Summary
Background and objectives
Poor linear growth is a well described complication of chronic kidney disease (CKD). This study evaluated whether abnormal birth history defined by low birth weight (LBW; <2500 g), prematurity (gestational age <36 weeks), small for gestational age (SGA; birth weight <10th percentile for gestational age), or intensive care unit (ICU) at birth were risk factors for poor growth outcomes in children with CKD.
Design, setting, participants, & measurements
Growth outcomes were quantified by age-sex-specific height and weight z-scores during 1393 visits from 426 participants of the Chronic Kidney Disease in Children Study, an observational cohort of children with CKD. Median baseline GFR was 42.9 ml/min per 1.73 m2, 21% had a glomerular diagnosis, and 52% had CKD for ≥90% of their lifetime.
Results
A high prevalence of LBW (17%), SGA (14%), prematurity (12%), and ICU after delivery (40%) was observed. Multivariate analyses demonstrated a negative effect of LBW (−0.43 ± 0.14; P < 0.01 for height and −0.37 ± 0.16; P = 0.02 for weight) and of SGA (−0.29 ± 0.16; P = 0.07 for height and −0.41 ± 0.19; P = 0.03 for weight) on current height and weight. In children with glomerular versus nonglomerular diagnoses, the effect of SGA (−1.08 versus −0.18; P = 0.029) on attained weight was more pronounced in children with a glomerular diagnosis.
Conclusions
LBW and SGA are novel risk factors for short stature and lower weight percentiles in children with mild to moderate CKD independent of kidney function.
Introduction
Linear growth and weight gain are impaired in children with chronic kidney disease (CKD) through various mechanisms (1). Causes of poor growth in children with CKD include renal osteodystrophy, metabolic acidosis, and inadequate nutrition (1). CKD also disrupts the growth hormone-IGF-I axis, a cause of growth failure in CKD that can be effectively addressed via administration of recombinant human growth hormone (2–4).
Among the general population of children, birth history is known to influence linear growth and adult height. Most otherwise healthy children who are small for gestational age (SGA) have rapid catch-up growth, with most occurring during the first 6 months of life (5). Nevertheless, approximately 10% of such children are >2 SD below the mean for height throughout childhood and as adults (6–8). In contrast, appropriate-for-gestational-age premature infants usually demonstrate catch-up growth during the first 2 years of life and attain a normal adult height.
The effect of an abnormal birth history on linear growth and weight in children with CKD has rarely been studied. The goal of this study was to determine if a child's birth history, specifically low birth weight (LBW), prematurity, SGA, or intensive care unit (ICU) stay in the neonatal period influence stature and weight in children with CKD. To accomplish this, we utilized data acquired during multiple visits from study participants of the Chronic Kidney Disease in Children (CKiD) Study, an observational study of a large cohort of children and adolescents with mild to moderate CKD.
Materials and Methods
Study Design and Population
From April 2005 through September 2009, the CKiD study enrolled 586 children with mild to moderate CKD into a multicenter, prospective cohort study at 48 pediatric nephrology centers across North America. Details of the CKiD study design have been previously published (9). Briefly, eligible children were aged 1 to 16 years and had a Schwartz-estimated GFR between 30 and 90 ml/min per 1.73 m2 (10,11). At the baseline visit, we used the plasma disappearance of iohexol to generate a four-point GFR (12). Exclusion criteria included renal, other solid-organ, bone marrow, or stem cell transplantation; dialysis treatment within the past 3 months; HIV diagnosis; cancer/leukemia diagnosis/treatment within past 12 months; current pregnancy or pregnancy within the past 12 months; history of structural heart disease; genetic syndromes involving the central nervous system; and history of severe to profound mental retardation.
The study design and conduct for CKiD was approved by an external advisory committee appointed by the National Institutes of Health and by the internal review boards for each participating center.
Primary Outcomes
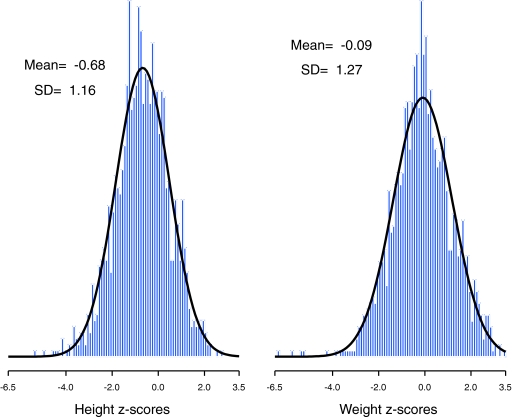
At each CKiD visit, height and weight were determined as the mean of two independent measurements at 99.6% of visits (the remainder used a single measurement). Age-sex-specific height and weight z-scores were calculated (13) using 2000 Centers for Disease Control standard growth charts for U.S. children (14). Each z-score represents how many SD the participant is above or below the expected mean value for their age and sex. Figure 1 shows the approximate normality of the distribution of age-sex-specific height (left panel) and weight z-scores (right panel).
