Summary
Background and objectives
Methicillin-resistant Staphylococcus aureus (MRSA) nasal carriage is a recognized risk factor for subsequent endogenous infections. However, the association between MRSA carriage and patient survival in hemodialysis patients has not been established.
Design, setting, participants, & measurements
In March 2007, this prospective cohort study enrolled 306 outpatients under maintenance hemodialysis from a hospital-based dialysis center in Taiwan. They received two consecutive weekly nasal swab cultures at the beginning of the study. Patients having at least one positive culture of MRSA were defined as MRSA carriers. Subjects were followed up until December 31, 2008. The primary outcome was all-cause mortality. Main secondary outcomes were infection-related mortality and morbidity.
Results
We identified 29 MRSA carriers (9.48%) at study entry. After a median of 613 days of follow-up, Kaplan-Meier analysis showed significant survival differences between MRSA carriers and noncarriers (log-rank P = 0.02). Compared with noncarriers, MRSA carriers had a 2.46-fold increased risk of dying from any cause, after adjusting for covariates at the start of follow-up. The adjusted hazard ratios of infection-related mortality and occurrence of subsequent S. aureus infection in carriers were 4.99 and 4.31, respectively.
Conclusions
A major limitation is the relatively small sample size of MRSA carriers. Nevertheless, we demonstrated that there may be an association between MRSA nasal carriage and poor clinical outcomes in an outpatient hemodialysis population. This underscores the need for routine surveillance of MRSA nasal carriage and should alert the physicians of a group at high risk of morbidity and mortality.
Introduction
Infection remains a major cause of morbidity and mortality among patients with ESRD. Staphylococcus aureus accounts for the majority of bacterial infections. S. aureus-related infections in patients with ESRD are frequently accompanied by multiple complications and incur high costs and prolong hospital stays (1,2). In addition, S. aureus has become increasingly resistant to antimicrobials worldwide. Patients with ESRD are at increased risk for infection and/or colonization with methicillin-resistant S. aureus (MRSA) because they are repeatedly exposed to the healthcare environment and often receive prolonged courses of antibiotics, besides being immunocompromised (3,4). The incidence of invasive MRSA infection among dialysis patients in the United States has been reported to be more than 100 times higher than in the general population (5).
Nasal carriage of MRSA is a recognized risk factor for subsequent infection of endogenous origin (6–9). Among nursing home residents and patients in intensive care units, past studies found an association between MRSA colonization and all-cause mortality (8,10,11). These findings brought recommendations of active surveillance for colonization, despite the lack of evidence that routine surveillance has an effect on clinical outcome (12). Furthermore, the bulk of evidence concerning the risk of MRSA nasal carriage was limited to hospitalized patients. There were very few studies addressing the clinical effect of MRSA nasal carriage on hemodialysis (HD) patients, most of whom were freely ambulatory in the general community (4,13,14). For the establishment of public health intervention policy in this vulnerable population, we were interested in determining the effect of MRSA nasal carriage on all-cause mortality.
We hypothesized that MRSA nasal carriage among outpatient HD patients adversely affects patient survival. This study aimed to prospectively examine whether the detection of MRSA nasal carriage in HD patients could predict higher mortality. Special attention was focused on the characteristics of infection-related morbidity and mortality.
Materials and Methods
Design and Population
This observational cohort study was conducted in the hospital-based outpatient HD unit of Far Eastern Memorial Hospital, a tertiary medical center in northern Taiwan. The dialysis unit is composed of two wards with 40 and 26 beds, respectively, and a separated four-bed isolation room. In March 2007, all of the prevalent adult patients on maintenance HD were invited to participate.
Baseline demographic and medical data were collected from medical records. The causes of ESRD of the patients were identified from dialysis registry of the unit. Vascular accesses for HD and comorbidities (diabetes mellitus, hypertension, and coronary artery disease) of the subjects were recorded at the study entry. Malignancy was defined as having a history of any malignancy. The patients' Karnofsky performance scale, designed to measure functional status of daily life, was rated by nursing staff in the HD center. The Karnofsky scale runs from 100 to 0, where 100 is “perfect” health and 0 is death. Concurrent active infection was defined as having any kind of clinical infection at study entry. We also assessed whether patients had a history of recent hospitalization and any use of antibiotics in the 3 months previous to study entry. Monthly laboratory data were also collected, and the most recent results before the date the cultures were obtained were recorded.
This cohort was prospectively observed for each outcome, in which patients were classified by the initial MRSA nasal carriage states (MRSA carriers and MRSA noncarriers). We obtained information about the patients' clinical histories and medical care from detailed medical records. Subjects who were transferred to other HD centers and those who received kidney transplantations were censored on the day of transfer or operation. Follow-up with the patients continued until December 31, 2008.
