection, hypertension, and recurrent disease may be confounding factors (3). Also, in most studies, kidney biopsies were performed for clinical indications, making comparisons of relative toxicities difficult to interpret. In a study of renal allograft patients randomized to CSA or TAC, 24% of the TAC and 17% of the CSA patients had CNI nephrotoxicity in 2-year protocol biopsies (16). Chronic allograft nephropathy [or “interstitial fibrosis and tubular atrophy” (IFTA)] incidence and lesion scores were also similar in the two groups (16). However, as mentioned, CNI chronic toxicity and IFTA from other etiologies may be indistinguishable (3,13). There are several native kidney biopsy studies during CSA therapy (6–8,22–24), but little information for TAC. Similar renal biopsy findings have been reported in CSA- and TAC-treated liver transplant patients (6). However, performed for clinical indications, these biopsies cannot be fully representative of the treated population. Finally, most studies have been limited by the absence of a baseline kidney biopsy; liver transplant patients, for example, may have pre-existing renal lesions consequent to diabetes, hypertension, hepatitis C (6), α1-antitripsin deficiency (7), and other conditions. Thus, whether the severity of TAC and CSA induced renal lesions is different was previously untested.
The present study, with protocol research kidney biopsies before and 5 years after PTA is unique, allowing for direct comparison of the development of CSA and TAC renal lesions under nearly identical conditions. Moreover, the systematic unbiased morphometric measurements provided quantitative tools that could detect relatively small structural differences, should they exist. None of the follow-up biopsies showed acute CNI toxicity, such as isometric vacuolization of tubules or thrombotic microangiopathy, perhaps to be expected in these stable patients on relatively low doses of these drugs 5 years after PTA. We previously reported that tubular, interstitial, and GS lesions worsened in CSA patients 5 years after PTA (8) and that the increases in interstitial fractional volume significantly correlated with CSA dose and blood levels during the first year post-PTA (8). There was also a significant correlation between the increase in interstitial fractional volume at 5 years and the decrease in GFR at 1 year post-PTA (8). In our previous studies, diabetic patients with similar severity of diabetic nephropathy lesions at baseline who did not undergo PTA did not have progression of interstitial or GS lesions 5 years later (8). This clearly demonstrates that the progression in renal injury in CNI-treated patients was not consequent to pre-existing diabetic nephropathy per se.
Baseline clinical, renal functional, and structural parameters in the CSA and TAC PTA groups in the present study were very similar. The GFR decline and the increase in interstitial fractional volume, tubular atrophy, and %GS in the TAC and CSA groups were similar in both absolute and relative terms. Moreover, there was a significant correlation between the GFR decline and the worsening in interstitial fibrosis and tubular atrophy in the TAC group, similar to what we previously reported in the CSA patients (8). However, unlike CSA, these changes did not correlate with any of the TAC doses or blood levels, probably because of the wider range of doses among the CSA as compared with the TAC patients or because of greater heterogeneity in individual susceptibility to nephrotoxicity in the TAC patients. Importantly, only about 30% of the variability of the increase in Vv(Int/cortex) was explained by CSA doses and levels (8), this consistent with heterogeneity in susceptibility to CNI nephrotoxicity. Moreover, only a subset of nonrenal solid organ transplant patients develops ESRD in the first 10 posttransplant years (2,4). Recent reports suggest a genetic basis for CNI nephrotoxicity susceptibility (5,25); an association of tubular P-glycoprotein expression gene polymorphism with susceptibility to TAC functional nephrotoxicity has been described in liver transplant children (25).
Arteriolar hyalinosis, common in diabetic nephropathy (9,11) and CNI nephrotoxicity (1,14–17), was present at baseline and did not change in either group. Given this, it is not surprising that there were no significant correlations between changes in IAH and the other structural parameters. We reported worsening in these arteriolar lesions over 5 years in T1DM patients (8,11,26). In fact, the IAH method used in the present studies was able to detect increases over 5 years in this parameter as small as 5% (26). Possibly our failure to demonstrate worsening of arteriolar hyalinosis in the present and previous (8) studies may be because CNI and normoglycemia influences on these lesions were in offsetting directions.