Figure 1.
(Left) Histogram of 1393 age-sex-specific height z-scores with mean −0.68 and SD 1.16 with the density function from a Gaussian distribution with mean −0.68 and SD 1.16 superimposed. (Right) Histogram of 1383 age-sex-specific weight z-scores with mean −0.09 and SD 1.27 with the density function from a Gaussian distribution with mean −0.09 and SD 1.27 superimposed.
Primary Exposures
At the baseline visit, a parent questionnaire was used to collect data on history of birth abnormalities, which included LBW (birth weight <2500 g), prematurity (gestational age <36 weeks), SGA (birth weight <10th percentile for gestational age), (15) and neonatal ICU immediately after delivery. At the baseline visit, a question to determine whether the child was premature (defined as <36 weeks gestation) was administered and if premature, by how many weeks before the due date. To determine the exact number of weeks for gestational age, we refined the questionnaire and at the first follow-up visit ascertained how many weeks the child was born before the due date.
For the participants on whom we only had the information at the baseline visit, we assigned a gestational age of 39 weeks to those who answered that their gestational age was ≥36 weeks.
Covariates
The diagnoses of CKD were categorized as glomerular or nonglomerular (see footnotes of Table 1 for the specific CKD diagnoses). The percentage of a participants' life with CKD was determined as the time between CKD diagnosis and a given visit divided by age. Mid-parental height was determined as the average height of the child's biologic mother and father (measured at a study visit). Current growth hormone use was ascertained at the baseline visit and all annual visits, but not at the second component of the baseline visit, which occurred 6 months after the initial visit. Hence, this variable was not included in the full analysis because its inclusion would have reduced the number of study visits for analysis by 26%. Moreover, when we repeated the final regression analyses with and without growth hormone use as a covariate among only the person-visits at which growth hormone use was assessed, there was no difference in the result.
Table 1.
Characteristics at baseline and at birth of the study population of 426 CKiD study participants overall and stratified by CKD diagnosis
| Characteristics | Overall (n = 426) | Glomerulara (n = 89) | Nonglomerularb (n = 337) | P Valuec Comparing Glomerular and Nonglomerular |
|---|---|---|---|---|
| Primary outcomes | ||||
| age-sex-specific height z-score | −0.73 (−1.45 to 0.11) | −0.51 (−1.18 to 0.34) | −0.83 (−1.48 to 0.02) | 0.006 |
| age-sex-specific weight z-score | −0.10 (−0.90 to 0.73) | 0.42 (−0.32 to 1.28) | −0.27 (−1.04 to 0.53) | <0.001 |
| Primary exposures | ||||
| LBW, birth weight <2500 g | 17% | 13% | 18% | 0.365 |
| premature, GA < 36 wk | 12% | 9% | 13% | 0.297 |
| SGA, birth weight <10th percentile for GA | 14% | 17% | 13% | 0.356 |
| ICU immediately after delivery | 40% | 9% | 48% | <0.001 |
| Covariates | ||||
| male | 62% | 51% | 65% | 0.015 |
| white | 73% | 61% | 76% | 0.004 |
| Percent of life with CKD | <0.001 | |||
| <50% | 28% | 65% | 18% | |
| 50% to <90% | 20% | 28% | 18% | |
| ≥90% | 52% | 7% | 64% | |
| age, years | 10.7 (7.1 to 14.2) | 14.2 (10.8 to 15.8) | 10.0 (6.4 to 13.4) | <0.001 |
| mid-parental height, m | 1.71 (1.68 to 1.75) | 1.70 (1.66 to 1.75) | 1.71 (1.68 to 1.75) | 0.160 |
| growth hormoned | 14% | 6% | 16% | 0.015 |
All values are median (interquartile range) or percentage. GA, gestational age.
Glomerular diagnoses included 25 (28%) with focal segmental glomerulosclerosis, 21 (24%) with hemolytic uremic syndrome, 8 (9%) with familial nephritis, 7 (8%) with IgA nephropathy, 6 (7%) with systemic immunological disease, 3 (3%) with chronic GN (unclassified), 3 (3%) with membranoproliferative GN type I, 3 (3%) with idiopathic crescentic GN, 3 (3%) with Henoch Schönlein purpura, 2 (2%) with congenital nephrotic syndrome, 2 (2%) with membranoproliferative GN type II, 1 (1%) with membranous nephropathy, and 5 (6%) with other glomerular diagnoses.
