Summary
Background and objectives
South Asians (SAs) comprise 25% of all Canadian visible minorities. SAs constitute a group at high risk for cardiovascular disease in the general population, but the risk in SA kidney transplant recipients has never been studied.
Design, setting, participants, & measurements
In a cohort study of 864 kidney recipients transplanted from 1998 to 2007 and followed to June 2009, we identified risk factors including ethnicity associated with major cardiac events (MACEs, a composite of nonfatal myocardial infarction, coronary intervention, and cardiac death) within and beyond 3 months after transplant. Kaplan-Meier methodology and multivariate Cox regression analysis were used to determine risk factors for MACEs.
Results
There was no difference among SAs (n = 139), whites (n = 550), blacks (n = 65), or East Asians (n = 110) in baseline risk, including pre-existing cardiac disease. Post-transplant MACE rate in SAs was 4.4/100 patient-years compared with 1.31, 1.16, and 1.61/100 patient-years in whites, blacks, and East Asians, respectively (P < 0.0001 versus each). SA ethnicity independently predicted MACEs along with age, male gender, diabetes, systolic BP, and prior cardiac disease. SAs also experienced more MACEs within 3 months after transplant compared with whites (P < 0.0001), blacks (P = 0.04), and East Asians (P = 0.006). However, graft and patient survival was similar to other groups.
Conclusions
SA ethnicity is an independent risk factor for post-transplant cardiac events. Further study of this high-risk group is warranted.
Introduction
South Asians (SAs) are individuals whose ethnic roots originate from the Indian subcontinent, a region of the world that includes India, Pakistan, Sri Lanka, Nepal, and Bangladesh (1). In Canada, there were approximately 5 million visible minority individuals in 2008, 25% of who had documented SA ethnicity. SAs constitute >3% of the total Canadian population (2). At the end of 2005, there were 12,654 Canadian kidney transplant recipients (3), and it is estimated that 3% of Canadian patients with ESRD are of SA origin (4). Thus SAs constitute a significant population group within Canadian transplant practice.
Canadian SAs in the general population possess an increased risk for cardiovascular disease (5). North American SAs have higher coronary heart disease mortality rates than individuals of either European or Chinese descent (6), have more severe disease (1), and may also present with disease at an earlier age (7). In the United Kingdom, a country with a large SA population, SA kidney transplant recipients show graft function and survival and patient survival rates that are comparable to whites (8). Traditional cardiovascular risk factor prevalence is similar, with the exception of diabetes (8). Unfortunately, American transplant registry studies do not report SAs as a separate group (9), and SA recipient cardiovascular outcomes have not been compared with other major ethnic groups to date. There is thus a distinct need to understand post-transplant outcomes in this large population group in North America (9). Toronto, which is the largest city in Canada, together with its suburbia, has a population in excess of 4 million, of whom >50% are foreign-born (10). In Toronto, we thus have the ability to comparatively study cardiovascular disease and its risk factors in SA kidney transplant recipients and other ethnic groups.
Materials and Methods
The primary hypothesis of the study was that SA ethnic background would have a significant independent influence on major post-transplant cardiovascular outcomes.
St. Michael's Hospital is a major urban university-affiliated tertiary care medical–surgical center that currently performs about 120 adult single-organ kidney transplants annually and currently (as of December 2009) provides post-transplant care to approximately 1250 recipients. After initial discharge, all patients are seen in the transplant clinic weekly to the end of month 1, biweekly during month 2, monthly to month 6, and then every 3 months to month 12, every 6 months to month 24, every 9 months to month 60, and annually thereafter. All recipient-related information is maintained in secure electronic and paper format. Data on all cardiovascular outcomes have been collected prospectively since 2004 from the main hospital database, with supplementation by patient interview at each visit and family physician contact where necessary to capture events occurring at other hospitals. Professional interpreters are used wherever necessary. In this study, we included all kidney transplant recipients ever followed at our center and transplanted between January 1, 1998 and December 31, 2007. Demographic and cardiovascular event data on patients transplanted before July 1, 2004 were collected retrospectively to that date and prospectively thereafter. For this analysis, data were collected to June 30, 2009. Research ethics board approval was available for the study.
