Summary
Background and objectives
Translocated endotoxin derived from intestinal bacteria has a wide range of adverse effects on cardiovascular (CV) structure and function, driving systemic inflammation, atherosclerosis and oxidative stress. This study's aim was to investigate endotoxemia across the spectrum of chronic kidney disease (CKD).
Design, setting, participants, & measurements
Circulating endotoxin was measured in 249 patients comprising CKD stage 3 to 5 and a comparator cohort of hypertensive patients without significant renal impairment. Patients underwent extended CV assessment, including pulse wave velocity and vascular calcification. Hemodialysis (HD) patients also received detailed echocardiographic-based intradialytic assessments. Patients were followed up for 1 year to assess survival.
Results
Circulating endotoxemia was most notable in those with the highest CV disease burden (increasing with CKD stage), and a sharp increase was observed after initiation of HD. In HD patients, predialysis endotoxin correlated with dialysis-induced hemodynamic stress (ultrafiltration volume, relative hypotension), myocardial stunning, serum cardiac troponin T, and high-sensitivity C-reactive protein. Endotoxemia was associated with risk of mortality.
Conclusions
CKD patients are characteristically exposed to significant endotoxemia. In particular, HD-induced systemic circulatory stress and recurrent regional ischemia may lead to increased endotoxin translocation from the gut. Resultant endotoxemia is associated with systemic inflammation, markers of malnutrition, cardiac injury, and reduced survival. This represents a crucial missing link in understanding the pathophysiology of the grossly elevated CV disease risk in CKD patients, highlighting the potential toxicity of conventional HD and providing a novel set of potential therapeutic strategies to reduce CV mortality in CKD patients.
Introduction
Systemic inflammation is well recognized to be associated with an increased CV disease risk in patients with and without chronic kidney disease (CKD) (1). However, the mechanisms linking the observed associations are still largely unelucidated. Bacterial endotoxin is a lipopolysaccharide (LPS) and the major glycolipid component of the outer membrane of gram-negative bacteria, which comprise 70% of the total bacteria in the healthy human gut.
Exposure to endotoxin, a profoundly proinflammatory stimulus, results in release of a wide variety of proinflammatory cytokines and binding via CD14 to systemic immune competent cells (2). It results in a broad range of negative cardiovascular (CV) effects including peripheral vasodilation and reduction in cardiac contractile performance (3).
Endotoxin (without sepsis) was initially proposed as a stimulus for immune activation in the proinflammatory state of congestive heart failure (4). Endotoxin is released by bacterial cell wall breakdown within and beyond the gut lumen, from effective host defense mechanisms and by autolysis. Endotoxin enters the circulation via bacterial translocation (passage of intact bacteria and macromolecules such as endotoxin across the intestinal barrier [5]), with bowel edema and hypoperfusion being the two main factors influencing bowel wall permeability in congestive heart failure (6).
Dialysis patients are characteristically volume overloaded. Hemodialysis (HD) in combination with ultrafiltration results in significant systemic hemodynamic perturbation and clinically significant reduction of regional perfusion in critical organs such as the heart (7,8). Such repeated ischemic injury to this vulnerable vascular bed results in acute cardiac injury, long-term myocardial damage, and increased mortality (9,10). It has been shown previously that patients on long-term maintenance HD have evidence of mucosal ischemia (11) and ultrafiltration causes a reduction in splanchnic blood volume (12) despite preserved blood pressure (BP) (13). Mesenteric ischemia results in disrupted gut mucosal structure and function, with increased gut permeability (14).
Endotoxin contamination of dialysis water has long been recognized as a cause of CV instability during dialysis (15). Elevated levels in earlier-stage CKD patients, potentially because of altered gut permeability, have been commented on in an earlier study (16), but otherwise this area has remained largely unexplored.
We hypothesize that previously unappreciated significant translocation of intestinal endotoxin may occur in patients with CKD and is aggravated by dialysis.
Materials and Methods
Cross-Sectional Description of Endotoxemia in CKD Patients
We studied patients with a wide range of CKD severity; patient characteristics in the six groups are summarized in Table 1. HD and peritoneal dialysis (PD) patients were recruited from the prevalent dialysis population, CKD patients in stages 3 to 5 from nephrology outpatients, and non-CKD patients from primary care. Patients were assessed for conventional bloods and extensive additional investigations at a single dedicated study visit to characterize CV structure and function as well as systemic inflammation. Adult patients receiving HD (n = 120) were followed up over a 1-year period to assess survival.
Table 1.