Nonglomerular diagnoses included 90 (27%) with obstructive uropathy, 71 (21%) with reflux nephropathy, 69 (20%) with aplastic/hypoplastic/dysplastic kidneys, 14 (4%) with autosomal recessive polycystic kidney disease, 14 (4%) with renal infarct, 10 (3%) with syndrome of agenesis of abdominal musculature, 8 (2%) with pyelonephritis/interstitial nephritis, 8 (2%) with cystinosis, 5 (1%) with medullary cystic disease, 4 (1%) with oxalosis, 2 (1%) with Wilms' tumor, 1 (<1%) with autosomal dominant polycystic kidney disease, and 41 (12%) with other nonglomerular diagnosis.
P value from Wilcoxon rank sum test when medians are compared, and P value from Pearson χ2 when percentages are compared.
Missing in 12 children (1 with glomerular diagnosis and 11 with nonglomerular diagnosis).
Statistical Methods
The unit of analysis was a person-visit with values of growth outcomes (age-sex-specific height and weight z-scores), age, and percentage of life with CKD updated at each visit. The two z-scores were compared between exposed and unexposed groups over time using separate linear regression models for each of four primary exposures (LBW, premature, SGA, and ICU at birth) with generalized estimating equations to account for the statistical dependence incurred by repeated measures of each outcome on the same individual (16). In addition to the primary exposure, each adjusted regression model included sex (male versus female [reference]), race (non-white versus white [reference]), age (centered at a value of 11.0 years), percentage of life with CKD (<50%, 50 to <90%, ≥90% [reference]), and mid-parental height (centered at a value of 1.71 m). The linear regression model was of the form z-score = a + bX + cZ + ε, where X is an indicator (0 = no, 1 = yes) of one of the four primary exposures (LBW, prematurity, SGA, ICU), Z is a constellation of covariates (i.e., sex, race, age, percentage of life with CKD, and mid-parental height), and ε follows a normal distribution with mean 0 and variance σ2. Therefore, a represents the expected z-score for individuals who do not have the specific birth history in question, are female, white, have an attained age of 11.0 years, have had CKD ≥90% of their lifetime, and have parents with an average mid-parental height of 1.71 m. The primary aim was to test whether b was different than zero, because this coefficient represents the effect of the primary exposure in individuals of comparable sex, race, age, CKD duration, and mid-parental height.
We conducted the same multivariate analyses separately for children with glomerular and nonglomerular diagnosis. The primary aims were to (1) test whether bG or bNG were different than zero, because these coefficients represent the effect of the primary exposure in individuals of comparable sex, race, age, CKD duration, and mid-parental height among those with glomerular and nonglomerular diagnoses, respectively; and (2) test whether bG ≠ bNG to determine whether the effect of the primary exposures was different in those with glomerular and nonglomerular diagnoses, which was accomplished by standard methods for comparing two independent normally distributed variates.
Results
Of the 586 participants enrolled as of September 2009, we were able to analyze the 426 (73%) who had data on each of the four primary exposures, type of CKD diagnosis, both of the study outcomes, and all covariates of interest.
Table 1 provides descriptive statistics at baseline for the entire study population and by CKD diagnosis. The lower median values of the height and weight z-scores in children with a nonglomerular diagnosis were due in large part to the longer duration with CKD. There is evidence of selection by indication whereby children with a nonglomerular diagnosis, who are on average shorter than children with a glomerular diagnosis, are also more likely to use growth hormone. The overall prevalence of LBW, prematurity, and SGA did not differ by diagnosis. Those with a glomerular diagnosis were significantly less likely than those with a nonglomerular diagnosis to be in the ICU at birth.
Table 2 presents descriptive statistics of the outcomes and covariates at all person-visits (i.e., the unit of analysis) stratified by CKD diagnosis and indicators of abnormal birth history. Among participants with a glomerular diagnosis, LBW and SGA were associated with lower height and weight. Among participants with a nonglomerular diagnosis, LBW, prematurity, and SGA were associated with lower height whereas ICU was associated with lower weight.
Table 2a.