We defined cardiovascular events as one or more of the following: a hospital diagnosis of acute coronary syndrome (including unstable angina, abnormal exercise or pharmacologic stress test, coronary angiography showing significant stenosis in at least one major epicardial artery, percutaneous coronary intervention (angioplasty with or without stenting), coronary artery bypass surgery, death caused by a cardiac event, and stroke. We further defined a primary endpoint of “major cardiac event (MACE)” as a composite endpoint of nonfatal myocardial infarction, coronary intervention, and cardiac death based on prior trials in kidney transplant recipients (11). All events were verified independently by a chart review of physician outpatient plus inpatient notes and discharge summaries performed by at least two investigators. Patients were classified by ethnicity based on patient self-report and/or pretransplant assessment records as follows: white, any ancestry from Europe; black, any ancestry from Africa; East Asian, ancestry from China, Mongolia, Japan, North or South Korea, Taiwan, Myanmar, Thailand, Laos, Cambodia, Vietnam, Malaysia, Singapore, Indonesia, or Philippines; SA, ancestry from India, Pakistan, Bangladesh, Sri Lanka, Nepal, Maldives, or Bhutan. In case of mixed ancestry, patient self-report was used for assignment to a single category. Other demographic data that were collected included all relevant pre-, peri-, and post-transplant variables, including acute rejection, which was defined by Banff97 criteria or the use of intravenous methylprednisolone therapy any time after initial hospital discharge, and delayed graft function, defined as the need for dialysis in the first post-transplant week. Graft loss was defined as a permanent return to dialysis or repeat transplantation, and patient death was ascertained from hospital records. New-onset diabetes (NODAT) was defined on the basis of the 2008 Canadian Diabetes Association guidelines (12). The estimated GFR (eGFR) was calculated from the abbreviated Modification of Diet in Renal Disease equation (13). Previous cardiac disease was defined in a similar manner to post-transplant events as described above, with the important exception that all transplant candidates necessarily undergo pretransplant screening through cardiac stress testing, unlike post-transplant, where testing is performed by indication only.
Because cardiovascular events may be divided into those occurring early, where the major contributing risk factors post-transplant may be related to pre-existing disease burden compounded by the operative procedure itself, and those occurring later, where more traditional risk factors are predominant, a proportionality of hazards was assumed starting from 3 months after transplant. The primary analysis therefore included all events occurring beyond 3 months after transplant, with any events before 3 months counted as previous cardiac disease. NODAT within 3 months was combined with pre-existing diabetes to create a single diabetes variable. The value or status for all other traditional Framingham variables (14) at 3 months after transplant was used in the analysis. A secondary analysis of MACEs occurring before 3 months was also performed, along with death-censored graft survival and patient survival with a functioning graft starting at 3 months.
Kaplan-Meier survival curves for MACEs by ethnic group were constructed with the starting point at 3 months after transplant, and these were compared by the log-rank test. Patients were censored at the time of their first MACE event or June 30, 2009, whichever was earlier. In case of loss to follow-up, patients were censored on the date of their last clinic visit. Univariate comparisons between SAs and other ethnic groups was performed using ANOVA or χ2 testing as appropriate. A Cox regression model was developed to estimate hazard ratios by using the traditional Framingham cardiovascular risk factors (age, gender, systolic BP, total cholesterol, HDL cholesterol, diabetes status, and current smoking status). To this a priori model, the following were added in succession: ethnicity, previous cardiac disease, LDL cholesterol, and any differences between SAs and other groups identified by univariate analysis. A backward elimination method was also used in which variables with P > 0.20 were successively removed until a final stable model was attained. All data are reported as mean ± SD unless otherwise stated. P < 0.05 was taken as implying statistical significance. SAS version 9.2 (SAS Institute, Cary, NC) was the statistical software package used for the analysis.
Results
There were 872 kidney transplant recipients in total followed at our center who were transplanted during the defined study period (1998 to 2007), of whom 550 (63%) were white, 65 (7%) were black, 110 (13%) were East Asian, and 139 (16%) were SA. Their demographic characteristics are provided in Table 1. Eight patients could not be classified under any of the four major ethnic groups and were not studied further. Among patients transplanted before July 1, 2004, for which time data were collected retrospectively, 315 (69%) were white, 31 (6%) were black, 47 (10%) were East Asian, and 57 (12%) were SA (P = 0.01 versus on or after July 1, 2004). Several differences were noted between SA recipients and those from other groups. Compared with whites, SA had shorter follow-up after transplant and had more ESRD of unestablished etiology but less ESRD from polycystic kidney disease. Compared with blacks, SAs had a higher eGFR at 3 months after transplant and were less likely to be smokers. Compared with East Asians, SAs had a higher body mass index (BMI), lower BP, more living donors, and less ESRD from documented glomerulonephritis (Table 1). Compared with whites, SAs did not have an additional burden from traditional risk factors such as age, BP, hyperlipidemia, or pre-existing diabetes. Cardiac risk factors along with cardioprotective and immunosuppressive medication use at 3 months are provided in Table 2. SAs were more likely to be on cyclosporine than tacrolimus, but there was no difference in the use of cardioprotective medications. Likewise, there was no difference in BMI, NODAT, or most importantly, prior cardiovascular disease burden. Twenty-eight patients (3.2%) were lost to follow-up over the entire study period, with no differences noted by ethnicity.