Baseline patient characteristics, renal-related factors, biochemical data, and vascular assessment in all patients and in groups depending on CKD stage and dialysis modality
| Parameter | All Patients (n = 249) | Dialysis |
CKD |
Controls (n = 14) | P | |||
|---|---|---|---|---|---|---|---|---|
| HD (n = 120) | PD (n = 25) | Stage 5 (n = 25) | Stage 4 (n = 49) | Stage 3 (n = 16) | ||||
| Age (years; mean ± SD) | 65 ± 14 | 62 ± 15 | 61 ± 14 | 60 ± 14 | 68 ± 12 | 75 ± 5 | 76 ± 5 | <0.001 |
| Male:female | 163:86 | 83:37 | 16:10 | 17:9 | 32:17 | 8:8 | 9:5 | NS |
| eGFR (ml/min; mean ± SD) | – | NA | NA | 12 ± 2 | 19 ± 4 | 47 ± 11 | 85 ± 10 | <0.001 |
| Dialysis adequacy (Kt/V;a mean ± SD) | – | 1.2 ± 0.3 | 2.5 ± 0.5 | NA | NA | NA | NA | – |
| Dialysis vintage (months; median [IQR]) | 30 (15 to 60) | 34 (15 to 60) | 27 (13 to 52) | NA | NA | NA | NA | NS |
| Diabetes mellitus (n [%]) | 69 (28%) | 39 (33%) | 8 (31%) | 7 (27%) | 15 (31%) | 0 (0%) | 0 (0%) | 0.02 |
| Previous CV comorbiditiesb (n [%]) | 80 (32%) | 39 (33%) | 11 (42%) | 8 (31%) | 17 (35%) | 1 (6%) | 3 (21%) | NS |
| Smoker (n [%]) | 48 (19%) | 12 (10%) | 4 (15%) | 7 (27%) | 21 (44%) | 3 (19%) | 1 (7%) | 0.001 |
| Ethnicity | NS | |||||||
| Caucasian | 234 (94%) | 110 (92%) | 25 (100%) | 21 (84%) | 48 (98%) | 16 (100%) | 14 (100%) | |
| Afro-Caribbean | 5 (2%) | 4 (3%) | 0 (0%) | 1 (4%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Asian | 10 (4%) | 6 (5%) | 0 (0%) | 3 (12%) | 1 (2%) | 0 (0%) | 0 (0%) | |
| Etiologies (n = 235) | NA | NS | ||||||
| diabetic nephropathy | 55 (23%) | 30 (25%) | 8 (32%) | 5 (20%) | 12 (25%) | 0 (0%) | ||
| glomerular disease | 43 (18%) | 25 (21%) | 4 (16%) | 5 (20%) | 8 (16%) | 1 (6%) | ||
| APKD | 14 (6%) | 8 (7%) | 1 (4%) | 2 (8%) | 3 (6%) | 0 (0%) | ||
| urological | 21 (9%) | 12 (10%) | 4 (16%) | 2 (8%) | 3 (6%) | 0 (0%) | ||
| renovascular | 18 (8%) | 8 (7%) | 2 (8%) | 1 (4%) | 5 (10%) | 2 (13%) | ||
| other | 30 (13%) | 15 (12%) | 4 (16%) | 3 (12%) | 6 (12%) | 2 (13%) | ||
| unknown | 54 (23%) | 22 (18%) | 2 (8%) | 7 (28%) | 12 (25%) | 11 (68%) | ||
| Albumin (g/L) | 35 ± 5 | 35 ± 4 | 27 ± 4 | 35 ± 1 | 36 ± 3 | 39 ± 3 | 40 ± 3 | <0.001c |
| Phosphate (mmol/L) | 1.52 ± 0.40 | 1.65 ± 0.46 | 1.57 ± 0.24 | 1.62 ± 0.29 | 1.33 ± 0.23 | 1.19 ± 0.17 | 1.14 ± 0.15 | <0.001d |
| Calcium (mmol/L) | 2.42 ± 0.14 | 2.44 ± 0.13 | 2.51 ± 0.11 | 2.36 ± 0.14 | 2.33 ± 0.10 | 2.41 ± 0.08 | 2.43 ± 0.16 | <0.001e |
| PWV (m/s; mean ± SD) | – | 10.8 ± 3.6f | 10.5 ± 3.2 | 9.2 ± 2.9 | 9.1 ± 3.1 | 8.7 ± 2.6 | ||
| SFA CaSc (median [IQR]) | – | 142 (245 to 622)g | 29 (68 to 264) | 12 (46 to 198)g | 0 (7 to 176) | 0 (9 to 154) | ||
eGFR, estimated GFR; APKD, adult polycystic kidney disease; SFA CaSc, superficial femoral artery calcification score. Results analyzed using one-way ANOVA and Kruskal–Wallis test where appropriate, with Tukey post-test for normally distributed data and χ2 test for nonparametric data.
Kt/V in HD is per single session and in PD is weekly.