Descriptive statistics of primary outcomes and covariates at all person-visits of subjects with a glomerular CKD diagnosis stratified by the presence of indicators of abnormal birth history
| Glomerular CKD Diagnosisb (n = 286 person-visits) |
||||||||
|---|---|---|---|---|---|---|---|---|
| LBW |
Premature |
SGA |
ICU |
|||||
| No (n = 245) | Yes (n = 41) | No (n = 260) | Yes (n = 26) | No (n = 235) | Yes (n = 51) | No (n = 257) | Yes (n = 29) | |
| Mean height z-scorea | −0.25 | −1.02 | −0.39 | −0.05 | −0.21 | −1.05 | −0.36 | −0.36 |
| Mean weight z-scorea | 0.57 | −0.34 | 0.43 | 0.44 | 0.65 | −0.55 | 0.41 | 0.64 |
| Male | 49% | 41% | 50% | 27% | 50% | 35% | 47% | 48% |
| White | 62% | 76% | 64% | 62% | 64% | 63% | 65% | 59% |
| Percent of attained life with CKD | ||||||||
| <50% | 62% | 63% | 63% | 50% | 61% | 67% | 63% | 55% |
| 50% to <90% | 31% | 29% | 30% | 38% | 32% | 27% | 32% | 21% |
| ≥90% | 7% | 7% | 6% | 12% | 7% | 6% | 5% | 24% |
| Mean attained age, years | 13.9 | 13.9 | 13.9 | 14.0 | 13.7 | 14.7 | 13.9 | 13.6 |
| Mean mid-parental height, m | 1.71 | 1.68 | 1.70 | 1.71 | 1.71 | 1.67 | 1.70 | 1.71 |
| Growth hormonec | 4% | 17% | 5% | 11% | 4% | 14% | 5% | 14% |
z-scores were age- and sex-specific; bolded mean z-scores indicate significant differences (P < 0.05) between those with birth abnormality compared to those without the indicated birth abnormality using generalized estimating equations to account for the statistical dependence incurred by repeated measures of each outcome on the same individual.
A total of 89 children with a glomerular CKD diagnosis contributed a total of 286 person-visits (9 [10%] contributed one visit, 11 [12%] contributed two visits, 31 [35%] contributed three visits, 29 [33%] contributed four visits, 8 [9%] contributed five visits, and 1 [1%] contributed six visits).
Growth hormone use was missing at 77 person-visits.
Table 2b.
Descriptive statistics of primary outcomes and covariates at all person-visits of subjects with a nonglomerular CKD diagnosis stratified by the presence of indicators of abnormal birth history
| Nonglomerular CKD Diagnosisa (n = 1107 person-visits) |
||||||||
|---|---|---|---|---|---|---|---|---|
| LBW |
Premature |
SGA |
ICU |
|||||
| No (n = 910) | Yes (n = 197) | No (n = 964) | Yes (n = 143) | No (n = 953) | Yes (n = 154) | No (n = 576) | Yes (n = 531) | |
| Mean height z-scoreb | −0.64 | −1.29 | −0.69 | −1.22 | −0.68 | −1.25 | −0.69 | −0.84 |
| Mean weight z-scoreb | −0.17 | −0.50 | −0.18 | −0.58 | −0.20 | −0.39 | −0.10 | −0.37 |
| Male | 67% | 52% | 65% | 57% | 68% | 39% | 64% | 64% |
| White | 79% | 69% | 77% | 80% | 79% | 68% | 81% | 73% |
| Percent of attained life with CKD | ||||||||
| <50% | 17% | 17% | 18% | 12% | 17% | 18% | 25% | 8% |
| 50% to <90% | 17% | 19% | 17% | 21% | 17% | 19% | 24% | 10% |
| ≥90% | 66% | 64% | 65% | 67% | 66% | 62% | 51% | 81% |
| Mean attained age, years | 10.9 | 10.0 | 10.9 | 9.4 | 10.6 | 11.2 | 11.3 | 10.1 |
| Mean mid-parental height, m | 1.71 | 1.70 | 1.71 | 1.72 | 1.72 | 1.69 | 1.71 | 1.71 |
| Growth hormonec | 13% | 18% | 13% | 21% | 15% | 10% | 14% | 15% |
A total of 337 children with a nonglomerular diagnosis contributed a total of 1107 person-visits (25 [7%] contributed one visit, 50 [15%] contributed two visits, 106 [31%] contributed three visits, 117 [35%] contributed four visits, 38 [11%] contributed five visits, and 1 [<1%] contributed six visits).
z-scores were age and sex-specific; bolded mean z-scores indicate significant differences (P < 0.05) between those with birth abnormality compared to those without the indicated birth abnormality using generalized estimating equations to account for the statistical dependence incurred by repeated measures of each outcome on the same individual.
Growth hormone use was missing at 289 person-visits.
Table 3 presents the effect of each abnormal birth history exposure on age-sex-specific height and weight z-scores adjusted for sex, race, age, percentage of life with CKD, and mid-parental height. Even among children with a normal birth history, height and weight z-scores were significantly less than zero. There was an additional negative effect on height z-score in children with a history of LBW (−0.43 ± 0.14; P < 0.01) or of SGA (−0.29 ± 0.16; P = 0.07); there was no additional deleterious effect of prematurity or spending time in the ICU after delivery. The analysis of weight also demonstrated a significant additional negative effect of LBW (−0.37 ± 0.16; P = 0.02) and SGA (−0.41 ± 0.19; P = 0.03). Although some of the comparisons of growth outcomes between those with and without each of the birth abnormalities failed to reach conventional statistical significance, it is noteworthy that all of the regression coefficients were negative, indicating the overall negative effect abnormal birth history had on attained growth.