Table 1.
Baseline demographic characteristics of study cohort (n = 864)
| Variable | SA (n = 139) | White (n = 550) | Black (n = 65) | East Asian (n = 110) | P |
|---|---|---|---|---|---|
| Years since transplant | 4.09 ± 3.1a | 5.54 ± 3.1 | 5.29 ± 3.1 | 5.07 ± 2.9 | <0.0001 |
| Age (years) | 48.4 ± 12 | 47.1 ± 13 | 44.9 ± 13 | 49.4 ± 11 | 0.063 |
| Gender (male/female) | 106/33b | 346/204 | 34/31 | 67/43 | 0.0006 |
| Donor source (N, % live) | 69 (49%)c | 341 (62%) | 22 (33%) | 32 (29%) | 0.001 |
| Cause of ESRD [N (%)] | |||||
| diabetes | 23 (16%) | 71 (13%) | 8 (12%) | 15 (14%) | 0.264 |
| hypertension | 20 (14%)b | 39 (7%) | 26 (41%) | 14 (13%) | <0.0001 |
| glomerulonephritis | 55 (40%)c | 207 (37%) | 24 (37%) | 63 (57%) | 0.005 |
| PCKD | 5 (3.6%)a | 108 (20%) | 1 (1.5%) | 1 (0.9%) | <0.0001 |
| interstitial nephritis | 2 (1.5%)a | 46 (8%) | 1 (1.5%) | 1 (0.9%) | 0.002 |
| urogenital anomalies | 4 (2.9%)b | 27 (5%) | 0 (0%) | 1 (0.9%) | 0.212 |
| others/unknown | 30 (22%)a | 52 (10%) | 5 (7%) | 14 (13%) | <0.0001 |
| Current smoking [N (%)] | 11 (7%)b | 91 (16%) | 15 (23%) | 12 (10%) | 0.002 |
| No. of transplants (1/2/3) | 134/5/0 | 518/31/1 | 61/3/1 | 102/8/0 | 0.195 |
| Prior cardiac disease [N (%)] | 27 (19%) | 107 (19%) | 15 (23%) | 20 (18%) | 0.547 |
P value column refers to overall group difference. Specific between-group comparisons are provided below. PCKD, polycystic kidney disease.
P < 0.0001 for SA versus white.
P < 0.05 for SA versus black.
P < 0.01 for SA versus East Asian.
Table 2.
Risk factors and use of cardioprotective and immunosuppressive medications at 3 months after transplant (n = 825)a
| Variable Name | SA (n = 127) | White (n = 529) | Black (n = 62) | East Asian (n = 107) | P |
|---|---|---|---|---|---|
| BMI (kg/m2) | 26.6 ± 5d | 26.5 ± 5 | 26.4 ± 5 | 24.3 ± 4 | 0.0003 |
| Systolic BP (mmHg) | 127 ± 13d | 128 ± 15 | 128 ± 18 | 132 ± 13 | 0.040 |
| Diastolic BP (mmHg) | 77 ± 9 | 77.8 ± 9 | 77.7 ± 12 | 81 ± 9d | 0.040 |
| Total cholesterol (mmol/L) | 4.69 ± 1.2 | 4.71 ± 1.1 | 4.56 ± 1.2 | 4.61 ± 1.0 | 0.645 |
| HDL | 1.31 ± 0.5 | 1.30 ± 0.4 | 1.18 ± 0.4 | 1.25 ± 0.3 | 0.250 |
| LDL | 2.56 ± 0.8b | 2.63 ± 0.8 | 2.57 ± 0.9 | 2.61 ± 0.9 | 0.465 |
| Triglycerides | 1.89 ± 0.9 | 1.78 ± 0.9 | 1.77 ± 0.8 | 1.66 ± 0.6 | 0.112 |
| Renal function | |||||
| serum creatinine (μmol/L) | 137.5 ± 78c | 151.8 ± 116 | 174.8 ± 124 | 135.8 ± 73 | 0.058 |
| eGFR (ml/min per 1.73 m2) | 61.5 ± 28c | 55.6 ± 27 | 46.3 ± 23 | 53.9 ± 23 | 0.013 |
| acute rejection [N (%)] | 13 (9%) | 66 (12%) | 10 (15%) | 15 (13%) | 0.204 |
| delayed graft function [N (%)] | 15 (10%) | 47 (8%) | 13 (20%) | 14 (12%) | 0.074 |
| NODAT [N (%)] | 10 (7%) | 31 (5%) | 5 (7%) | 11 (10%) | 0.428 |
| Medications | |||||
| statin [N (%)] | 51 (40%) | 192 (36%) | 14 (22%) | 33 (31%) | 0.052 |
| ACEI [N (%)] | 26 (20%)b | 153 (29%) | 20 (32%) | 18 (17%) | 0.021 |
| ARB [N (%)] | 20 (16%) | 72 (13%) | 13 (20%) | 14 (13%) | 0.226 |
| NDCCB [N (%)] | 47 (37%) | 157 (29%) | 23 (37%) | 28 (26%) | 0.195 |
| DCCB [N (%)] | 34 (26%) | 120 (22%) | 13 (21%) | 26 (25%) | 0.618 |