Defined as any previous description of ischemic heart disease, heart failure, cerebrovascular disease, or peripheral vascular disease recorded in the patient's medical notes.
Albumin: comparison of HD with controls and CKD stage 3, P < 0.001; comparison of PD with all other groups, P < 0.001; comparison of CKD stage 5 with controls and CKD stage 3, P = 0.003.
Phosphate: comparison of HD with controls, CKD stages 3 and 4, P < 0.001, comparison of PD with controls, P = 0.007, with CKD stage 3, P = 0.016, comparison of CKD stage 5 with controls, P = 0.002, with CKD stage 4, P = 0.021, with CKD stage 3, P = 0.004.
Corrected calcium: comparison of HD with CKD stage 4, P < 0.001, with CKD stage 5, P < 0.023; comparison of PD with CKD stage 4 and 5, P < 0.001.
Sixty-eight of 120 HD patients underwent PWV measurement.
Sixty-eight of 120 HD patients and 56 of 74 CKD stages 4 and 5 patients had SFA calcification scoring performed.
A further cohort of 12 children established on maintenance HD was included to gain some insight into circulating endotoxin levels in patients who, although subjected to sustained uremia, did not have significant large-vessel atheromatous arterial disease, history of diabetes, or lifestyle issues such as smoking or significant alcohol use.
The study was approved by the local regional ethics committee and written consent was obtained from all patients or their guardians in the pediatric cohort.
Blood Samples
Blood samples were collected at regular clinic visits for PD and CKD patients in stages 4 to 5 and monthly for HD patients. A time-averaged value is given for baseline (averaged over the 6 months before the study). Nondialysis patients were staged based on the four-variable Modification of Diet in Renal Disease estimated GFR. A single sample was taken from each patient to allow cross-sectional comparison of endotoxin levels. Serum lipopolysaccharide quantification was performed using a Limulus Amebocyte assay (Cambrex, Verviers, Belgium) as described previously (17). All samples were run in duplicate and background subtracted.
Assessment of Vascular Calcification and Pulse Wave Velocity
Multislice computerized tomography was used to quantify vascular calcification (VC) in a standardized section of the superficial femoral artery as described previously (18). Carotid-femoral pulse wave velocity (PWV) was measured using a SphygmoCor (AtCor Medical Pty, Ltd., Australia). All PD, CKD stages 3 to 4 patients, and the non-CKD comparator group had VC and PWV assessed. Fifty-six percent of HD patients (68 of 120) were similarly assessed. All nondialyzed CKD stage 5 patients had PWV measured and 64% (16 of 25) had VC measured.
Effect of Dialysis on Endotoxemia
A group of 66 prevalent HD patients were recruited from a single hospital-based HD unit to undergo more detailed evaluation of factors associated with endotoxemia, including intradialytic BP and intradialytic myocardial stunning, with blood samples (including endotoxin levels) pre- and postdialysis. Twelve pediatric HD patients were also studied. Patients were excluded if they had pre-existing severe systolic dysfunction (New York Heart Association class III to IV) or inadequate echocardiographic windows (one patient excluded). All studies were conducted after the first 2-day interdialytic period. Adult patients were dialyzed using dual-pass water treatment with undetectable levels of endotoxin. Patients were categorized as intradialytic hypotension prone if experiencing a systolic BP (SBP) <100 mmHg or a SBP fall of >40 mmHg.
Echocardiographic Assessment
As described previously, two-dimensional echocardiography was performed before commencement (pre-HD), during HD at 2 and 4 hours, and 30 minutes into the recovery period (post-HD) to evaluate the presence and extent of HD-induced regional wall motion abnormalities (19,20). Measurement of segmental fractional shortening was made subsequently (Echo-CMS; MEDIS, The Netherlands) with new regional wall motion abnormalities classified as segments showing a decline in percent segmental fractional shortening >20% from baseline. Regions with evidence of functional recovery in the postdialysis period were classed as stunned segments. Left atrial volume (LAV) was calculated by biplane disc method and indexed to height2.7 (LAVI).
Statistical Analyses and Sample Size Calculation
The primary endpoint was to detect a 50% difference in circulating endotoxin levels between patients receiving dialysis and those not. A sample size of at least 40 patients in each of the comparison groups was needed to detect this difference at 90% power. Final sample size was larger to allow for further investigation of factors relating to endotoxemia and patient dropout.
Group data are presented as mean ± SD unless otherwise stated. All data were tested for normality. Analysis was performed using SPSS version 12.0.1 (SPSS, Inc., Chicago, IL). Categorical data were compared using χ2 test, continuous data using paired or unpaired t test, or one-way ANOVA with Tukey's correction as appropriate. Correlation between continuous variables was examined by Spearman's rank correlation coefficient. Factors independently associated with circulating endotoxin levels were further explored by a multiple linear regression model with backwards-stepwise analysis. A P value of <0.05 was considered significant. All probabilities were two-tailed.