Table 3.
Multivariate repeated measures analyses of abnormal birth history exposures on age-sex-specific height and weight z-scores (n = 1393 person visits contributed by 426 participants
| Height z-Score | Weight z-Score | |
|---|---|---|
| LBW | ||
| no (reference) | −0.96 ± 0.11a | −0.40 ± 0.12a |
| yes versus no | −0.43 ± 0.14a | −0.37 ± 0.16b |
| Premature | ||
| no (reference) | −0.99 ± 0.11a | −0.44 ± 0.12a |
| yes versus no | −0.31 ± 0.18 | −0.23 ± 0.19 |
| SGA | ||
| no (reference) | −0.99 ± 0.11a | −0.39 ± 0.11a |
| yes versus no | −0.29 ± 0.16 | −0.41 ± 0.19b |
| ICU | ||
| no (reference) | −0.93 ± 0.13a | −0.34 ± 0.14a |
| yes versus no | −0.21 ± 0.12 | −0.25 ± 0.13 |
All analyses were adjusted for gender (males versus female [reference]), race (non-white versus white [reference]), age (centered at 11.0 years), percentage of life with CKD (<50%, 50% to <90%, ≥90% [reference]), and mid-parental height (centered at 1.7 m). Data are regression coefficient ± SEM, where the regression coefficients for rows “no (reference)” and “yes versus no” correspond to a and b in model shown in Materials and Methods.
P < 0.01 for regression coefficient being different from zero.
0.01 ≤ P < 0.05 for regression coefficient being different from zero.
Among the covariates, being male, of older age, and having taller parents were significantly associated with increased height; race and duration of CKD were not significantly associated with height (results not shown). Each additional year of age and being non-white were associated with increased weight. Compared with those with CKD for ≥90% of their lifetime, those with CKD for <50% or between 50% and <90% of their lifetime had higher weight z-scores.
To describe the joint effect of LBW, prematurity, and SGA on height, we used a regression tree analytical approach and found four distinguishable subgroups of children with respect to height z-scores according to the joint presence of LBW, prematurity, and SGA. Specifically, children with none or just one of the abnormalities had an average height z-score of −0.93, children born <2500 g and who were premature had an average z-score of −1.35, children born <2500 and who were also SGA had an average z-score of −1.44, and children who had all three abnormalities had an average height z-score of −1.73. The differences in the last three groups with respect to the first had significance levels of 0.026, 0.014, and 0.146, respectively.
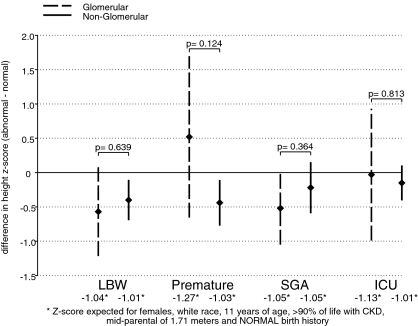
Figures 2 and 3 graphically depict the effect of a given abnormal birth exposure on age-sex-specific height (Figure 2) and weight (Figure 3) z-scores after adjustment and stratified by type of CKD diagnosis. Among those with a nonglomerular diagnosis, those born <2500 g, premature, or SGA had height z-scores that were on average 0.40 (95% confidence interval [CI]: −0.69 to −0.11), 0.44 (95% CI: −0.76 to −0.11), or 0.22 (95% CI: −0.59 to 0.16) SD lower than those born ≥2500 g, not premature, or not SGA, respectively. Those with a glomerular CKD diagnosis and also born <2500 g, SGA, or were in the ICU immediately after birth had nonsignificantly lower height z-scores than those not born with the specific abnormality. There was no significant differential birth abnormality effect of CKD diagnosis on height z-scores.
Figure 2.
Multivariate CKD diagnosis-specific repeated measures analyses of abnormal birth history exposures on age-sex-specific height z-scores. Point estimates of the mean differences (bG) and 95% CIs in age-sex-specific height z-scores comparing those with the indicated abnormal birth exposure to those without the birth abnormality among those with a glomerular diagnosis of CKD are indicated by the diamond-shaped points and dashed vertical lines, respectively. Point estimates of the mean differences (bNG) and 95% CIs in age-sex-specific height z-scores comparing those with the indicated abnormal birth exposure to those without the birth abnormality among those with a nonglomerular diagnosis of CKD are indicated by the diamond-shaped points and continuous vertical lines, respectively. The P values indicate the significance of any differential effects of the primary exposure on height z-score in those with a glomerular diagnosis of CKD compared with those with a nonglomerular diagnosis (i.e., is bG ≠ bNG). All analyses were adjusted for sex, race, age, percentage of life with CKD, and mid-parental height.