This study was approved by the Research Ethics Committee and was monitored by the Institutional Review Board of Far Eastern Memorial Hospital. All of the participants gave their informed consent before study entry.
Definition of Carriage States
The screening procedure for S. aureus nasal carriage, described by Nouwen et al. (15), was carried out by performing two consecutive nasal swab cultures obtained at a 1-week interval at the beginning of the study. Those who had at least one positive culture of S. aureus were defined as S. aureus carriers, whereas all of the others were defined as S. aureus noncarriers. An individual was classed as a methicillin-susceptible S. aureus (MSSA) carrier if the grown S. aureus strain was susceptible to oxacillin. If the grown strain was MRSA, the corresponding patient was defined as a MRSA carrier. Both S. aureus noncarriers and MSSA carriers were assigned as MRSA noncarriers. A person was classed as a persistent carrier when both of the cultures were positive. When only one of the cultures was positive, the person was considered an intermittent carrier.
Microbiological Study
For the preparation of the nasal cultures, a sterile swab (BBL CultureSwab Plus; Becton-Dickinson) was rotated in the anterior 1.5 cm of the nasal vestibule of both of the patient's nares, placed into the amine gel transport medium, and then plated onto a blood agar plate (Columbia CNA agar with 5% sheep blood; Becton-Dickinson). One trained medical technologist performed all of the nasal swab cultures. The plates were incubated at 35°C for 48 hours, after which the grown colonies were streaked and examined. Identification of S. aureus was on the basis of on the basis of the morphology of colonies grown on the agar plate, a Gram stain, and a positive coagulase test. To identify MRSA, oxacillin susceptibility was tested using the disk-diffusion method, in accordance with the Clinical and Laboratory Standards Institute guidelines (16).
MRSA Contact Isolation Policy and Decolonization Therapy
Our center followed universal precautions for all medical care to all patients. In addition, we followed the multidrug-resistant organisms infection and colonization control policy of Far Eastern Memorial Hospital for managing patients with MRSA colonization and infection, mainly modified from the Society of Healthcare Epidemiology of America's guidelines (17). In brief, isolated HD by contact isolation in a separated room during dialysis was performed for MRSA-infected patients who (1) had MRSA cultures from sputum, dirty wounds, or open wounds and (2) had MRSA cultures from urine or blood, combined with nasal swabs. On the other hand, HD in the patient's original bed with standard precautions was applied for (1) MRSA nasal carriers without infection and (2) MRSA-infected patients who had MRSA cultures from urine or blood, but not from nasal swabs concurrently.
Up until this study began in March 2007, the literature did not show solid evidence of overall mortality or non-S. aureus infection risk reduction by S. aureus decolonization therapy (18–20). Therefore, our dialysis unit did not apply routine decolonization therapy for S. aureus nasal carriers before or at the initiation of the study.
Primary and Secondary Outcomes
The primary outcome was death from any cause. Other observed secondary end points included death caused by infection, first hospitalization for any cause, first infection episode caused by any microorganism, and first occurrence of S. aureus-related infection. Death was deemed to be infection-related if there was active infection at the time of death and there was no other cause of death. Infection episodes were diagnosed clinically and/or microbiologically based upon the criteria of National Nosocomial Infections Surveillance System (21). All infection episodes were described according to the type of infection (21), associated pathogen(s) from culture results, and antibiotic usage.
Statistical Analyses
Characteristics between the groups were compared with the use of χ2 tests, Fisher's exact tests, two-sample t tests, or Wilcoxon rank sum tests as indicated. A logistic regression model was used for determining associated factor(s) of MRSA carriage. Analyses of outcomes were performed with the use of time-to-event methods. Kaplan-Meier estimates were used to obtain the proportion of patients who had an event during follow-up. The log-rank test was used to compare the equality of survival distribution. We used a Cox proportional hazard model to assess the effect of MRSA carriage on the study end points. Unadjusted hazard ratios were further adjusted in regression models by adding prespecified variables: age, gender, as well as the covariates (vascular access type, the presence of diabetes, recent hospitalization, recent use of antibiotics, and serum albumin level) that have been reported to have an association with MRSA carriage or infection in dialysis populations (4,13,14,22,23). All of the tests were two-tailed with significance defined by P values of less than 0.05. STATA software (STATA 10.0; StataCorp) was used for the statistical analysis.
Results
Demographic and Clinical Characteristics of Patients
A total of 350 eligible ESRD outpatients on maintenance HD were invited for the study in March 2007. Among them, 306 consented to participate. They were aged between 23 and 86 years at entry (mean age, 60.15 ± 12.12 years) with 147 men (48.04%) and 158 diabetes patients (51.63%). Two hundred twelve patients (69.28%) were dialyzed through a native arteriovenous fistula, 76 patients (24.84%) were dialyzed through a synthetic graft, and the remaining 18 patients (5.88%) were dialyzed through a central venous catheter. Another 44 invited patients refused to participate in this study. Participants were similar to nonparticipants with respect to baseline demographic and clinical characteristics (all P values >0.05).