We compared the association of the baseline measures of endotoxemia with outcomes by using unadjusted and adjusted Cox proportional hazards models. Covariates specified a priori in the adjusted models included age, race, sex, smoking, history of diabetes or CV disease, body mass index, LDL and HDL cholesterol, SBP, diastolic BP (DBP), HD-induced myocardial stunning, calcification score, and PWV.
Results
Cross-Sectional Description of Endotoxemia in CKD Patients
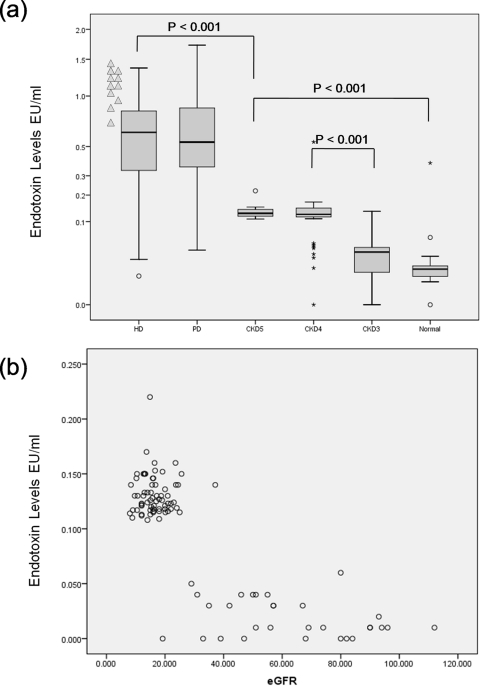
There were gradated increases in endotoxemia with increasing CKD stage (Figure 1a). Endotoxin levels were not statistically significantly different between CKD stage 3 and non-CKD controls, but they were significant between the other groups and controls. There was no evidence of a linear relationship between eGFR and endotoxin levels (Figure 1b). Patients receiving maintenance dialysis had grossly elevated levels (0.64 EU/ml in HD and 0.56 EU/ml in PD, P = 0.06). Pediatric HD patients were particularly endotoxemic (1.12 EU/ml, P = 0.008 compared with adult patients, Figure 1a). Serum endotoxin levels were nearly 6 times higher in CKD patients receiving dialysis compared with those that were not (0.62 ± 0.37 versus 0.11 ± 0.68 EU/ml, P < 0.001).
Figure 1.
(a)Distribution of circulating endotoxin levels across the spectrum of CKD patients at baseline. Pediatric HD patients are illustrated separately (▲). (b) Association of estimated GFR and circulating endotoxin levels in nondialysis-dependent CKD patients.
Multiple linear regression analyses were performed separately for dialysis and nondialyzed patients. In nondialyzed patients, the overall model yielded an r2 of 0.48, P < 0.001. Only CKD stage and serum albumin were identified as independent determinants (univariate analysis, r = − 0.58, P < 0.001 and r = −0.49, P < 0.001, respectively). In HD patients, the overall model yielded an r2 of 0.43, P < 0.001, with ultrafiltration volume displacing all other factors.
There was no association between endotoxemia and degree of VC, PWV, or any other factors relating to peripheral CV structure or function. There was no association between any significant group of drug use and circulating endotoxin levels. Despite a positive correlation with corrected calcium levels (r = 0.157, P < 0.001) and phosphate levels (r = 0.226, P < 0.001), there was no effect of phosphate binder use (76% of the population were prescribed phosphate binders, of which just over half were taking sevelamer).
Effect of HD on Endotoxemia
Eighteen patients (all with no previous HD use or vascular access other than native arteriovenous fistulas) commenced HD from the studied low-clearance population during the study period. Initial and second measurements of circulating endotoxin were taken no longer than 12 weeks before or after starting HD. All patients commenced HD electively with eGFRs of 10 to 15 ml/min. HD initiation was associated with a significant increase in circulating endotoxin level, rising from 0.13 ± 0.3 EU/ml to 0.34 ± 0.42 EU/ml (P = 0.002). Dialysis vintage in established HD patients did not correlate to endotoxin levels, suggesting that the increment postdialysis initiation is not related to loss of residual renal function.
Forty-one of 66 of established HD patients studied exhibited significant levels of dialysis-induced myocardial stunning. Twenty-one of 66 patients were defined as suffering significant intradialytic hypotension. Predialysis circulating endotoxin levels showed a statistically significant correlation with myocardial stunning severity (r = 0.44, P = 0.035) and maximum reduction in SBP and DBP during HD (r = 0.45, P = 0.032). Levels were significantly correlated with evidence of dialysis-related cardiac injury, with a significant correlation with predialysis cardiac troponin T (r = 0.34, P = 0.005) in the whole HD group.