| β blocker [N (%)] | 53 (41%) | 188 (35%) | 25 (40%) | 32 (29%) | 0.177 |
| α blocker [N (%)] | 4 (3%) | 32 (6%) | 4 (6%) | 2 (2%) | 0.152 |
| loop diuretic [N (%)] | 6 (4%)b | 56 (10%) | 9 (14%) | 4 (4%) | 0.01 |
| thiazide diuretic [N (%)] | 4 (3%) | 36 (7%) | 2 (3%) | 0 (0%) | 0.077 |
| other anti-hypertensives [N (%)] | 5 (3%) | 19 (3%) | 1 (1%) | 2 (2%) | 0.419 |
| insulin [N (%)] | 26 (20%)b | 51 (10%) | 8 (13%) | 14 (13%) | 0.002 |
| ASA [N (%)] | 18 (14%)d | 91 (17%) | 6 (10%) | 4 (4%) | 0.012 |
| allopurinol [N (%)] | 4 (3%) | 26 (5%) | 2 (3%) | 3 (3%) | 0.322 |
| colchicine [N (%)] | 0 (0%) | 7 (1%) | 0 (0%) | 0 (0%) | 0.199 |
| CsA [N (%)] | 54 (42%)b | 126 (23%) | 31 (50%) | 28 (26%) | 0.002 |
| tacrolimus [N (%)] | 71 (56%)a | 373 (71%) | 28 (45%) | 70 (65%) | <0.0001 |
| MMF [N (%)] | 93 (73%)c | 379 (72%) | 48 (77%) | 76 (71%) | 0.084 |
| MPA [N (%)] | 6 (4%) | 23 (4%) | 2 (3%) | 3 (3%) | 0.532 |
| azathioprine [N (%)] | 4 (3%)b | 8 (1%) | 1 (1%) | 1 (1%) | 0.264 |
| sirolimus [N (%)] | 5 (3%)b | 44 (8%) | 2 (3%) | 8 (7%) | 0.064 |
| prednisone [N (%)] | 104 (81%) | 389 (74%) | 45 (72%) | 86 (80%) | 0.26 |
ACEI, angiotensin converting-enzyme inhibitor; ARB, angiotensin II receptor blocker; ASA, acetylsalicylic acid; CsA, cyclosporine A; DCCB, dihydropyridine calcium channel blocker; MMF, mycophenolate mofetil; MPA, mycophenolic acid; NDCCB, non-dihydropyridine calcium channel blocker.
Excludes patients censored before 3 months as the result of an event or loss to follow-up.
P < 0.01 for SA versus white.
P < 0.05 for SA versus black.
P < 0.01 for SA versus East Asian.
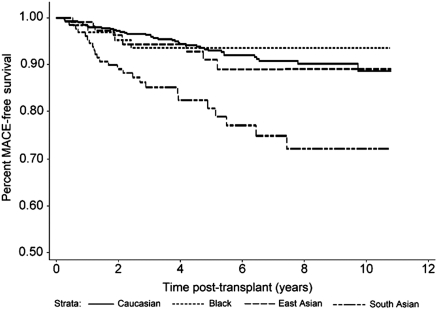
Kaplan-Meier survival curves for MACEs classified by ethnic group starting from 3 months after transplant are provided in Figure 1. SA ethnicity was associated with a markedly increased MACE rate versus each of the other groups (P < 0.0001 for each). This difference appeared early and continued for up to 10 years after transplant. Univariate associations for risk factors associated with MACEs are provided in Table 3. The following variables besides ethnicity were entered in to the initial multivariate model: Framingham variables including age, gender, smoking, diabetes, systolic BP, fasting HDL cholesterol and total cholesterol, plus nontraditional and transplant-specific variables including BMI, fasting triglycerides and LDL cholesterol, pre-existing cardiac disease, eGFR, and number of transplants. Results of the final forward multivariate model are provided in Table 4. Although both pre-existing diabetes and NODAT were significantly associated with MACEs in the univariate analysis, only combined diabetes was associated with MACEs in the multivariate analysis. Results from the backward elimination model were very similar. Post hoc addition of calcineurin inhibitor type did not influence the main result. By both methods, SA ethnicity was an independent, powerful predictor of post-transplant MACEs in addition to traditional Framingham risk factors, with the exception of smoking and cholesterol.
