Summary
Background and objectives
Clinical and experimental data have shown that differences in nephron endowment result in differences in renal mass and predisposition to chronic renal failure, hypertension, and proteinuria. We hypothesized that a significant proportion of the variance in GFR, as estimated by serum creatinine, is attributable to differences in renal size in normal children.
Design, setting, participants, & measurements
A total of 1748 normal renal ultrasounds that were performed in children older than 6 months were reviewed. For each ultrasound, serum creatinine, serum blood urea nitrogen, and systolic and diastolic office BP were recorded. Renal size was evaluated as a function of renal length and thickness. All data were normalized for height, weight, age, and gender.
Results
When expressed as SD scores, a significant correlation was found between kidney size and serum creatinine (P < 0.0001) and between kidney size and serum blood urea nitrogen (P < 0.002). When dividing kidney size data per quintiles, a difference of 0.51 SD score in serum creatinine was observed between the lowest and highest quintile. No significant correlation was found with office BP measurements.
Conclusions
These data show that, even in the normal pediatric population, differences in renal function are significantly explained by differences in renal mass. Methodologic limitations of this study are likely to underestimate this relationship.
Introduction
The mean number of nephrons in the normal population has been estimated to be 600,000 to 800,000 per kidney (1,2). This number can vary widely among individuals, ranging from 300,000 to 1,800,000 glomeruli per kidney (3,4).
Since the early 1990s, much interest has been placed on the determinants of nephron number in normal subjects and on the impact that nephron endowment at birth has on renal and cardiovascular functions later in life.
Current knowledge indicates that intrauterine events and genetic factors influence fetal programming of nephron number. Consequently, a significant proportion of individuals are born with fewer glomeruli, which undergo a process of glomerular hypertrophy and develop glomerular hypertension. This initiates with time a cascade of events leading to glomerular sclerosis and further loss of nephrons (5,6). Other factors such as alterations in kidney sodium transport or perturbations in the renin–angiotensin system also play an important role (7).
Several experimental and clinical observations have now established that decreased number of nephrons predispose to hypertension, proteinuria, and chronic renal failure (summarized in ref 8).
Currently, no reliable tests or studies allow measuring the number of glomeruli in vivo. Autopsy data, however, suggest that subjects with fewer nephrons tend to have smaller kidneys that contain hypertrophic glomeruli (9). A direct correlation between kidney weight and nephron number has been also reported in infants (10).
In this study, we hypothesized that, in the normal pediatric population, part of the physiologic variance in BP and in GFR, as assessed by the serum creatinine, is related to differences in renal size. For this purpose, we reviewed 1748 renal ultrasounds that were performed in children without evidence of acute or chronic renal disease and analyzed the relationship between renal size, renal function, and BP.
Materials and Methods
Patient Inclusion Criteria
We retrospectively reviewed all renal ultrasounds that were performed at our unit between 1995 and 2006 and were labeled as “normal.” Of these, studies that met the following criteria were selected: subjects were ≥6 months of age; measurement of renal length and renal thickness were recorded separately for each kidney; height, weight, serum creatinine, serum blood urea nitrogen (BUN), systolic BP (SBP), and diastolic BP (DBP) were measured when the ultrasound was performed; and absence of diseases that are known to influence renal size and other measured parameters. Overall, 1748 ultrasounds were retained for analysis.
Subjects were divided in four groups, based on their underlying diagnosis or indication for the ultrasonographic examination. These include (1) patients with a first episode of lower urinary tract infection that did not have an active infection at the time of ultrasound, (2) patients with idiopathic hypercalciuria and/or previous nephrolithiases, (3) patients with enuresis and/or mild to moderate bladder dysfunction, and (4) patients in whom no abnormalities were found at the end of the work-up (labeled as “control subjects”).
