Summary
Background and objectives
Proliferative GN with monoclonal IgG deposits (PGNMID) is a newly described entity resembling immune complex GN. Its potential to recur in the allograft is undefined.
Design, setting, participants, & measurements
The first cases of recurrent PGNMID in the allograft are reported.
Results
The cohort includes four Caucasians (3 women, 1 man) with a mean age 58.5 years. No patient had M spike or hematologic malignancy. Recurrence was first documented by biopsy at a mean of 3.8 months posttransplant for indications of renal insufficiency in four patients, proteinuria in three patients, and microhematuria in three patients. Monoclonal IgG deposits (3 IgG3κ and 1 IgG3λ) in the transplants had identical heavy- and light-chain isotypes as in the native kidneys. In two patients, a pattern of endocapillary GN was identified in the native and transplant biopsies, whereas two patients with membranoproliferative GN in the native kidney developed endocapillary or mesangial GN in the transplant. Recurrence was treated with combined high-dose prednisone plus rituximab (n = 3) or plus cyclophosphamide (n = 1). After a mean posttransplant follow-up of 43 months, all four patients achieved reduction in proteinuria and three had reduction in creatinine. Repeat biopsies showed reduced histologic activity after treatment.
Conclusions
PGNMID can recur in the transplant despite the absence of a serum M spike. Recurrence is heralded by proteinuria, hematuria, and allograft dysfunction and manifests diverse histologic patterns. Although the pathogenesis remains unknown, early immunosuppressive therapy appears to stabilize the course.
Introduction
Various glomerular diseases can be caused by deposition of monoclonal IgG. These conditions include Randall-type light- and heavy-chain deposition disease (1), light- and heavy-chain amyloid (2), type 1 cryoglobulinemic GN (3), immunotactoid GN, and rarely fibrillary GN (4). We recently reported a novel form of glomerular injury related to monoclonal IgG deposition that could not be assigned to any of the previously defined categories that we termed “proliferative GN with monoclonal IgG deposits” (PGNMID) (5,6). Light microscopy (LM) in PGNMID usually exhibits predominantly endocapillary proliferative GN or membranoproliferative GN (MPGN), with or without membranous features. Electron microscopy (EM) shows granular, nonorganized deposits, typically in a subendothelial and mesangial distribution. The glomerular deposits on immunofluorescence (IF) are monoclonal, staining for a single light-chain isotype and a single heavy-chain subtype, most commonly IgG3κ. PGNMID is most common in older Caucasian women. Thirty percent of patients have a detectable circulating monoclonal protein with the same heavy- and light-chain isotypes as the glomerular deposits; however, the presence of an underlying hematologic malignancy is rare. Patients typically present with nephritic or nephrotic syndrome. The prognosis of this disease in the native kidney is variable. On follow-up available in 32 of our 37 previously reported patients, 37.5% recovered renal function, 37.5% developed persistent renal dysfunction, and 22% progressed to ESRD (6). Over 40 additional patients with PGNMID in the native kidney have been reported by other groups (7–16). Except for a single case reported in abstract form (13), recurrent PGNMID in the renal allograft has not been described in the literature. Here we present the clinical-pathologic features and outcome of four patients with recurrent PGNMID in the renal allograft.
Materials and Methods
Three of the four patients (#1, 3, and 4) were followed at Mayo Clinic, Rochester, Minnesota, where protocol allograft biopsies are generally performed at implantation, 4 months, and 1, 2 and 5 years after transplantation. A total of 12 allograft biopsies were performed in these three patients. The remaining patient (#2) was followed at St. Mary's Health Care, Grand Rapids, Michigan, where she underwent two clinically indicated transplant biopsies. Standard processing of the renal biopsies included LM, IF, and EM. For LM, all cases were stained with hematoxylin and eosin, periodic acid–Schiff, Masson's trichrome, and Jones methenamine silver. For IF, 3-μm cryostat sections were stained with polyclonal FITC-conjugated antibodies to IgG, IgM, IgA, C3, C1q, kappa, lambda, fibrinogen, and albumin. Transplant biopsies were also stained for C4d. Determination of the IgG subclass was performed on 3-μm cryostat sections using monoclonal FITC-conjugated antibodies to IgG1 (clone 8c/6 to 39), IgG2 (clone HP6014), IgG3 (clone HP6050), and IgG4 (clone HP6025; Sigma-Aldrich, St. Louis, MO). IF staining intensity was graded on a semiquantitative scale (0 to 3+).
Previously defined diagnostic criteria for PGNMID (5) include renal biopsy findings of GN with the following: (1) glomerular immune deposits staining positive for γ heavy chain (IgG), with negativity for α (IgA) and μ (IgM) heavy-chains, indicating restriction to a single (γ) Ig class; (2) positive staining for a single γ (IgG) subclass (IgG1, IgG2, IgG3, or IgG4); (3) positive staining for a single light-chain isotype (κ or λ), indicating monoclonality; (4) predominantly granular electron-dense deposits in mesangial, subendothelial, and/or subepithelial locations by EM, resembling immune complex GN; and (5) no clinical or laboratory evidence of cryoglobulinemia.