Figure 1.
Major cardiac event-free survival beyond 3 months after renal transplantation (P < 0.0001 for South Asian versus each of the other groups). Number of person-years at risk: South Asian, 568; white, 3047; black, 344; East Asian, 557. (Also see Appendix.)
Table 3.
Summary of univariate hazard ratios for risk factors associated with major cardiac events beyond 3 months after transplant, arranged in decreasing order of statistical significance
| Variable | P | Hazard Ratio |
|---|---|---|
| Age (per 10 years) | <0.0001 | 1.903 |
| SA ethnicity (versus white) | <0.0001 | 3.209 |
| All diabetes | <0.0001 | 2.913 |
| Pre-existing diabetes | <0.0001 | 2.770 |
| Systolic BP (per 10 mmHg) | <0.0001 | 1.320 |
| Previous cardiac disease | <0.0001 | 3.904 |
| Gender (male) | 0.0002 | 2.988 |
| New-onset diabetes | 0.014 | 1.961 |
| Smoking | 0.012 | 1.936 |
| Triglycerides (per 1 mmol/L) | 0.046 | 1.141 |
| Total cholesterol (per 1 mmol/L) | 0.049 | 1.249 |
| Number of transplants | 0.126 | 0.218 |
| LDL cholesterol (per 0.5 mmol/L) | 0.231 | 1.091 |
| eGFR (per 10 ml/min per 1.73 m2) | 0.479 | 0.967 |
| HDL cholesterol (per 0.1 mmol/L) | 0.637 | 1.014 |
| Delayed graft function | 0.735 | 1.127 |
| Acute rejection | 0.832 | 1.074 |
| BMI (per kg/m2) | 0.910 | 0.997 |
Table 4.
Final multivariate Cox regression model for major cardiac events beyond 3 months after transplant
| Variable | Parameter Estimate | SE | χ2 | P | Hazard Ratio | 95% Hazard Ratio Confidence Limits |
|
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Age (per 10 years) | 0.492 | 0.103 | 22.47 | <0.0001 | 1.63 | 1.33 | 2.00 |
| Gender (male) | 0.789 | 0.308 | 6.54 | 0.010 | 2.20 | 1.20 | 4.03 |
| SA ethnicity (versus white) | 1.239 | 0.251 | 24.26 | <0.0001 | 3.45 | 2.10 | 5.65 |
| Diabetes | 0.482 | 0.244 | 3.91 | 0.048 | 1.62 | 1.00 | 2.61 |
| Systolic BP (per 10 mmHg) | 0.216 | 0.063 | 11.82 | 0.0006 | 1.24 | 1.09 | 1.40 |
| Previous cardiac disease | 0.788 | 0.245 | 10.34 | 0.0013 | 2.20 | 1.36 | 3.55 |
Appendix.
| Year |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| Figure 1 | ||||||||||
| white | 522 | 489 | 444 | 388 | 347 | 292 | 236 | 185 | 115 | 56 |
| black | 60 | 60 | 58 | 46 | 424 | 35 | 30 | 26 | 16 | 4 |
| East Asian | 105 | 102 | 92 | 87 | 75 | 70 | 61 | 42 | 29 | 14 |
| SA | 127 | 121 | 118 | 111 | 100 | 92 | 87 | 71 | 51 | 18 |
| Figure 3 | ||||||||||
| white | 522 | 477 | 435 | 384 | 348 | 298 | 244 | 191 | 120 | 57 |
| black | 60 | 57 | 56 | 47 | 42 | 36 | 33 | 28 | 18 | 4 |
| East Asian | 102 | 97 | 91 | 87 | 76 | 72 | 63 | 43 | 31 | 14 |
| SA | 125 | 124 | 113 | 109 | 97 | 89 | 83 | 68 | 47 | 17 |
| Figure 4 | ||||||||||
| white | 522 | 489 | 444 | 388 | 347 | 292 | 236 | 185 | 115 | 56 |
| black | 60 | 60 | 58 | 46 | 42 | 35 | 30 | 26 | 16 | 4 |
| East Asian | 105 | 92 | 87 | 75 | 75 | 70 | 61 | 42 | 29 | 14 |
| SA | 125 | 124 | 118 | 111 | 100 | 92 | 87 | 71 | 51 | 18 |
MACE rate was 4.4/100 patient-years of follow-up for SAs compared with 1.31, 1.16, and 1.61/100 patient-years in whites, blacks, and East Asians, respectively. There were a total of 56 MACE events beyond 3 months in whites (10.2%), 7 in blacks (10.7%), 10 in East Asians (9.0%), and 35 in SAs (25.1%). The myocardial infarction (both ST segment [40%] and non-ST segment elevation [60%]) event rates were 48 (8.7%), 5 (7.6%), 8 (7.2%), and 30 (21.5%), and cardiac death rates were 14 (2.5%), 3 (4.6%), 1 (1%), and 5 (3.6%) in the four groups, respectively, with no difference by ethnicity noted. Because information was obtained from hospital records, there was no appreciable difference in access to hospital care. Stroke rates were low, with four, one, two, and two events in the four groups, respectively. Unstable angina, which was not counted toward MACEs, was more prevalent, with 53, 3, 11, and 35 events in each group, respectively. In a post hoc Kaplan-Meier calculation to determine if the difference in MACE rates was caused by heightened ascertainment in more recent years, when data were collected prospectively, events in patients transplanted 2004 or after were compared separately. Within that subgroup, SAs had a higher MACE rate (20 events in 82 patients) compared with whites (25 events in 235 patients; P = 0.0021 for difference). There was also no MACE rate difference between SA patients transplanted before or after 2004 (P = 0.74).