Renal Ultrasound Technique
All ultrasound studies were performed by staff radiologists, who are fully trained in the procedure. Kidney length was determined as the maximum longitudinal dimension, and kidney thickness was determined as the shortest distance from the renal sinus fat to the renal capsule on a mid-kidney transverse section. In most cases, measurements were performed using a posterior approach with the patient in prone position. In small children and infants, better measurements are obtained in supine decubitus or using a posterior approach with the patient in lateral decubitus. Information on the technique that was used for renal measurements was not routinely available in the ultrasound reports.
Serum Creatinine, Serum BUN, and BP Measurements
Serum creatinine was measured with the alkaline picrate colorimetric method, and BUN was determined with the urease/glutamate dehydrogenase (GLDH) enzymatic method, both on a Modular P800 analyzer (Hoffmann-La Roche, Basel, Switzerland).
In most cases, BP was measured during an outpatient visit on the same day of the renal ultrasound. A minority of patients had renal ultrasound performed while they were admitted to the hospital. BP was measured with a Critikon Dinamap 1846SX NIBP Monitor before 2002 and with a GE Dinamap ProCare 120 thereafter (GE Healthcare, Chalfont St. Giles, UK). Appropriate BP cuffs for arm size were used. We routinely measured BP three times in resting children and recorded the last reading in the medical chart.
Data Analyses
As opposed to adult data, pediatric data need to be normalized for growth parameters. Because the goal of this study was to test whether renal size correlates with renal function or BP, the entire cohort was treated as a unique population, and data were expressed in SD scores (SDSs) following steps that are listed below and that are detailed in the online supplementary material. Data analyses were performed using Microsoft Excel spreadsheets and SPSS 11.0 software (SPSS, Chicago, IL).
Briefly, for each patient, kidney length (mm), kidney thickness (mm), serum creatinine (mg/dl), serum BUN (mg/dl), SBP (mmHg), DBP (mmHg), and age (years) were recorded. To correct for differences in renal geometry, a least square method was used to generate a best-fitting formula that incorporates renal length and renal thickness. This formula corresponds to the weighted sum of right + left kidney lengths (coefficient = 0.58) and right + left kidney thickness (coefficient = 0.42). For clarity, we will refer to this result as “kidney size” (KS).
Nonlinear transformations were applied to express all dependent variables (kidney size, serum creatinine, serum BUN, SBP, and DBP) as a function of height, weight, age, and gender. All Pearson correlation coefficients between dependent and independent variables were highly significant before data normalization and were close to zero after data normalization (see online supplementary material). At the end of this process, each dependent variable was expressed as a function of all four independent variables. Data were corrected for heteroscedasticity, allowing expressing them as SDSs.
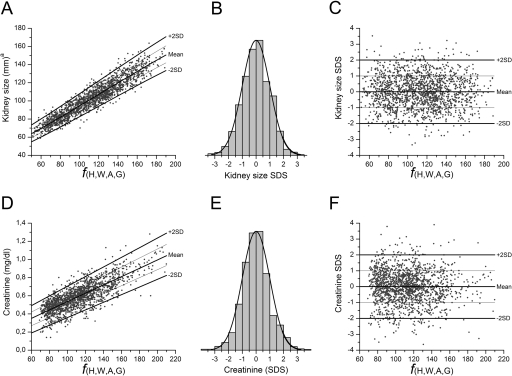
This process is shown in Figure 1. Figure 1, A and D, shows data dispersion for KS and serum creatinine as a function of height, weight, age, and gender (ƒ[H,W,A,G]); Figure 1, B and E, confirms that data follow a normal distribution; Figure 1, C and F, shows that SDSs obtained after data normalization are independent of height, weight, age, and gender.
Figure 1.