Results
Clinical Features of Native Disease
Clinical features of native PGNMID are shown in Table 1. The cohort consisted of four Caucasian adults including three women and 1 man. The mean age at diagnostic biopsy of the native kidney was 56.7 years (range 38 to 74 years). Patient #1 had a history of ankylosing spondylitis that had been treated with anti-TNF agents (Etanercept then Adalimumab) in the past. His disease has been asymptomatic on no anti-TNF therapy since 11 months before transplant. He has had no reactivation of ankylosing spondylitis posttransplant. Patient #2 had a history of Graves disease that was treated 2 years before clinical onset of native renal disease with radioactive iodine, which resulted in hypothyroidism requiring continuous hormonal replacement. Posttransplant, she remains on levothyroxine with no evidence of reactivation of autoimmune thyroiditis. Patient #3 carried a history of rheumatoid arthritis (RA) that had been treated with methotrexate and low-dose prednisone in the past. Her RA has been quiescent on no methotrexate therapy since starting dialysis 9 months before transplant. She has had no reactivation of RA posttransplant, despite recurrence of PGNMID.
Table 1.
Clinical data of the native disease
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Age at native biopsy | 55 | 60 | 74 | 38 |
| Gender | Male | Female | Female | Female |
| Race | White | White | White | White |
| Associated conditions | Ankylosing spondylitis | Graves disease, endometrial carcinoma | RA | Hypertension |
| Parameters at the time of first native biopsy | ||||
| serum creatinine (mg/dl) | 3.6 | 2.2 | 1.6 | 3.5 |
| proteinuria (g/24 h) | 3.9 | 10.6 | 1.4 | 7.0 |
| serum albumin | 4.0 | 2.3 | 3.0 | 3.5 |
| edema | No | Yes | No | No |
| hematuria | Yes | Yes | Yes | Yes |
| serum complement | Normal C3 and C4 | Normal C3 and C4 | Normal C3 and C4, then low C4 and normal C3 4 months postbiopsy | Normal C3 and C4 |
| serum paraprotein | Negative | Not done | Negative | Negative |
| urine paraprotein | Negative | Not done | Negative | Negative |
| serum cryoglobulin | Negative | Not done | Negative | Negative |
| hepatitis C antibody | Negative | Negative | Negative | Negative |
| ANA | Not done | Negative | Negative | Negative |
| Treatment of native disease | PRED for 4 months and MMF for 9 months after first biopsy PRED/PO CYT for 5 months after second biopsy | PRED/CYA for 11 months | PRED/CYA for 6 months Plasmapheresis | PRED for 12 months (also PRED for 4 months for relapse) |
| Time from biopsy to dialysis in months | 8 | 18 | 4 | NAa |
| Duration of dialysis before transplant in months | 12 | 6 | 9 | NAa |
CYA, cyclosporine; CYT, cyclophosphamide; NA, not applicable; PO, oral; PRED, prednisone.
Pre-emptive transplant. Time from biopsy to transplant was 30 months.
All patients presented with renal insufficiency, proteinuria, and hematuria. Mean serum creatinine at the time of native kidney biopsy was 2.7 mg/dl (range 1.6 to 3.6 mg/dl). Three patients had nephrotic-range proteinuria, including one with full nephrotic syndrome, and one had subnephrotic proteinuria. The mean 24-hour urine protein was 5.7 g (range 1.4 to 10.6 g). The mean serum albumin was 3.2 g/dl (range 2.3 to 4.0 g/dl). One patient exhibited peripheral edema. Serum complements C3 and C4 were normal at the time of biopsy in all patients. In one patient (#3), C4 was found to be depressed on repeat testing 4 months later. Hepatitis C antibody and hepatitis B surface antigen were negative in all patients. Serum cryoglobulin and anti-nuclear antibody (ANA), tested in three patients, were negative. Serum and urine immunofixation, performed in three patients, were negative for monoclonal protein. Bone marrow biopsy was not performed in any of the four patients. All patients received immunosuppressive therapy, including steroids alone in one patient; steroids and cyclosporine in two patient; and steroids, cyclophosphamide, and mycophenolate mofetil (MMF) in one patient. One patient also received plasmapheresis. Three patients progressed to ESRD within 18 months after the diagnostic native renal biopsy and were maintained on hemodialysis for 6 to 12 months before receiving a kidney transplant. The fourth patient received a pre-emptive transplant 30 months after the diagnostic native renal biopsy (Table 1).
Clinical Presenting Features and Outcome of Recurrent Disease (Table 2)
Table 2.