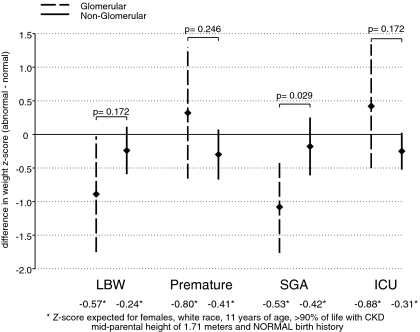
Figure 3.
Multivariate CKD diagnosis-specific repeated measures analyses of abnormal birth history exposures on age-sex-specific weight z-scores. Point estimates of the mean differences (bG) and 95% CIs in age-sex-specific weight z-scores comparing those with the indicated abnormal birth exposure to those without the birth abnormality among those with a glomerular diagnosis of CKD are indicated by the diamond-shaped points and dashed vertical lines, respectively. Point estimates of the mean differences (bNG) and 95% CIs in age-sex-specific weight z-scores comparing those with the indicated abnormal birth exposure to those without the birth abnormality among those with a nonglomerular diagnosis of CKD are indicated by the diamond-shaped points and continuous vertical lines, respectively. The P values indicate the significance of any differential effects of the primary exposure on weight z-score in those with a glomerular diagnosis of CKD compared with those with a nonglomerular diagnosis (i.e., is bG ≠ bNG). All analyses were adjusted for sex, race, age, percentage of life with CKD, and mid-parental height.
As shown in Figure 3, children with a glomerular CKD diagnosis who were born <2500 g or SGA had significantly lower weight z-scores relative to those born ≥2500 g or who were not SGA, respectively. None of the birth abnormalities were significantly associated with weight in children with a nonglomerular CKD diagnosis. In children with glomerular versus nonglomerular diagnoses, the effect of SGA (−1.08 versus −0.18; P = 0.029) on weight was more pronounced in children with a glomerular diagnosis.
Discussion
Abnormal birth history was quite common in our large cohort of North American children with CKD. LBW was reported in 17% of our cohort; this contrasts with an overall rate in the United States that ranged from 7.4% to 8.3% between 1996 and 2006 (17). Furthermore, SGA, defined as a birth weight <10th percentile, occurred in 14% of the cohort and 40% of the cohort spent time in a neonatal ICU after delivery. This finding that such a large percentage of children in the CKiD cohort had an abnormal birth history raises the possibility that birth history may be an important exposure when looking at growth-related and other types of outcomes. An abnormal birth history is a risk factor for various short- and long-term medical and developmental problems (18,19). Prematurity is a risk factor for chronic lung disease, cognitive impairment, and developmental abnormalities (19–21). Children who are SGA are known to be at increased risk for developmental delay (18). Moreover, a growing body of evidence supports the Barker hypothesis, which contends that LBW places individuals at increased risk for development of various chronic problems (22). Hence, future analysis of outcomes in the CKiD cohort should consider the effects of a child's birth history.
We found that children with CKD who were born with LBW or SGA were shorter than children whose birth weight was ≥2500 g or who had a birth weight >10th percentile for their gestational age. This suggests that LBW and SGA should be added to the list of risk factors for short stature in children with CKD. The effect of SGA on stature in children with CKD was surprising because most children who are SGA have rapid catch-up growth and achieve a normal height by 2 years of age (5); only 10% of SGA children have a persistent height deficit (6–8). LBW, in the absence of SGA, is not known to be a risk factor for short stature. There are several possible explanations for these two findings. First, the negative effect of CKD may prevent the normal catch-up in growth among children who are SGA or LBW that would likely otherwise be observed. Second, in some of our study participants, SGA or LBW may be secondary to the underlying kidney disease, thus selecting for a group of children with more severe CKD. Third, as discussed below, SGA or LBW may be a risk factor for the development of CKD. It is possible that children who have CKD due to SGA or LBW are more likely to have other consequences, such as short stature.
In multivariate analyses of the total cohort, LBW and SGA were associated with lower weight z-scores. When analyzed by diagnostic group, there was a significant negative effect of LBW and SGA on weight in the glomerular group, but not in the nonglomerular group; perhaps most interestingly, the negative effect of SGA on weight was significantly worse in those with glomerular diagnosis compared with those with a nonglomerular diagnosis. This may be explained by the Barker hypothesis, which postulates that in utero events, with SGA as the initial manifestation, increase the risk of various medical problems postnatally (22). We speculate that the in utero events that led to SGA in the children with glomerular disease place these patients at risk for poor weight gain and glomerular kidney disease (e.g., focal segmental glomerulosclerosis). In contrast, abnormal birth history in the nonglomerular group is more likely to be a consequence of the kidney disease rather than a cause of the kidney disease.