Our studies were not designed to elucidate the pathogenesis of CSA and TAC renal lesions. Nonetheless, the remarkable similarity in the nature and pace of these lesions, despite the marked differences in CSA and TAC molecular structure and target binding proteins (27,28), suggests the involvement of processes downstream to the CNI inhibition of the phosphatase activity.
We previously reported that, although GFR decline from baseline to 12 months correlated with CSA dose, GFR remained stable thereafter, despite a lowering of CSA dose and blood levels (8). Importantly, there was relatively little CNI-related changes in renal biopsies performed 1.5 to 2.5 years after PTA (8). Thus, serious CNI lesions can develop while after the early decline, GFR remains stable. The failure of GFR to improve, as CNI doses were gradually decreased over 5 years, might reflect the progression of the underlying renal lesions. The finding that lesions are often progressing without further GFR loss supports the view that baseline and follow-up renal biopsies may be useful in the management of long-term CNI-treated patients.
In conclusion, in PTA patients, the chronic nephrotoxic effects of TAC and CSA are similar in terms of both renal function and renal structure.
Disclosures
None.
Acknowledgments
This work was supported by grants from the National Institutes of Health (DK13083), the National Center for Research Resources (MO1-RR00400), an endowment from the Kroc Research Foundation, and an academic grant from Astellas. During the initial stages of this work, Dr. Fioretto was supported by Research Fellowship and Career Development Awards from the Juvenile Diabetes Research Foundation International. The authors are grateful to Ms. Adrienne Kari and Cathy Bagne for clinical coordinator assistance and to Ms. Susan Sisson-Ross and Isabella Barzon for technical assistance. We thank Ms. Patricia L. Erickson for assistance in manuscript preparation. We are most indebted to the patients who generously participated in these studies.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Access to UpToDate on-line is available for additional clinical information at http://www.cjasn.org/
References
- 1. Williams D, Lukas H: Calcineurin nephrotoxicity. Adv Chronic Kidney Dis 13: 47–55, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, Arndorfer J, Christensen L, Merion RM: Chronic renal failure after transplantation of a non-renal organ. N Engl J Med 349: 931–940, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Liptak P, Ivanyi B: Primer: Histopathology of calcineurin-inhibitor toxicity in renal allografts. Nat Clin Pract Nephrol 2: 389–404, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Gruessner RWG, Sutherland DER, Kandaswamy R, Gruessner AC: Over 500 solitary pancreas transplants in nonuremic patients with brittle diabetes mellitus. Transplantation 85: 42–47, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Naesens M, Kuypers DRJ, Sarwall M: Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol 4: 481–508, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Pillebout E, Nochy D, Hill G, Conti F, Antoine C, Calmus Y, Glotz D: Renal histopathological lesions after orthotopic liver transplantation. Am J Transplant 5: 1120–1129, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Needham M, Stockley RA: α1-antitripsin deficiency 3: Clinical manifestations and natural history. Torax 59: 441–445, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fioretto P, Steffes MW, Mihatsch MJ, Strøm EH, Sutherland DER, Mauer M: Cyclosporine-associated lesions in native kidneys of diabetic pancreas transplant recipients. Kidney Int 48: 489–495, 1995 [DOI] [PubMed] [Google Scholar]
- 9. Harris RD, Steffes MW, Bilous RW, Sutherland DER, Mauer SM: Global glomerular sclerosis and glomerular arteriolar hyalinosis in insulin dependent diabetes. Kidney Int 40: 107–114, 1991 [DOI] [PubMed] [Google Scholar]