Data normalization process. The data normalization process is shown for KS and for serum creatinine. Nonlinear equations were used to calculate the best fitting function of each parameter as a function of height, weight, age, and gender, and residuals were corrected for heteroscedasticity (A and D). This allowed expressing data as SDS that followed a normal distribution (B and E). Once expressed as SDS, values were homogeneously distributed on both sides of the mean, independently of height, weight, age, and gender (C and F). Thinner lines in A, C, D, and F indicate +1SD and −1SD. The same process was used to generate SDS for BUN, SBP, and DBP (data not shown). aKS was calculated using a best-fitting model (equation 1 in online supplementary material).
Finally, we verified that the four subgroups of patients were comparable (Table 1). The ANOVA analysis showed only small, but statistically significant, differences for DBP. No differences were observed in KS, serum creatinine, BUN, and SBP.
Table 1.
Patient characteristics: renal and blood pressure values after data normalization
| Control Subjects | UTI | Nephrolithiasis Hypercalciuria | Enuresis Bladder Dysf. | Total | ANOVA |
||
|---|---|---|---|---|---|---|---|
| F | P | ||||||
| Patient characteristics | |||||||
| number of patients | 257 | 776 | 556 | 159 | 1748 | ||
| gender (male:female) | 0.53 | 0.47 | 0.53 | 0.40 | 0.49 | 3.84 | 0.009 |
| age (years) | 6.7 ± 5.2 | 4.0 ± 3.8 | 8.2 ± 4.5 | 7.4 ± 3.5 | 6.0 ± 4.6 | 117.1 | <0.0001 |
| SDS after data normalization | |||||||
| KS | 0.07 ± 1.06 | −0.01 ± 0.97 | −0.03 ± 1.01 | 0.05 ± 0.98 | 0.00 ± 1.00 | 0.87 | 0.458 |
| creatinine | 0.04 ± 1.13 | −0.04 ± 0.99 | 0.03 ± 0.96 | 0.02 ± 0.98 | 0.00 ± 1.01 | 2.07 | 0.101 |
| BUN | −0.03 ± 1.05 | 0.00 ± 1.01 | 0.01 ± 0.98 | 0.03 ± 0.96 | 0.00 ± 1.00 | 0.18 | 0.908 |
| SBP | −0.10 ± 1.02 | 0.02 ± 1.02 | −0.02 ± 0.99 | 0.1 ± 0.87 | 0.00 ± 1.00 | 1.87 | 0.132 |
| DBP | −0.16 ± 1.04 | 0.06 ± 1.02 | −0.03 ± 0.96 | 0.04 ± 0.96 | 0.00 ± 1.00 | 3.30 | 0.020 |
Age and normalized data are indicated as mean ± SD. UTI, urinary tract infection; bladder dysf., mild to moderate bladder dysfunction.
Results
The data elaboration described in the Materials and Methods section allowed to select 1748 “normal” renal ultrasound scans and to express, for each patient, KS, serum creatinine, serum BUN, SBP, and DBP as a function of height, weight, age, and gender. As a result of this process, all values were expressed as SDSs and had a mean value of 0 and an SD value of 1 (Table 1).
To test the initial hypothesis, correlation studies were performed and are reported in Table 2. As indicated, a strong correlation was observed between KS and serum creatinine and between KS and serum BUN. As expected, serum creatinine and serum BUN correlated among them, as well as SBP and DBP. No correlation was observed between BP data and KS.
Table 2.
Correlation table
| Kidney Size SDS | Creatinine SDS | BUN SDS | SBP SDS | DBP SDS | ||
|---|---|---|---|---|---|---|
| Kidney Size SDS | R | −0.17 | −0.07 | 0.00 | −0.03 | |
| p | <0.0001 | 0.002 | 0.84 | 0.19 | ||
| Creatinine SDS | R | −0.17 | 0.22 | −0.01 | 0.00 | |
| p | <0.0001 | <0.0001 | 0.67 | 0.88 | ||
| BUN SDS | R | −0.07 | 0.22 | −0.01 | −0.02 | |
| p | 0.002 | <0.0001 | 0.81 | 0.45 | ||
| SBP SDS | R | 0.00 | −0.01 | −0.01 | 0.61 | |
| p | 0.84 | 0.67 | 0.81 | <0.0001 | ||
| DBP SDS | R | −0.03 | 0.00 | −0.02 | 0.61 | |
| p | 0.19 | 0.88 | 0.45 | <0.0001 |
Correlation analysis between parameters of renal function, blood pressure and renal size. R, Pearson correlation coefficient.