At the beginning of the study, 29 patients (9.48%) were identified as MRSA carriers: 13 persistent carriers and 16 intermittent carriers. Demographic characteristics of MRSA carriers and noncarriers are summarized in Table 1. Serum albumin levels were significantly lower in MRSA carriers (P = 0.01). All other baseline characteristics, including their types of vascular access, were similar between the two groups. Using logistic regression analysis, only serum albumin level was significantly associated with MRSA carriage (OR, 0.4; 95% CI = 0.19 to 0.82).
Table 1.
Demographics and clinical characteristics of the patients by MRSA carriage
| Characteristic | MRSA Noncarrier (n = 277) | MRSA Carrier (n = 29) | Pa |
|---|---|---|---|
| Age (years) | 60.07 (11.88) | 60.97 (14.48) | 0.7 |
| Gender, men (%) | 130 (46.93) | 17 (58.62) | 0.2 |
| Marriage (%) | 0.4 | ||
| couple | 246 (88.81) | 24 (82.76) | |
| single | 31 (11.19) | 5 (17.24) | |
| Time on dialysis (months) | 126.13 (120.75) | 153.90 (147.12) | 0.2 |
| Cause of ESRD (%) | 0.3 | ||
| glomerulonephritis | 84 (30.32) | 11 (37.93) | |
| diabetes | 121 (43.6) | 9 (31.03) | |
| hypertension | 31 (11.19) | 2 (6.9) | |
| other | 41 (14.8) | 7 (24.14) | |
| Vascular access (%) | 0.9 | ||
| fistula | 192 (69.31) | 20 (68.97) | |
| graft | 69 (24.91) | 7 (24.14) | |
| catheter | 16 (5.78) | 2 (6.9) | |
| Diabetes (%) | 147 (53.07) | 11 (37.93) | 0.1 |
| Hypertension (%) | 216 (77.98) | 23 (79.31) | 0.9 |
| Coronary artery disease (%) | 34 (12.27) | 4 (13.79) | 0.8 |
| Malignancy (%) | 11 (3.97) | 3 (10.34) | 0.1 |
| Karnofsky performance scale | 75.70 (18.37) | 71.03 (20.41) | 0.2 |
| Concurrent active infection (%)b | 1 (3.45) | 4 (1.44) | 0.4 |
| Recent hospitalization (%)c | 28 (10.11) | 6 (20.69) | 0.09 |
| Recent antibiotics use (%)d | 49 (17.69) | 7 (24.14) | 0.4 |
| Albumin (g/dl) | 4.06 (0.42) | 3.84 (0.61) | 0.01 |
| Serum creatinine (mg/dl) | 10.4 (2.27) | 10.14 (2.32) | 0.6 |
| Total cholesterol (mg/dl) | 175.46 (43.93) | 163.9 (46.53) | 0.2 |
| Triglyceride (mg/dl) | 173.7 (142.57) | 206.86 (246) | 0.3 |
| Hemoglobin (g/dl) | 10.93 (1.58) | 10.55 (2.1) | 0.2 |
| WBC count (1000 cells/mm3) | 7.45 (2.58) | 7.27 (2.39) | 0.7 |
| hsCRP (mg/dl) | 0.99 (2.08) | 1.51 (1.75) | 0.2 |
| Single pool Kt/Ve | 1.56 (0.27) | 1.50 (0.18) | 0.2 |
| Follow-up duration (days) | 613 (598, 639) | 612 (406.5, 639) | 0.2 |
| Censored (%)f | 28 (10.1) | 1 (3.4) | 0.3 |
The data are presented as the means (standard deviation) or number (%). Follow-up duration is presented as median (25th/75th percentile). WBC, white blood cell; hsCRP, high-sensitivity C-reactive protein.
The values represent comparisons between the MRSA noncarrier and MRSA carrier groups. Two-sample t tests were used for the comparison of continuous variables other than follow-up duration. χ2 tests or Fisher's exact tests were used for comparison of categorical variables. The Wilcoxon rank sum test was used for comparison of follow-up duration.
Defined as having clinical infection at study entry.
Defined as hospitalization ≦3 months before study entry.
Defined as any antibiotic use ≦3 months before study entry.
Calculated using the Daugirdas formula.
Censored because of receiving kidney transplantation (n = 5, all were MRSA noncarriers) or transferral to other hemodialysis centers before December 31, 2008.