- 10. Fioretto P, Sutherland DER, Najafian B, Mauer M: Remodeling of renal interstitial and tubular lesions in pancreas transplant recipients. Kidney Int 69: 907–912, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Drummond K, Mauer M. for the International Diabetic Nephropathy Study Group (IDNSG): The early natural history of nephropathy in type 1 diabetes. II. Early renal structural changes in type 1 diabetes. Diabetes 51: 1580–1587, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Fioretto P, Steffes MW, Sutherland DER, Goetz FC, Mauer M: Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med 339: 69–75, 1998 [DOI] [PubMed] [Google Scholar]
- 13. Nambham N, Nagarajan S, Shah S, Li L, Salvatierra O, Sarwal MM: A novel, semiquantitative, clinically correlated calcineurin inhibitor toxicity score for renal allograft biopsies. Clin J Am Soc Nephrol 2: 135–142, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Finn WF: FK506 nephrotoxicity. Ren Fail 21: 319–329, 1999 [DOI] [PubMed] [Google Scholar]
- 15. Nankivell BL, Borrows RJ, Fung CLS, O'Connell PJ, Chapman JR, Allen DM: Calcineurin inhibitor nephrotoxicity: Longitudinal assessment by protocol histology. Transplantation 78: 557–565, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Solez K, Vincenti F, Filo RS: Histopathologic findings from 2-year protocol biopsies from US multicenter kidney transplant trial comparing tacrolimus versus cyclosporine. Transplantation 66: 1736–1740, 1999 [DOI] [PubMed] [Google Scholar]
- 17. Mihatsch MJ, Thiel G, Ryffel B: Histopathology of cyclosporine nephrotoxicity. Transplant Proc 20: 759–771, 1988 [PubMed] [Google Scholar]
- 18. Pirsch JD, Miller J, Deierhoi MH, Vincenti F, Filo RS: A comparison of tacrolimus (FK506) and cyclosporine for immunosuppression after cadaveric renal transplantation. FK506 Kidney Transplant Study Group. Transplantation 63: 977–983, 1997 [DOI] [PubMed] [Google Scholar]
- 19. Platz KP, Mueller AR, Blumhardt G, Bachmann S, Bechstein WO, Kahl A, Neuhaus P: Nephrotoxicity following orthotopic liver transplantation. A comparison between cyclosporine and FK506. Transplantation 58: 170–178, 1994 [PubMed] [Google Scholar]
- 20. Schmitz V, Laudi S, Moeckel F, Puhl G, Stockmann M, Tran ZV, Kahl A, Neumann U, Neuhaus P: Chronic renal dysfunction following liver transplantation. Clin Transplant 22: 333–340, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Klein IH, Abrahams A, van Ede T, Hene' RJ, Koomans HA, Ligtenberg G: Different effects of tacrolimus and cyclosporine on renal hemodynamics and blood pressure in healthy subjects. Transplantation 73: 732–736, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Myers BD: Cyclosporine nephrotoxicity. Kidney Int 30: 964–974, 1986 [DOI] [PubMed] [Google Scholar]
- 23. Myers BD, Sibley R, Newton L, Tomlanovich SJ, Boshkos C, Stinson E, Luetscher JA, Whitney DJ, Krasny D, Coplon NS, Perlroth MG: The long-term course of cyclosporine-associated chronic nephropathy. Kidney Int 33: 590–600, 1988 [DOI] [PubMed] [Google Scholar]
- 24. Falkenhain ME, Cosio FG, Sedmak DD: Progressive histologic injury in kidneys from heart and liver transplant recipients receiving cyclosporine. Transplantation 62: 364–370, 1996 [DOI] [PubMed] [Google Scholar]
- 25. Hawwa AF, McKiernan PJ, Shields M, Millership JS, Collier PS, McElnay JC: Influence of ABCB1 polymorphism and haplotypes on tacrolimus nephrotoxicity and dosage requirements in children with liver transplant. Br J Clin Pharmacol 68: 413–421, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mauer M, Zinman B, Gardiner R, Suissa S, Sinaiko A, Strand T, Drummond K, Donnelly S, Goodyer P, Gubler MC, Klein R: Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med 361: 40–51, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ho S, Clipstone N, Timmermann L, Northrop J, Graef I, Fiorentino D, Nourse J, Crabtree GR: The mechanism of action of cyclosporin A and FK506. Clin Immunol Immunopathol 80: S40–S45, 1996 [DOI] [PubMed] [Google Scholar]
- 28. Hemenway CS, Heitman J: Calcineurin. Structure, function, and inhibition. Cell Biochem Biophys 30: 115–151, 1999 [DOI] [PubMed] [Google Scholar]