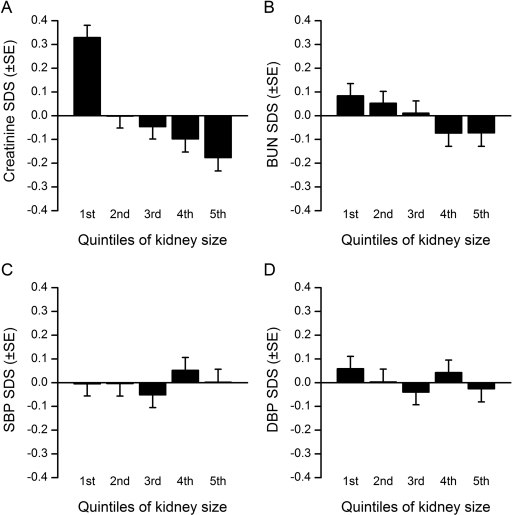
To show these results, the population was divided in quintiles for KS. As shown in Figure 2A, a difference in serum creatinine of 0.51 SDS was observed between the lowest and the highest quintile, indicating that serum creatinine was more elevated in smaller kidneys. Although statistically significant, this difference was less marked for serum BUN (0.16 SDS) (Figure 2B). No trend was observed when dividing SBP or DBP data per quintiles of KS (Figure 2, C and D).
Figure 2.
Distribution of SDS for serum creatinine, serum BUN, SBP, and DBP per quintiles of KS. Quintiles are expressed in SDS (see Figure 1B): 1st quintile, < −0.85; 2nd quintile, from −0.85 to −0.22; 3rd quintile, from −0.23 to +0.24; 4th quintile, from +0.25 to +0.84; 5th quintile, > +0.84.
Discussion
In humans, nephrogenesis begins around the 5th week of gestation and proceeds until approximately the 35th week (11). The number of glomeruli at birth depends therefore on intrauterine and genetic factors that regulate nephrogenesis.
In the past 15 years, the importance of nephron endowment has been emphasized by several experimental and clinical studies. Taken together, these studies indicate that low nephron number leads to glomerular hyperfiltration and hypertrophy, glomerular sclerosis, and eventually further loss of nephrons and development of chronic renal failure (12). This process is exacerbated by hypertension and decreased renal ability to excrete sodium, which may result directly from decreased nephron mass and/or may represent parallel effects of the same intrauterine and genetic factors that influence nephrogenesis (12).
To date, no clinical accessible method exists to measure nephron number. Despite compensatory glomerular hypertrophy, renal mass has been generally shown to be proportional to the number of nephrons per kidney (13). Measuring renal volume has therefore been proposed as a surrogate for measuring nephron endowment in humans (14).
This approach is based in part on autopsy studies showing a strong correlation between renal weight, glomerular number, glomerular hypertrophy, and clinical signs of hypertension (left ventricular mass) (15). Likewise, intrauterine growth retardation is associated with decreased renal mass and low nephron number (16,17). For example, Australian Aboriginal children have, on average, lower birth weights and smaller kidneys than white children (18). This population has been shown to have kidneys with fewer nephrons and to more frequently develop hypertension, proteinuria, and chronic renal failure (18,19). Similarly, Zhang et al. (10) have shown that a common variant of the RET gene is associated with reduced KS in newborns and in the number of glomeruli, as measured in autopsy specimens of children that died before the age of 3 months.