Clinical data of the recurrent disease
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Age at transplant | 57 | 62 | 75 | 40 |
| Kidney source | Living-unrelated donor | Deceased donor | Living-related donor | Living-unrelated donor |
| HLA mismatch | 4 of 6 HLA antigens | 0 of 6 HLA antigens | 2 of 6 HLA antigens | 4 of 6 HLA antigens |
| Percent PRAa | 1% for class I | 0% for class I | 0% for class I | 0% for class I |
| 13% for class II | 0% for class II | 0% for class II | 0% for class II | |
| Maintenance immunosuppressive regimen | FK506/PRED/MMF | FK506/PRED/Myfortic acid | FK506/PRED/MMF | FK506/PRED/MMF |
| Time from transplant to diagnosis of recurrent disease | 3 | 4 | 5 | 3 |
| Baseline serum creatinine (mg/dl) | 1.9 | 1.2 | 1.4 | 0.9 |
| Parameters at the time of first biopsy showing recurrence | ||||
| serum creatinine (mg/dl) | 2.8 | 3.7 | 4.8 | 1.2 |
| 24-hour urine protein | 0.790 | 7.4 | 5.8 | 0.061 |
| serum albumin | 3.5 | 2.0 | 3 | 4.4 |
| edema | Yes | Yes | Yes | No |
| hematuria | Yes | Yes | Yes | No |
| serum complement | Normal C3 and C4 then low C3 and C4 3 months postbiopsy | Low C3 and C4 | Low C3 and C4 | Normal C3 and C4 |
| serum paraprotein | Negative (also normal serum free K:L) | Negative | Negative (also normal serum free K:L) | Negative |
| urine paraprotein | Negative | Negative | Negative | Not done |
| serum cryoglobulin | Negative | Negative | Negative | Not done |
| hepatitis C antibody | Negative | Negative | Negative | Not done |
| ANA | Negative | Negative | Negative | Not done |
| Treatment of recurrent disease | PRED and PO CYT for 6 months, after the third biopsy | M-PRED/PO PRED for 8 months/RIT ×2/lisinopril for 6 months | M-PRED/RIT ×1/PLX ×4/HD ×3 | M-PRED for ACR/RIT ×2 |
| Duration of posttransplant follow-up in months | 15 | 11 | 83 | 63 |
| Outcome | Final serum creatinine: 2.3 mg/dl | Final serum creatinine: 1.1 mg/dl | Final serum creatinine: 1.3 mg/dl | Final serum creatinine: 2.3 mg/dl |
| Final 24-hour urine protein: 0.128 g | Final 24-hour urine protein: 0.390 g | Final 24-hour urine protein: 0.629 g | Final 24-hour urine protein: 0.059 g |
PRA, panel reactive antibody; FK506, tacrolimus; HD, hemodialysis; K:L, kappa:lambda; M-PRED, methyl-prednisolone; PLX, plasmapheresis; RIT, rituximab.
Measured in pretransplant serum.
The donor kidney source for transplantation was a living-unrelated donor in two patients, a living-related donor in one patient, and a deceased donor in one patient. All patients had negative crossmatch, but one (#3) received an ABO-incompatible transplant. The maintenance immunosuppression regimen consisted of tacrolimus, MMF, and prednisone in three patients and tacrolimus, mycophenolic acid, and prednisone in the remaining patient. The mean time from transplant to the first allograft biopsy showing recurrent disease was 3.8 months (range 3 to 5 months). At the time of recurrence, all patients had worsening renal function with a mean serum creatinine at recurrence of 3.1 mg/dl (range 1.2 to 4.8 mg/dl), which was increased from a mean baseline serum creatinine of 1.4 mg/dl (range 0.9 to 1.9 mg/dl). Two patients had full nephrotic syndrome. Of the remaining two patients, one (#1) had mild proteinuria (0.79 g/d) that increased to 2.8 g/d 6 months later. The remaining patient (#4) did not have proteinuria at the time of initial allograft biopsy but developed minimal proteinuria (162 mg/d, negative <150 mg/d) 20 months later. Three patients had microhematuria. Serum complements C3 and C4 were depressed in two patients (#2, 3) and normal in one patient (#4). The fourth patient (#1) had normal C3 and C4 at biopsy, but these levels became depressed 3 months later. Hepatitis C antibody, serum cryoglobulin, and ANA were negative in all three patients tested. Serum immunofixation, performed in all patients, was negative for a monoclonal protein. Both patients tested with serum free light-chain assay had a normal free kappa:lambda ratio. Urine immunofixation, performed in three patients, was negative for monoclonal protein. Bone marrow biopsy was not performed posttransplant in any of the four patients.
The recurrent disease was treated with high-dose prednisone and rituximab in three patients (#2 to #4) and with high-dose prednisone and cyclophosphamide in one patient (#1). Patient 3 was also treated with three treatments of plasmapheresis and acute dialysis. Patient 2 also received lisinopril. None of the other three patients were treated with renin-angiotensin system blockade posttransplant. Duration of posttransplant follow-up ranged from 11 to 83 months (mean 43 months) (Table 2). The degree of proteinuria has improved in all four patients with a mean 24-hour urine protein of 0.302 g (range 0.059 to 0.629 g). Three patients (#1 to #3) had a decrease in serum creatinine to a mean of 1.6 mg/dl (range 1.1 to 2.3 mg/dl). The remaining patient (#4), who experienced two episodes of acute cellular rejection, had worsening serum creatinine from 1.2 mg/dl at the time of first detection of disease recurrence to 2.3 mg/dl at the last follow-up.