All-cause Mortality and Its Association with Initial MRSA Nasal Carriage
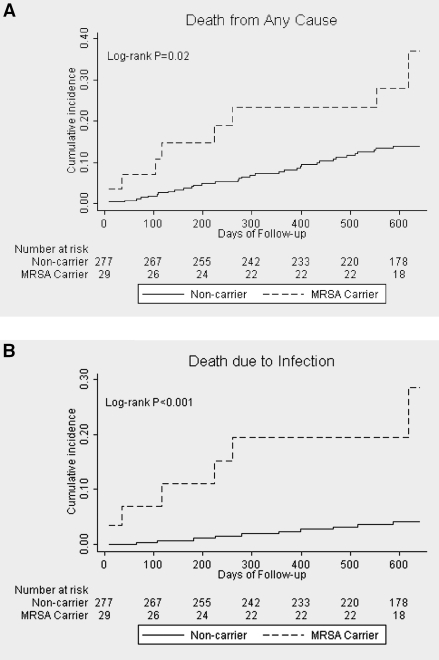
The median duration of follow-up of all patients was 613 days (with 25th/75th percentile of 598/639 days). Follow-up days and the frequency of being censored before the end of the study were not significantly different between MRSA carriers and noncarriers. The primary outcome of all-cause mortality occurred in eight MRSA carriers and 34 noncarriers (Table 2A). Kaplan-Meier analysis showed significant survival differences between MRSA carriers and noncarriers (P = 0.02) (Figure 1A). Unadjusted Cox proportional hazard analysis revealed that MRSA nasal carriage might affect the time to death [hazard ratio (HR) 2.45, 95% CI = 1.13 to 5.3]. After adjusting for age, gender, diabetes, vascular access type, recent hospitalization, recent use of antibiotics, and serum albumin level at the start of follow-up, MRSA carriers still had a 2.46-fold (95% CI = 1.08 to 5.62) increased risk of all-cause mortality compared with noncarriers (Table 3).
Table 2.
Causes of death of the patients (A) Distribution of different death causes between groups
| Cause of Death | MRSA Noncarrier (n = 34) | MRSA Carrier (n = 8) |
|---|---|---|
| Infection | 10 | 6 |
| Cardiovascular events | 17 | 1 |
| Malignancy | 4 | 1 |
| Others | 3 | 0 |
| (B) Associated Pathogens and types of fetal infection episodes | ||
|---|---|---|
| Associated Pathogen | MRSA Noncarrier (n = 10) | MRSA Carrier (n = 6) |
| MRSA | 0 | vascular access-related BSI (1) osteomyelitis (1) |
| MSSA | IAI (1) vascular access-related BSI (3) | 0 |
| Other than S. aureus | BSI (1) IAI (2) pneumonia (1) | pneumonia (2) |
| Uncertain | IAI (1) pneumonia (1) | clinical sepsis (1) IAI (1) |
BSI, bloodstream infection; IAI, intra-abdominal infection.
Figure 1.
Kaplan-Meier curves for the death from any cause (A) and death due to infection (B).
Table 3.
Cox regression models for outcome hazard ratios by MRSA nasal carriage
| Outcome | MRSA Noncarrier (n = 277)a | MRSA Carrier (n = 29)a | Unadjusted |
Adjustedb |
||
|---|---|---|---|---|---|---|
| HR | Pc | HR | Pc | |||
| Primary outcome | ||||||
| all-cause mortality | 34 (12.27) | 8 (27.59) | 2.45 (1.13–5.3) | 0.02 | 2.46 (1.08–5.62) | 0.03 |
| Secondary outcome | ||||||
| infection-related death | 10 (3.61) | 6 (20.69) | 6.2 (2.25–17.07) | <0.001 | 4.99 (1.52–16.39) | 0.008 |
| hospitalization due to any cause | 104 (37.55) | 13 (44.83) | 1.32 (0.74–2.36) | 0.3 | 1.38 (0.76–2.52) | 0.3 |
| any infection | 60 (21.66) | 10 (34.48) | 1.84 (0.94–3.6) | 0.07 | 2.09 (1.02–4.28) | 0.04 |
| S. aureus infection | 18 (6.5) | 6 (20.69) | 3.69 (1.46–9.29) | 0.01 | 4.31 (1.55–11.97) | 0.01 |
The values indicate the number of patients (%).
Adjusted for age, gender, diabetes, vascular access type, recent hospitalization, recent antibiotics use, and serum albumin level.
The Cox proportional hazards model was used to assess the effect of MRSA nasal carriage on study end points.
MRSA Nasal Carriage and Infection-related Death
Six MRSA carriers and 10 noncarriers died from infection (Table 2). MRSA nasal carriage was associated with a higher risk of infection-related death compared with noncarriers (adjusted HR 4.99; 95% CI = 1.52 to 16.39) (Figure 1B and Table 3).