In clinical practice, the renal parenchyma endowment of each individual represents its reserve in GFR, which may impact significantly on the prognosis of acquired renal diseases and on the response to treatments aimed at decreasing intraglomerular pressure and renal fibrosis, such as angiotensin-converting enzyme inhibitors and angiotensin receptor blockers.
Differences between individuals in renal mass are probably important since childhood. Small-for-gestational age children with steroid-dependent nephrotic syndrome, for example, tend to develop a more severe course of disease (20). Likewise, it has been shown that small-for-gestational age pediatric patients with IgA nephropathy are at higher risk of developing chronic renal failure (21).
The normal range for GFR is relatively large in the normal population, ranging from 95 to 145 ml/min per 1.73 m2. In the clinical setting, GFR is most frequently estimated by creatinine clearance, which correlates with serum creatinine levels. Serum creatinine increases during childhood as a consequence of increased muscular mass, whereas creatinine clearance (ml/min per 1.73 m2), increases rapidly during the first year of life and stabilizes thereafter (22).
In this study, we hypothesized that variations in renal mass, as a surrogate parameter of nephron endowment, explain a significant proportion of the variance in serum creatinine levels that is observed in normal children. Pediatric data are more complex to analyze than data from adult subjects because they require normalization to account for changes during growth. Conversely, they are influenced by fewer factors such as essential hypertension, smoking, or diabetes mellitus that typically influence adult data and may provide a better estimate of the impact of renal size on normal renal function.
This study has limitations. It is a retrospective study, and patients underwent a renal ultrasound scan for medical reasons, although these were not expected to significantly influence the parameters of interest. This limitation was partially overcome because a significant number of subjects were not diagnosed with a renal or urologic disease, and they served as controls. Although there was a reason in the first place for the ultrasound to be performed, the low invasiveness of this technique has probably led to performing these studies in essentially normal children. Previous urinary tract infections or nephrolithiases, idiopathic hypercalciuria, enuresis, and mild/moderate bladder dysfunction had no significant effect on the analyzed parameters, except on DBP. We do not have a convincing explanation for this finding, which was modest. Most likely, the large sample size of our cohort conferred high statistical power to the analysis, which was able to detect small changes of questionable clinical relevance.
A second limitation of this study is related to the fact that we assessed renal function using serum creatinine levels. Although creatinine clearance based on a 24-hour urine collection was available in a significant proportion of the studied population, it is often hampered in small incontinent children by unreliable urine collections. Calculation of GFR by the Schwartz formula (23), which uses height and serum creatinine in a first-degree ratio, would not have added to our results, because serum creatinine was normalized for height during the normalization process and would have introduced a statistical redundancy. We also performed the analyses using GFR calculated with the Schwartz formula and obtained the same results as with serum creatinine (data not shown). Because measurements of creatinine clearance allow reducing the variance related to differences in body muscle mass, it is likely that our analysis has underestimated the relationship between renal function and renal mass by using serum creatinine levels.
Finally, the accurateness of ultrasound measurements is influenced by intra- and interobserver variability (24), and measurements of renal volume were not available in most of our patients. We used length and thickness to estimate KS. In fact, data in children with renal dysplasia have shown that correlation between ultrasonographic measurements and renal function increase as KS is expressed as kidney length, kidney area, or kidney volume (25). Here again, the unavailability of kidney volume measurements and intra- and interobserver variations in ultrasonographic measurements probably resulted in underestimating the impact of renal mass on GFR.
In contrast, we were able to study a very large number of patients, which significantly powered the analysis and allowed us to normalize data to treat the entire population as a whole.
Our results confirmed the initial hypothesis that serum creatinine levels are higher in patients with smaller kidneys. As expected, correlation with BUN was weaker, because this parameter is influenced by other variables, such as nutrition and hydration status.