Pathologic Findings (Table 3)
Table 3.
Pathologic findings
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| First native biopsy | ||||
| glomerular pattern on LM | MPGN | Diffuse endocapillary proliferative GN | Focal endocapillary proliferative GN with mild membranoproliferative features | MPGN |
| IF | 2+ IgG, 3+ C3, 2+ κ | 2+ IgG, 3+ C3, 2+ κ | 2+ IgG, 3+ C3, 2+ λ | 3+ IgG, 2+ C3, 3+ κ |
| − C1q, − IgA, − IgM, − λ | 1+ C1q, ± IgM, − IgA, − λ | 2+ C1q, ± IgM, − IgA, − κ | 3+ C1q, − IgM, − IgA, − λ | |
| 3+ IgG3, − IgG1, − IgG2, − IgG4 | ||||
| EM | Granular mesangial deposits | Granular mesangial and subendothelial deposits | Granular mesangial and subendothelial deposits | Granular mesangial and subendothelial deposits |
| First allograft biopsy showing recurrence | ||||
| glomerular pattern on LM | Mesangial proliferative GN | Diffuse endocapillary proliferative and exudative GN | Diffuse endocapillary proliferative and exudative GN with mild membranoproliferative features | Minimal mesangial proliferative GN |
| IF | 1+ IgG, 3+ C3, 2+ κ | 2+ IgG, 3+ C3, 2+ κ | 3+ IgG, 3+ C3, 3+ λ | 2+ IgG, 2+ C3, 2+ κ |
| − C1q, − IgA, − IgM, ± λ | 1+ C1q, − IgM, − IgA, − λ − C4d in PTC |
3+ C1q, ± IgM, − IgA, − κ, | 3+ C1q, ± IgM, − IgA, − λ | |
| 2+ IgG3, − IgG1, − IgG2, − IgG4 | 3+ IgG3, − IgG1, − IgG2, − IgG4 | 3+ IgG3, − IgG1, − IgG2, − IgG4 | ||
| − C4d in PTC | − C4d in PTC | C4d staining not done | ||
| EM | Granular mesangial deposits | Not performed | Granular subendothelial deposits | Granular mesangial deposits |
| Final allograft biopsy | ||||
| glomerular pattern on LM | Focal endocapillary proliferative GN | Focal endocapillary proliferative GN | Mesangial proliferative GN with mild membranoproliferative features | Mesangial proliferative GN with mild membranoproliferative features |
| IF | 2+ IgG, 3+ C3, 2+ κ | 3+ IgG, 3+ C3, 2+ κ | 2+ IgG, 2+ C3, 2+ λ | 2+ IgG, 3+ C3, 2+ κ |
| − C1q, − IgA, − IgM, − λ − C4d in PTC | 1+ C1q, ± IgM, − IgA, − λ | 2+ C1q, ± IgM, − IgA, − κ 1+ C4d in PTCa | 2+ C1q, − IgM, − IgA, − λ − C4d in PTC | |
| 3+ IgG3, − IgG1, − IgG2, − IgG4 | ||||
| − C4d in PTC | ||||
| EM | Granular mesangial and subendothelial deposits | Granular mesangial and subendothelial deposits | Granular mesangial and subendothelial deposits | Granular mesangial and subendothelial deposits |
PTC, peritubular capillaries.
ABO-incompatible transplant.
Patient 1.
The native kidney biopsy in patient #1 showed a mild MPGN pattern with segmental duplication of the glomerular basement membrane (GBM), mesangial interposition and hypercellularity, and only mild and focal endocapillary hypercellularity. A subsequent native kidney biopsy, performed 9 months later, showed a similar MPGN pattern but with cellular crescents in three of five glomeruli sampled. IF in both biopsies revealed bright granular glomerular staining for IgG, C3, and kappa, with negative IgM, IgA, C1q, and lambda. EM, performed on the first biopsy, showed granular mesangial electron-dense deposits. Disease recurrence was documented in four allograft biopsies performed at 3, 6, 9, and 10 months posttransplant. The glomerular pattern on LM was mesangial proliferative GN in the first two biopsies, diffuse endocapillary proliferative GN (diffuse endocapillary hypercellularity without GBM duplication or mesangial interposition) in the third biopsy (Figure 1A), and focal endocapillary proliferative GN in the fourth biopsy (Figure 1B), which was performed after therapy. On EM, only granular mesangial electron-dense deposits were seen in the first two transplant biopsies, whereas abundant granular mesangial and subendothelial deposits were seen in the two subsequent transplant biopsies (Figure 2). Similar to the native biopsies, all transplant biopsies showed monoclonal IgG-kappa deposits on IF (Figure 3). IF staining for IgG subtypes performed on the first transplant biopsy revealed that the deposits were monotypic, staining for IgG3 only, with negative IgG1, IgG2, and IgG4. All transplant biopsies also showed mild tubular atrophy and interstitial fibrosis without rejection.