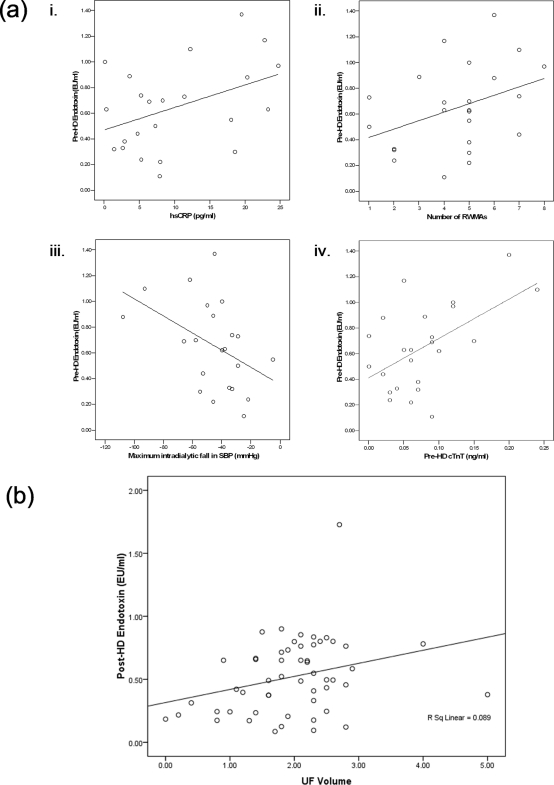
There was little evidence of volume overload being significantly associated with the degree of endotoxemia. In addition to the lack of significant correlation of endotoxemia with N-terminal pro-brain naturetic peptide (as a composite biochemical marker for volume overload), there was no positive correlation with LAVI (as an echocardiogram-based measure of volume overload). The degree of endotoxemia positively correlated with high-sensitivity C-reactive protein (hsCRP; r = 0.42, P = 0.047) but not with IL-6 levels (Figure 2a).
Figure 2.
(a) In HD patients with intradialytic hypotension, predialysis endotoxin levels were significantly correlated with (i) inflammation; (ii) the number of myocardial stunned segments; (iii) intradialytic hypotension; and (iv) predialysis cardiac troponin T, a marker of myocardial damage. (b) Postdialysis endotoxin levels were significantly correlated with ultrafiltration volume.
In patients dialyzing with polysulfone membranes, circulating mean endotoxin levels fell over the period of the HD treatment (0.68 ± 0.30 EU/ml versus 0.53 ± 0.30 EU/ml, P = 0.004). Post-HD circulating endotoxin levels were still significantly correlated with fluid removal (r = 0.24, P = 0.001) (Figure 2b). There was an increase in circulating endotoxin level during the HD session in 8 of the 12 pediatric patients who dialyzed using cellulose acetate-based membranes (0.72 ± 0.02 versus 0.86 ± 0.017, P = 0.054).
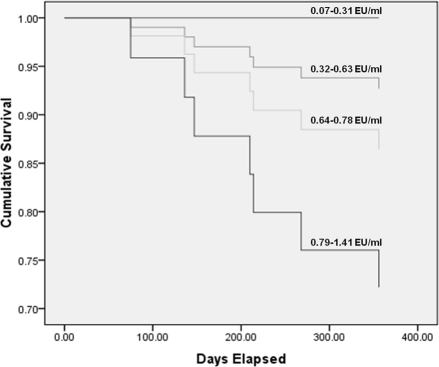
Survival was assessed in the HD patients because they had the largest range of circulating endotoxin levels and the highest number of deaths. Endotoxemia was strongly associated with an increased risk of death (P = 0.034) (Figure 3). This association disappeared when corrected for adverse dialysis-related CV factors (myocardial stunning, ultrafiltration volume, and intradialytic fall in BP).
Figure 3.
Unadjusted Cox proportional hazard of mortality over a 1-year period. Population is divided into quartiles of baseline circulating endotoxin level (P = 0.034).
Discussion
We have demonstrated that significant endotoxemia is common in patients with advanced CKD. Endotoxemia appears to be aggravated by initiation of dialysis and is higher in those HD patients with the greatest degree of dialysis-induced hemodynamic instability, who also exhibit high degrees of dialysis-induced myocardial stunning. Elevated levels of circulating endotoxin are significantly associated with reduced survival.