The identified associated pathogens of infection-related deaths are listed in Table 2B. Two MRSA carriers died because of confirmed MRSA-associated infection, and two others died from infection without documented pathogens. Of note, none of the four patients had ever received decolonization therapy for S. aureus before their fatal infection episodes. None of the MRSA noncarriers died from MRSA-associated infections, but four of them died due to MSSA sepsis. Only one of these four patients had been identified as a S. aureus carrier at the beginning of the study, and none of them had ever received decolonization therapy for S. aureus.
MRSA Nasal Carriage and Other Secondary Outcomes
Table 3 shows that the risks of all-cause hospitalization and infection episodes due to any microorganism were not significantly different between MRSA carriers and noncarriers. In contrast, MRSA carriers faced a 4.31-fold higher risk of subsequent occurrence of S. aureus infection. During follow-up, 24 patients had 28 subsequent S. aureus infection episodes: six patients (with seven episodes) were MRSA carriers at study entry, and 18 patients (with 21 episodes) were MRSA noncarriers at study entry. When focusing on the MRSA infection, 11 patients had 12 subsequent MRSA infections. Among the 29 MRSA carriers at study entry, five patients (17.24%) had six subsequent MRSA infections: four vascular access-related infections, one osteomyelitis, and one pneumonia. Among the 277 MRSA noncarriers at study entry, six patients (2.17%) had six subsequent MRSA infections: three cellulitis, two vascular access-related infections, and one osteomyelitis.
Additional Analyses Not Specified a Priori
It is not known whether nasal carriages of S. aureus with different persistence or with different oxacillin susceptibility have different effects on all-cause mortality in HD population. As a result, we performed additional analyses to examine the correlation of survival or death with different methods for distinguishing carriage patterns: S. aureus nasal carriage, persistent S. aureus nasal carriage, S. aureus nasal carriage with different oxacillin susceptibility, MRSA nasal carriage, or persistent MRSA nasal carriage (Table 4). Among these methods, only MRSA nasal carriage was significantly correlated with the all-cause mortality (P = 0.02).
Table 4.
Summary of the correlation to method for distinguishing S. aureus nasal carriage patterns related to all-cause mortality
| Method for Distinguishing Carriage Patterns | Died | Survived | Pa |
|---|---|---|---|
| By S. aureus nasal carriage | 0.3 | ||
| S. aureus carriers (n = 75) | 13 (17.3) | 62 (82.7) | |
| S. aureus noncarriers (n = 231) | 29 (12.6) | 202 (87.4) | |
| By persistent S. aureus nasal carriage | 0.9 | ||
| persistent S. aureus carriers (n = 36) | 5 (13.9) | 31 (86.1) | |
| patients other than persistent S. aureus carriers (n = 270)b | 37 (13.7) | 233 (86.3) | |
| By S. aureus nasal carriage with different oxacillin susceptibility | 0.07 | ||
| MSSA carriers (n = 46) | 5 (10.9) | 41 (89.1) | |
| MRSA carriers (n = 29) | 8 (27.6) | 21 (72.4) | |
| S. aureus noncarriers (n = 231) | 29 (12.6) | 202 (87.4) | |
| By MRSA nasal carriage | 0.02 | ||
| MRSA carriers (n = 29) | 8 (27.6) | 21 (72.4) | |
| MRSA noncarriers (n = 277) | 34 (12.3) | 243 (87.7) | |
| By persistent MRSA nasal carriage | 0.4 | ||
| persistent MRSA carriers (n = 13) | 3 (23.1) | 10 (76.9) | |
| patients other than persistent MRSA carriers (n = 293)c | 39 (13.3) | 254 (86.7) |
The data are presented as number (% of each carriage state).
χ2 tests or Fisher's exact tests were used for comparison.
Combined S. aureus intermittent and noncarriers.
Combined MRSA intermittent and noncarriers.
Discussion
Several studies have reported an increased risk for colonization and infection with MRSA in patients with ESRD (4,5,13,14,24). However, there are limited data on the clinical consequences of MRSA nasal carriage in HD patients (4,13,14), and no reports have measured mortality as the study outcome. Unlike for acute care settings, the United States Centers for Disease Control and Prevention do not have recommendations of routine MRSA screening for ambulatory healthcare facilities, including hospital-based outpatient clinics and free-standing dialysis centers. As the first study to evaluate the effect of MRSA carriage on mortality in an outpatient HD population, we found there may be an association between MRSA nasal carriage and all-cause mortality. Because of the observational nature of this study, the association may not represent a causal relationship. Nevertheless, it underscores the need for routine surveillance of MRSA nasal carriage in the HD population from the viewpoint of public health.
There are two possible mechanisms that may explain how MRSA nasal carriage predicts mortality in outpatient HD patients. First, MRSA nasal colonization predisposed patients to subsequent endogenous infections and led to further untoward outcomes. This intuitive sense is supported by our observations that MRSA carriers were susceptible to subsequent S. aureus infection and infection-related death. Accordingly, half of the MRSA carriers' deaths (two MRSA-associated deaths and two infection-related deaths without identified pathogens) might have been prevented if preventative decolonization therapy had been applied.