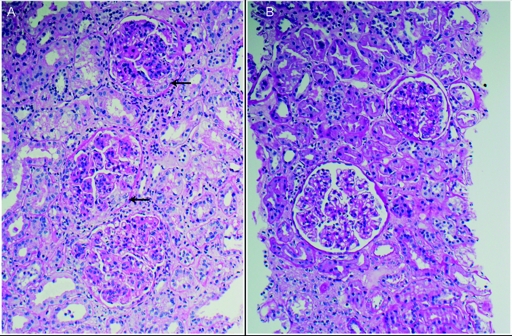
Figure 1.
LM findings. (A) The third transplant biopsy in patient #1 showed diffuse endocapillary proliferative GN. The three glomeruli depicted in this representative image show global endocapillary hypercellularity. Small segmental cellular crescents are present in two glomeruli (arrows) (hematoxylin and eosin; ×200). (B) The fourth transplant biopsy performed after therapy showed less disease activity. The two glomeruli depicted show mesangial hypercellularity, one of which also exhibits minimal segmental endocapillary hypercellularity (hematoxylin and eosin; ×200).
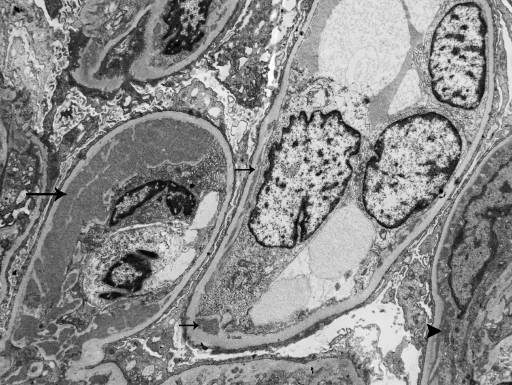
Figure 2.
This representative electron micrograph from the third transplant biopsy in patient #1 shows segmental granular subendothelial electron-dense deposits that range from small (small arrows) to large (large arrow). Minute paramesangial electron-dense deposits are also apparent (arrowhead). Podocytes exhibit marked foot process effacement (×4200).
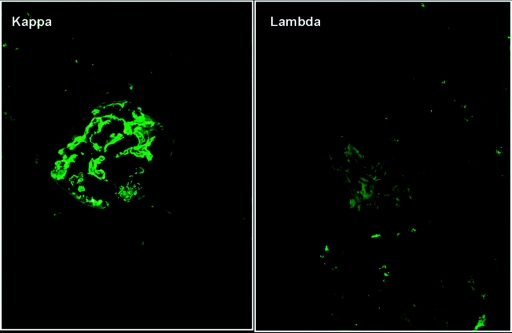
Figure 3.
The third transplant biopsy in patient #1 shows strong glomerular staining for kappa light chain with negative staining for lambda light chain (IF micrograph ×400).
Patient 2.
The native kidney biopsy showed diffuse endocapillary proliferative GN. On IF, there was bright (2 to 3+) granular glomerular staining for IgG, C3, and kappa, with negative IgA, ± IgM, 1+ C1q, and negative lambda. EM showed prominent granular mesangial and subendothelial electron-dense deposits. The patient underwent two allograft biopsies at 4 and 12 months posttransplant, both revealing an endocapillary proliferative GN, which was diffuse and exudative in the first biopsy and focal in the second biopsy performed after therapy. The first transplant biopsy also showed focal acute pyelonephritis. Similar to the native biopsy, both transplant biopsies had monoclonal IgG-kappa glomerular deposits on IF with granular mesangial and subendothelial electron-dense deposits on EM. IF staining for IgG subtypes performed on the native biopsy and the second transplant biopsy revealed monotypic glomerular deposits, staining for IgG3 only. Both transplant biopsies also had mild tubular atrophy and interstitial fibrosis without rejection.
Patient 3.
The native kidney biopsy showed focal endocapillary proliferative GN with only rare areas of GBM duplication. A second native kidney biopsy performed 4 months later showed a similar pattern but with 30% crescents, and a third native kidney biopsy 5 months later showed a similar pattern but with extensive fibrous crescents and global glomerulosclerosis. IF in all three biopsies showed 2 to 3+ granular glomerular staining for IgG, C3, and lambda, with negative IgA, ± IgM, 1 to 2+ C1q, and negative kappa. EM, performed on the first two biopsies, showed segmental granular mesangial and subendothelial electron-dense deposits. Disease recurrence was documented in three allograft biopsies performed at 5, 12, and 23 months posttransplant. The glomerular pattern on LM was diffuse endocapillary proliferative and exudative GN with only rare areas of GBM duplication in the first biopsy and mesangial proliferative GN with rare GBM duplication in the two subsequent biopsies performed after therapy. Similar to the native biopsies, the transplant biopsies showed monoclonal IgG-lambda glomerular deposits on IF. IF staining for IgG subtypes performed on the first transplant biopsy revealed monotypic IgG3 deposits (Figure 4). EM performed on the first and third transplant biopsies showed granular subendothelial electron-dense deposits in the former and granular mesangial and subendothelial electron-dense deposits in the latter. The degree of tubular atrophy and interstitial fibrosis was mild in the first and second transplant biopsies and moderate in the third biopsy.