In contrast, no correlation was found between KS and BP. Several studies have shown that office measurements of BP in children are poor estimates of average BP, as assessed by ambulatory BP measurements (26,27). Even with this limitation, we anticipated at the beginning of the study to detect at least a trend correlating BP and KS, given the very large sample size of our cohort. This hypothesis was based on multiple clinical and experimental evidences. A systematic review of the literature that included >50 studies, for example, has shown a strong correlation between systolic BP and birth weight (28). Similarly, diet modification in rats resulting in low birth weight is associated with decreased nephron number, decreased GFR, and higher BP (29). On the other hand, low nephron number is not always associated with elevated BP, and a second hit may be needed for this association to be expressed (30). The present data suggest that renal mass does not influence substantially BP control during childhood.
In conclusion, this study showed a strong correlation between renal function and renal mass among normal children. Technical limitations of this study may have underestimated this correlation. Because genetic studies are increasingly used to evaluate risk factors for renal disease, accurate measurements of renal volume by ultrasonography may prove to have equally significant prognostic value in patients with acquired renal disease. In addition, this study provided a rationale for population-based genetic studies aimed at identifying genes that influence renal development.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental information for this article is available online at www.cjasn.org.
References
- 1. Nyengaard J, Bendtsen T: Glomerular number and size in relation to age, kidney weight, and body surface in normal man. Anat Rec 232: 194–201, 1992 [DOI] [PubMed] [Google Scholar]
- 2. Samuel T, Hoy WE, Douglas-Denton R, Hughson MD, Bertram JF: Determinants of glomerular volume in different cortical zones of the human kidney. J Am Soc Nephrol 16: 3102–3109, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Fulladosa X, Moreso F, Narvaez JA, Grinyò JM, Seròn D: Estimation of total glomerular number in stable renal transplants. J Am Soc Nephrol 14: 2662–2668, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Hoy WE, Douglas-Denton RN, Hughson MD, Cass A, Johnson K, Bertram JF: A stereological study of number and volume: Preliminary findings in a multiracial study of kidneys at autopsy. Kidney Int Suppl 83: S31–S37, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Vikse BE, Irgens LM, Leivestad T, Hallan S, Iversen BM: Low birth weight increases risk for end-stage renal disease. J Am Soc Nephrol 19: 151–157, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hoy WE, Bertram JF, Denton RD, Zimanyi M, Samuel T, Hughson MD: Nephron number, glomerular volume, renal disease and hypertension. Curr Opin Nephrol Hypertens 17: 258–265, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Baum M: Role of the kidney in the prenatal and early postnatal programming of hypertension. Am J Physiol Renal Physiol 298: 235–247, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brenner BM, Chertow GM: Congenital oligonephropathy and the etiology of adult hypertension and progressive renal injury. Am J Kidney Dis 23: 171–175, 1994 [PubMed] [Google Scholar]
- 9. Amann K, Plank C, Dötsch J: Low nephron number: A new cardiovascular risk factor in children? Pediatr Nephrol 19: 1319–1323, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Zhang Z, Quinlan J, Hoy W, Hughson MD, Lemire M, Hudson T, Hueber PA, Benjamin A, Roy A, Pascuet E, Goodyer M, Raju C, Houghton F, Bertram J, Goodyer P: A common RET variant is associated with reduced newborn kidney size and function. J Am Soc Nephrol 19: 2027–2034, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Saxén L, Sariola H: Early organogenesis of the kidney. Pediatr Nephrol 1: 385–392, 1987 [DOI] [PubMed] [Google Scholar]