Second, the predictive role for death of MRSA carriage may be attributed to the selection of a population of patients who become MRSA carriers. We have observed that MRSA carriers had significantly lower serum albumin levels and trends of higher percentages of recent hospitalizations and antibiotic use. Protein malnutrition may increase the patients' susceptibility to colonization with resistant bacterial strains (23). It may also reflect inflammation or additional cardiovascular comorbidities, which predispose patients to antibiotic exposure or hospitalization (25). One study among HD patients also reported that, compared with noncarriers, S. aureus carriers may have impaired cytokine and cellular immune response (3). Furthermore, in our study, using S. aureus carriers as an indicator was not associated with a risk of death. This is because there were no significant differences between MSSA carriers and S. aureus noncarriers with respect to all-cause mortality (adjusted HR 1.35, 95% CI = 0.5 to 3.6; data not shown in Results section). However, data regarding the hypothesis of increased virulence of MRSA over MSSA are conflicting (2,26). We inferred that MRSA nasal carriage in HD patients may be a surrogate marker of a poor general condition that is vulnerable to fetal infection caused by any microorganism and all-cause mortality.
In any case, identifying MRSA nasal carriers in the HD population is important. Compared with other studies that screened MRSA carriage by a single culture undertaken in Taiwan (4,24,27), the prevalence of MRSA carriers detected by performing two consecutive weekly cultures in our patients (9.48%) was higher. Negative cultures can be found in true noncarriers but also in patients who intermittently carry S. aureus. In our study, cultures were negative in seven intermittent carriers in the first exam and in nine intermittent carriers in the second exam; the exams were taken 1 week apart. Using one exam failed to identify 24 or 31% of MRSA carriers. Moreover, we found that persistent MRSA carriers were not associated with higher all-cause mortality than the others (i.e. combined MRSA intermittent and noncarriers) (Table 4). This could be attributed to the inadequate power because there were only 13 persistent carriers. Another possibility is that intermittent MRSA carriage is also an important predictor of death. Risks associated with intermittent MRSA carriers adversely affect the outcomes of the whole “patients other than persistent MRSA carrier” group. This contradicts a previous study in peritoneal dialysis patients (28). As a result, screening of MRSA nasal carriage in HD patients should be carried out by at least two consecutive weekly nasal swab cultures to maximize the probability of identifying all MRSA carriers.
Mupirocin is generally highly effective in eradication of S. aureus nasal carriage in dialysis patients (14,18–20,29) Studies treating all S. aureus carriers (both MRSA and MSSA) in dialysis populations have shown that eradicating the S. aureus carrier state can lower rates of S. aureus-related infections but not overall infections (18–20). Furthermore, recolonization of S. aureus is not uncommon (19,20,29). Thus, more data are required to validate the efficacy of S. aureus decolonization in patients with ESRD. Targeting intervention to high-risk groups is needed, and on the basis of our study, MRSA carriers are reasonable candidates. Long-term follow-up studies are warranted to elucidate the effect of MRSA decolonization therapy on mortality in HD patients.
Our study had limitations. First, although the total cohort included 306 patients, the sample size of MRSA carriers was relative small. We were unable to fully adjust all of the potential confounders, and the association we found might not imply causality. Second, we did not monitor the patients' carriage status periodically. In a proportion of dialysis patients, colonization with S. aureus is a dynamic process (28). This might have underestimated the prevalence of carriers and biased the carriage-mortality relationship. However, the culture rule we used was reported to be able to predict S. aureus carriage state accurately, for at least 12 weeks (15). Sanford et al. had shown that MRSA may be carried in the nares for a mean of 42 months (30). Other authors also reported that once being identified as MRSA carriers, those patients might face a prolonged heightened risk of subsequent MRSA infections, even extended beyond 1 year (6,9). As a result, without decolonization, MRSA carriers might harbor that pathogen for a long period of time, resulting in prolonged risk.
Third, we did not utilize PCR-based methods to detect MRSA carriers. These methods are rapid and more sensitive than traditional culture (31,32). However, they are costly in general practice and are not proven practically in dialysis populations. Furthermore, molecular studies and genotyping of S. aureus isolates were not performed. Accordingly, molecular epidemiology and the frequency of community-associated MRSA could not be analyzed. Finally, S. aureus decolonization therapy was not routinely applied. During follow-up, some selected patients did receive mupirocin intranasally without a predefined protocol, which may have altered the final results. However, if decolonization therapy is really effective in reducing mortality, the predictive value of MRSA carriage should be more significant than we observed, as decolonization therapy was not applied at all. A sensitivity analysis excluding these patients still showed a heightened total mortality risk associated with MRSA nasal carriage (adjusted HR 3.29, 95% CI = 1.25 to 8.69).