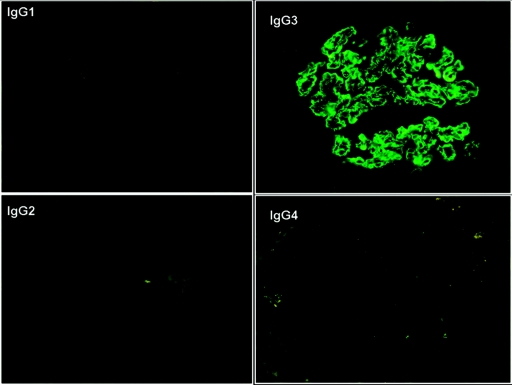
Figure 4.
IF staining for the IgG subtypes performed on the first transplant biopsy with recurrence in patient #3 shows intense glomerular positivity for IgG3 with negative staining for IgG1, IgG2, and IgG4 (×400).
Patient 4.
The native kidney biopsy showed a well developed MPGN pattern on LM with diffuse and global duplication of GBM, mesangial interposition, and mesangial expansion by increased mesangial cellularity; bright granular global glomerular staining for IgG, C1q, kappa, and C3 with negative IgM, IgA, and lambda on IF; and global granular mesangial and subendothelial electron-dense deposits on EM. Disease recurrence was detected in five allograft biopsies performed at 3, 12, 23, 24, and 34 months posttransplant; the first two showed minimal mesangial proliferative GN and the later biopsies showed mild mesangial proliferative GN with only rare GBM duplications. Similar to the native biopsy, the transplant biopsies showed monoclonal IgG-kappa glomerular deposits on IF. IF staining for IgG subtypes performed on the first and third transplant biopsies revealed that the deposits were monotypic, staining for IgG3 only. EM showed granular mesangial electron-dense deposits in the first and second transplant biopsies and granular mesangial and subendothelial electron-dense deposits in the later biopsies. The third and fifth transplant biopsies also showed concurrent acute cellular rejection, Banff grade 1A. The degree of tubular atrophy and interstitial fibrosis was mild in the first and second biopsies and moderate in the later biopsies.
Discussion
Albawardi et al. recently described in abstract form 16 cases of PGNMID, two of which occurred in the renal transplant, one as de novo disease, and one as recurrent disease (13). The de novo disease was diagnosed 13 years posttransplant in a patient with ESRD secondary to polycystic kidney disease. The patient with recurrent disease lost his native kidney 3 years after the diagnosis of PGNMID of the IgG3 kappa subtype. One year posttransplant, he presented with heavy proteinuria (8 to 12 g/d) and a transplant biopsy showed recurrent disease with similar IgG3 kappa glomerular deposits. No other patients with recurrent or de novo PGNMID have been reported in the literature.
The study presented here provides the first detailed report of recurrent PGNMID in the renal allograft. Patients' demographics were similar to the previously reported patients with PGNMID: all four were Caucasians, 75% were women, and 75% were ≥55 years of age at the time of native biopsy. No patient had a detectable M spike in serum or urine. Similar to other recurrent glomerular diseases, PGNMID tends to recur early posttransplant, within 5 months in all four patients. The recurrent disease manifested clinically as allograft dysfunction and proteinuria. The degree of proteinuria and the presence of hematuria correlated with the LM pattern: The three patients with endocapillary proliferative GN (#1 to #3) had hematuria and ≥2.8 g/d proteinuria, including two with full nephrotic syndrome, whereas patient #4 with mild mesangial proliferative GN and only rare GBM duplications developed minimal proteinuria 23 months posttransplant without hematuria. Three patients developed hypocomplementemia.
The use of protocol biopsies in our medical center allowed us to make an earlier histologic diagnosis of recurrent PGNMID and to study the evolution over time and the response to therapy. The monoclonal protein deposited in the glomeruli of the transplanted and native kidneys was identical with respect to light- and heavy-chain isotype restriction (IgG-kappa in three patients and IgG-lambda in one patient), whereas the LM pattern was variable. In two patients (#2 and #3), the native and transplant biopsies showed similar endocapillary proliferative GN, whereas the two patients with a membranoproliferative pattern in the native kidney developed an endocapillary or mesangial pattern in the allograft. In patient #4, the milder pathologic and clinical features of the recurrence compared with the original disease are possibly due to the early disease detection by protocol biopsies and the therapeutic effects of the maintenance anti-rejection immunosuppression. Of note, despite the improvement of the LM pattern of recurrent disease on repeat biopsies in three patients (#1 to #3) after therapy, the deposits on IF and EM persisted.