Epidemiologic studies have shown that LBW is a risk factor for the development of CKD in adults (23,24). Many of the children with nonglomerular causes of CKD in this cohort may have had LBW secondary to their underlying kidney disease. However, participants with glomerular disease, which was diagnosed on average 7.5 years after birth (compared with a nonglomerular disease which was diagnosed on average 0.1 years after birth), also had a higher than expected rate of LBW and SGA. This raises the intriguing possibility that LBW may be a risk factor for the development of CKD, even during childhood.
There are limited data on abnormal birth history in children with CKD. In a single-center study, 26 of 101 children were preterm (25), but this study focused on children who developed CKD during infancy and is thus likely heavily biased toward abnormal birth history. The study presented here provides the first multicenter study of birth history in a large group of children with CKD.
The findings of this report should be interpreted in light of the following limitations. First, this report uses exposures collected from parent recall of their child's birth history. Although there is evidence that parent recall is quite accurate for birth weight and hospitalizations (26–28), we must acknowledge the possibility of misclassification of abnormal birth exposures. Second, the z-scores for height and weight departed from a normal distribution because of heavier tails and a negative skewness because of a greater frequency of extremely low z-scores relative to the frequency of extremely high z-scores. However, given the reasonable fit of the normal distribution as depicted in Figure 1, we opted to base our inferences on standard linear regression models. Lastly, as with all observational cohort studies, our findings are subject to possible unmeasured confounding. It is possible that the children with abnormal birth history had more comorbidities or feeding difficulties early in life that impaired linear growth and weight gain.
In conclusion, this analysis of more than 400 children has shown that an abnormal birth history is more common in children with CKD than in the general population. LBW and SGA were associated with lower weight, especially in children with a glomerular etiology of CKD. Moreover, children with a history of LBW or SGA, regardless of type of CKD diagnosis, were disproportionately short in stature. Hence, we have identified LBW and SGA as novel risk factors for abnormal growth in children with CKD.
Disclosures
None.
Acknowledgments
The CKiD prospective cohort study is funded by the National Institute of Diabetes and Digestive and Kidney Diseases, with additional funding from the National Heart, Lung, and Blood Institute, the National Institute of Child Health and Human Development, and the National Institute of Neurologic Disorders and Stroke. The CKiD study has been supported by participating institutional General Clinical Research Centers and Clinical Translational Research Centers. Research at Emory is supported in part by PHS Grant UL1 RR025008 from the Clinical and Translational Science Award program and PHS Grant M01 RR0039 from the General Clinical Research Center program, National Institutes of Health, and the National Center for Research Resources. The CKiD prospective cohort study has clinical coordinating centers (principal investigators) at Children's Mercy Hospital and the University of Missouri–Kansas City (Bradley Warady, MD; U01-DK-66143) and at Johns Hopkins School of Medicine (Susan Furth, MD, Ph.D.; U01-DK-66174), a data coordinating center at Johns Hopkins Bloomberg School of Public Health (Alvaro Muñoz, Ph.D.; U01-DK-66116), and the Central Biochemistry Laboratory at the University of Rochester Medical Center (George J. Schwartz, MD; U01-DK82194).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Greenbaum LA, Warady BA, Furth SL: Current advances in chronic kidney disease in children: Growth, cardiovascular, and neurocognitive risk factors. Semin Nephrol 29: 425–434, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Haffner D, Schaefer F, Nissel R, Wuhl E, Tonshoff B, Mehls O: Effect of growth hormone treatment on the adult height of children with chronic renal failure. German Study Group for Growth Hormone Treatment in Chronic Renal Failure. N Engl J Med 343: 923–930, 2000 [DOI] [PubMed] [Google Scholar]
- 3. Fine RN, Kohaut EC, Brown D, Perlman AJ: Growth after recombinant human growth hormone treatment in children with chronic renal failure: Report of a multicenter randomized double-blind placebo-controlled study. Genentech Cooperative Study Group. J Pediatr 124: 374–382, 1994 [DOI] [PubMed] [Google Scholar]
- 4. Greenbaum LA, Del Rio M, Bamgbola F, Kaskel F: Rationale for growth hormone therapy in children with chronic kidney disease. Adv Chronic Kidney Dis 11: 377–386, 2004 [PubMed] [Google Scholar]