In summary, our study demonstrated the predictive role of MRSA nasal carriage on all-cause mortality, infection-related death, and subsequent S. aureus infections in an outpatient HD population. This indicates that identifying MRSA nasal carriers in the HD population is needed. It should alert the physicians of a group of patients with a higher probability of morbidity and mortality.
Disclosures
None.
Acknowledgments
The authors would like to thank Mei-Yu Wu for her assistance in swab culture sampling. We are also grateful to Hua-Ru Shung for her help in data acquisition. The authors thank the Ta-Tung Kidney Foundation and Mrs. Hsiu-Chin Lee Kidney Research Fund for supporting this study.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Li Y, Friedman JY, O'Neal BF, Hohenboken MJ, Griffiths RI, Stryjewski ME, Middleton JP, Schulman KA, Inrig JK, Fowler VG, Jr., Reed SD: Outcomes of Staphylococcus aureus infection in hemodialysis-dependent patients. Clin J Am Soc Nephrol 4: 428–434, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vandecasteele SJ, Boelaert JR, De Vriese AS: Staphylococcus aureus infections in hemodialysis: What a nephrologist should know. Clin J Am Soc Nephrol 4: 1388–1400, 2009 [DOI] [PubMed] [Google Scholar]
- 3. Koziol-Montewka M, Chudnicka A, Ksiazek A, Majdan M: Rate of Staphylococcus aureus nasal carriage in immunocompromised patients receiving haemodialysis treatment. Int J Antimicrob Agents 18: 193–196, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Lu PL, Tsai JC, Chiu YW, Chang FY, Chen YW, Hsiao CF, Siu LK: Methicillin-resistant Staphylococcus aureus carriage, infection and transmission in dialysis patients, healthcare workers and their family members. Nephrol Dial Transplant 23: 1659–1665, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Centers for Disease Control: Invasive Methicillin-Resistant Staphylococcus aureus Infections Among Dialysis Patients, United States, 2005. MMWR Morb Mortal Wkly Rep 56: 197–199, 2007 [PubMed] [Google Scholar]
- 6. Huang SS, Platt R: Risk of methicillin-resistant Staphylococcus aureus infection after previous infection or colonization. Clin Infect Dis 36: 281–285, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Davis KA, Stewart JJ, Crouch HK, Florez CE, Hospenthal DR: Methicillin-resistant Staphylococcus aureus (MRSA) nares colonization at hospital admission and its effect on subsequent MRSA infection. Clin Infect Dis 39: 776–782, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Patel M, Weinheimer JD, Waites KB, Baddley JW: Active surveillance to determine the impact of methicillin-resistant Staphylococcus aureus colonization on patients in intensive care units of a Veterans Affairs Medical Center. Infect Control Hosp Epidemiol 29: 503–509, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Datta R, Huang SS: Risk of infection and death due to methicillin-resistant Staphylococcus aureus in long-term carriers. Clin Infect Dis 47: 176–181, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Suetens C, Niclaes L, Jans B, Verhaegen J, Schuermans A, Van Eldere J, Buntinx F: Methicillin-resistant Staphylococcus aureus colonization is associated with higher mortality in nursing home residents with impaired cognitive status. J Am Geriatr Soc 54: 1854–1860, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Ridenour GA, Wong ES, Call MA, Climo MW: Duration of colonization with methicillin-resistant Staphylococcus aureus among patients in the intensive care unit: Implications for intervention. Infect Control Hosp Epidemiol 27: 271–278, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Weber SG, Huang SS, Oriola S, Huskins WC, Noskin GA, Harriman K, Olmsted RN, Bonten M, Lundstrom T, Climo MW, Roghmann MC, Murphy CL, Karchmer TB: Legislative mandates for use of active surveillance cultures to screen for methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci: Position statement from the Joint SHEA and APIC Task Force. Am J Infect Control 35:73–85, 2007 [DOI] [PubMed] [Google Scholar]
- 13. Saxena AK, Panhotra BR, Venkateshappa CK, Sundaram DS, Naguib M, Uzzaman W, Al Mulhim K: The impact of nasal carriage of methicillin-resistant and methicillin-susceptible Staphylococcus a ureus (MRSA & MSSA) on vascular access-related septicemia among patients with type-II diabetes on dialysis. Ren Fail 24: 763–777, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Lederer SR, Riedelsdorf G, Schiffl H: Nasal carriage of methicillin resistant Staphylococcus aureus: The prevalence, patients at risk and the effect of elimination on outcomes among outclinic haemodialysis patients. Eur J Med Res 12: 284–288, 2007 [PubMed] [Google Scholar]