In three of the four patients with recurrent PGNMID in this series, there was a prompt and sustained response to aggressive immunosuppressive treatment, which consisted of combined high-dose steroids and rituximab in two patients and high-dose steroids and cyclophosphamide in one patient, leading to a reduction in proteinuria from a mean of 4.7 to 0.302 g/d and a decrease in serum creatinine from a mean of 3.8 to 1.6 mg/dl. This response is best illustrated in patient #3, who presented 5 months posttransplant with nephrotic syndrome (with a 24-hour urine protein of 5.8 g) and acute renal failure (with a serum creatinine of 4.8 mg/dl). A kidney biopsy revealed recurrent PGNMID with diffuse endocapillary proliferative and exudative GN. She was treated acutely with methylprednisolone, rituximab, plasmapheresis, and hemodialysis. Three months later, her serum creatinine had decreased to 1.6 mg/dl and 24-hour urine protein had fallen to 0.941 g. Two subsequent biopsies, 5 and 18 months later, showed histologic improvement to a predominantly mesangial proliferative GN. The only patient with deteriorating graft function on follow-up (#4) had also experienced two episodes of acute cellular rejection without significant increase in proteinuria or histologic progression of GN, suggesting that the deteriorating renal function was largely due to the rejection. The excellent response to therapy is encouraging and may be due in part to early initiation of therapy. Larger studies with longer follow-up are needed to determine the risk factors for recurrence of PGNMID, the effect of recurrence on graft survival, and the optimal therapeutic regimen, including the role of rituximab.
Recurrent PGNMID is possibly an under-recognized disease, particularly if IF and EM are not routinely performed on allograft biopsies. Without IF or EM, the disease could be misinterpreted as transplant glomerulopathy. Even with IF and EM and knowledge of hypocomplementemia, the differential diagnosis would include de novo postinfectious GN and recurrent or de novo primary MPGN, which may have atypical histologic features in the allograft (17).
The pathogenesis of PGNMID remains unknown. The recurrence in the allograft favors that the disease is caused by persistent circulating factors in the recipient. Our previous demonstration that all three constant domains of the IgG molecule are present suggests that there is glomerular deposition of a circulating nondeleted monoclonal IgG molecule followed by fixation of complement and activation of downstream inflammatory mediators that promote glomerular leukocyte infiltration and proliferation (5,16). In all four patients with PGNMID reported here, the IgG subtype deposited in glomeruli was IgG3, which is the isoform identified in most native kidney biopsies of PGNMID (6,13). This subtype, which comprises only 8% of IgG in the serum of normal individuals, has the unique physicochemical property of self-aggregability via Fc-Fc interactions (18,19). In addition, compared with other IgG subtypes, it has the highest molecular weight, the greatest complement-fixing ability, and the most positive charge, properties that may make it intrinsically “nephritogenic” (18,19). None of our patients had an identifiable serum monoclonal protein by conventional immunochemical analysis (i.e., serum protein electrophoresis and immunofixation), which is in agreement with our prior finding in the native kidney that a demonstrable circulating paraprotein is more common in patients with monoclonal IgG1 or IgG2 deposits (6). We hypothesized that the inability to detect a serum monoclonal IgG3 protein by immunofixation may be due to its high affinity for the negatively charged GBM and rapid aggregability, which probably is favored by the high intracapillary concentrations reached during glomerular filtration (6). Whether the glomerular deposition of IgG3 (as opposed to other IgG subtypes) is in itself a risk factor for recurrence requires further study. Our findings are in contrast to other forms of recurrent MPGN associated with dysproteinemia, for which the presence of a serum monoclonal protein increases the likelihood of recurrence (17).
It is interesting that three of our patients carried a history of underlying autoimmune disease (RA in one, ankylosing spondylitis in one, and Graves disease in one) in contrast to our previously reported 37 patients with PGNMID in the native kidney, in whom a history of autoimmune disease was lacking. In these three patients, features that argue against autoimmune disease-associated GN are the monoclonal and monotypic deposits on IF detected on multiple native and transplant biopsies, the absence of endothelial tubuloreticular inclusions, the negative ANA, and the lack of any relationship between recurrent PGNMID and a reactivation of the autoimmune disease. In these three patients, it is possible that during an immune response to autoantigens, one or more clones of B cells proliferate and produce monoclonal IgG3 molecules, although no patient had a clinical reactivation of autoimmune disease posttransplantation. Low serum complements are common in PGNMID patients without evidence of autoimmune disease. Moreover, no patient had membranous glomerulopathy, which is the most common pattern of glomerular disease in patients with RA and autoimmune thyroiditis, and there was no evidence of IgA nephropathy, the most common immune complex GN in ankylosing spondylitis. Whether the presence of an underlying autoimmune disease in PGNMID patients is a predisposing factor for recurrence will require additional studies.