The more severe degree of renal impairment is associated with significantly greater levels of circulating endotoxin. This has been previously reported in PD patients (21) but has otherwise been largely unappreciated (22,23). A follow-up PD study that focused on this highly selected patient cohort with limited access to HD did not find a negative effect of endotoxemia on survival (24). The biologic fate of plasma-free endotoxin is well known and relies on humoral inactivation and uptake into liver and mononuclear phagocyte cells (25–28) rather than renal clearance. Endotoxins are complex, amphiphilic macromolecules of up to 1000 kD in molecular weight, making free glomerular filtration unlikely, supported by research showing endotoxin is not present in sterile urine (29).
The levels seen in patients receiving dialysis are extremely high, comparable with those reported in severe liver disease (30), gut irradiation (31), and severe decompensated heart failure (4). Significant heart failure (New York Heart Association class III to IV) was an exclusion criterion and, in the HD patients, endotoxin levels did not correlate with markers of volume overload. The greater CV disease burden characteristic of patients with more severe CKD may be a critical factor predisposing to demand ischemia in the gut. The relative contribution of the factors likely to influence endotoxin translocation (intestinal bacterial load, membrane permeability, gut edema, and ischemic intestinal injury) may vary across the spectrum of CKD.
In CKD patients, low serum albumin appears to clearly correlate with the degree of endotoxemia. This is in keeping with the only previous report (in PD patients) (21). There was a lack of association with hsCRP in the patient group as a whole, in contrast to the association in HD patients (with a generally higher degree of systemic inflammation). This may be attributable to the narrow range of hsCRP values or differences in immunoreactivity. Tachyphylactic response to endotoxin has been previously described. There was no observed association with VC or markers of arterial stiffness.
The complex biology resulting in systemic endotoxemia in dialysis patients requires further elucidation because dialysis modality may contribute to endotoxin translocation by different underlying mechanisms. We postulate venous congestion and edema are dominant in PD, in contrast to recurrent regional ischemia in HD. However, further assessment of these putative mechanisms is required.
HD itself appears to be responsible for increasing exposure to translocated intestinal endotoxin, as evidenced by a large difference between patients with very severe CKD stage 5 but not yet started on dialysis and those receiving dialysis. Predialysis CKD stage 5 patients are very similar for demographic factors and comorbidities when compared to patients established on HD. After commencing HD, patients swiftly demonstrated a marked increase in endotoxemia, potentially resulting from dialysis-induced splanchnic hypoperfusion.
In the HD patients who underwent echocardiography, left ventricular ejection fraction was relatively well preserved with no relationship between degree of endotoxemia and markers of volume overload (N-terminal pro-brain naturetic peptide, LAVI). Children (with low prevalence of CV comorbidities and little evidence of increased left ventricular mass) exhibited the highest levels.
In contrast, there was direct evidence of the severity of the hemodynamic insult being related to the severity of the endotoxemic state. In patients who were unstable during HD, there was a direct correlation between magnitude of the fall in SBP and DBP with level of circulating endotoxin. HD is well described as being capable of inducing recurrent cardiac ischemic injury, associated with reduced segmental myocardial perfusion (8). Circulating endotoxin levels were highly correlated with cardiac troponin T as a biochemical marker of cardiac injury. The enteric circulation is exposed to a very similar set of predisposing factors to demand ischemia, including large-vessel atheroma, microcirculatory disturbances, increasing shear stress with ultrafiltration, and a significant demand on overall cardiac output (32). There was a significant correlation between severity of HD-induced cardiac stunning and endotoxin level. It is therefore possible that the observed changes in cardiac function might be a result of, or aggravated by, the direct myocardial effects of endotoxin (3).
The time course and interaction of CV, hemodynamic, and enteric responses when patients are repeatedly exposed to HD-induced systemic circulatory stress require further study to elucidate events in the potentially self-propagating cycle of regional ischemia, increased gut permeability, inflammation, and cardiac dysfunction.
We did not observe an increase in circulating levels of endotoxin during HD therapies, although post-HD levels of endotoxin still significantly correlated with ultrafiltration volume. Translocation may occur predominantly in the postdialytic period, which we did not have access to samples from. Other possibilities are that there was sequestration of endotoxin during the HD treatment as an effect of monocyte activation, which is commonly seen during extracorporeal circulation, or by direct adsorption onto the dialysis membrane. The polysulfone material used in most of these treatments is well described as having a potent ability to adsorb endotoxin and a wide variety of other circulating substances (33), deriving its high biocompatibility status from the ability to buffer complement and other factors within the reactive cascade. Cellulose acetate does not share the same potent binding characteristics as polysulfone (34) and was associated with an increase in circulating endotoxin over a dialysis session. Heparin (used to maintain the extracorporeal circuit) is also associated with a dose-dependent inhibition of the endotoxin assay.