- 15. Nouwen JL, Ott A, Kluytmans-Vandenbergh MF, Boelens HA, Hofman A, van Belkum A, Verbrugh HA: Predicting the Staphylococcus aureus nasal carrier state: Derivation and validation of a “culture rule.” Clin Infect Dis 39: 806–811, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Clinical Laboratory and Standards Institute: Performance standards for antimicrobial susceptibiity testin: 17th informational supplement. M100–S17 PA, Clinical and Laboratory Standards Institute, 2007 [Google Scholar]
- 17. Muto CA, Jernigan JA, Ostrowsky BE, Richet HM, Jarvis WR, Boyce JM, Farr BM: SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and enterococcus. Infect Control Hosp Epidemiol 24: 362–386, 2003 [DOI] [PubMed] [Google Scholar]
- 18. Kluytmans JA, Wertheim HF: Nasal carriage of Staphylococcus aureus and prevention of nosocomial infections. Infection 33: 3–8, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Herwaldt LA: Reduction of Staphylococcus aureus nasal carriage and infection in dialysis patients. J Hosp Infect 40 Suppl B: S13–S23, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Laupland KB, Conly JM: Treatment of Staphylococcus aureus colonization and prophylaxis for infection with topical intranasal mupirocin: An evidence-based review. Clin Infect Dis 37: 933–938, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Horan TC, Gaynes RP: Survellance of nosocomial infection. In: Hospital Epidemiology and Infection Control, 3rd ed., edited by Mayhall CG, Philadelphia, Lippinocott Williams & Wilkins, 2004, pp 1659–1702 [Google Scholar]
- 22. Reed SD, Friedman JY, Engemann JJ, Griffiths RI, Anstrom KJ, Kaye KS, Stryjewski ME, Szczech LA, Reller LB, Corey GR, Schulman KA, Fowler VG, Jr.: Costs and outcomes among hemodialysis-dependent patients with methicillin-resistant or methicillin-susceptible Staphylococcus aureus bacteremia. Infect Control Hosp Epidemiol 26: 175–183, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Hadley AC, Karchmer TB, Russell GB, McBride DG, Freedman BI: The prevalence of resistant bacterial colonization in chronic hemodialysis patients. Am J Nephrol 27: 352–359, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Lu PL, Chin LC, Peng CF, Chiang YH, Chen TP, Ma L, Siu LK: Risk factors and molecular analysis of community methicillin-resistant Staphylococcus aureus carriage. J Clin Microbiol 43: 132–139, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pecoits-Filho R, Lindholm B, Stenvinkel P: The malnutrition, inflammation, and atherosclerosis (MIA) syndrome: The heart of the matter. Nephrol Dial Transplant 17[Suppl 11] 28–31, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Rozgonyi F, Kocsis E, Kristof K, Nagy K: Is MRSA more virulent than MSSA? Clin Microbiol Infect 13: 843–845, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Wang CY, Wu VC, Chen YM, Su CT, Wu KD, Hsueh PR: Nasal carriage of methicillin-resistant Staphylococcus aureus among patients with end-stage renal disease. Infect Control Hosp Epidemiol 30: 93–94, 2009 [DOI] [PubMed] [Google Scholar]
- 28. Nouwen JL, Fieren MW, Snijders S, Verbrugh HA, van Belkum A: Persistent (not intermittent) nasal carriage of Staphylococcus aureus is the determinant of CPD-related infections. Kidney Int 67: 1084–1092, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Pena C, Fernandez-Sabe N, Dominguez MA, Pujol M, Martinez-Castelao A, Ayats J, Gudiol F, Ariza J: Staphylococcus aureus nasal carriage in patients on haemodialysis: Role of cutaneous colonization. J Hosp Infect 58: 20–27, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Sanford MD, Widmer AF, Bale MJ, Jones RN, Wenzel RP: Efficient detection and long-term persistence of the carriage of methicillin-resistant Staphylococcus aureus. Clin Infect Dis 19: 1123–1128, 1994 [DOI] [PubMed] [Google Scholar]
- 31. Daeschlein G, Assadian O, Daxboeck F, Kramer A: Multiplex PCR-ELISA for direct detection of MRSA in nasal swabs advantageous for rapid identification of non-MRSA carriers. Eur J Clin Microbiol Infect Dis 25: 328–330, 2006 [DOI] [PubMed] [Google Scholar]
- 32. Wolk DM, Picton E, Johnson D, Davis T, Pancholi P, Ginocchio CC, Finegold S, Welch DF, de Boer M, Fuller D, Solomon MC, Rogers B, Mehta MS, Peterson LR: Multicenter evaluation of the Cepheid Xpert methicillin-resistant Staphylococcus aureus (MRSA) test as a rapid screening method for detection of MRSA in nares. J Clin Microbiol 47: 758–764, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]