- 5. Albertsson-Wikland K, Karlberg J: Postnatal growth of children born small for gestational age. Acta Paediatr Scand Suppl 423: 193–195, 1997 [DOI] [PubMed] [Google Scholar]
- 6. Karlberg JP, Albertsson-Wikland K, Kwan EY, Lam BC, Low LC: The timing of early postnatal catch-up growth in normal, full-term infants born short for gestational age. Horm Res 1: 17–24, 1997 [DOI] [PubMed] [Google Scholar]
- 7. Leger J, Levy-Marchal C, Bloch J, Pinet A, Chevenne D, Porquet D, Collin D, Czernichow P: Reduced final height and indications for insulin resistance in 20 year olds born small for gestational age: Regional cohort study. BMJ 315: 341–347, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee PA, Chernausek SD, Hokken-Koelega AC, Czernichow P. International Small for Gestational Age Advisory B: International Small for Gestational Age Advisory Board consensus development conference statement: Management of short children born small for gestational age, April 24-October 1, 2001 Pediatrics 111: 1253–1261, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Furth SL, Cole SR, Moxey-Mims M, Kaskel F, Mak R, Schwartz G, Wong C, Munoz A, Warady BA: Design and methods of the Chronic Kidney Disease in Children (CKiD) prospective cohort study. Clin J Am Soc Nephrol 1: 1006–1015, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schwartz GJ, Haycock GB, Edelmann CM, Jr, Spitzer A: A simple estimate of glomerular filtration rate in children derived from body length and plasma creatinine. Pediatrics 58: 259–263, 1976 [PubMed] [Google Scholar]
- 11. Schwartz GJ, Gauthier B: A simple estimate of glomerular filtration rate in adolescent boys. J Pediatr 106: 522–526, 1985 [DOI] [PubMed] [Google Scholar]
- 12. Schwartz GJ, Furth S, Cole SR, Warady B, Munoz A: Glomerular filtration rate via plasma iohexol disappearance: Pilot study for chronic kidney disease in children. Kidney Int 69: 2070–2077, 2006 [DOI] [PubMed] [Google Scholar]
- 13. Pere A: Comparison of two methods for transforming height and weight to normality. Ann Hum Biol 27: 35–45, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL: CDC growth charts for the United States: Methods and development. Vital Health Stat 11: 1–190, 2002 [PubMed] [Google Scholar]
- 15. Alkalay AL, Graham JM, Jr, Pomerance JJ: Evaluation of neonates born with intrauterine growth retardation: Review and practice guidelines. J Perinatol 18: 142–151, 1998 [PubMed] [Google Scholar]
- 16. Diggle P, Heagerty P, Liang K-Y, Zeger S: Analysis of Longitudinal Data, 2nd Ed., New York, Oxford University Press, 2002 [Google Scholar]
- 17.March of Dimes Perinatal Data Center. [Accessed August 10, 2009]. Available online at http://www.marchofdimes.com/peristats.
- 18. Fang S: Management of preterm infants with intrauterine growth restriction. Early Hum Dev 81: 889–900, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Valero De Bernabe J, Soriano T, Albaladejo R, Juarranz M, Calle ME, Martinez D, Dominguez-Rojas V: Risk factors for low birth weight: A review. Eur J Obstet Gynecol Reprod Biol 116: 3–15, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Aylward GP: Neurodevelopmental outcomes of infants born prematurely. J Dev Behav Pediatr 26: 427–440, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Colvin M, McGuire W, Fowlie PW: Neurodevelopmental outcomes after preterm birth. BMJ 329: 1390–1393, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Barker DJ: The developmental origins of well-being. Philos Trans R Soc Lond B Biol Sci 359: 1359–1366, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vikse BE, Irgens LM, Leivestad T, Hallan S, Iversen BM: Low birth weight increases risk for end-stage renal disease. J Am Soc Nephrol 19: 151–157, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. White SL, Perkovic V, Cass A, Chang CL, Poulter NR, Spector T, Haysom L, Craig JC, Salmi IA, Chadban SJ, Huxley RR: Is low birth weight an antecedent of CKD in later life? A systematic review of observational studies. Am J Kidney Dis 54: 248–261, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Kari JA, Gonzalez C, Ledermann SE, Shaw V, Rees L: Outcome and growth of infants with severe chronic renal failure. Kidney Int 57: 1681–1687, 2000 [DOI] [PubMed] [Google Scholar]
- 26. Seidman DS, Slater PE, Ever-Hadani P, Gale R: Accuracy of mothers' recall of birthweight and gestational age. Br J Obstet Gynaecol 94: 731–735, 1987 [DOI] [PubMed] [Google Scholar]
- 27. O'Sullivan JJ, Pearce MS, Parker L: Parental recall of birth weight: How accurate is it? Arch Intern Med 82: 202–203, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pless CE, Pless IB: How well they remember. The accuracy of parent reports. Arch Pediatr Adolesc Med 149: 553–558, 1995 [DOI] [PubMed] [Google Scholar]