In conclusion, this is the first study to systematically examine endotoxemia across the spectrum of CKD. The key findings of endotoxemia potentially driving interlinked malnutrition, inflammation, and CV disease in CKD patients represent an important advance in understanding the pathophysiology of grossly elevated CV mortality rates in this population. We have identified the role HD plays inducing systemic circulatory stress, predisposing to reduction of intestinal perfusion and exposure to sustained significant endotoxemia (with resultant increase in risk of mortality). Not only do these insights highlight further the potential toxicity of conventional dialysis, but they also provide a justification for a frame shift in the search for potential therapeutic targets to reduce CV attrition. These might include a focus on dialysis-related interventions to reduce circulatory stress, reduction of gut venous congestion/edema, and reduction/sequestration of the intestinal reservoir of bacterially derived endotoxin.
Disclosures
None.
Acknowledgments
We gratefully acknowledge the participation of patients and staff at Royal Derby Hospital in this study. Author contributions are as follows:
C.W.M.—study conception and design; data acquisition, analysis, and interpretation; manuscript drafting and revision; and final responsibility for the analyses and manuscript content.
L.E.A.H., M.T.E., and H.J.J.—literature search, data analyses and interpretation, and figure and manuscript preparation.
S.G.J., M.K.S., J.O.B., D.H., S.K., P.J.O.—study design and execution, sample preparation, data acquisition, analyses, and manuscript preparation.
C.C.S., K.B.L., P.K.T.L.—endotoxin assay, data collection, and manuscript preparation.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Wanner C, Drechsler C, Krane V: C-reactive protein and uremia. Semin Dial 22: 438–441, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Yang RB, Mark MR, Gray A, Huang A, Xie MH, Zhang M, Goddard A, Wood WI, Gurney AL, Godowski PJ: Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature 395: 284–288, 1998 [DOI] [PubMed] [Google Scholar]
- 3. Kumar A, Haery C, Parillo JE: Myocardial dysfunction in septic shock. Crit Care Clin 16: 251–287, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Anker SD, Egerer KR, Volk HD, Kox WJ, Poole-Wilson PA, Coats AJ: Elevated soluble CD14 receptors and altered cytokines in chronic heart failure. Am J Cardiol 79: 1426–1430, 1997 [DOI] [PubMed] [Google Scholar]
- 5. Kotanko P, Carter M, Levin NW: Intestinal bacterial microflora—A potential source of chronic inflammation in patients with chronic kidney disease. Nephrol Dial Transplant 21: 2057–2060, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Krack A, Sharma R, Figulla HR, Anker SD: The importance of the gastrointestinal system in the pathogenesis of heart failure. Eur Heart J 26: 2368–2374, 2005 [DOI] [PubMed] [Google Scholar]
- 7. McIntyre CW. Effects of hemodialysis on cardiac function: Kidney Int 76: 371–375, 2009 [DOI] [PubMed] [Google Scholar]
- 8. McIntyre CW, Burton JO, Selby S, Leccisotti L, Korsheed S, Baker CS, Camici P: Haemodialysis-induced cardiac dysfunction is associated with an acute reduction in global and segmental myocardial blood flow. Clin J Am Soc Nephrol 3: 19–26, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burton JO, Jefferies HJ, Selby NM, McIntyre CW: Hemodialysis-induced cardiac injury: Determinants and associated outcomes. Clin J Am Soc Nephrol 4: 914–920, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Burton JO, Jefferies HJ, Selby NM, McIntyre CW: Hemodialysis-induced repetitive myocardial injury results in global and segmental reduction in systolic cardiac function. Clin J Am Soc Nephrol 4: 1925–1931, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Diebel L, Kozol R, Wilson RF, Mahajan S, Abu-Hamdan D, Thomas D: Gastric intramucosal acidosis in patients with chronic kidney failure. Surgery 113: 520–526, 1993 [PubMed] [Google Scholar]
- 12. Yu AW, Nawab ZM, Barnes WE, Lai KN, Ing TS, Daugirdas JT: Splanchnic erythrocyte content decreases during hemodialysis: A new compensatory mechanism for hypovolemia. Kidney Int 51: 1986–1990, 1997 [DOI] [PubMed] [Google Scholar]
- 13. Jakob SM, Ruokonen E, Vuolteenaho O, Lampainen E, Takala J: Splanchnic perfusion during hemodialysis: Evidence for marginal tissue perfusion. Crit Care Med 29: 1393–1398, 2001 [DOI] [PubMed] [Google Scholar]