In summary, our data indicate that PGNMID may recur in the transplant despite the absence of a detectable serum M spike. The recurrent GN may respond to early aggressive immunosuppressive therapy, including a regimen of high-dose prednisone and rituximab.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Lin J, Markowitz GS, Valeri AM, Kambham N, Sherman WH, Appel GB, D'Agati VD: Renal monoclonal immunoglobulin deposition disease: The disease spectrum. J Am Soc Nephrol 12: 1482–1492, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Nasr SH, Colvin R, Markowitz GS: IgG1 lambda light and heavy chain renal amyloidosis. Kidney Int 70: 7, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Nasr SH, Markowitz GS, Reddy BS, Maesaka J, Swidler MA, D'Agati VD: Dysproteinemia, proteinuria, and glomerulonephritis. Kidney Int 69: 772–775, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Rosenstock JL, Markowitz GS, Valeri AM, Sacchi G, Appel GB, D'Agati VD: Fibrillary and immunotactoid glomerulonephritis: Distinct entities with different clinical and pathologic features. Kidney Int 63: 1450–1461, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Nasr SH, Markowitz GS, Stokes MB, Seshan SV, Valderrama E, Appel GB, Aucouturier P, D'Agati VD: Proliferative glomerulonephritis with monoclonal IgG deposits: A distinct entity mimicking immune-complex glomerulonephritis. Kidney Int 65: 85–96, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Nasr SH, Satoskar A, Markowitz GS, Valeri AM, Appel GB, Stokes MB, Nadasdy T, D'Agati VD: Proliferative glomerulonephritis with monoclonal IgG deposits. J Am Soc Nephrol 20: 2055–2064, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Evans DJ, Macanovic M, Dunn MJ, Pusey CD: Membranous glomerulonephritis associated with follicular B-cell lymphoma and subepithelial deposition of IgG1-kappa paraprotein. Nephron Clin Pract 93: c112–c118, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Lee JG, Moon KC, Lee JE, Kim P, Lee JG, Kim JH, Lee KY: A case of proliferative glomerulonephritis with monoclonal IgG deposits. Korean J Nephrol 23: 987–991, 2004 [Google Scholar]
- 9. Komatsuda A, Masai R, Ohtani H, Togashi M, Maki N, Sawada K, Wakui H: Monoclonal immunoglobulin deposition disease associated with membranous features. Nephrol Dial Transplant 23: 3888–3894, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Bridoux F, Zanetta G, Mougenot B, Goujon JM, Vanhille P, Bauwens M, Chevet D, Ronco P, Preud'homme JL, Touchard G: Glomerulopathy with non-organized and non-Randall type monoclonal immunoglobulin deposits: A rare entity [Abstract]. J Am Soc Nephrol 12: 94A, 2001 [Google Scholar]
- 11. Geldenhuys L, Jones B: Glomerulonephritis with monoclonal immunoglobulin deposits [Abstract]. J Am Soc Nephrol 19: 671A, 2008 [Google Scholar]
- 12. Masai R, Wakui H, Komatsuda A, Togashi M, Maki N, Ohtani H, Oyama Y, Sawada K: Characteristics of proliferative glomerulonephritis with monoclonal IgG deposits associated with membranoproliferative features. Clin Nephrol 72: 46–54, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Albawardi A, Sataskar A, Brodsky S, Nadasdy GM, Nadasdy T: Proliferative glomerulonephritis with monoclonal IgG deposits recurs or may develop de novo in renal allografts [Abstract]. Modern Pathology 23: 337A, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Alpers CE, Tu WH, Hopper J, Jr, Biava CG: Single light chain subclass (kappa chain) immunoglobulin deposition in glomerulonephritis. Hum Pathol 16: 294–304, 1985 [DOI] [PubMed] [Google Scholar]
- 15. Komatsuda A, Wakui H, Ohtani H, Nimura T, Sawada K: Steroid-responsive nephrotic syndrome in a patient with proliferative glomerulonephritis with monoclonal IgG deposits with pure mesangial proliferative features. NDT Plus 3: 357–359, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Seigneux S, Bindi P, Debiec H, Alyanakian MA, Aymard B, Callard P, Ronco P, Aucouturier P: Immunoglobulin deposition disease with a membranous pattern and a circulating monoclonal immunoglobulin G with charge-dependent aggregation properties. Am J Kidney Dis 56: 117–121, 2010 [DOI] [PubMed] [Google Scholar]
- 17. Lorenz EC, Sethi S, Leung N, Dispenzieri A, Fervenza FC, Cosio FG: Recurrent membranoproliferative glomerulonephritis after kidney transplantation. Kidney Int 77: 721–728, 2010 [DOI] [PubMed] [Google Scholar]
- 18. Grey HM, Hirst JW, Cohn M: A new mouse immunoglobulin: IgG3. J Exp Med 133: 289–304, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Capra JD, Kunkel HG: Aggregation of gamma-G3 proteins: Relevance to the hyperviscosity syndrome. J Clin Invest 49: 610–621, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]