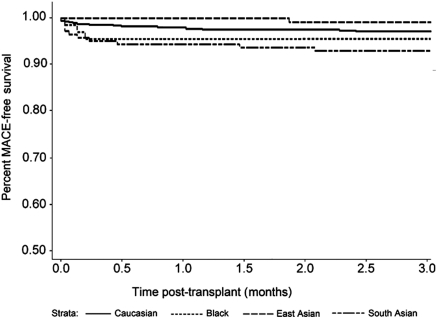
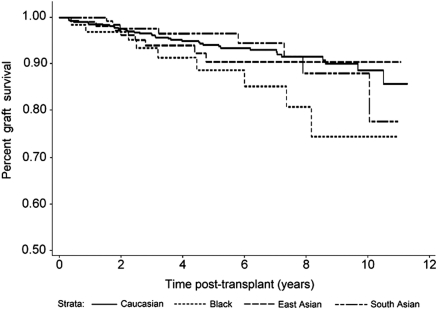
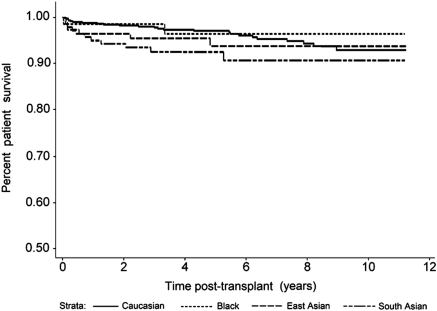
SA kidney transplant recipients also experienced more MACEs in the first 3 months after transplant compared with whites (P < 0.0001), blacks (P = 0.04), and East Asians (P = 0.006; Figure 2). By contrast, long-term graft survival in SAs was no different from whites (P = 0.98) or East Asians (P = 0.71), although a trend was noted toward improved graft survival compared with blacks (P = 0.10; Figure 3). Death-censored graft survival was influenced by both acute rejection and delayed graft function (P < 0.0001 for each). Overall patient survival was similar for SAs compared with the other ethnic groups (Figure 4; P = not significant for each).
Figure 2.
Major cardiac event-free survival in the first 3 months after renal transplantation (P < 0.0001 for South Asian versus white, P = 0.04 for South Asian versus black, and P = 0.006 for South Asian versus East Asian).
Figure 3.
Death-censored graft survival beyond 3 months after renal transplantation (P = NS for South Asian versus all other groups). (Also see Appendix.)
Figure 4.
All-cause patient mortality beyond 3 months after renal transplantation, with graft loss counted as a censoring event (P = not significant for South Asian versus all other groups). (Also see Appendix.)
Discussion
This bidirectional cohort study showed that SA kidney transplant recipients carry an increased post-transplant risk for major cardiac events, despite baseline cardiovascular morbidity similar to other ethnic groups. In particular, documented pretransplant cardiovascular disease prevalence was similar, as shown by uniform pretransplant screening practice; moreover, traditional, Framingham risk factor prevalence rates were similar, with only minor, possibly random, differences noted. In addition to SA ethnicity, independent risk factors for MACEs included many of these traditional factors including age, gender, diabetes, and BP, besides previous cardiac disease.