- 12. Luyckx VA, Brenner BM: Low birth weight, nephron number, and kidney disease. Kidney Int Suppl 97: S68–S77, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Abitbol CL, Ingelfinger JR: Nephron mass and cardiovascular and renal disease risks. Semin Nephrol 29: 445–454, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Hughson M, Farris AB, III, Douglas-Denton R, Hoy WE, Bertram JF: Glomerular number and size in autopsy kidneys: The relationship to birth weight. Kidney Int 63: 2113–2122, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Keller G, Zimmer G, Mall G, Ritz E, Amann K: Nephron number in patients with primary hypertension. N Engl J Med 348: 101–108, 2003 [DOI] [PubMed] [Google Scholar]
- 16. Hinchliffe SA, Lynch MR, Sargent PH, Howard CV, Van Velzen D: The effect of intrauterine growth retardation on the development of renal nephrons. Br J Obstet Gynaecol 99: 296–301, 1992 [DOI] [PubMed] [Google Scholar]
- 17. Manalich R, Reyes L, Herrera M, Melendi C, Fundora I: Relationship between weight at birth and the number and size of renal glomeruli in humans: A histomorphometric study. Kidney Int 58: 770–773, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Spencer J, Wang Z, Hoy W: Low birth weight and reduced renal volume in Aboriginal children. Am J Kidney Dis 37: 915–920, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Hoy WE, Hughson MD, Bertram JF, Douglas-Denton R, Amann K: Nephron number, hypertension, renal disease, and renal failure. J Am Soc Nephrol 16: 2557–2564, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Plank C, Ostreicher I, Dittrich K, Waldherr R, Voigt M, Amann K, Rascher W, Dötsch J: Low birth weight, but not postnatal weight gain, aggravates the course of nephrotic syndrome. Pediatr Nephrol 22: 1881–1889, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Zidar N, Cavić MA, Kenda RB, Koselj M, Ferluga D: Effect of intrauterine growth retardation on the clinical course and prognosis of IgA glomerulonephritis in children. Nephron 79: 28–32, 1998 [DOI] [PubMed] [Google Scholar]
- 22. Atiyeh BA, Dabbagh SS, Gruskin AB: Evaluation of renal function during childhood. Pediatr Rev 17: 175–180, 1996 [DOI] [PubMed] [Google Scholar]
- 23. Schwartz GJ, Furth SL: Glomerular filtration rate measurement and estimation in chronic kidney disease. Pediatr Nephrol 22: 1839–1848, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Geelhoed JJ, Kleiburg-Linkers VE, Snijders SP, Lequin M, Nauta J, Steegers EA, van der Heijden AJ, Jaddoe WW: The Generation R Study Group. Reliability of renal ultrasound measurements in children. Pediatr Nephrol 24: 1345–1353, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Uroz-Tristán J, Pérez Candela V, García-Anguiano Duque F, Busto Ferrer C, Domínguez Ortega F, Arteaga García R, Sanchís Solera L, de la Iglesia Iñigo S, Valenciano Fuentes B: Renal volumetric echography in the newborn infant with an agenetic, dysplastic or obstructive contralateral kidney. Cir Pediatr 7: 124–127, 1994 [PubMed] [Google Scholar]
- 26. Zhuo S, Wen W, Li-Yuan M, Shu-Yu W, Yi-Xin W: Home blood pressure measurement in prehypertension and untreated hypertension: Comparison with ambulatory blood pressure monitoring and office blood pressure. Blood Press Monit 14: 245–250, 2009 [DOI] [PubMed] [Google Scholar]
- 27. Stergiou GS, Salgami EV, Tzamouranis DG, Roussias LG: Masked hypertension assessed by ambulatory blood pressure versus home blood pressure monitoring: Is it the same phenomenon? Am J Hypertens 18: 772–778, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Huxley RR, Shiell AW, Law CM: The role of size at birth and postnatal catch-up growth in determining systolic blood pressure: A systematic review of the literature. J Hypertens 18: 815–831, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Vehaskari VM, Woods LL: Prenatal programming of hypertension: Lessons from experimental models. J Am Soc Nephrol 16: 2545–2556, 2005 [DOI] [PubMed] [Google Scholar]
- 30. Nenov VD, Taal MW, Sakharova OV, Brenner BM: Multi-hit nature of chronic renal disease. Curr Opin Nephrol Hypertens 9: 85–97, 2000 [DOI] [PubMed] [Google Scholar]