- 14. Khanna A, Rossman JE, Fung HL, Caty MG: Intestinal and hemodynamic impairment following mesenteric ischemia/reperfusion. J Surg Res 99: 114–119, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Raij L, Shapiro FL, Michael AF: Endotoxemia in febrile reactions during hemodialysis. Kidney Int 4: 57–60, 1973 [DOI] [PubMed] [Google Scholar]
- 16. Gonçalves S, Pecoits-Filho R, Perreto S, Barberato SH, Stinghen AE, Lima EG, Fuerbringer R, Sauthier SM, Riella MC: Associations between renal function, volume status and endotoxaemia in chronic kidney disease patients. Nephrol Dial Transplant 21: 2788–2794, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar BR, Rodriguez B, Teixeira-Johnson L, Landay A, Martin JN, Hecht FM, Picker LJ, Lederman MM, Deeks SG, Douek DC: Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med 12: 1365–1371, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Sigrist M, Bungay P, Taal MW, McIntyre CW: Vascular calcification and cardiovascular function in chronic kidney disease. Nephrol Dial Transplant 21: 707–714, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Selby NM, Lambie SH, Camici PG, Baker CS, McIntyre CW: Occurrence of regional left ventricular dysfunction in patients undergoing standard and biofeedback dialysis. Am J Kidney Dis 47: 830–841, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Selby NM, Burton JO, Chesterton LJ, McIntyre CW: Dialysis induced regional left ventricular dysfunction is ameliorated by cooling the dialysate. Clin J Am Soc Nephrol 1: 1216–1225, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Szeto CC, Kwan BC, Chow KM, Lai KB, Chung KY, Leung CB, Li PK: Endotoxemia is related to systemic inflammation and atherosclerosis in peritoneal dialysis patients. Clin J Am Soc Nephrol 3: 431–436, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nisbeth U, Hallgren R, Eriksson O, Danielson BG: Endotoxemia in chronic renal failure. Nephron 45: 93–97, 1987 [DOI] [PubMed] [Google Scholar]
- 23. Markum HM, Suhardjono, Pohan HT, Suhendro, Lydia A, Inada K: Endotoxin in patients with terminal renal failure undergoing dialysis with re-processing dialyser. Acta Med Indones 36: 93–96, 2004 [PubMed] [Google Scholar]
- 24. Szeto CC, Kwan BC, Chow KM, Lai KB, Pang WF, Chung KY, Leung CB, Li PK: Endotoxemia is associated with better clinical outcome in incident Chinese peritoneal dialysis patients: A prospective cohort study. Perit Dial Int 30: 178–186, 2010 [DOI] [PubMed] [Google Scholar]
- 25. Caridis DT, Reinhold RB, Woodruff PWH, Fine J: Endotoxaemia in man. Lancet 299: 1381–1386, 1972 [DOI] [PubMed] [Google Scholar]
- 26. Casey WF, Hauser GJ, Hannallah RS, Midgley FM, Khan WN: Circulating endotoxin and tumour necrosis factor during pediatric cardiac surgery. Crit Care Med 20: 1090–1096, 1992 [DOI] [PubMed] [Google Scholar]
- 27. Freudenberg MA, Galanos C: Metabolism of LPS in vivo. In: Bacterial Endotoxic Lipopolysaccharides, edited by Morrison DC, Ryan JL, Boca Raton, FL, CRC Press, 1992, pp 275–294 [Google Scholar]
- 28. Skarnes RC: In vivo distribution and detoxification of endotoxins. In: Handbook of Endotoxins, Vol. 3, edited by Berry LJ, Amsterdam, Elsevier, Amsterdam, 1985, pp 56–81 [Google Scholar]
- 29. Matsumoto T, Tanaka M, Ogata N, Mizunoe Y, Takahashi K, Kumazawa J: Significance of urinary endotoxin concentration in patients with urinary tract infection. Urol Res 19: 293–295, 1991 [DOI] [PubMed] [Google Scholar]
- 30. Lumsden AB, Henderson JM, Kutner MH: Endotoxin levels measured by a chromogenic assay in portal, hepatic and peripheral venous blood in patients with cirrhosis. Hepatology 8: 232–236, 1988 [DOI] [PubMed] [Google Scholar]
- 31. Maxwell A, Gaffin SL, Wells MT: Radiotherapy, endotoxemia and nausea. Lancet 327: 1148–1149, 1986 [DOI] [PubMed] [Google Scholar]
- 32. Grum CM: Tissue oxygenation in low flow states and during hypoxaemia. Crit Care Med 21: S44–S49, 1993 [DOI] [PubMed] [Google Scholar]
- 33. Takemoto Y, Nakatani T, Sugimura K, Yoshimura R, Tsuchida K: Endotoxin adsorption of various dialysis membranes: In vitro study. Artif Organs 27: 1134–1137, 2003 [DOI] [PubMed] [Google Scholar]
- 34. Ureña P, Herbelin A, Basile C, Zingraff J, Man NK, Drueke T: In vitro studies of endotoxin transfer across cellulosic and noncellulosic dialysis membranes. I. Radiolabeled endotoxin. Contrib Nephrol 74: 71–78, 1989 [DOI] [PubMed] [Google Scholar]