The striking cardiovascular risk imposed on transplant recipients by virtue of ethnic background cannot be explained by traditional risk factors alone, although they are indeed contributory. This finding is in conformity to the general population (15). A previous study (8) showed that post-transplant BP and lipid levels in SAs are comparable to whites, although diabetes rates are higher. Our center tailors immunosuppressive regimen to NODAT risk (16), which may have served to equalize this risk factor as well. Many SA patients with pre-existing diabetes received tacrolimus after transplant, whereas those with glucose intolerance received cyclosporine to prevent NODAT. It is possible that there may be ethnic differences in glucose tolerance, which was not tested after transplant. Also, total event rate may have permitted combined diabetes, but not prediabetes and NODAT, to be a determinant of MACEs. In the general population, the INTERHEART study (17) has shown that traditional risk factors do play an important role in myocardial infarction prediction in SAs. Despite this observation, some authors contend that the risk factor profile is actually lower in SAs (18,19). Thus, attention to traditional risk factors alone will not be sufficient for this high-risk group. In the Canadian health care system, where both pretransplant and post-transplant care is universally provided, the demonstration of cardiovascular risk differences by ethnicity assumes a particular significance. Immunosuppressive medication coverage is universal, and cardioprotective medication use is similar. In this study, SAs had shorter post-transplant follow-up time, in keeping with the changing demographic of the Canadian population. The greater use of cardioprotective medication in the transplant population over the course of the 2000s in response to, for example, the Assessment of LEscol in Renal Transplantation (ALERT) study (11), and from which SAs would therefore be expected to have benefited and reduced their risk, further exemplifies the need for urgent study of high cardiovascular risk subgroups within the transplant population.
Reasons for the increased risk faced by SA transplant recipients must be necessarily speculative at this time. For example, the higher rate in SAs compared with East Asians may be because of their higher BMI (Table 2). Post-transplant insulin resistance may be under-recognized in the absence of glucose tolerance testing, similar to other populations (5), but not adequately reflected by BMI assessments (20). In nontransplant populations, C-reactive protein levels (21) and leptin levels (22) are higher, whereas adiponectin levels are lower (23), and microalbuminuria is more prevalent (24) in SAs. Other proposed “novel” risk factor differences worthy of further study include lipoprotein (a), homocysteine, and plasminogen activator inhibitor-1 (1,25). Any extrapolation of these findings to the SA transplant population must be discouraged and investigation encouraged, keeping in mind that the underlying risk factor profiles and their relationship with outcomes may be atypical (26). Once obtained, this information may be useful both in the evaluation and counseling of transplant candidates and the determination of the need for more intense post-transplant monitoring, as well as cardiovascular risk factor modification. We did not have information about the prevalence of left ventricular hypertrophy after transplantation. However, until more is known about the pathophysiologic basis for this increased risk in SA, identifying high-risk patients in SAs and other groups by conventional methods seems prudent.
In summary, SA ethnicity may be a distinct, independent risk factor for major adverse cardiovascular events after renal transplantation.
Disclosures
None.
Acknowledgments
This study was funded in part by Heart and Stroke Foundation of Ontario Grant PEA-6532. This study was reported as an oral presentation at the American Transplant Congress 2010 in San Diego, CA.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Gupta M, Singh N, Verma S: South Asians and cardiovascular risk. What clinicians should know. Circulation 113: e924–e929, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Gupta M, Brister S: Is South Asian ethnicity an independent cardiovascular risk factor? Can J Cardiol 22: 193–197, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Canadian Institute for Health Information: Treatment of End-Stage Organ Failure in Canada, 1998 to 2007-Canadian Organ Replacement Register 2009 Annual Report, Ottawa, Canadian Institute for Health Information, 2009 [Google Scholar]
- 4. Tonelli M, Hemmelgarn B, Gill JS, Chou S, Culleton B, Klarenbach S, Manns B, Wiebe N, Gourishankar S; Alberta Kidney Disease Network: Patient and allograft survival of Indo Asian and East Asian dialysis patients treated in Canada. Kidney Int 72: 499–504, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Anand SS, Yusuf S, Vuksan V, Devanesen S, Teo KK, Montague PA, Kelemen L, Yi C, Lonn E, Gerstein H, Hegele RA, McQueen M: Differences in risk factors, atherosclerosis, and cardiovascular disease between ethnic groups in Canada: The Study of Health Assessment and risk in Ethnic groups (SHARE). Lancet 356: 279–284, 2000 [DOI] [PubMed] [Google Scholar]
- 6. Sheth T, Nair C, Nargundkar M, Anand S, Yusuf S: Cardiovascular and cancer mortality among Canadians of European, South Asian and Chinese origin from 1979 to 1993: an analysis of 12 million deaths. CMAJ 161: 132–138, 1999 [PMC free article] [PubMed] [Google Scholar]
- 7. Enas EA, Garg A, Davidson MA, Nair VM, Huet BA, Yusuf S: Coronary heart disease and its risk factors in first-generation immigrant Asian Indians to the United States of America. Indian Heart J 48: 343–353, 1996 [PubMed] [Google Scholar]
- 8. Dooldeniya MD, Dupont PJ, He X, Johnson RJ, Joshi T, Basra R, Johnston A, Warrens AN: Renal transplantation in Indo-Asian patients in the U.K. Am J Transplant 6: 761–769, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Prasad GV: Renal transplantation for ethnic minorities in Canada: Inequity in access and outcomes? Kidney Int 72: 390–392, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Prasad GV, Zaltzman JS, Huang M: Renal transplantation among South Asians: A North American perspective. Transplantation 79: 855, 2005 [DOI] [PubMed] [Google Scholar]
- 11. Holdaas H, Fellstrom B, Jardine AG, Holme I, Nyberg G, Fauchald P, Gronhagen-Riska C, Madsen S, Neumayer HH, Cole E, Maes B, Ambuhl P, Olsson AG, Hartmann A, Solbu DO, Pedersen TR; Assessment of LEscol in Renal Transplantation (ALERT) Study Investigators: Effect of fluvastatin on cardiac outcomes in renal transplant recipients: A multicentre, randomized, placebo-controlled trial. Lancet 361: 2024–2031, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Canadian Diabetes Association Clinical Practice Guidelines Expert Committee: Canadian Diabetes Association 2008 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes 32[Suppl 1]: S1–S201, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F; Chronic Kidney Disease Epidemiology Collaboration: Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145: 247–254, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB: Prediction of coronary heart disease using risk factor categories. Circulation 97: 1837–1847, 1998 [DOI] [PubMed] [Google Scholar]
- 15. Forouhi NG, Sattar N, Tillin T, McKeigue PM, Chaturvedi N: Do known risk factors explain the higher coronary heart disease mortality in South Asian compared with European men? Prospective follow-up of the Southall and Brent studies, UK. Diabetologia 49: 2580–2588, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Prasad GV, Huang M, Bandukwala F, Nash MM, Rapi L, Montada-Atin T, Meliton G, Zaltzman JS: Pretransplantation glucose testing for predicting new-onset diabetes mellitus after renal transplantation. Clin Nephrol 71: 140–146, 2009 [PubMed] [Google Scholar]
- 17. Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L; The INTERHEART Study Investigators: Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART Study): Case-control study. Lancet 364: 937–952, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Patel JV, Vyas A, Cruickshank JK, Prabhakaran D, Hughes E, Reddy KS, Mackness MI, Bhatnagar D, Durrington PN: Impact of migration on coronary heart disease risk factors: Comparison of Gujaratis in Britain and their contemporaries in villages of origin in India. Atherosclerosis 185: 297–306, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Pais P, Pogue J, Gerstein H, Zachariah E, Savitha D, Jayprakash S, Nayak PR, Yusuf S: Risk factors for acute myocardial infarction in Indians: A case-control study. Lancet 348: 358–363, 1996 [DOI] [PubMed] [Google Scholar]
- 20. McKeigue PM, Shah B, Marmot MG: Relation of central obesity and insulin resistance with high diabetes prevalence and cardiovascular risk in South Asians. Lancet 337: 382–386, 1991 [DOI] [PubMed] [Google Scholar]
- 21. Anand SS, Razak F, Yi Q, Davis B, Jacobs R, Vuksan V, Lonn E, Teo K, McQueen M, Yusuf S: C-reactive protein as a screening test for cardiovascular risk in a multiethnic population. Arterioscler Thromb Vasc Biol 24: 1509–1515, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Abate N, Chandalia M, Snell PG, Grundy SM: Adipose tissue metabolites and insulin resistance in nondiabetic Asian Indian men. J Clin Endocrinol Metab 89: 2750–2755, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Raji A, Gerhard-Herman MD, Warren M, Silverman SG, Raptopoulos V, Mantzoros CS, Simonson DC: Insulin resistance and vascular dysfunction in nondiabetic Asian Indians. J Clin Endocrinol Metab 89: 3965–3972, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Fischbacher CM, Bhopal R, Rutter MK, Unwin NC, Marshall SM, White M, Alberti KG: Microalbuminuria is more frequent in South Asian than in European origin populations: A comparative study in Newcastle, UK Diabet Med 20: 31–36, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Hoogeveen RC, Gambhir JK, Gambhir DS, Kimball KT, Ghazzaly K, Gaubatz JW, Vaduganathan M, Rao RS, Koschinsky M, Morrisett JD: Evaluation of Lp (a) and other independent risk factors for CHD in Asian Indians and their USA counterparts. J Lipid Res 42: 631–638, 2001 [PubMed] [Google Scholar]
- 26. Jardine AG, Fellstrom B, Logan JO, Cole EH, Nyberg G, Gronhagen-Riska C, Madsen S, Neumayer HH, Maes B, Ambuhl P, Olsson AG, Pedersen T, Holdaas H: Cardiovascular risk and renal transplantation: Post hoc analyses of the Assessment of Lescol in Renal Transplantation (ALERT) Study. Am J Kidney Dis 46: 529–536, 2005 [DOI] [PubMed] [Google Scholar]